Submitted:
26 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
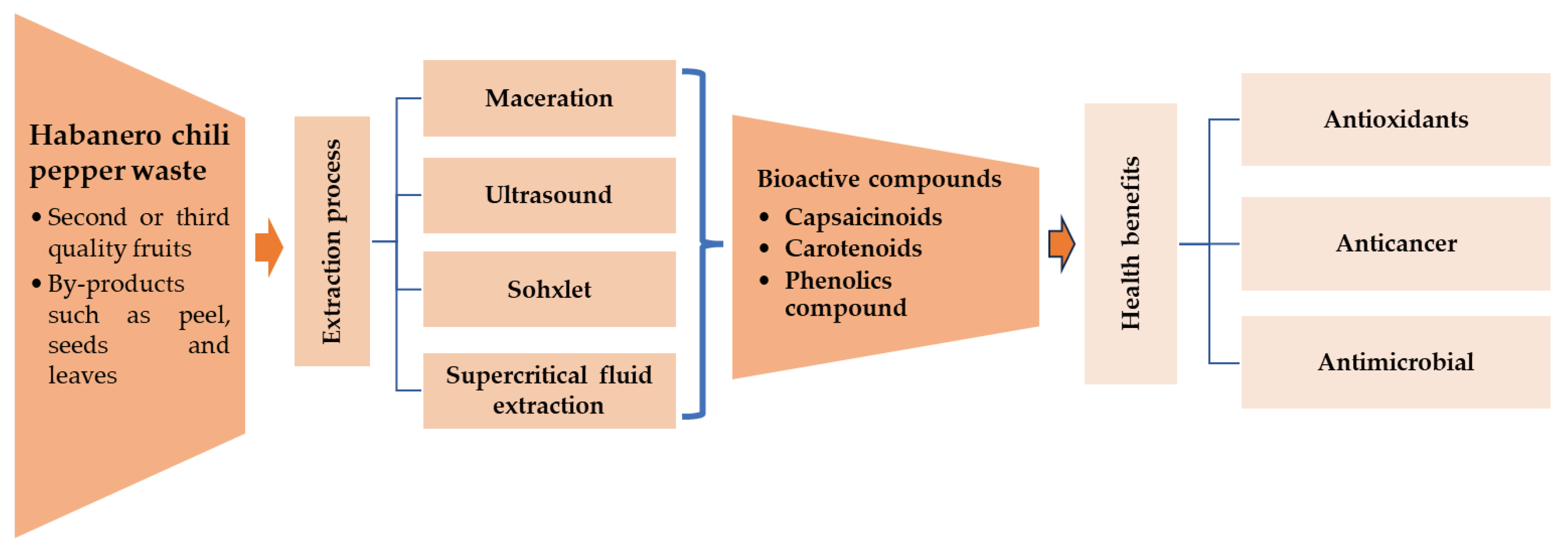
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Extractions Experiments
2.3.1. Maceration Extraction (ME)
2.3.2. Soxhlet Extraction (SE)
2.3.3. Maceration Extraction Assisted by Ultrasound (US)
2.3.4. Supercritical Fluid Extraction (SFE and SFEC)
2.4. Extraction Efficiency
2.5. Analysis
2.5.1. Determination of Extractable Solids
2.5.2. Determination of Total Capsaicinoids
2.5.3. Determination of Total Carotenoids
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physical Characteristics of Red Habanero Chili Pepper Extract
3.2. Extraction Yield from Different Extraction Processes
3.3. Bioactive Compound of Red Habanero Chili Pepper Extract from Different Extraction Processes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro-Concha, L.A.; Tuyub-Che, J.; Moo-Mukul, A.; Vazquez-Flota, F.A.; Miranda-Ham, M.L.; Bekatorou, A.; Tariq, A.; Tripathi, N.K. Antioxidant Capacity and Total Phenolic Content in Fruit Tissues from Accessions of Capsicum Chinense Jacq. (Habanero Pepper) at Different Stages of Ripening. 2014. [Google Scholar] [CrossRef]
- Joshi, D.D.; Changkija, S.; Sujata, W.; Somkuwar, B.G.; Rana, V.S.; Talukdar, N.C. Nutraceutical from Capsicum Chinense Fruits in Shelf-Stable Herbal Matrix. Innovative Food Science and Emerging Technologies 2017, 42, 130–137. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P.J.; Menichini, F. The Influence of Fruit Ripening on the Phytochemical Content and Biological Activity of Capsicum Chinense Jacq. Cv Habanero. Food Chem 2009, 114, 553–560. [Google Scholar] [CrossRef]
- 4. Diario Oficial de la Federación NORMA Oficial Mexicana NOM-189-SCFI-2012, Chile Habanero de La Península de Yucatán (Capsicum Chinense Jacq.)-Especificaciones y Métodos de Prueba. 2012. Disponible en URL: http://www. dof. gob. mx/normasOficiales/4730/seeco2/seeco2.htm.
- Borges-Gómez, L.; Cervantes Cárdenas, L.; Ruiz Novelo, J.; Soria Fregoso, M.; Reyes Oregel, V.; Villanueva Couoh, E. Capsaicinoides En Chile Habanero (Capsicum Chinense Jacq.) Bajo Diferentes Condiciones de Humedad y Nutrición. Terra latinoamericana 2010, 28, 35–41. [Google Scholar]
- Maldonado Astudillo, Y.I.; Jimenez Hernandez, J.; Salazar Lopez, R. Fisiología y Tecnología Postcosecha Del Chile Habanero (Capsicum Chinense Jacq. ). 2020. [Google Scholar]
- Zapata-Aguilar, J.A.; Pérez-Akaki, P.; Moo-Novelo, C.A. Análisis de La Cadena de Comercialización Del Chile Habanero de Yucatán y Su Denominación de Origen. Revista CEA 2020, 6, 109–125. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; de la Mora, Z.V.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum Annum l.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J Agric Food Chem 2022, 70, 6787–6804. [Google Scholar] [CrossRef]
- Cortés-Ferré, H.E.; Guajardo-Flores, D.; Romero-De La Vega, G.; Gutierrez-Uribe, J.A. Recovery of Capsaicinoids and Other Phytochemicals Involved with TRPV-1 Receptor to Re-Valorize Chili Pepper Waste and Produce Nutraceuticals. Front Sustain Food Syst 2021, 4, 588534. [Google Scholar] [CrossRef]
- Oğuzkan, S.B. Extraction of Capsinoid and Its Analogs From Pepper Waste of Different Genotypes. Nat Prod Commun 2019, 14, 1934578X19865673. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour Bioprocess 2017, 4, 18. [Google Scholar] [CrossRef]
- Campos, M.R.S.; Gómez, K.R.; Ordoñez, Y.M.; Ancona, D.B. Polyphenols, Ascorbic Acid and Carotenoids Contents and Antioxidant Properties of Habanero Pepper (<I>Capsicum Chinense</I>) Fruit. Food Nutr Sci 2013, 04. [Google Scholar] [CrossRef]
- De Lourdes Reyes-Escogido, M.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and Pharmacological Aspects of Capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Mosquera, M.I.; Hornero-Méndez, D. Separation and Quantification of the Carotenoid Pigments in Red Peppers (Capsicum Annuum L.), Paprika, and Oleoresin by Reversed-Phase HPLC. J Agric Food Chem 1993, 41. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary Metabolites of Capsicum Species and Their Importance in the Human Diet. J Nat Prod 2013, 76, 783–793. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum Varieties by Evaluation of Their Carotenoid Profile and Pungency Determination. Food Chemistry 2013, 140. [Google Scholar] [CrossRef]
- Aizawa, K.; Inakuma, T. Dietary Capsanthin, the Main Carotenoid in Paprika (Capsicum Annuum), Alters Plasma High-Density Lipoprotein-Cholesterol Levels and Hepatic Gene Expression in Rats. British Journal of Nutrition 2009, 102. [Google Scholar] [CrossRef]
- Olguín-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; Ferreiro-González, M.; del Carmen Rodríguez-Jimenes, G.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum Chinense Jacq. Agronomy 2019, 9. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Jiménez-Fernández, M.; Azuara, E. Oleoresins from Capsicum Spp.: Extraction Methods and Bioactivity. Food Bioproc Tech 2017, 10. [Google Scholar] [CrossRef]
- Fernández-Trujillo, J.P. Extracción Convencional de Oleorresina de Pimentón Dulce y Picante II. Peligros y Puntos de Control Críico y Requerimientos Comerciales. Grasas y Aceites 2007, 58. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J Food Eng 2013, 117. [Google Scholar] [CrossRef]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Sathiya Seelan, J.S. Value-Added Metabolites from Agricultural Waste and Application of Green Extraction Techniques. Sustainability 2021, 13. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-Assisted Extraction of Capsaicinoids from Peppers. Talanta 2008, 75. [Google Scholar] [CrossRef]
- Chinn, M.S.; Sharma-Shivappa, R.R.; Cotter, J.L. Solvent Extraction and Quantification of Capsaicinoids from Capsicum Chinense. Food and Bioproducts Processing 2011, 89. [Google Scholar] [CrossRef]
- Dong, X.; Li, X.; Ding, L.; Cui, F.; Tang, Z.; Liu, Z. Stage Extraction of Capsaicinoids and Red Pigments from Fresh Red Pepper (Capsicum) Fruits with Ethanol as Solvent. LWT - Food Science and Technology 2014, 59. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Gontarek-Castro, E.; Jafari, S.M. Up-to-Date Strategies and Future Trends towards the Extraction and Purification of Capsaicin: A Comprehensive Review. Trends Food Sci Technol 2022, 123, 161–171. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Viganó, J.; da Silva Anthero, A.G.; Dias, A.L.B.; Hubinger, M.D.; Martínez, J. Supercritical Fluids and Fluid Mixtures to Obtain High-Value Compounds from Capsicum Peppers. Food Chem X 2022, 13, 100228. [Google Scholar] [CrossRef] [PubMed]
- Fabela-Morón, M.F.; Cuevas-Bernardino, J.C.; Ayora-Talavera, T.; Pacheco, N. Trends in Capsaicinoids Extraction from Habanero Chili Pepper (Capsicum Chinense Jacq.): Recent Advanced Techniques. Food Reviews International 2020, 36, 105–134. [Google Scholar] [CrossRef]
- Weinhold, T. de S.; Bresciani, L.F.V.; Tridapalli, C.W.; Yunes, R.A.; Hense, H.; Ferreira, S.R.S. Polygala Cyparissias Oleoresin: Comparing CO2 and Classical Organic Solvent Extractions. Chemical Engineering and Processing: Process Intensification 2008, 47. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Rodríguez, M.; Martínez De La Ossa, E. Extraction of Antioxidant Compounds from Different Varieties of Mangifera Indica Leaves Using Green Technologies. Journal of Supercritical Fluids 2012, 72. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.D.; Oney-Montalvo, J.E.; Rodríguez-Buenfil, I.M. Phytochemical Characterization of By-Products of Habanero Pepper Grown in Two Different Types of Soils from Yucatán, Mexico. Plants 2021, 10, 779. [Google Scholar] [CrossRef]
- Cortés-Ferré, H.E.; La Vega, D.; Romero, G.; Antunes-Ricardo, M.; Gutierrez, J. Ultrasound Assisted Extraction of Capsaicinoids from By-Products of Chili Pepper Industry and Effects on Macrophage Cells Viability. food 2022. [Google Scholar] [CrossRef]
- Herrera-Pool, E.; Ramos-Díaz, A.L.; Lizardi-Jiménez, M.A.; Pech-Cohuo, S.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pacheco, N. Effect of Solvent Polarity on the Ultrasound Assisted Extraction and Antioxidant Activity of Phenolic Compounds from Habanero Pepper Leaves (Capsicum Chinense) and Its Identification by UPLC-PDA-ESI-MS/MS. Ultrason Sonochem 2021, 76, 105658. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Betanzos, K.A.; Oney-Montalvo, J.E.; Cauich-Rodríguez, J.V.; González-Ávila, M.; Scampicchio, M.; Morozova, K.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Antioxidant Capacity, Vitamin C and Polyphenol Profile Evaluation of a Capsicum Chinense By-Product Extract Obtained by Ultrasound Using Eutectic Solvent. Plants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Chel-Guerrero, L.D.; Castañeda-Corral, G.; López-Castillo, M.; Scampicchio, M.; Morozova, K.; Oney-Montalvo, J.E.; Ferrentino, G.; Acevedo-Fernández, J.J.; Rodríguez-Buenfil, I.M. In Vivo Anti-Inflammatory Effect, Antioxidant Activity, and Polyphenolic Content of Extracts from Capsicum Chinense by-Products. Molecules 2022, 27, 1323. [Google Scholar] [CrossRef]
- Cortes-Ferre, H.E.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A. Enzyme-Assisted Extraction of Anti-Inflammatory Compounds from Habanero Chili Pepper (Capsicum Chinense) Seeds. Front Nutr 2022, 9, 942805. [Google Scholar] [CrossRef]
- Olguín Rojas, J.A.; Vázquez-León, L.A.; Salgado-Cervantes, M.A.; Fernandez-Barbero, G.; Díaz-Pacheco, A.; García-Alvarado, M.A.; Rodriguez-Jimenes, G.C. Water and Phytochemicals Dynamic during Drying of Red Habanero Chili Habanero Pepper (Capsicum Chinense) Slices. Rev Mex Ing Quim 2019, 18, 851–864. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical Carbon Dioxide Extraction of Capsicum Peppers: Global Yield and Capsaicinoid Content. Journal of Supercritical Fluids 2013, 81. [Google Scholar] [CrossRef]
- Vázquez-León, L.A.; Olguín-Rojas, J.A.; Páramo-Calderón, D.E.; Palma, M.; Barbero, G.F.; Robles-Olvera, V.J.; García-Alvarado, M.A.; Rodríguez-Jimenes, G.C. Modeling of Counter-Current Multistage Extraction of Moringa Oleifera Leaves Using a Mechanistic Model. Food and Bioproducts Processing 2019, 115. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Olguín-Rojas, J.A.; Fayos, O.; v. González-de-Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; G. Barroso, C.; F. Barbero, G.; Garcés-Claver, A.; Palma, M. Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum Chinense Jacq.). Agronomy 2020, 10, 252. [Google Scholar] [CrossRef]
- Carciochi, R.A.; D’Alessandro, L.G.; Vauchel, P.; Rodriguez, M.M.; Nolasco, S.M.; Dimitrov, K. Valorization of Agrifood By-Products by Extracting Valuable Bioactive Compounds Using Green Processes. In Ingredients extraction by physicochemical methods in food; Elsevier, 2017; pp. 191–228. [Google Scholar]
- Fernández-Ronco, M.P.; Gracia, I.; De Lucas, A.; Rodríguez, J.F. Extraction of Capsicum Annuum Oleoresin by Maceration and Ultrasound-Assisted Extraction: Influence of Parameters and Process Modeling. J Food Process Eng 2013, 36. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, G.; Yang, B.; Wu, Y.; Du, M.; Kan, J. Insights into the Stability of Carotenoids and Capsaicinoids in Water-Based or Oil-Based Chili Systems at Different Processing Treatments. Food Chem 2021, 342, 128308. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Osorio-Tobón, J.F.; Silva, L.P.S.; Barbero, G.F.; Martínez, J. Economic Analysis of Oleoresin Production from Malagueta Peppers (Capsicum Frutescens) by Supercritical Fluid Extraction. J Supercrit Fluids 2018, 133, 86–93. [Google Scholar] [CrossRef]
- Shi, J.; Yi, C.; Ye, X.; Xue, S.; Jiang, Y.; Ma, Y.; Liu, D. Effects of Supercritical CO2 Fluid Parameters on Chemical Composition and Yield of Carotenoids Extracted from Pumpkin. LWT - Food Science and Technology 2010, 43. [Google Scholar] [CrossRef]
- Duranova, H.; Valkova, V.; Gabriny, L. Chili Peppers (Capsicum Spp.): The Spice Not Only for Cuisine Purposes: An Update on Current Knowledge. Phytochemistry Reviews 2022, 21, 1379–1413. [Google Scholar] [CrossRef]
- Avilés-Betanzos, K.A.; Scampicchio, M.; Ferrentino, G.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Evaluation of the Capsaicinoid Extraction Conditions from Mexican Capsicum Chinense Var. Mayapan with Supercritical Fluid Extraction (SFE). Processes 2023, 11, 2272. [Google Scholar] [CrossRef]
- Jarén-Galán, M.; Nienaber, U.; Schwartz, S.J. Paprika (Capsicum Annuum) Oleoresin Extraction with Supercritical Carbon Dioxide. J Agric Food Chem 1999, 47. [Google Scholar] [CrossRef] [PubMed]
- Richins, R.D.; Hernandez, L.; Dungan, B.; Hambly, S.; Holguin, F.O.; O’Connell, M.A. A “Green” Extraction Protocol to Recover Red Pigments from Hot Capsicum Fruit. HortScience 2010, 45. [Google Scholar] [CrossRef]
- Li, G.; Song, C.; You, J.; Sun, Z.; Xia, L.; Suo, Y. Optimisation of Red Pepper Seed Oil Extraction Using Supercritical CO2 and Analysis of the Composition by Reversed-Phase HPLC-FLD-MS/MS. Int J Food Sci Technol 2011, 46. [Google Scholar] [CrossRef]
| Habanero chili pepper waste | Extraction process | Bioactive compounds recovery | Reference |
|---|---|---|---|
| Leaves, peduncles, and stems | Ultrasound extraction | Phenolic compounds, carotenoids, and capsaicinoids | [32] |
| Wasted pulp and seeds | Ultrasound extraction | Capsaicinoids | [33] |
| Leaves | Ultrasound extraction | Phenolic compounds | [34] |
| Leaves and stems | Ultrasound extraction using NADES | Phenolic compounds and vitamin C | [35] |
| Leaves and stems | Maceration, Soxhlet, and SFE | Phenolic compounds | [36] |
| Seeds | Enzyme-assisted extraction | Phenolic compounds andcapsaicinoids | [37] |
| Extractionprocess | Extractable solids concentration* | Recovery from raw material* | Concentration in extract** | |||
|---|---|---|---|---|---|---|
| Total capsaicinoids | Total carotenoids | Total capsaicinoids | Total carotenoids | |||
| ME | 40.22 ± 2.53a | 30.82 ± 0.24a | 9.26 ± 0.16b | 77.52 ± 1.19bc | 3.1 ± 0.07a | 25.99 ± 0.55a |
| SE | 1.55 ± 0.07b | 4.6 ± 0.01c | 10.44 ± 1.15b | 111.36 ± 7.85b | 0.79 ± 0.13b | 8.38 ± 0.08c |
| US | 42.5 ± 1.67a | 31.49 ± 3.63a | 10.64 ± 0.2b | 97.24 ± 0.51bc | 3.29 ± 0.17a | 30.27 ± 2.6a |
| SFE | ND | 18.68 ± 0.03b | 8.56 ± 1.27b | 56.53 ± 2.08c | 3.34 ± 0.37a | 17.43 ± 0.14c |
| SFEC | 3.95 ± 0.01b | 29.24 ± 0.07a | 14.91 ± 0.38a | 292.09 ± 10.59a | 0.48 ± 0.01b | 9.35 ± 0.82b |
| Extractionprocess | Capsaicin* | Dihidrocapsaicin* | Capsanthin** | Zeaxanthin** | β-carotene** |
|---|---|---|---|---|---|
| ME | 2.70 ± 0.03a | 0.40 ± 0.03a | 15.50 ± 0.67a | 9.15 ± 0.13a | 1.34 ± 0.32c |
| SE | 0.60 ± 0.09b | 0.19 ± 0.03c | 4.79 ± 0.03c | 0.91 ± 0.06b | 2.68 ± 0.08b |
| US | 2.84 ± 0.14a | 0.45 ± 0.02a | 18.05 ± 1.56a | 10.33 ± 0.93a | 1.85 ± 0.16bc |
| SFE | 3.05 ± 0.21a | 0.29 ± 0.05b | 10.59 ± 0.07b | 0.97 ± 0.01b | 5.87 ± 0.04a |
| SFEC | 0.42 ± 0.01b | 0.06 ± 0.03d | 5.31 ± 0.28c | 0.60 ± 0.03c | 3.44 ± 0.27b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





