1. Introduction
Malaria is a serious global health problem causing over 600,000 deaths in 2021 [
1]. Correct diagnosis is necessary for prevention, control, and suitable treatment of malaria, and commonly performed by microscopy, rapid diagnostic tests (RDTs), and molecular methods [
2]. Success of a molecular method for malaria diagnosis depends critically on the quality of genomic DNA being used for examination, which in turn depends on accuracy of the employed isolation procedure.
Multitudes of different DNA extraction methods have been published [
3] since the first DNA isolation in 1869 [
4], and each method must overcome specific extraction problems such as DNA shearing, background contamination, low purity, and low yield. Of these, dried blood spot (DBS) is one of the most common methods used to collect and extract DNA in field studies of malaria molecular epidemiology, where transport and storage conditions are often suboptimal. This technique is minimally invasive (requiring only microliters of typically peripheral blood), inexpensive, and easy to multiplex and automate [
5]. DBS samples are compatible with many bioanalytical methods, among them chromatography, mass spectrometry, DNA, and immunoassays. However, DNA extraction is particularly challenging in resource-limited regions, where scissors are used to cut filter papers, possibly introducing contamination between samples due to poor scissor cleaning.
Many DBS methods use 70% ethanol to clean the scissors, but it is unknown whether ethanol or other cleaning solutions may be a source of sample contamination. We drafted this letter to shed light on concerns regarding contamination of samples extracted by scissors which may impact interpretation of molecular epidemiology results.
2. Methods
2.1. DNA Extraction from DBS
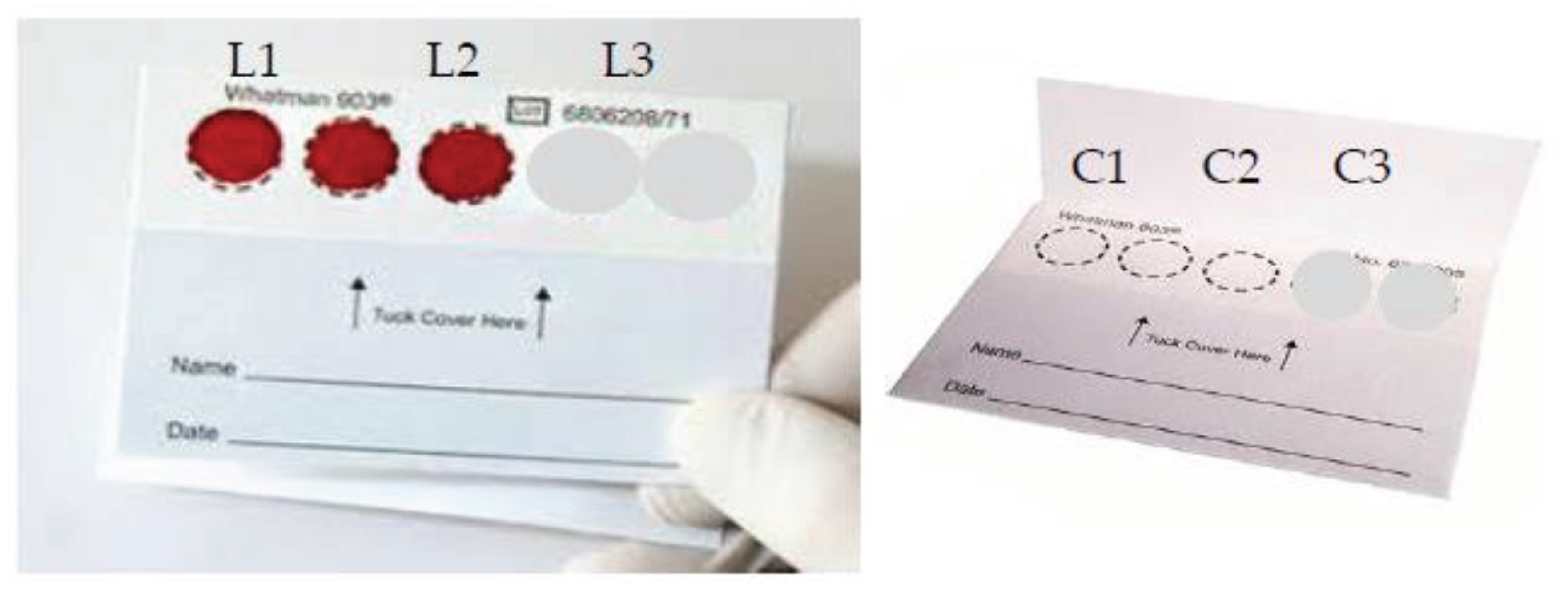
DBS were prepared from 3 drops of blood from the same culture, spotted on Whatman FTA cards (Whatman Inc., Brentford, UK), then dried and stored for 24 hours at room temperature (
Figure 1). DBS on cards were cut into small pieces with scissors and transferred into 1.5-mL microtubes. Parasite DNA was extracted using QIAamp DNA blood mini kits (Qiagen, Valencia, CA USA) according to the manufacturer’s instructions and final eluted volume was 30 mL. DNA samples were stored at −20°C until use. The concentration of DNA was achieved using a NanoDrop2000.
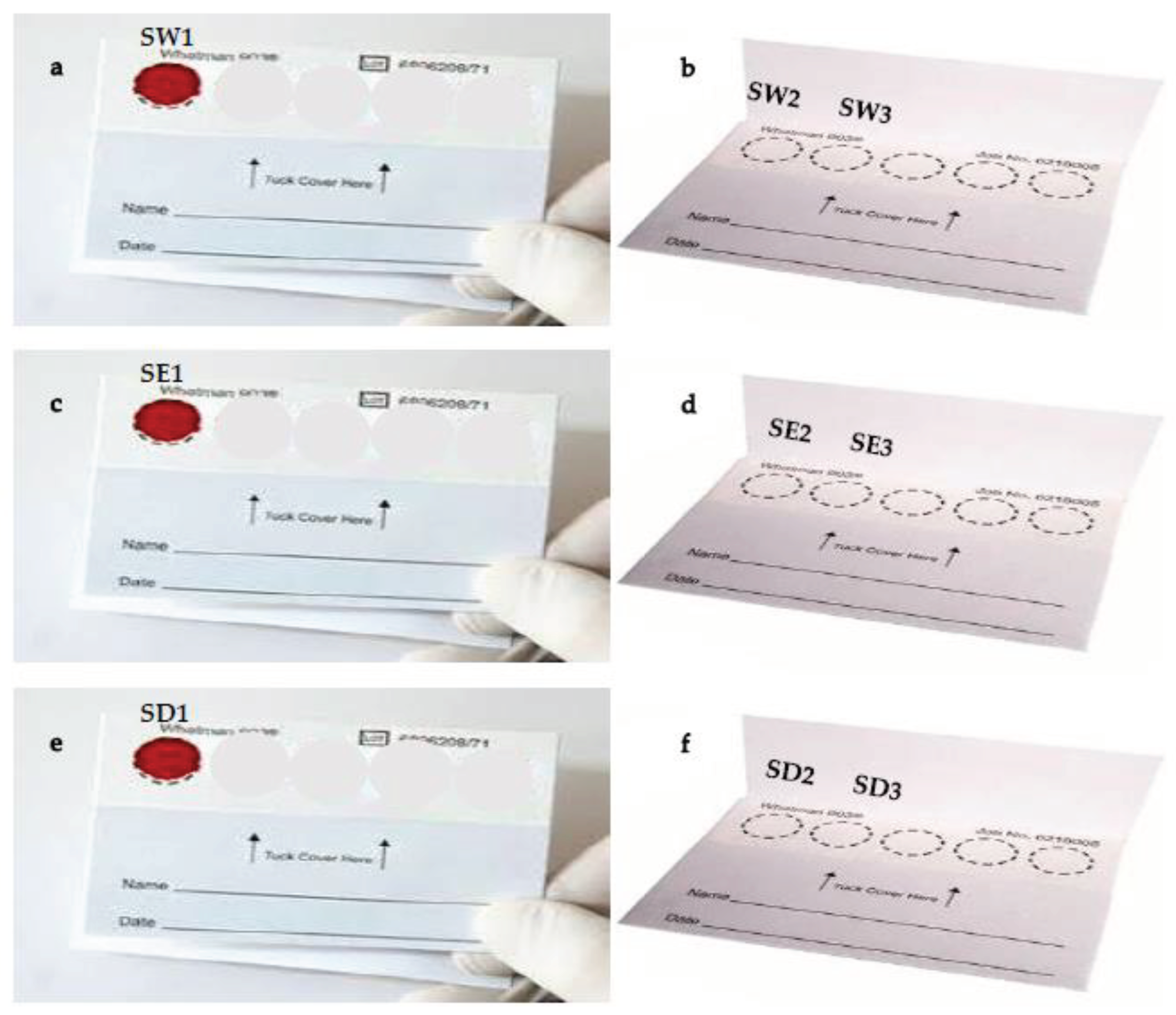
DBS were prepared from 3 drops of blood from two
Plasmodium falciparum cultures (20-0119-0 and 20-2091-0 isolates) spotted on filter paper (
Figure 2). All samples were dried for 24 hours at room temperature. We examined three solutions (water, ethanol, and DNase) to clean scissors between spots during DNA extraction. For each cleaning solution, two blank confetti were used as negative controls. DBS were cut into small pieces with scissors and transferred into 1.5-mL microtubes. Residual water (RW), ethanol (RE) and DNase (RD) after scissor washing were also amplified.
2.2. PCR Assay of DBS
Parasite-infected and negative control samples were analyzed by PCR-based genotyping of P. falciparum merozoite surface proteins 1 & 2 (msp-1 and msp-2), and P. falciparum chloroquine resistance transporter (Pfcrt) genes. A total of 15 samples were analyzed. Based on msp1 and msp2 amplification (3 for each cleaning solution, n=9), P. falciparum was detected on blank confetti when scissors were washed with ethanol. Based on Pfcrt amplification (2 for each cleaning solution, n=6), DNA of P. falciparum was detected on blank confetti when scissors were washed with ethanol or water. Genotyping using amplification of msp-1 and msp-2 markers were performed on paired samples obtained.
PCR primers sequences and cycles have been previously described [
6]. Gels were stained with ethidium bromide and visualized under UV illumination. Band sizes of PCR products across the two markers were measured visually and compared. PCR products visualized on 1.5% agarose gel in 1×Tris-acetate-EDTA (TAE) and stained with ethidium bromide (Sigma-Aldrich, Sydney, NSW, Australia). Length differences were determined using a 100 bp molecular weight ladder (Promega). Electrophoresis conditions were 100 mV for 30 min. Allele-specific positive controls and DNA-free negative controls were included in each round of reactions. Primers for
msp-1 and
msp-2 were synthesized by Integrated DNA Technologies, Coralville, IA, USA.
3. Results and Discussion
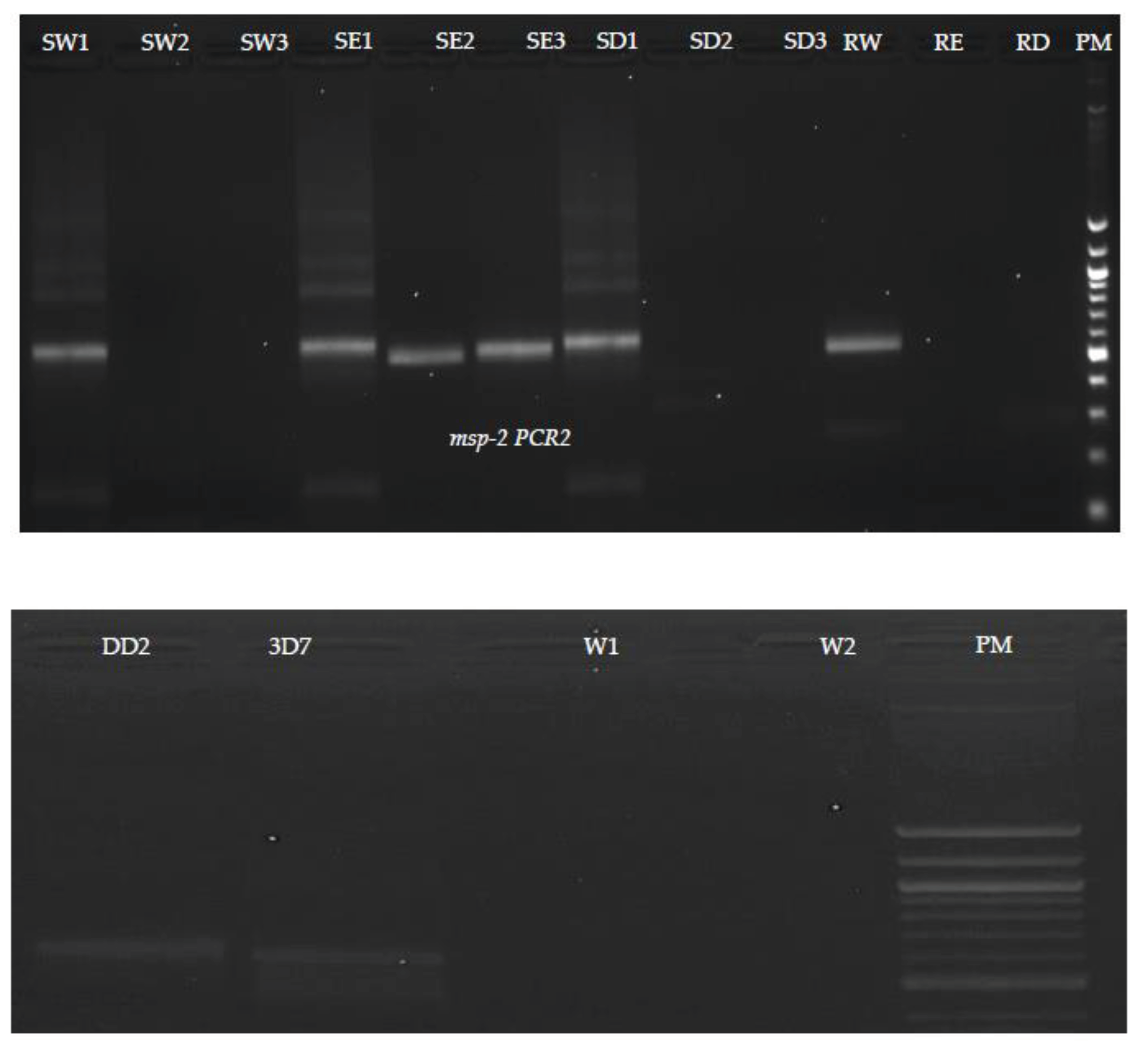
Results of the nested PCR revealed that no bands were detected in samples washed with DNase. Schematic representation of agarose gel electrophoresis of nested PCR products from SW1, SE1 and SD1 show the presence of bands at 565 bp and 534 bp in
msp1 (
Figure 3) and
msp-2 (
Figure 4) respectively. In the nested PCR assay using
msp-1 and
msp-2, no band was detected in SW1, SW2, SE2, SD1, SD2, RE and RD tested. We detected bands in SE2 in
msp-1 and SE1 and SE2 in
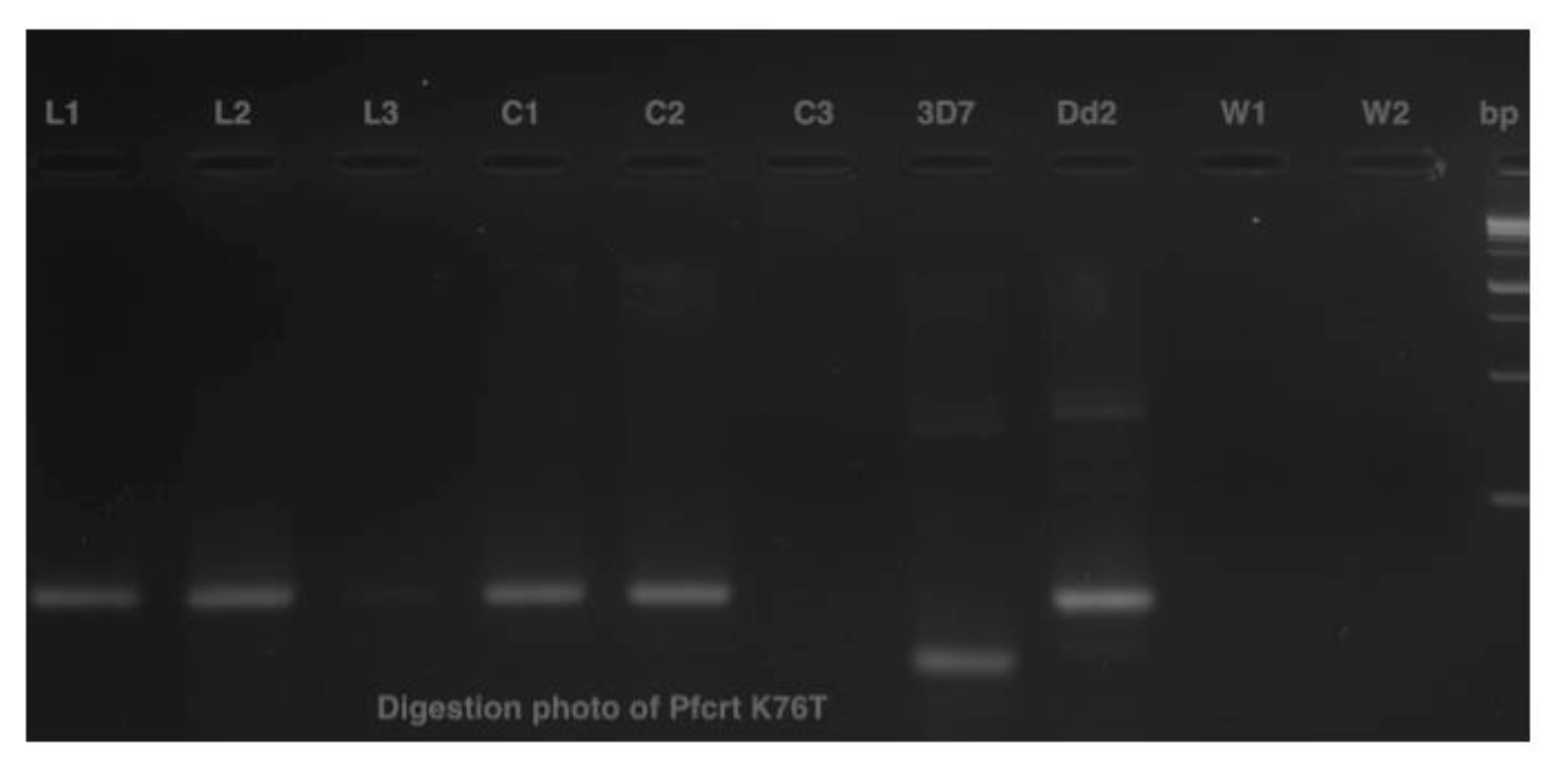
msp-2, respectively. Only blank confetti catted by scissors washed with ethanol presented bands, detected in C1 and C2 on agarose gel electrophoresis of digestion PCR products, but no band was detected with C3 (
Figure 5).
Ethanol is a non-additive precipitant fixative that shifts isoelectric points of proteins less than other fixatives [
7]. It fixes proteins by dehydration and precipitation, the degree of which being dependent on the amount of water present and the solubility of materials in the mixture. The basic aims of fixation are to preserve the tissue nearest to its living state, and to prevent any change in shape and size of tissue at the time of processing. This could possibly explain how pieces of blood-stained confetti attached to scissors after being dipped in ethanol. In addition, we observed one band from residual of water (RW) in
msp-2. Water is by far the most studied chemical compound [
8] and called the universal solvent since it can dissolve more substances than any other liquid [
9]. After dipping in water, particles of confetti on scissors detached themselves and remained at the bottom of the water. DNase is an endonuclease that digests single and double-stranded DNA; it hydrolyzes phosphodiester bonds producing mono- and oligodeoxyribonucleotides with 5’-phosphate and 3’OH groups. The enzyme activity is strictly dependent on Ca2+ and is activated by Mg2+ or Mn2+ ions: 1) in the presence of Mg2+, DNase cleaves each strand of dsDNA independently in a statistically random fashion [
10]; 2) In the presence of Mn 2+, the enzyme cleaves both. DNA strands at approximately the same site, producing DNA fragments with blunt ends or with one or two nucleotide overhangs [
10]. We did not detect any band with
msp-1 and
msp-2 in scissors washed with DNase.
4. Conclusions
Scissors cleaned with DNase prevented cross-contamination between samples during processing of dried blood spots. Ethanol cleaning of scissors, which is commonly used, failed to avert cross-contamination. Sample cross-contamination can reduce diagnosis accuracy; we recommend DNase wash as the standard cleaning solution to improve DNA extraction.
Funding
This work supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Hamma Maiga was the recipient of African Postdoctoral Training Initiative (APTI) Scholarship (##APT-18-01). The content is solely the responsibility of the authors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank Justin Yai Doritchamou of LMIV for providing the blood sample, and the NIH Fellows Editorial Board for critical editing.
Conflicts of Interest
The authors declare no competing interest.
References
- WHO: World Malaria Report. In WHO, editor. Geneva, Switzerland: World Health Organizaiton; 2022.
- Fontecha GA, Mendoza M, Banegas E, Poorak M, De Oliveira AM, Mancero T, Udhayakumar V, Lucchi NW, Mejia RE: Comparison of molecular tests for the diagnosis of malaria in Honduras. Malar J 2012, 11:119. [CrossRef]
- Ramona KuhnJörg BK, KrahlIsaac Mbir, BryantMarion Martienssen: Comparison of ten different DNA extraction procedures with respect to their suitability for environmental samples. Journal of Microbiological Methods; December 2017.
- Dahm R: Discovering DNA: Friedrich Miescher and the early years of nucleic acid research. Hum Genet 2008, 122:565-581. [CrossRef]
- Demirev PA: Dried blood spots: analysis and applications. Anal Chem 2013, 85:779-789. [CrossRef]
- de Radigues X, Diallo KI, Diallo M, Ngwakum PA, Maiga H, Djimde A, Sacko M, Doumbo O, Guthmann JP: Efficacy of chloroquine and sulfadoxine/pyrimethamine for the treatment of uncomplicated falciparum malaria in Koumantou, Mali. Trans R Soc Trop Med Hyg 2006, 100:1013-1018. [CrossRef]
- aker JR: Principles of Biological Microtechnique. 3rd edn edn. John Wiley, New York.1958.
- Greenwood NNE, Alan Chemistry of the Elements 2nd ed edn: Butterworth-Heinemann; 1997.
-
U.S. Department of the Interior. usgs.gov (website). .
- Sambrook J, Russell, D.W.,: Molecular Cloning: A Laboratory Manual. the third edition edn. New York: Cold Spring Harbor Laboratory Press, Cold Spring Harbor; 2001.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).