Submitted:
23 February 2024
Posted:
26 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and methods
- Population: Human and animal population in the Amazon region.
- Exposure: Exposure to mercury (Hg).
- Comparator: Different levels of mercury exposure or comparisons between different species or regions, and the values allowed by health organizations.
- Outcome: Hg contamination in animal tissues and humans relative to diet.
| Arguments | Search syntax |
| mercury in Amazon | amazon* AND (mercury* OR hg OR MeHg OR methyl* OR "total mercury") |
| activity | AND (mining OR artisanal OR asgm OR "gold mining*" OR garimp* OR alluvial OR contamination OR "inorganic contaminant*" OR "trace mineral*" OR trophic) |
| specimen | AND (fish OR species OR population OR aquatic OR bioaccumulation OR community OR riverine OR "human hair" OR indigenous OR human OR nonhuman OR animal) |
| conditions | AND (exposure OR accumulation OR concentration OR content OR consumption) |
| biomarker | AND (tissue OR muscle OR protein OR hair OR blood) |
| exclude ex-situ | NOT (farm OR agriculture) |
- Study Characteristics: DOI, title, and author(s) of the study.
- Population Characteristics: Whether study focuses on human or non-human species, the scientific nomenclature of the studied species, and their feeding guild.
- Collection Details: Sample collection location (in decimal degrees) and the period during which the samples were collected.
- Mercury Exposure Details: Type of tissue sampled and total amount of mercury found in these tissues.
- Additional Data: Any mention of indigenous regions, hydroelectric dams, or Artisanal and Small-Scale Gold Mining (ASGM), as well as details ofmercury analysis.
2.1. Geoprocessing
2.2. Statistical Analysis
3. Results
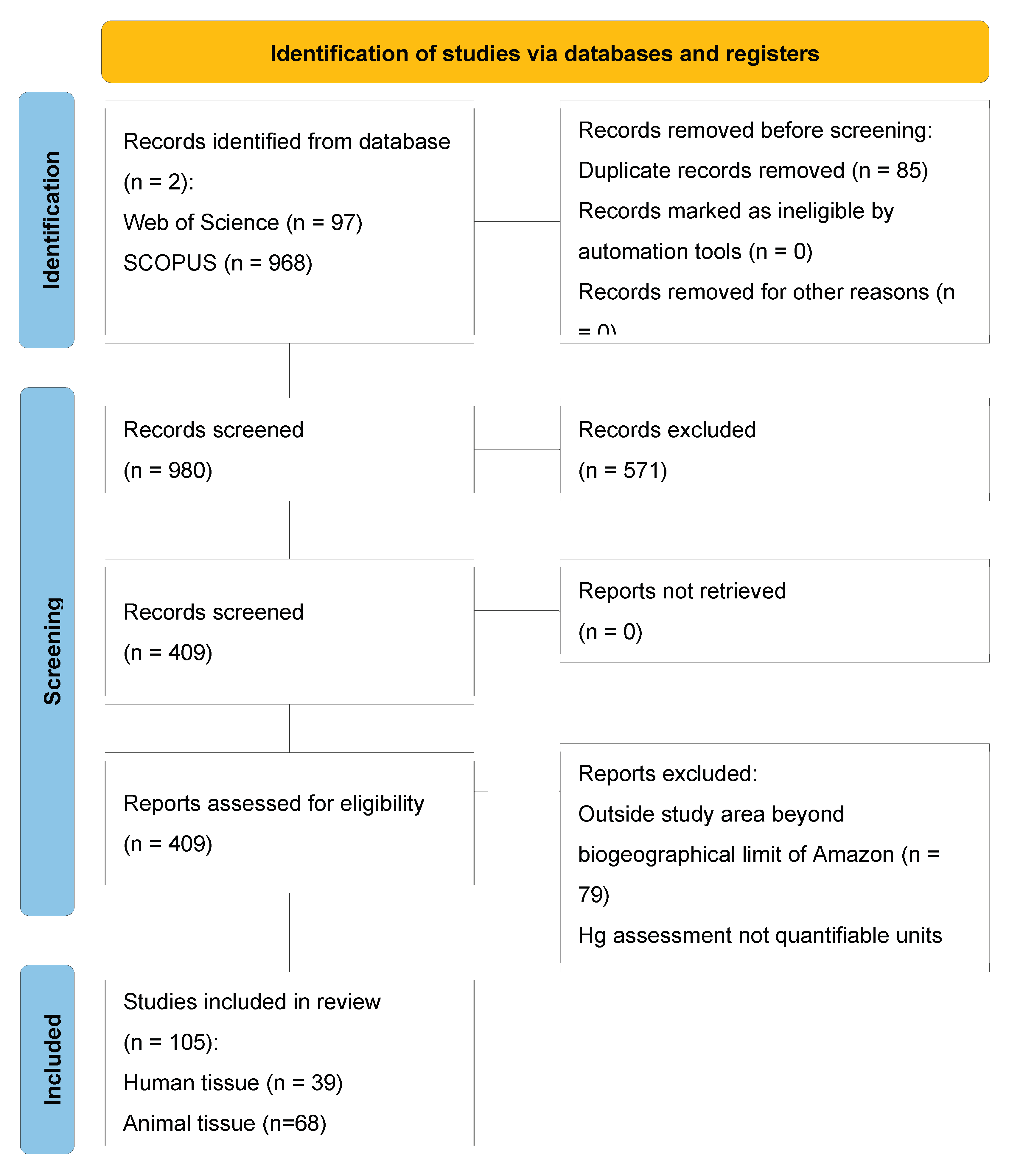
3.1. Study characteristics
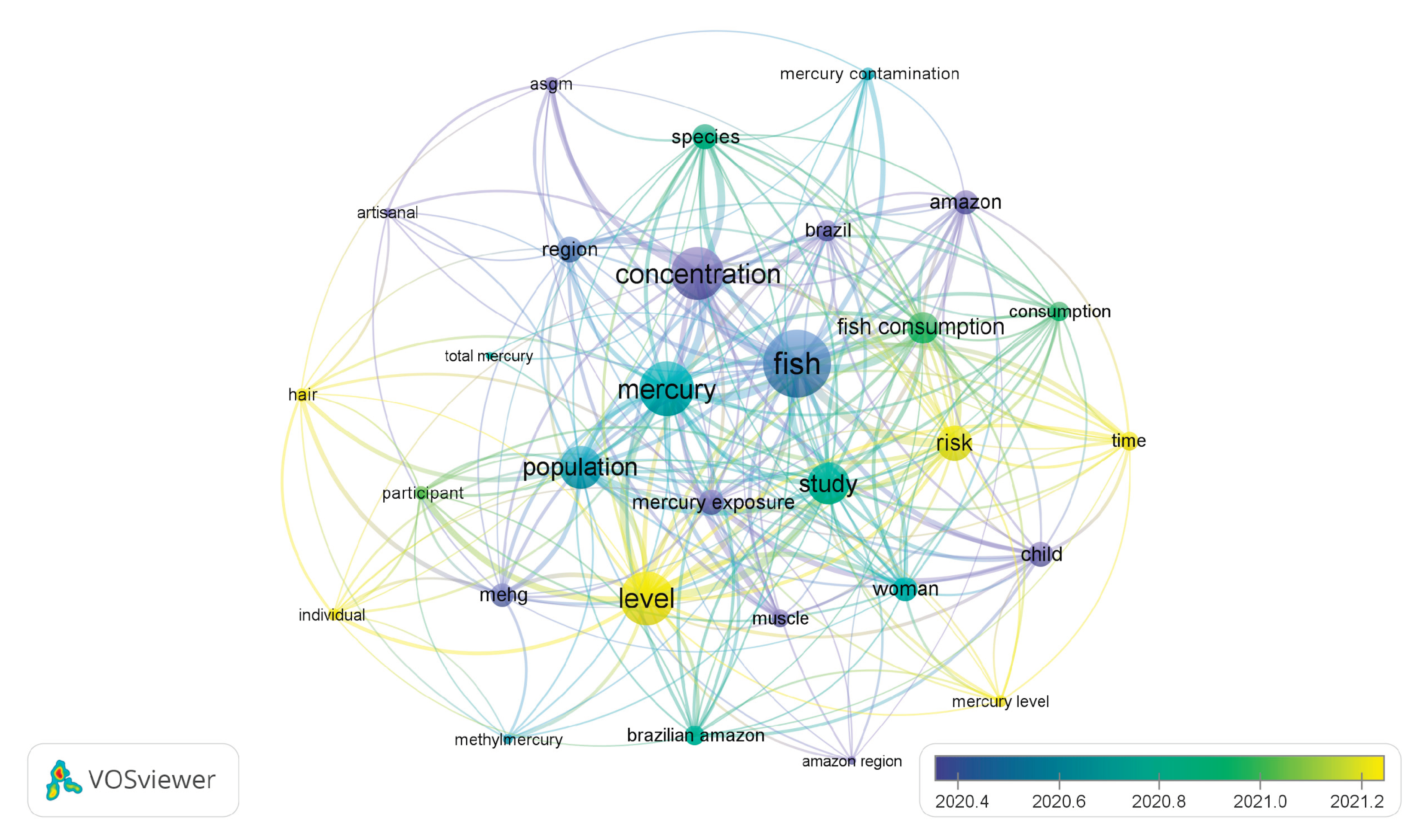
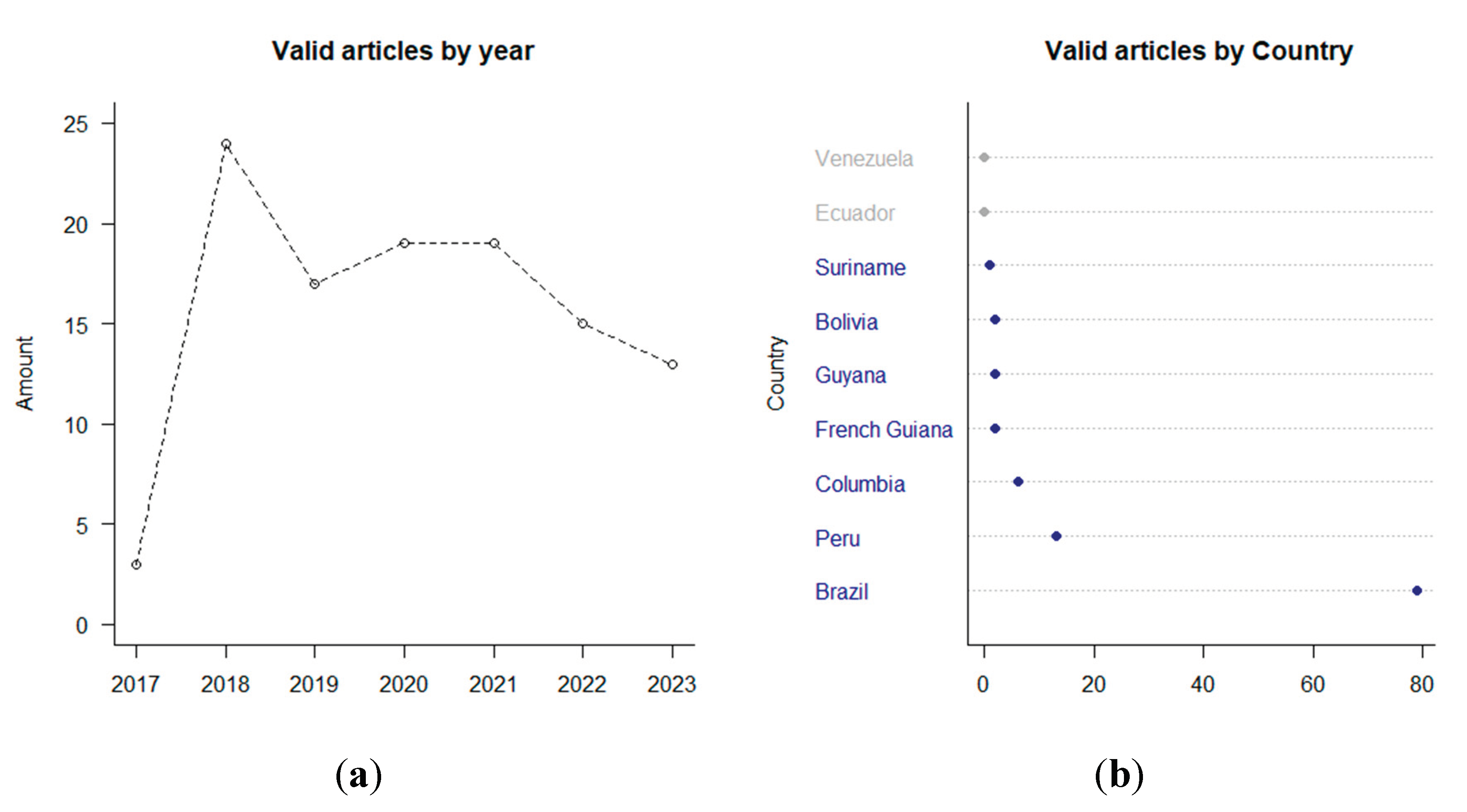
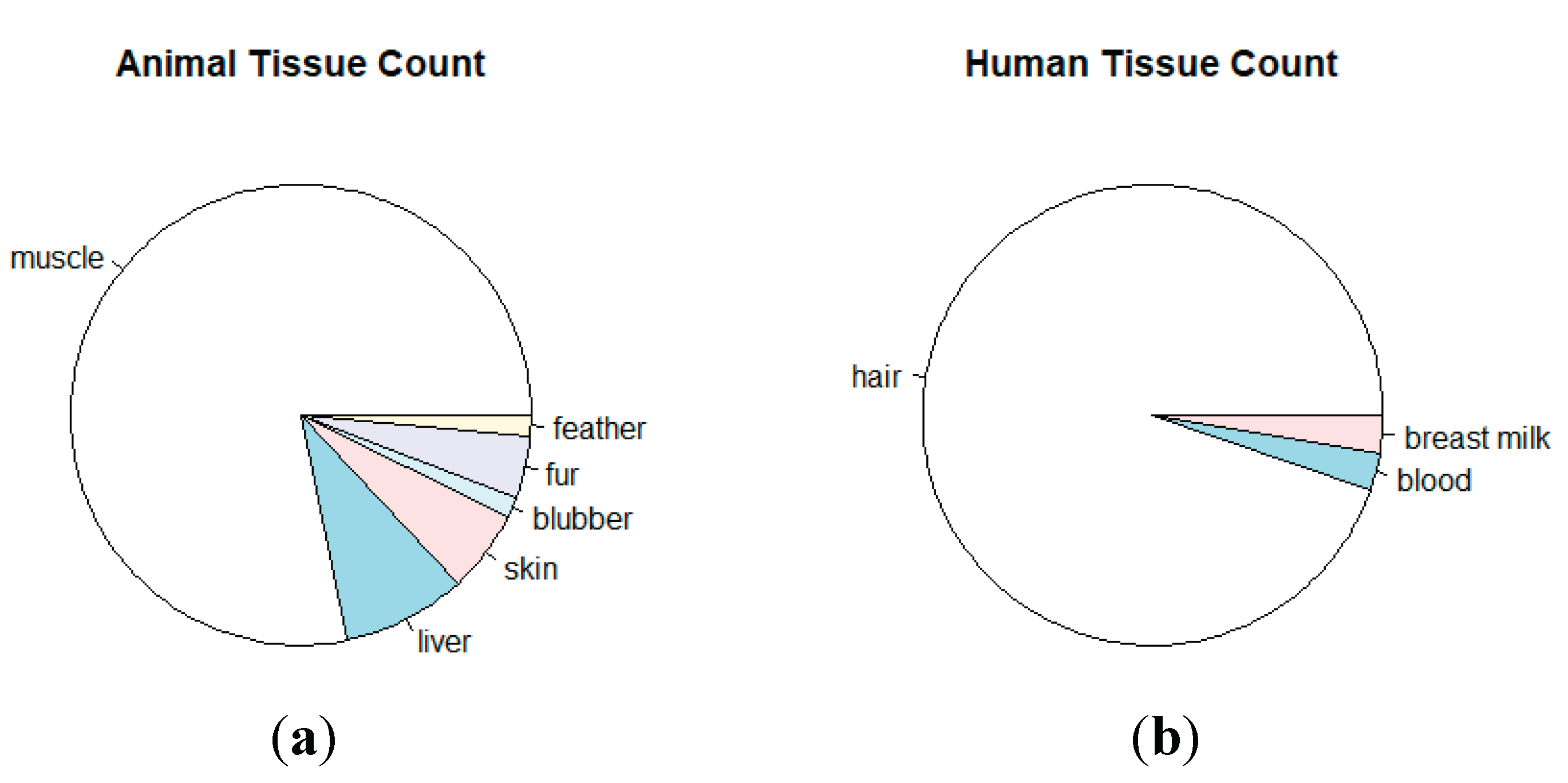
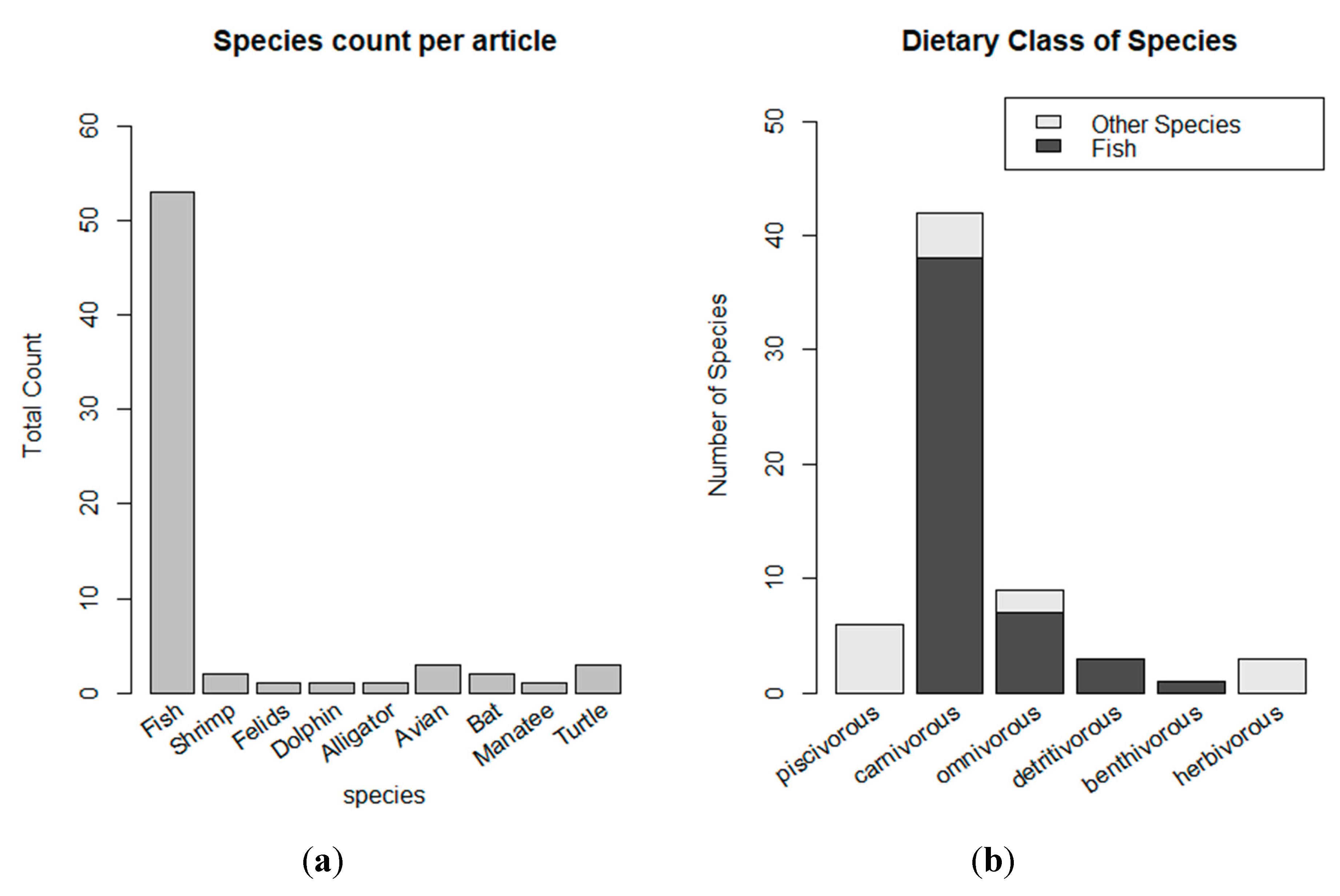
3.2. Overview of THg levels in human research
| Hydrographic basin * | Mentioned mining | Country | Study | Species/feeding guild | THg (μg/g) based on mean/median † |
| A | No | Brazil | [66,67]** | Hoplias malabaricus, carnivore | 0.947† |
| A | No | Brazil | [68] | Carcharhinus acronotus, carnivore | 1.120 |
| A, T | Yes | Brazil | [69] | Arapaima sp., carnivore | 0.375 |
| A,T | No | Brazil | [70] | Plagioscion squamosisYesus, carnivore | 1.510 |
| A | Yes | Brazil | [71] | Pseudoplatystoma tigrinum, carnivore | 0.920 |
| A | No | Brazil | [72] | Ageneiosus inermis, carnivore | 0.691 |
| A | No | Brazil | [73] | Acestrorhyncus falcirostris, carnivore | 1.490 |
| A | No | Brazil | [74] | Colomesus asellus, omnivore | 0.350 |
| T | Yes | Brazil | [75] | Carnivores Group: Cichla monoculus , Plagioscion squamosisYesus , Serrasalmus calmoni. | 0.668 |
| T | Yes | Brazil | [76] | Plagioscion squamosisYesus, carnivore | 0.730 |
| T | Yes | Brazil | [77] | Cichla pinima, carnivore | 1.172 |
| T | No | Brazil | [78] | Serrasalmus rhombeus, carnivore | 0.088 |
| M | No | Brazil | [79] | Carnivorous Group: Plasgioscion squamosisYesus, Calophysus macropterus, Cichla pleiozona and Hoplias malabaricus. | 0.970 |
| M | No | Brazil | [80] | Prochilodus nigricans, detritivore | 0.064 |
| M | No | Brazil | [81] | Serrasalmus rhombeus, carnivore | 0.263 |
| M | Yes | Brazil | [82] | Triportheus angulatus, omnivore | 0.290 |
| M | No | Brazil | [83] | Triportheus albus, omnivore | 0.029 |
| M | No | Brazil | [84] | Pinirampus pirinampu, benthivore | 0.060 |
| M | No | Brazil | [85] | Arapaima gigas, carnivore | 0.153 |
| M | No | Brazil | [86] | Serrasalmus rhombeus, carnivore | 1.640 |
| M | No | Brazil | [87] | Plagioscion squamosisYesus, carnivore and Colossoma macropomum, omnivore | 0.086 |
| M | No | Brazil | [88] | Brachyplatystoma filamentosum, carnivore | 0.402 |
| M | No | Brazil | [89] | Calophysus maropterus, carnivore | 1.400 |
| M | No | Peru/ Brazil (Border) | [90] | Calophysus maropterus, carnivore | 0.229 |
| M | No | Brazil | [91] | Cichla spp., carnivore | 0.128 |
| M | No | Brazil | [92] | Semaprochilodus spp.(Jaraqui), detritivore | 0.132 |
| M | No | Brazil | [93] | Serrasalmus rhombeus, carnivore | 0.268 |
| M | No | Brazil | [94] | Cichla spp. (Tucunaré), carnivore | 0.435 |
| M | Yes | Brazil | [95] | Serrasalmus rhombeus, carnivore | 0.417 |
| X | No | Brazil | [96] | Hemiodus unimaculatus, omnivore | 0.480 |
| Tp | No | Brazil | [97] | Serrasalmus rhombeus, carnivore | 0.304 |
| Tp | No | Brazil | [98] | Brycon falcatus, omnivore | 0.052 |
| Ar | Yes | Brazil | [12] | Carnivorous Group: Ageneiosus inermis, Boulengerella cuvieri, Cichla monoculus, and Hoplias aimara | 0.580 |
| Ar | Yes | Brazil | [99] | Curimata incompta, detritivore | 0.370 |
| Ar | Yes | Brazil | [100] | Plagioscion squamosisYesus, carnivore | 0.320† |
| J | No | Brazil | [101] | Plagioscion squamosisYesus, carnivore | 1.090 |
| MD | Yes | Peru | [102] | Serrasalmus spp., carnivore | 0.280 |
| MD | Yes | Peru | [103] | Serrasalmus spp., carnivore | 3.720 |
| B | No | Bolivia | [104] | Brycon amazonicus, omnivore | 0.700 |
| Br | Yes | Brazil | [105] | Pinirampus pirinampu, carnivore | 0.869 |
| Br | Yes | Brazil | [33] | Pygocentrus nattereri, carnivore | 1.215 |
| M | Yes | Brazil | [106] | Serrasalmus rhombeus, carnivore | 0.283 |
| Su | Yes | Suriname | [107] | Multiple, high THg in carnivores: Acestrorhynchus microlepsis, Hoplias malabaricus, Cichla ocellaris, Serrasalmus rhombeus,and Pristobrycon eigenmanni. | 2.528 |
| C | Yes | French Guiana | [108] | Hoplias aimara and Boulengerella cuvieri, carnivore | 2.900 |
| Mz | Yes | Guyana | [109] | Ageneiosus ucayalensis, carnivore | 5.920 |
| Location | Mentioned mining | Study | Species | Tissue | Mean THg (μg/g) |
| Madeira River, Rondônia, Brazil | No | [110] | Arapaima gigas, fish, carnivore | hepatic | 17.420 |
| Araguari River, Amapá, Brazil | No | [111] | Anodus orinocensis, fish, omnivore | hepatic | 0.500 |
| Madeira River, Brazil | No | [112] | Macrobrachium amazonicum, shrimp, omnivore | muscle | 0.610 |
| Atlantic Coast, Ilha dos Caranguejos, Brazil | No | [113] | Sciades herzbergii, fish, omnivore | muscle | 0.033 |
| Mamirauá, Amazonas, Brazil | No | [114] | Panthera onca, mammal, carnivore | pelage | 17.900 |
| Guaporé River, Brazil | No | [115] | Inia boliviensis, mammal, piscivore | adipose | 1.323 |
| Beni River, Bolívia | No | [116] | Caiman yacare, reptile, piscivore | muscle | 0.150 |
| Madeira River, Brazil | No | [117] | Ardea cocoi, bird, carnivore | feather | 4.046 |
| Biological Station Cocha Cashu, Peru | No | [118] | Rhynconycteris naso, mammal, carnivore | pelage | 7.440 |
| Madre de Dios, Peru | Yes | [119] | Phyllostomus elongatus, mammal, carnivore | pelage | 0.660 |
| Figueiredo, Amazonas, Brazil | No | [120] | Trichechus inunguis, mammal, herbivore | muscle | 0.059 |
| Arauca River and Orinoco River, Colombia | Yes | [121] | Inia sp. and Sotalia sp., mammal, piscivore | muscle | 0.870 |
| Itapuru mirim Lagoon, Brazil | No | [122] | Podocnemis unifilis, reptile, herbivore | muscle | 0.011 |
| Xingu and Teles Pires’ Rivers, Brazil | No | [123] | Podocnemis unifilis, reptile, herbivore | muscle | 0.134 |
| Uatumã River, Balbina Brazil | No | [124] | Podocnemis expansa, reptile, omnivore | muscle | 0.109 |
| Teles Pires, Brazil | No | [125] | Chloroceryle amazona, bird, piscivore | feather | 11.570 |
| Teles Pires, Brazil | No | [126] | Chloroceryle amazona, bird, piscivore | feather | 4.000 |
| Madeira River, Brazil | No | [127] | Macrobrachium depresYesanum, Macrobrachium jelskii, shrimp, omnivore | muscle | 0.022 |

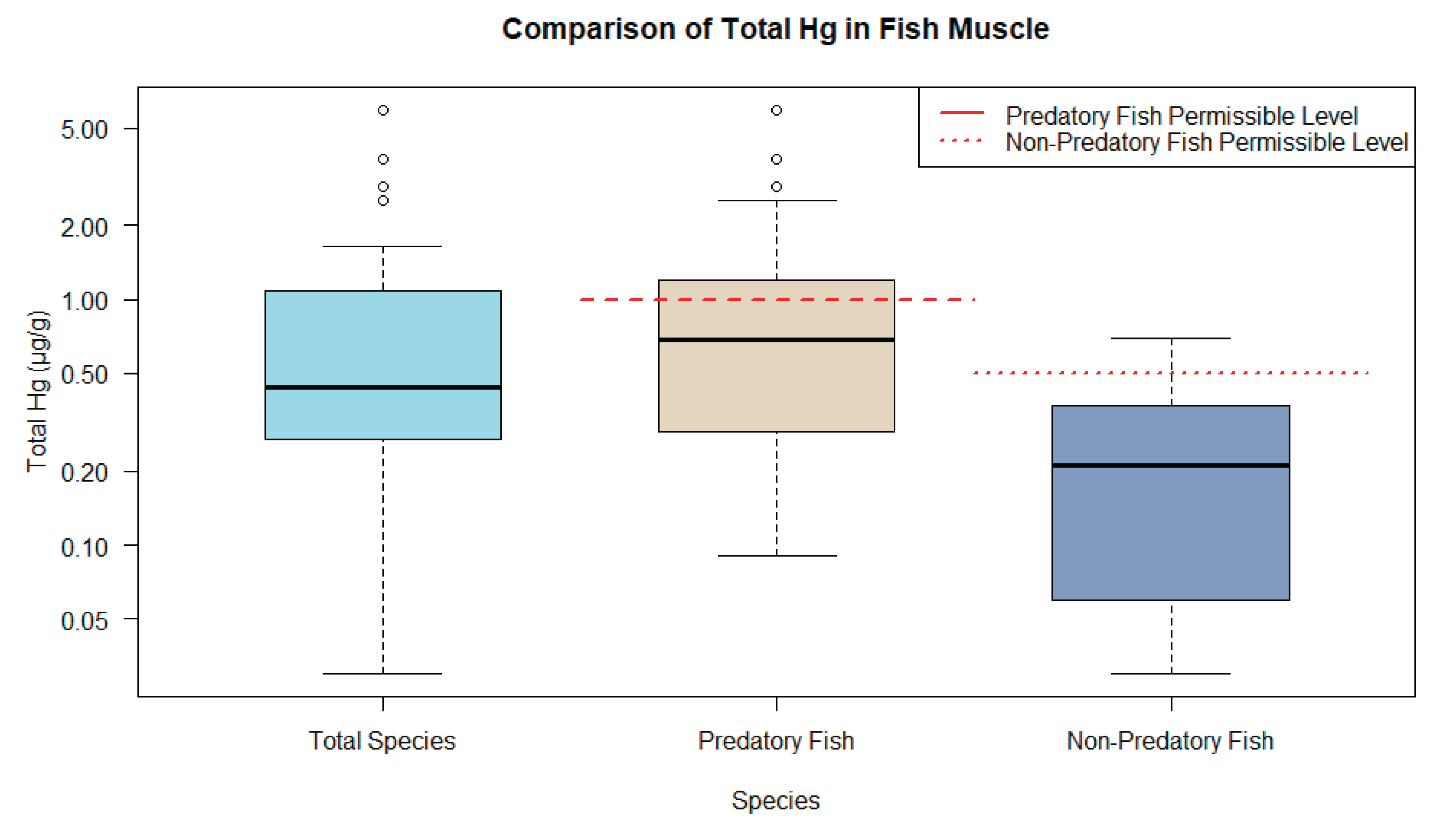
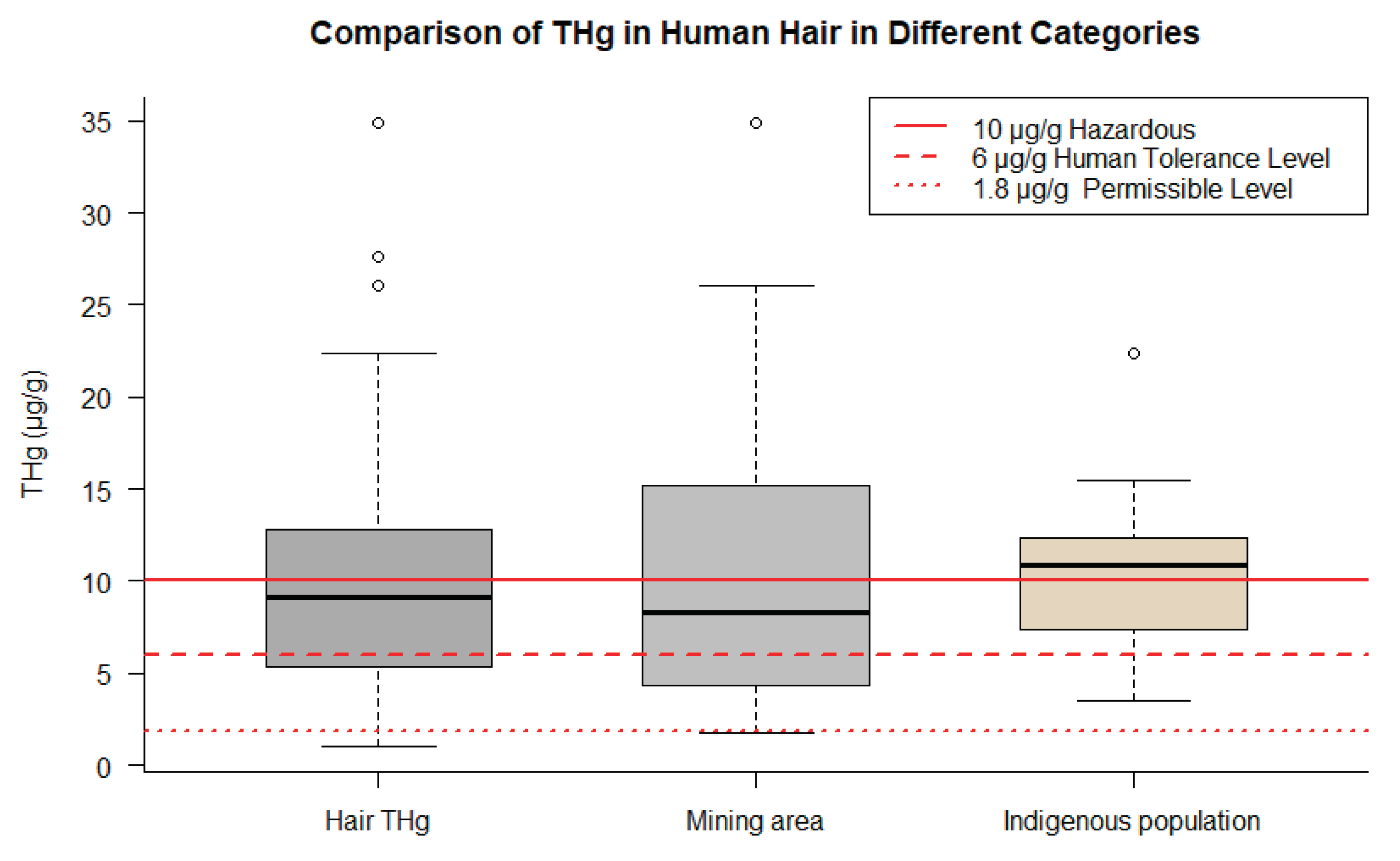
3.3. Overview of THg levels in human research
| Hydrographic basin * | Mining mentioned | Country | Study | Community/Population | THg (μg/g) in mean /median † |
| To | Yes | Brazil | [128] | Adults (18-70), fished as a staple food, near the reservoir of Tucuruí Dam. | 10.900 |
| U | Yes | Brazil | [129] | Age groups and comparison of various villages. Yanomami indigenous reserve, with a high diet of fish and mining activity. | 15.500† |
| M | Yes | Brazil | [130] | Age groups of adults ranging from 17 to 92 years. | 26.030 |
| To, Ta | Yes | Brazil | [131] | Adult riverside dwellers only (18 to 60 years old). | 4.500 |
| Ta | Yes | Brazil | [132] | Riverside dwellers only; adult women (13 to 53 years old). | 9.150 |
| Ta | Yes | Brazil | [133] | Munduruku Indigenous Reserve. Comparison between villages. | 7.400 |
| M, Ta | No | Brazil | [76] | Pregnant women (18 to 40 years old). Fish Diet. | 6.070 |
| MD | Yes | Peru | [64] | Urban and rural demographic comparison with a focus on fish diets. | 1.740 |
| A | Yes | Colombia | [71] | Indigenous community in Puerto Nariño. Mean age ~ 35 years. Diet rich in fish. | 5.310 |
| Ta | No | Brazil | [11] | Munduruku Indigenous Reserve. Comparison between villages and age categorization with juvenile, childbearing age, and other adults | 11.500 |
| Ta | No | Brazil | [134] | Munduruku Indigenous Reserve. Comparison between villages and the exclusively juvenile population. | 11.800 |
| Ta | No | Brazil | [10] | Munduruku Indigenous Reserve. Comparison between villages. Ages > 12 years. | 7.400 |
| A, Cg, Ta | Yes | Brazil | [135] | Youth and adults in riverside communities. | 12.700 |
| MD | Yes | Peru | [136] | Matsigenka Indigenous community (years 1 to 65). | 11.830 |
| MD | No | Peru | [137] | Riverside communities. High fish diet. | 4.800 |
| MD | No | Peru | [138] | Comparison of various dwellings in the Amarakaeri Reserve, age categorization (under 5 years old, and 5 to 11 years old). | 1.030† |
| MD | Yes | Peru | [139] | Comparison of various dwellings in the Amarakaeri Reserve. | 4.150 |
| A | Yes | Brazil | [140] | Riverside population. Prenatal exposure, women of childbearing age (15 to 49 years) | 6.490 |
| To, Ta | No | Brazil | [141] | Riverside communities with ages between 19 and 70 years (high THg) | 15.900† |
| M, N | Yes | Brazil | [142] | Lactating women. | 2.120 |
| To | No | Brazil | [143] | Riverine populations in the Tucuruí Dam reservoir area. | 8.120† |
| CP | Yes | Colombia | [26] | Indigenous communities in Tarapacá village. | 17.800† |
| MD | Yes | Peru | [144] | Women of child-bearing age. | 5.500 |
| Ta, To | No | Brazil | [145] | Children from riverside villages; born to women aged between 25 and 40. Fish-rich diet and primary exposure to Hg. | 22.380 |
| M | No | Brazil | [146] | Children/adolescents aged 6 to 14 along the Madeira River. | 3.070 |
| MD | Yes | Peru | [147] | Comparison of various dwellings in the Amarakaeri Reserve. Indigenous Native Children (6 to 15 years old) | 2.060 |
| RK | Yes | Guyana | [148] | Indigenous people from the Rupununi region (15 to 78 years old). High fish diet. Comparison of mining area and control area. | 27.620 |
| Ta, To | Yes | Brazil | [149] | Riverine men and miners and THg among them. High fish diet. Itaituba and Serra Pelada. | 20.000 |
| MD | Yes | Peru | [150] | Comparison of various dwellings in the Amarakaeri Reserve. Women of childbearing age (15 to 49 years). | 3.500† |
| M | Yes | Brazil | [151] | Riverine, rural, mining and urban communities. Women of childbearing age. | 12.220† |
| To | No | Brazil | [152] | Adults (18–70 years), Riverine populations in the Tucuruí Dam reservoir area. | 7.900† |
| Ap | Yes | Colombia | [153] | Population in different locations in mining regions. High fish diet. | 14.920 |
| Ta | No | Brazil | [154] | Munduruku indigenous reserve. Comparison between villages and categorization. Diet rich in fish. Age over 12 years old. | 8.500 |
| Ap | Yes | Colombia | [15] | Indigenous population of the Yaigojé Apaporis National Natural Park. | 34.900 |
| Ta | No | Brazil | [55] | Adult riverside residents only (18 to 60 years old). High consumption of fish. | 10.800 |
| Co | Yes | French Guiana | [155] | Only pregnant women, ethnic groups considered (15 to 41 years old); tribal and indigenous communities of Wayana. | 12.800 |
| M | No | Brazil | [156] | Mothers and children in childbirth after pregnancy at 6, 24 and 59 months of age. | 11.610 |
| GLM | Logistic regression | ||
| AIC | 257.26 | 53.867 | |
| parameters | P-value | P-value | |
| THg | Children | 0.6055 | 0.416 |
| Indigenous | 0.4201 | 0.273 | |
| Maternity | 0.2364 | 0.661 | |
| Riverside | 0.6108 | 0.287 | |
| Mining | 0.2413 | 0.774 |
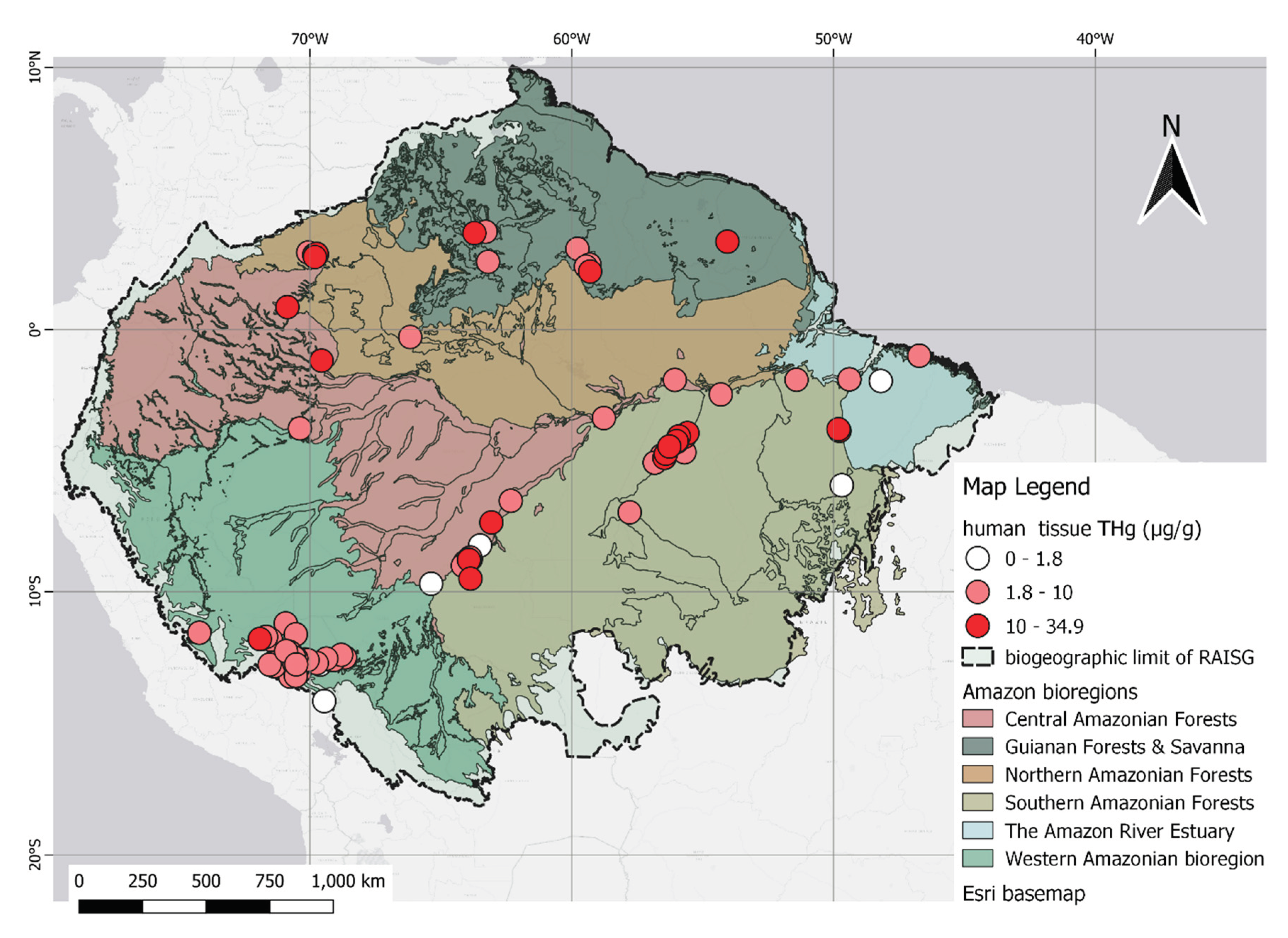
3.3. Mining
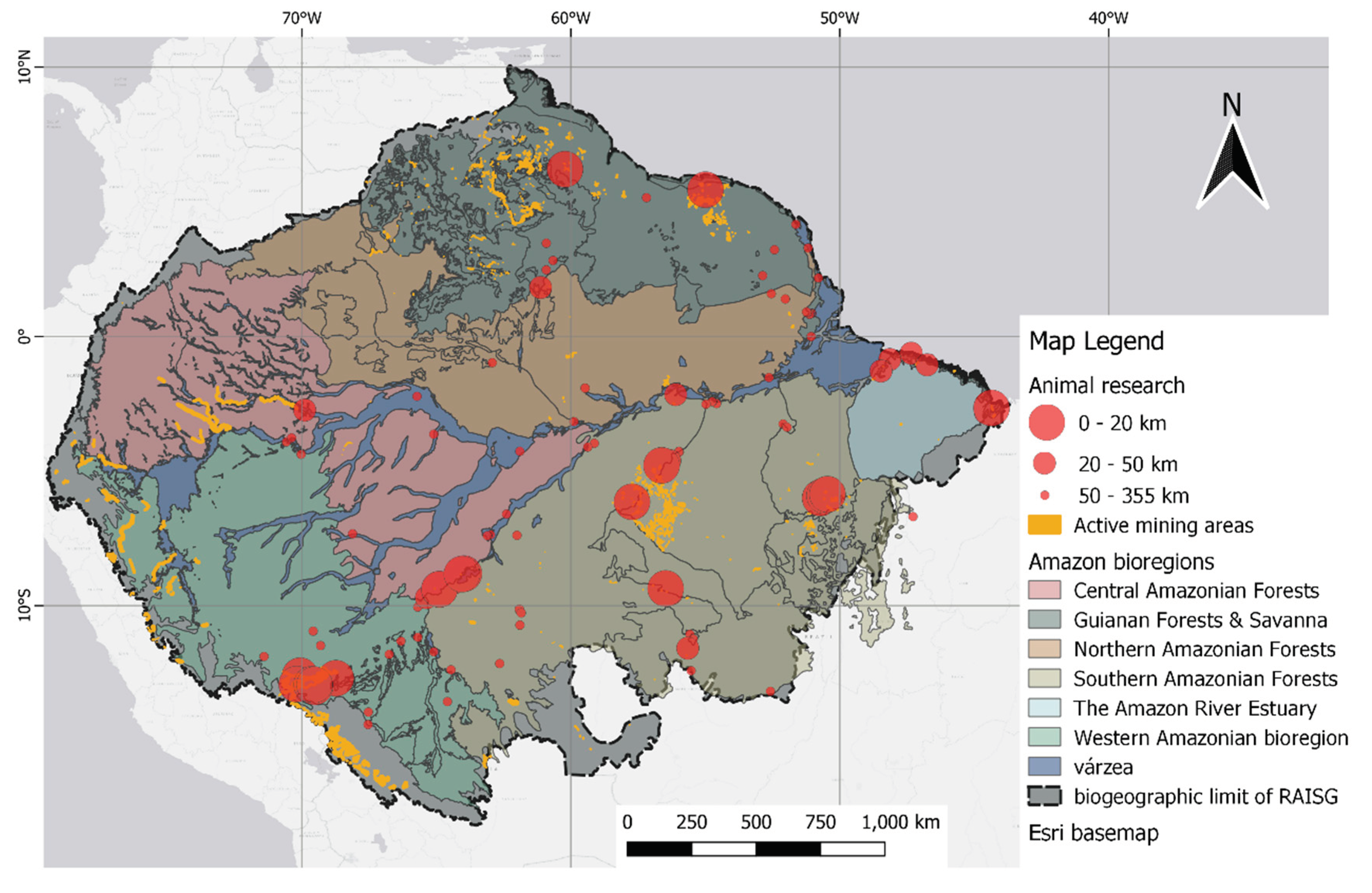
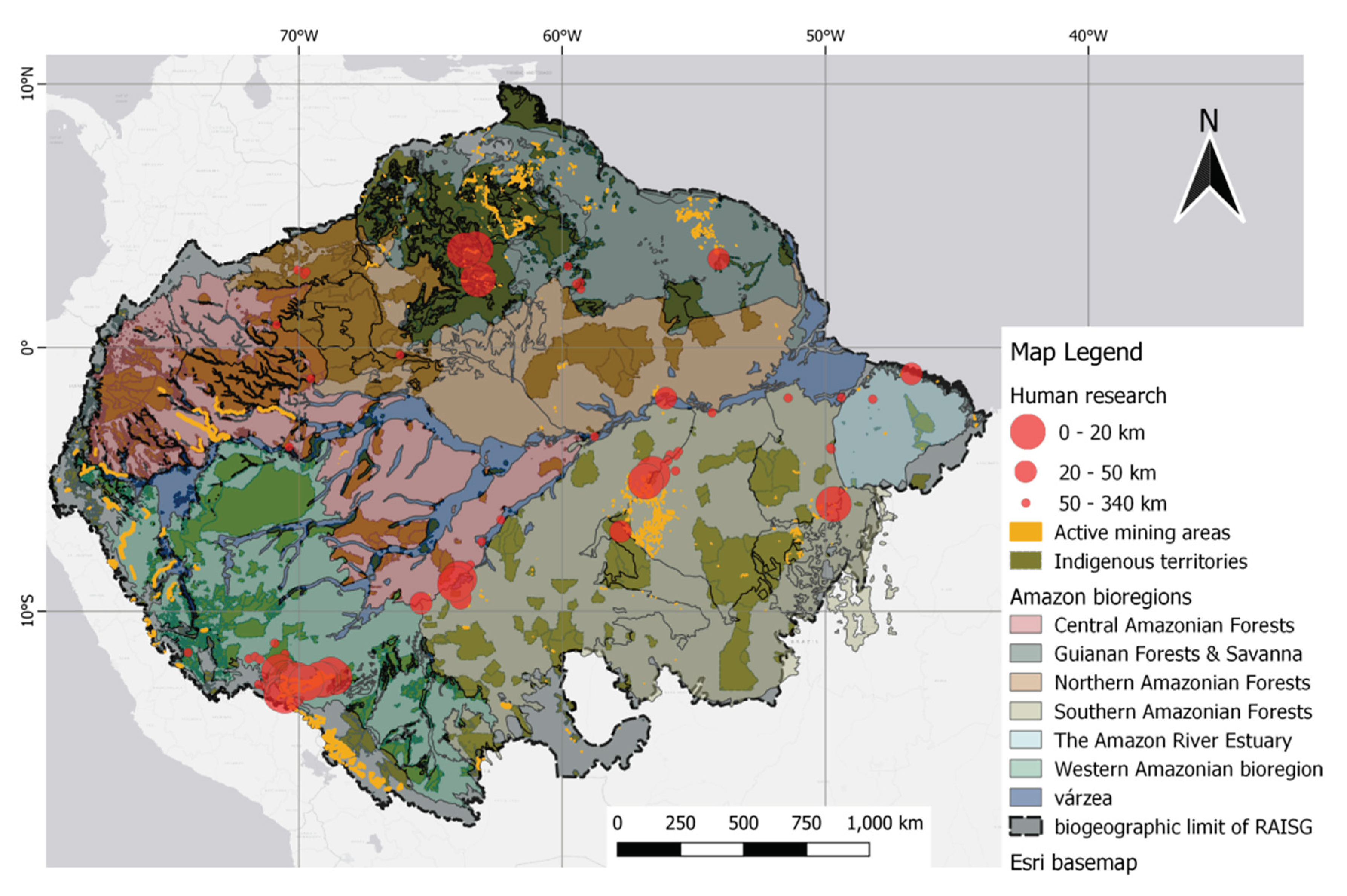
4. Discussion
4.1. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paiva, P.F.P.R.; de Lourdes Pinheiro Ruivo, M.; da Silva Júnior, O.M.; de Nazaré Martins Maciel, M.; Braga, T.G.M.; de Andrade, M.M.N.; dos Santos Junior, P.C.; da Rocha, E.S.; de Freitas, T.P.M.; da Silva Leite, T.V.; et al. Deforestation in Protect Areas in the Amazon: A Threat to Biodiversity. Biodiversity and Conservation 2020, 29, 19–38. [Google Scholar] [CrossRef]
- Garcia, B. The Amazon from an International Law Perspective. Cambridge University Press, 2011; 1-139-49668-9. [Google Scholar]
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharn, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia Is the Primary Source of Neotropical Biodiversity. Proceedings of the National Academy of Sciences 2018, 115, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Berenguer, E.; França, F.; Ferreira, J.; Lees, A.C.; Louzada, J.; Sayer, E.J.; Solar, R.; Smith, C.C.; Aragão, L.E.O.C.; et al. Linking Land-Use and Land-Cover Transitions to Their Ecological Impact in the Amazon. Proceedings of the National Academy of Sciences 2022, 119, 1–9. [Google Scholar]
- Matricardi, E.A.T.; Skole, D.L.; Costa, O.B.; Pedlowski, M.A.; Samek, J.H.; Miguel, E.P. Long-Term Forest Degradation Surpasses Deforestation in the Brazilian Amazon. Science 2020, 369, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Giljum, S.; Maus, V.; Kuschnig, N.; Luckeneder, S.; Tost, M.; Sonter, L.J.; Bebbington, A.J. A Pantropical Assessment of Deforestation Caused by Industrial Mining. Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2118273119. [Google Scholar] [CrossRef]
- Albert, J.S.; Carnaval, A.C.; Flantua, S.G.A.; Lohmann, L.G.; Ribas, C.C.; Riff, D.; Carrillo, J.D.; Fan, Y.; Figueiredo, J.J.P.; Guayasamin, J.M.; et al. Human Impacts Outpace Natural Processes in the Amazon. Science 2023, 379. [Google Scholar] [CrossRef]
- Vega, C.; Orellana, J.; Oliveira, M.; Hacon, S.; Basta, P. Human Mercury Exposure in Yanomami Indigenous Villages from the Brazilian Amazon. IJERPH 2018, 15, 1051. [Google Scholar] [CrossRef] [PubMed]
- Siqueira-Gay, J.; Sánchez, L.E. The Outbreak of Illegal Gold Mining in the Brazilian Amazon Boosts Deforestation. Regional Environmental Change 2021, 21, 1–5. [Google Scholar] [CrossRef]
- Achatz, R.W.; De Vasconcellos, A.C.S.; Pereira, L.; Viana, P.V.D.S.; Basta, P.C. Impacts of the Goldmining and Chronic Methylmercury Exposure on the Good-Living and Mental Health of Munduruku Native Communities in the Amazon Basin. IJERPH 2021, 18, 8994. [Google Scholar] [CrossRef]
- Basta, P.C.; De Sousa Viana, P.V.; De Vasconcellos, A.C.S.; Santos Périssé, A.R.; Hofer, C.B.; Paiva, N.S.; Kempton, J.W.; De Andrade, D.C.; De Oliveira, R.A.A.; Achatz, R.; et al. Mercury Exposure in Munduruku Indigenous Communities from Brazilian Amazon: Methodological Background and an Overview of the Principal Results. International Journal of Environmental Research and Public Health 2021, 18. [Google Scholar] [CrossRef]
- Hacon, S.D.S.; Oliveira-da-Costa, M.; Gama, C.D.S.; Ferreira, R.; Basta, P.C.; Schramm, A.; Yokota, D. Mercury Exposure through Fish Consumption in Traditional Communities in the Brazilian Northern Amazon. IJERPH 2020, 17, 5269. [Google Scholar] [CrossRef]
- Vasconcellos, A.C.S. de; Hallwass, G.; Bezerra, J.G.; Aciole, A.N.S.; Meneses, H.N. de M.; Lima, M. de O.; Jesus, I.M. de; Hacon, S. de S.; Basta, P.C. Health Risk Assessment of Mercury Exposure from Fish Consumption in Munduruku Indigenous Communities in the Brazilian Amazon. International Journal of Environmental Research and Public Health 2021, 18, 7940. [Google Scholar] [CrossRef]
- Pestana, I.A.; De Rezende, C.E.; Almeida, R.; De Lacerda, L.D.; Bastos, W.R. Let’s Talk about Mercury Contamination in the Amazon (Again): The Case of the Floating Gold Miners’ Village on the Madeira River. The Extractive Industries and Society 2022, 11, 101122. [Google Scholar] [CrossRef]
- Valdelamar-Villegas, J.; Olivero-Verbel, J. High Mercury Levels in the Indigenous Population of the Yaigojé Apaporis National Natural Park, Colombian Amazon. Biol Trace Elem Res 2020, 194, 3–12. [Google Scholar] [CrossRef]
- Calvimontes, J.; Massaro, L.; Araujo, C.H.X.; Moraes, R.R.; Mello, J.; Ferreira, L.C.; De Theije, M. Small-Scale Gold Mining and the COVID-19 Pandemic: Conflict and Cooperation in the Brazilian Amazon. The Extractive Industries and Society 2020, 7, 1347–1350. [Google Scholar] [CrossRef]
- Velásquez Ramírez, M.G.; Barrantes, J.A.G.; Thomas, E.; Gamarra Miranda, L.A.; Pillaca, M.; Tello Peramas, L.D.; Bazán Tapia, L.R. Heavy Metals in Alluvial Gold Mine Spoils in the Peruvian Amazon. Catena 2020, 189, 104454. [Google Scholar] [CrossRef]
- Adler Miserendino, R.; Guimarães, J.R.D.; Schudel, G.; Ghosh, S.; Godoy, J.M.; Silbergeld, E.K.; Lees, P.S.J.; Bergquist, B.A. Mercury Pollution in Amapá, Brazil: Mercury Amalgamation in Artisanal and Small-Scale Gold Mining or Land-Cover and Land-Use Changes? ACS Earth and Space Chemistry 2018, 2, 441–450. [Google Scholar] [CrossRef]
- Bullock, E.L.; Woodcock, C.E.; Souza, C.; Olofsson, P. Satellite-based Estimates Reveal Widespread Forest Degradation in the Amazon. Global Change Biology 2020, 26, 2956–2969. [Google Scholar] [CrossRef]
- Gallay, M.; Mora, A.; Martinez, J.; Gardel, A.; Laraque, A.; Sarrazin, M.; Beaucher, E.; Doudou, J.; Lagane, C. Dynamics and Fluxes of Organic Carbon and Nitrogen in Two Guiana Shield River Basins Impacted by Deforestation and Mining Activities. Hydrological Processes 2018, 32, 17–29. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Mora-Silva, D.; D’Orio, G.; Tapia-Segarra, E.; Gaibor, I.D.; Esparza Parra, J.F.; Chávez Velásquez, C.R.; Straface, S. Artisanal and Small-Scale Gold Mining (ASGM): Management and Socioenvironmental Impacts in the Northern Amazon of Ecuador. Sustainability 2022, 14, 6854. [Google Scholar] [CrossRef]
- Veiga, M.M.; Gunson, A.J. Gravity Concentration in Artisanal Gold Mining. Minerals 2020, 10, 1026. [Google Scholar] [CrossRef]
- Dethier, E.N.; Sartain, S.L.; Lutz, D.A. Heightened Levels and Seasonal Inversion of Riverine Suspended Sediment in a Tropical Biodiversity Hot Spot Due to Artisanal Gold Mining. Proceedings of the National Academy of Sciences of the United States of America 2019, 116, 23936–23941. [Google Scholar] [CrossRef]
- Esdaile, L.J.; Chalker, J.M. The Mercury Problem in Artisanal and Small-Scale Gold Mining. Chemistry - A European Journal 2018, 24, 6905–6916. [Google Scholar] [CrossRef]
- Espejo, J.C.; Messinger, M.; Román-Dañobeytia, F.; Ascorra, C.; Fernandez, L.E.; Silman, M. Deforestation and Forest Degradation Due to Gold Mining in the Peruvian Amazon: A 34-Year Perspective. Remote Sensing 2018, 10, 1–17. [Google Scholar] [CrossRef]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Olivero-Verbel, J. Mercury Exposure Assessment in Indigenous Communities from Tarapaca Village, Cotuhe and Putumayo Rivers, Colombian Amazon. Environ Sci Pollut Res 2019, 26, 36458–36467. [Google Scholar] [CrossRef]
- Cosio, C. Special Issue on Bioconversion, Bioaccumulation and Toxicity of Mercury in a Changing World. Applied Sciences 2020, 10, 6548. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Pereira, W.V.D.S.; Souza, E.S.D.; Ramos, S.J.; Dias, Y.N.; Lima, M.W.D.; De Souza Neto, H.F.; Oliveira, E.S.D.; Fernandes, A.R. Artisanal Gold Mining in the Eastern Amazon: Environmental and Human Health Risks of Mercury from Different Mining Methods. Chemosphere 2021, 284, 131220. [Google Scholar] [CrossRef]
- Fujimura, M.; Usuki, F. Cellular Conditions Responsible for Methylmercury-Mediated Neurotoxicity. International Journal of Molecular Sciences 2022, 23, 7218. [Google Scholar] [CrossRef]
- Vasconcellos, A.C.S.D.; Hallwass, G.; Bezerra, J.G.; Aciole, A.N.S.; Meneses, H.N.D.M.; Lima, M.D.O.; Jesus, I.M.D.; Hacon, S.D.S.; Basta, P.C. Health Risk Assessment of Mercury Exposure from Fish Consumption in Munduruku Indigenous Communities in the Brazilian Amazon. IJERPH 2021, 18, 7940. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Yuki Takeda, P.; Macchi, B. de M.; do Nascimento, J.L.M.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What Can We Learn from the Amazon? Environment International 2021, 146. [Google Scholar] [CrossRef]
- Crespo-López, M.E.; Augusto-Oliveira, M.; Santos-Sacramento, L.; Lopes-Araújo, A. Mercúrio Na Amazônia Uma Breve Contextualizaçao Do Problema. 2021.
- De Oliveira, D.F.; De Castro, B.S.; Do Nascimento Recktenvald, M.C.N.; Da Costa Júnior, W.A.; Da Silva, F.X.; De Menezes Alves, C.L.; Froehlich, J.D.; Bastos, W.R.; Ott, A.M.T. Mercury in Wild Animals and Fish and Health Risk for Indigenous Amazonians. Food Additives & Contaminants: Part B 2021, 14, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hacon, S. de S.; Oliveira-Da-costa, M.; Gama, C. de S.; Ferreira, R.; Basta, P.C.; Schramm, A.; Yokota, D. Mercury Exposure through Fish Consumption in Traditional Communities in the Brazilian Northern Amazon. International Journal of Environmental Research and Public Health 2020, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Baturin, G.; Gordeev, V. Geochemistry of Suspended Matter in the Amazon River Waters. Geochemistry International 2019, 57, 197–205. [Google Scholar] [CrossRef]
- Kasper, D.; Forsberg, B.R.; do Amaral Kehrig, H.; Amaral, J.H.F.; Bastos, W.R.; Malm, O. Mercury in Black-Waters of the Amazon; 2018; ISBN 978-3-319-90121-3.
- Fisher, J.A.; Schneider, L.; Fostier, A.-H.; Guerrero, S.; Guimarães, J.R.D.; Labuschagne, C.; Leaner, J.J.; Martin, L.G.; Mason, R.P.; Somerset, V.; et al. A Synthesis of Mercury Research in the Southern Hemisphere, Part 2: Anthropogenic Perturbations. Ambio 2023, 52, 918–937. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.C.; Forsberg, B.R.; Kasper, D.; Amaral, J.H.F.; De Vasconcelos, M.R.R.; De Sousa, O.P.; Cunha, F.A.G.; Bastos, W.R. The Influence of Inundation and Lake Morphometry on the Dynamics of Mercury in the Water and Plankton in an Amazon Floodplain Lake. Hydrobiologia 2017, 790, 35–48. [Google Scholar] [CrossRef]
- Salazar-Camacho, C.; Salas-Moreno, M.; Marrugo-Madrid, S.; Marrugo-Negrete, J.; Díez, S. Dietary Human Exposure to Mercury in Two Artisanal Small-Scale Gold Mining Communities of Northwestern Colombia. Environment International 2017, 107, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Diringer, S.E.; Berky, A.J.; Marani, M.; Ortiz, E.J.; Karatum, O.; Plata, D.L.; Pan, W.K.; Hsu-Kim, H. Deforestation Due to Artisanal and Small-Scale Gold Mining Exacerbates Soil and Mercury Mobilization in Madre de Dios, Peru. Environmental Science and Technology 2019. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sacramento, L.; Arrifano, G.P.; Lopes-Araújo, A.; Augusto-Oliveira, M.; Albuquerque-Santos, R.; Takeda, P.Y.; Souza-Monteiro, J.R.; Macchi, B.M.; do Nascimento, J.L.M.; Lima, R.R.; et al. Human Neurotoxicity of Mercury in the Amazon: A Scoping Review with Insights and Critical Considerations. Ecotoxicology and Environmental Safety 2021, 208. [Google Scholar] [CrossRef]
- Miguel V. M., Neto; Darlan, Q. Brito Mercury (Hg) Researches in Brazilian Biomes: A Scientometric Analysis between the Years 1991 and 2018. J Veterina Sci Res 2021, 01–13. [Google Scholar] [CrossRef]
- Basta, P.C.; De Vasconcellos, A.C.S.; Hallwass, G.; Yokota, D.; Pinto, D.D.O. d’El R.; De Aguiar, D.S.; De Souza, C.C.; Oliveira-da-Costa, M. Risk Assessment of Mercury-Contaminated Fish Consumption in the Brazilian Amazon: An Ecological Study. Toxics 2023, 11, 800. [Google Scholar] [CrossRef]
- Moulatlet, G.M.; Yacelga, N.; Rico, A.; Mora, A.; Hauser-Davis, R.A.; Cabrera, M.; Capparelli, M.V. A Systematic Review on Metal Contamination Due to Mining Activities in the Amazon Basin and Associated Environmental Hazards. Chemosphere 2023, 339, 139700. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, T.C.; Penteado, J.O.; dos Santos, M.; da Silva Júnior, F.M.R. Methylmercury in Fish from the Amazon Region—a Review Focused on Eating Habits. Water, Air, and Soil Pollution 2021, 232. [Google Scholar] [CrossRef]
- RAISG Amazon in Numbers 2022.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Systematic Reviews 2021, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; 2nd ed.; John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England: Chichester (UK), 2019.
- Kirby, A. Exploratory Bibliometrics: Using VOSviewer as a Preliminary Research Tool. Publications 2023, 11, 10. [Google Scholar] [CrossRef]
- Cohen Hubal, E.A.; Frank, J.J.; Nachman, R.; Angrish, M.; Deziel, N.C.; Fry, M.; Tornero-Velez, R.; Kraft, A.; Lavoie, E. Advancing Systematic-Review Methodology in Exposure Science for Environmental Health Decision Making. Journal of Exposure Science and Environmental Epidemiology 2020, 30, 906–916. [Google Scholar] [CrossRef] [PubMed]
- FAO. WHO GENERAL STANDARD FOR CONTAMINANTS AND TOXINS IN FOOD AND FEED. CODEX ALIMENTARIUS 2019, 1–13. [Google Scholar]
- WHO Chapter 6.9 Mercury General Description. Air Quality Guidelines 2000, 1–15.
- Ministério da Saúde RESOLUÇÃO - RDC No 42, DE 29 DE AGOSTO DE 2013 2013.
- Poulin, J.; Gibb, H.; Prüss-Üstün, A. World Health Organization Mercury: Assessing the Environmental Burden of Disease at National and Local Levels. 2008.
- Costa Junior, J.M.F.; da Silva, C.I.M.; Lima, A.A. da S.; Rodrigues Júnior, D.; Silveira, L.C. de L.; Souza, G. da S.; Pinheiro, M. da C.N. Teores de Mercúrio Em Cabelo e Consumo de Pescado de Comunidades Ribeirinhas Na Amazônia Brasileira, Região Do Tapajós. Ciencia e Saude Coletiva 2018, 23, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Yao, C.; Tian, X.; Wu, Y.; Xie, Q.; Wang, D. Mercury in Human Hair and Its Implications for Health Investigation. Current Opinion in Environmental Science and Health 2021, 22, 100271. [Google Scholar] [CrossRef]
- QGIS Association QGIS.Org 2023.
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef]
- Southern America | Realm & Subrealms | One Earth. Available online: https://www.oneearth.org/realms/southern-america/ (accessed on 16 November 2023).
- Tang, L.; Werner, T.T. Author Correction: Global Mining Footprint Mapped from High-Resolution Satellite Imagery. Commun Earth Environ 2023, 4, 163. [Google Scholar] [CrossRef]
- Yevugah, L.L.; Darko, G.; Bak, J. Does Mercury Emission from Small-Scale Gold Mining Cause Widespread Soil Pollution in Ghana? Environmental Pollution 2021, 284, 116945. [Google Scholar] [CrossRef] [PubMed]
- Goix, S.; Maurice, L.; Laffont, L.; Rinaldo, R.; Lagane, C.; Chmeleff, J.; Menges, J.; Heimbürger, L.-E.; Maury-Brachet, R.; Sonke, J.E. Quantifying the Impacts of Artisanal Gold Mining on a Tropical River System Using Mercury Isotopes. Chemosphere 2019, 219, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.R.; Szponar, N.; Zambrano, A.A.; Bergquist, B.; Broadbent, E.; Driscoll, C.T.; Erkenswick, G.; Evers, D.C.; Fernandez, L.E.; Hsu-Kim, H. Amazon Forests Capture High Levels of Atmospheric Mercury Pollution from Artisanal Gold Mining. Nature communications 2022, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Feingold, B.J.; Berky, A.; Hsu-Kim, H.; Rojas Jurado, E.; Pan, W.K. Population-Based Dietary Exposure to Mercury through Fish Consumption in the Southern Peruvian Amazon. Environmental Research 2020, 183, 108720. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2023.
- Silva, S.F.D.; Oliveira, D.C.; Pereira, J.P.G.; Castro, S.P.; Costa, B.N.S.; Lima, M.D.O. Seasonal Variation of Mercury in Commercial Fishes of the Amazon Triple Frontier, Western Amazon Basin. Ecological Indicators 2019, 106, 105549. [Google Scholar] [CrossRef]
- Ferreira Da Silva, S.; De Oliveira Lima, M. Mercury in Fish Marketed in the Amazon Triple Frontier and Health Risk Assessment. Chemosphere 2020, 248, 125989. [Google Scholar] [CrossRef] [PubMed]
- Souza-Araujo, J.; Souza-Junior, O.G.; Guimarães-Costa, A.; Hussey, N.E.; Lima, M.O.; Giarrizzo, T. The Consumption of Shark Meat in the Amazon Region and Its Implications for Human Health and the Marine Ecosystem. Chemosphere 2021, 265, 129132. [Google Scholar] [CrossRef]
- Albuquerque, F.E.A.; Minervino, A.H.H.; Miranda, M.; Herrero-Latorre, C.; Barrêto Júnior, R.A.; Oliveira, F.L.C.; Sucupira, M.C.A.; Ortolani, E.L.; López-Alonso, M. Toxic and Essential Trace Element Concentrations in Fish Species in the Lower Amazon, Brazil. Science of The Total Environment 2020, 732, 138983. [Google Scholar] [CrossRef]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Mercury and Selenium in Fishes from the Tapajós River in the Brazilian Amazon: An Evaluation of Human Exposure. Journal of Trace Elements in Medicine and Biology 2018, 48, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Olivero-Verbel, J. Biomonitoring of Mercury, Cadmium and Selenium in Fish and the Population of Puerto Nariño, at the Southern Corner of the Colombian Amazon. Arch Environ Contam Toxicol 2020, 79, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.F.; Pereira, J.P.G.; Oliveira, D.C.; Lima, M.D.O. Methylmercury in Predatory and Non-Predatory Fish Species Marketed in the Amazon Triple Frontier. Bull Environ Contam Toxicol 2020, 104, 733–737. [Google Scholar] [CrossRef] [PubMed]
- De Castro Paiva, T.; Dary, E.P.; Pestana, I.A.; Amadio, S.A.; Malm, O.; Kasper, D. Flood-Pulse and Trophic Position Modulate Mercury Concentrations in Fishes from an Amazon Floodplain Lake. Environmental Research 2022, 215, 114307. [Google Scholar] [CrossRef]
- Finoto Viana, L.; Damasceno De Souza, D.C.; Batista Da Silva, E.; Kummrow, F.; Lima Cardoso, C.A.; De Lima, N.A.; Crispim, B.D.A.; Barufatti, A.; Florentino, A.C. Bioaccumulation of Metals and Genotoxic Effects in Females of Colomesus Asellus Collected in an Amazon River Estuary, Amapá, Brazil. Limnetica 2023, 42, 1. [Google Scholar] [CrossRef]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Total and Methyl Mercury Distribution in Water, Sediment, Plankton and Fish along the Tapajós River Basin in the Brazilian Amazon. Chemosphere 2019, 235, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.B.D.; Silva, D.M.D.; Franco, T.S.B.S.; Vasconcelos, C.R.S.; Sousa, D.J.D.A.D.; Sarrazin, S.L.F.; Sakamoto, M.; Bourdineaud, J.-P. Fish Consumption Habits of Pregnant Women in Itaituba, Tapajós River Basin, Brazil: Risks of Mercury Contamination as Assessed by Measuring Total Mercury in Highly Consumed Piscivore Fish Species and in Hair of Pregnant Women. Archives of Industrial Hygiene and Toxicology 2022, 73, 131–142. [Google Scholar] [CrossRef]
- De Souza Azevedo, J.; Hortellani, M.A.; De Souza Sarkis, J.E. Organotropism of Total Mercury (THg) in Cichla Pinima, Ecological Aspects and Human Consumption in Fish from Amazon Region, Brazil. Environ Sci Pollut Res 2019, 26, 21363–21370. [Google Scholar] [CrossRef]
- Da Silva Montes, C.; Ferreira, M.A.P.; Giarrizzo, T.; Amado, L.L.; Rocha, R.M. The Legacy of Artisanal Gold Mining and Its Impact on Fish Health from Tapajós Amazonian Region: A Multi-Biomarker Approach. Chemosphere 2022, 287, 132263. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pestana, I.A.; Da Costa Nery, A.F.; Bastos, W.R.; Souza, C.M.M. Variation in Hg Accumulation between Demersal and Pelagic Fish from Puruzinho Lake, Brazilian Amazon. Ecotoxicology 2019, 28, 1143–1149. [Google Scholar] [CrossRef]
- Cavecci-Mendonça, B.; Cavalcante de Souza Vieira, J.; Monteiro de Lima, P.; Leite, A.L.; Buzalaf, M.A.R.; Zara, L.F.; de Magalhães Padilha, P. Study of Proteins with Mercury in Fish from the Amazon Region. Food Chemistry 2020, 309, 125460. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, J.V.; Cavecci-Mendonça, B.; Vieira, J.C.S.; Martins, R.A.; de Almeida Assunção, A.S.; Cavallini, N.G.; dos Santos, F.A.; de Magalhães Padilha, P. Metalloproteomic Strategies for Identifying Proteins as Biomarkers of Mercury Exposure in Serrasalmus Rhombeus from the Amazon Region. Biological Trace Element Research 2021, 199, 712–720. [Google Scholar] [CrossRef]
- Soares, J.M.; Gomes, J.M.; Anjos, M.R.; Silveira, J.N.; Custódio, F.B.; Gloria, M.B.A. Mercury in Fish from the Madeira River and Health Risk to Amazonian and Riverine Populations. Food Research International 2018, 109, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.S.; Pestana, I.A.; Nery, A.F.D.C.; Bastos, W.R.; Souza, C.M.M. Influence of the Flood Pulse on Mercury Accumulation in Detritivorous, Herbivorous and Omnivorous Fish in Brazilian Amazonia. Ecotoxicology 2019, 28, 478–485. [Google Scholar] [CrossRef]
- Vieira, J.C.S.; De Oliveira, G.; Cavallini, N.G.; Braga, C.P.; Adamec, J.; Zara, L.F.; Buzalaf, M.A.R.; De Magalhães Padilha, P. Investigation of Protein Biomarkers and Oxidative Stress in Pinirampus Pirinampu Exposed to Mercury Species from the Madeira River, Amazon-Brazil. Biol Trace Elem Res 2022, 200, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha Bataglioli, I.; De Queiroz, J.V.; Vieira, J.C.S.; Cavalline, N.G.; Braga, C.P.; Buzalaf, M.A.R.; Zara, L.F.; Adamec, J.; De Magalhães Padilha, P. Mercury Metalloproteomic Profile in Muscle Tissue of Arapaima Gigas from the Brazilian Amazon. Environ Monit Assess 2022, 194, 705. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pestana, I.A.; Almeida, M.G.; Ferreira Da Costa Nery, A.; Bastos, W.R.; Magalhães Souza, C.M. Mercury Biomagnification in an Ichthyic Food Chain of an Amazon Floodplain Lake (Puruzinho Lake): Influence of Seasonality and Food Chain Modeling. Ecotoxicology and Environmental Safety 2021, 207, 111249. [Google Scholar] [CrossRef]
- Bittarello, A.C.; Vieira, J.C.S.; Braga, C.P.; De Paula Araújo, W.L.; Da Cunha Bataglioli, I.; Da Silva, J.M.; Buzalaf, M.A.R.; Fleuri, L.F.; De Magalhães Padilha, P. Characterization of Molecular Biomarkers of Mercury Exposure to Muscle Tissue of Plagioscion Squamosissimus and Colossoma Macropomum from the Amazon Region. Food Chemistry 2019, 276, 247–254. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, J.V.; Vieira, J.C.S.; De Oliveira, G.; Braga, C.P.; Da Cunha Bataglioli, I.; Da Silva, J.M.; De Paula Araújo, W.L.; De Magalhães Padilha, P. Identification of Biomarkers of Mercury Contamination in Brachyplatystoma Filamentosum of the Madeira River, Brazil, Using Metalloproteomic Strategies. Biol Trace Elem Res 2019, 187, 291–300. [Google Scholar] [CrossRef]
- Mussy, M.H.; De Almeida, R.; De Carvalho, D.P.; Lauthartte, L.C.; De Holanda, I.B.B.; Almeida, M.G.D.; De Sousa-Filho, I.F.; De Rezende, C.E.; Malm, O.; Bastos, W.R. Evaluating Total Mercury and Methylmercury Biomagnification Using Stable Isotopes of Carbon and Nitrogen in Fish from the Madeira River Basin, Brazilian Amazon. Environ Sci Pollut Res 2022, 30, 33543–33554. [Google Scholar] [CrossRef]
- Reis, P.A.; Ozório, R.O.A.; Rodriguez, A.F.R.; Faria, F.S.E.D.V.; Furtado, C.M.; Ribeiro, R.A. Mercury Distribution in Two Commercial Fish Species (Pimelodus Maculatus and Calophysus Macropterus) - Case Study of River Acre (Acre State, Brazilian Amazon). Human and Ecological Risk Assessment 2020, 26, 1439–1448. [Google Scholar] [CrossRef]
- Vieira, J.C.S.; Braga, C.P.; Queiroz, J.V.D.; Cavecci-Mendonça, B.; Oliveira, G.D.; Freitas, N.G.D.; Fernandes, A.A.H.; Fernandes, M.D.S.; Buzalaf, M.A.R.; Adamec, J.; et al. The Effects of Mercury Exposure on Amazonian Fishes: An Investigation of Potential Biomarkers. Chemosphere 2023, 316, 137779. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.C.S.; Braga, C.P.; De Oliveira, G.; Padilha, C.D.C.F.; De Moraes, P.M.; Zara, L.F.; Leite, A.D.L.; Buzalaf, M.A.R.; Padilha, P.D.M. Mercury Exposure: Protein Biomarkers of Mercury Exposure in Jaraqui Fish from the Amazon Region. Biol Trace Elem Res 2018, 183, 164–171. [Google Scholar] [CrossRef]
- De Queiroz, J.V.; Vieira, J.C.S.; Da Cunha Bataglioli, I.; Bittarello, A.C.; Braga, C.P.; De Oliveira, G.; Do Carmo Federici Padilha, C.; De Magalhães Padilha, P. Total Mercury Determination in Muscle and Liver Tissue Samples from Brazilian Amazon Fish Using Slurry Sampling. Biol Trace Elem Res 2018, 184, 517–522. [Google Scholar] [CrossRef]
- Vieira, J.C.S.; Cavecci, B.; Queiroz, J.V.; Braga, C.P.; Padilha, C.C.F.; Leite, A.L.; Figueiredo, W.S.; Buzalaf, M.A.R.; Zara, L.F.; Padilha, P.M. Determination of the Mercury Fraction Linked to Protein of Muscle and Liver Tissue of Tucunaré (Cichla Spp.) from the Amazon Region of Brazil. Arch Environ Contam Toxicol 2015, 69, 422–430. [Google Scholar] [CrossRef]
- da Silva Montes, C.; Pantoja Ferreira, M.A.; Giarrizzo, T.; Amado, L.L.; Rocha, R.M. Evaluation of Metal Contamination Effects in Piranhas through Biomonitoring and Multi Biomarkers Approach. Heliyon 2020, 6, e04666. [Google Scholar] [CrossRef]
- Souza-Araujo, J.; Andrades, R.; Hauser-Davis, R.A.; Lima, M.O.; Giarrizzo, T. Before the Dam: A Fish-Mercury Contamination Baseline Survey at the Xingu River, Amazon Basin Before the Belo Monte Dam. Bull Environ Contam Toxicol 2022, 108, 861–866. [Google Scholar] [CrossRef]
- De Matos, L.S.; Silva Correa, A.S.A.; Da Silva, S.A.A.; Muniz, C.C.; Alves Ignacio, A.R. Mercury Concentrations in Fish and Human Health Assessment in Preflood Phase of a Hydro Dam in Teles Pires River, Southern Brazilian Amazon. Elementa 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Matos, L.S.D.; Silva, J.O.S.; Kasper, D.; Carvalho, L.N. Assessment of Mercury Contamination in Brycon Falcatus (Characiformes: Bryconidae) and Human Health Risk by Consumption of This Fish from the Teles Pires River, Southern Amazonia. Neotrop. ichthyol. 2018, 16. [Google Scholar] [CrossRef]
- Viana, L.F.; Kummrow, F.; Cardoso, C.A.L.; De Lima, N.A.; Do Amaral Crispim, B.; Barufatti, A.; Florentino, A.C. Metal Bioaccumulation in Fish from the Araguari River (Amazon Biome) and Human Health Risks from Fish Consumption. Environ Sci Pollut Res 2023, 30, 4111–4122. [Google Scholar] [CrossRef]
- Da Silva Costa, M.; Viana, L.F.; Lima Cardoso, C.A.; Gonar Silva Isacksson, E.D.; Silva, J.C.; Florentino, A.C. Landscape Composition and Inorganic Contaminants in Water and Muscle Tissue of Plagioscion Squamosissimus in the Araguari River (Amazon, Brazil). Environmental Research 2022, 208, 112691. [Google Scholar] [CrossRef]
- Costa, I.D.D.; Nascimento, E.L.D.; Faccheti, M.S.D.A.; Nunes, N.N.D.S.; Gomes, J.P.D.O.; Almeida, R.D.; Bastos, W.R. Mercury in Muscle and Liver of Plagioscion Squamosissimus (Acanthuriformes: Sciaenidae) from the Machado River, Brazilian Amazon. Acta Amaz. 2022, 52, 60–68. [Google Scholar] [CrossRef]
- Martinez, G.; McCord, S.; Driscoll, C.; Todorova, S.; Wu, S.; Araújo, J.; Vega, C.; Fernandez, L. Mercury Contamination in Riverine Sediments and Fish Associated with Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru. IJERPH 2018, 15, 1584. [Google Scholar] [CrossRef] [PubMed]
- Barocas, A.; Vega, C.; Alarcon Pardo, A.; Araujo Flores, J.M.; Fernandez, L.; Groenendijk, J.; Pisconte, J.; Macdonald, D.W.; Swaisgood, R.R. Local Intensity of Artisanal Gold Mining Drives Mercury Accumulation in Neotropical Oxbow Lake Fishes. Science of The Total Environment 2023, 886, 164024. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Levy, I.E.; Van Damme, P.A.; Carvajal-Vallejos, F.M.; Bervoets, L. Trace Element Accumulation in Different Edible Fish Species from the Bolivian Amazon and the Risk for Human Consumption. Heliyon 2022, 8, e11649. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcellos, A.C.S.; Ferreira, S.R.B.; De Sousa, C.C.; De Oliveira, M.W.; De Oliveira Lima, M.; Basta, P.C. Health Risk Assessment Attributed to Consumption of Fish Contaminated with Mercury in the Rio Branco Basin, Roraima, Amazon, Brazil. Toxics 2022, 10, 516. [Google Scholar] [CrossRef]
- Borges, A.C.; Da Silva Montes, C.; Barbosa, L.A.; Ferreira, M.A.P.; Berrêdo, J.F.; Martins Rocha, R. Integrated Use of Histological and Ultrastructural Biomarkers for Assessing Mercury Pollution in Piranhas (Serrasalmus Rhombeus) from the Amazon Mining Region. Chemosphere 2018, 202, 788–796. [Google Scholar] [CrossRef]
- Vreedzaam, A.; Ouboter, P.; Hindori-Mohangoo, A.D.; Lepak, R.; Rumschlag, S.; Janssen, S.; Landburg, G.; Shankar, A.; Zijlmans, W.; Lichtveld, M.Y.; et al. Contrasting Mercury Contamination Scenarios and Site Susceptibilities Confound Fish Mercury Burdens in Suriname, South America. Environmental Pollution 2023, 336, 122447. [Google Scholar] [CrossRef]
- Laffont, L.; Menges, J.; Goix, S.; Gentès, S.; Maury-Brachet, R.; Sonke, J.E.; Legeay, A.; Gonzalez, P.; Rinaldo, R.; Maurice, L. Hg Concentrations and Stable Isotope Variations in Tropical Fish Species of a Gold-Mining-Impacted Watershed in French Guiana. Environ Sci Pollut Res 2021, 28, 60609–60621. [Google Scholar] [CrossRef]
- Montaña, C.G.; Liverpool, E.; Taphorn, D.C.; Schalk, C.M. The Cost of Gold: Mercury Contamination of Fishes in a Neotropical River Food Web. Neotrop. ichthyol. 2021, 19, e200155. [Google Scholar] [CrossRef]
- Cunha Bataglioli, I.D.; Souza Vieira, J.C.; Vitor De Queiroz, J.; Da Silva Fernandes, M.; Bittarello, A.C.; Braga, C.P.; Rabelo Buzalaf, M.A.; Adamec, J.; Zara, L.F.; Magalhães Padilha, P.D. Physiological and Functional Aspects of Metal-Binding Protein Associated with Mercury in the Liver Tissue of Pirarucu (Arapaima Gigas) from the Brazilian Amazon. Chemosphere 2019, 236, 124320. [Google Scholar] [CrossRef]
- Viana, L.F.; Cardoso, C.A.L.; Lima-Junior, S.E.; Súarez, Y.R.; Florentino, A.C. Bioaccumulation of Metal in Liver Tissue of Fish in Response to Water Toxicity of the Araguari-Amazon River, Brazil. Environmental Monitoring and Assessment 2020, 192. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, F.E.A.; Minervino, A.H.H.; Miranda, M.; Herrero-Latorre, C.; Barrêto Júnior, R.A.; Oliveira, F.L.C.; Dias, S.R.; Ortolani, E.L.; López-Alonso, M. Toxic and Essential Trace Element Concentrations in the Freshwater Shrimp Macrobrachium Amazonicum in the Lower Amazon, Brazil. Journal of Food Composition and Analysis 2020, 86, 103361. [Google Scholar] [CrossRef]
- Pinheiro-Sousa, D.B.; da Costa Soares, S.H.; Torres, H.S.; de Jesus, W.B.; de Oliveira, S.R.S.; Bastos, W.R.; de Oliveira Ribeiro, C.A.; Carvalho-Neta, R.N.F. Sediment Contaminant Levels and Multibiomarker Approach to Assess the Health of Catfish Sciades Herzbergii in a Harbor from the Northern Brazilian Amazon. Ecotoxicology and Environmental Safety 2021, 208. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.C.B.; de CARVALHO, G.O.; Bernardo, R.R.; Macedo, J.; Lino, A.S.; Ramalho, E.E.; Kasper, D.; Meire, R.O.; Torres, J.P.M.; Malm, O. Total Mercury in Wild Felids Occurring in Protected Areas in the Central Brazilian Amazon. Acta Amazonica 2020, 50, 142–148. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Carvalho, D.P.; Gravena, W.; De Almeida, R.; Mussy, M.H.; Sousa, E.A.; Holanda, I.B.B.; De Sousa-Filho, I.F.; Bastos, W.R. Total Mercury and Methylmercury in River Dolphins (Cetacea: Iniidae: Inia Spp.) in the Madeira River Basin, Western Amazon. Environ Sci Pollut Res 2021, 28, 45121–45133. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Pammo, A.C.; Achá, D.; Miranda-Chumacero, G. Preferential Liver Accumulation of Mercury Explains Low Concentrations in Muscle of Caiman Yacare (Alligatoridae) in Upper Amazon. Bull Environ Contam Toxicol 2021, 106, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Dias Dos Santos, A.N.; Recktenvald, M.C.N. do N.; De Carvalho, D.P.; Bortoleto Puerta, E.L.; De Sousa-Filho, I.F.; Dórea, J.G.; Bastos, W.R. Mercury in Birds (Aquatic and Scavenger) from the Western Amazon. Environmental Research 2021, 201, 111574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Divoll, T.J.; Ganguli, P.M.; Trama, F.A.; Lamborg, C.H. Presence of Artisanal Gold Mining Predicts Mercury Bioaccumulation in Five Genera of Bats (Chiroptera). Environmental Pollution 2018, 236, 862–870. [Google Scholar] [CrossRef]
- Moreno-Brush, M.; Portillo, A.; Brändel, S.D.; Storch, I.; Tschapka, M.; Biester, H. Mercury Concentrations in Bats (Chiroptera) from a Gold Mining Area in the Peruvian Amazon. Ecotoxicology 2018, 27, 45–54. [Google Scholar] [CrossRef]
- Da Silva Júnior, F.J.T.M.; Ribeiro, J.D.N.; Da Silva, H.L.A.; Da Silva Carneiro, C.; De Jesus, E.F.O.; De Araújo, U.B.; Lazzarini, S.M.; Souza, A.R.; Simões, J.S.; Lopes, R.T.; et al. Study of Inorganic Elements in Different Organs and Tissues of Amazonian Manatee (Trichechus Inunguis) from Brazil. Environ Sci Pollut Res 2022, 29, 30486–30495. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Guerra, F.; Trujillo, F.; Parks, D.; Oliveira-da-Costa, M.; Van Damme, P.A.; Echeverría, A.; Franco, N.; Carvajal-Castro, J.D.; Mantilla-Meluk, H.; Marmontel, M.; et al. Mercury in Populations of River Dolphins of the Amazon and Orinoco Basins. EcoHealth 2019, 16, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Borges, Â.O.; Erickson, J.; Silva, L.A.D.; Fantin, C.; Domingos-Moreira, F.X.V. Mercury Bioaccumulation, Genotoxic and Biochemical Biomarkers Reveal the Health Status of Yellow-Spotted Amazon River Turtles (Podocnemis Unifilis) in an Environmental Protection Area in the Amazon. Acta Amaz. 2022, 52, 254–263. [Google Scholar] [CrossRef]
- Pignati, M.; Pezzuti, J.; Souza, L.; Lima, M.; Pignati, W.; Mendes, R. Assessment of Mercury Concentration in Turtles (Podocnemis Unifilis) in the Xingu River Basin, Brazil. IJERPH 2018, 15, 1185. [Google Scholar] [CrossRef] [PubMed]
- Targino, F.J.; Ribeiro, J.D.D.N.; Simões, J.S.; Carneiro, C.S.; Lazzarini, S.M.; Souza, A.R.; Ferreira, M.D.S.; Mano, S.B.; Mársico, E.T. Total Mercury Content in the Tissues of Freshwater Chelonium (Podocnemis Expansa) and a Human Health Risk Assessment for the Amazon Population in Brazil. IJERPH 2023, 20, 6489. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Ignácio, A.R.A.; Lázaro, W.L.; Díez, S.; Guimarães, J.R.D.; Santos-Filho, M. Green Kingfishers as Sentinel Species for Mercury Contamination in Amazon. Arch Environ Contam Toxicol 2023, 85, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, T.C.; De Medeiros Costa, G.; De Carvalho, G.S.; Brum, B.R.; Ignácio, Á.R.A. Mercury and Methylmercury Concentration in the Feathers of Two Species of Kingfishers Megaceryle Torquata and Chloroceryle Amazona in the Upper Paraguay Basin and Amazon Basin. Ecotoxicology 2023. [Google Scholar] [CrossRef] [PubMed]
- Galvão, R.C.F.; Holanda, I.B.B.; De Carvalho, D.P.; Almeida, R.; Souza, C.M.M.; Lacerda, L.D.; Bastos, W.R. Freshwater Shrimps (Macrobrachium Depressimanum and Macrobrachium Jelskii) as Biomonitors of Hg Availability in the Madeira River Basin, Western Amazon. Environ Monit Assess 2018, 190, 77. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Martín-Doimeadios, R.C.R.; Jiménez-Moreno, M.; Ramírez-Mateos, V.; Da Silva, N.F.S.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.O.; Macchi, B.M.; Do Nascimento, J.L.M.; et al. Large-Scale Projects in the Amazon and Human Exposure to Mercury: The Case-Study of the Tucuruí Dam. Ecotoxicology and Environmental Safety 2018, 147, 299–305. [Google Scholar] [CrossRef]
- Vega, C.; Orellana, J.; Oliveira, M.; Hacon, S.; Basta, P. Human Mercury Exposure in Yanomami Indigenous Villages from the Brazilian Amazon. IJERPH 2018, 15, 1051. [Google Scholar] [CrossRef]
- Feitosa-Santana, C.; Souza, G.D.S.; Sirius, E.V.P.; Rodrigues, A.R.; Cortes, M.I.T.; Silveira, L.C.D.L.; Ventura, D.F. Color Vision Impairment with Low-Level Methylmercury Exposure of an Amazonian Population – Brazil. NeuroToxicology 2018, 66, 179–184. [Google Scholar] [CrossRef]
- Dos Santos Freitas, J.; Da Costa Brito Lacerda, E.M.; Da Silva Martins, I.C.V.; Rodrigues, D.; Bonci, D.M.O.; Cortes, M.I.T.; Corvelo, T.C.O.; Ventura, D.F.; De Lima Silveira, L.C.; Da Conceição Nascimento Pinheiro, M.; et al. Cross-Sectional Study to Assess the Association of Color Vision with Mercury Hair Concentration in Children from Brazilian Amazonian Riverine Communities. NeuroToxicology 2018, 65, 60–67. [Google Scholar] [CrossRef]
- Costa Junior, J.M.F.; Lima, A.A.D.S.; Rodrigues Junior, D.; Khoury, E.D.T.; Souza, G.D.S.; Silveira, L.C.D.L.; Pinheiro, M.D.C.N. Manifestações Emocionais e Motoras de Ribeirinhos Expostos Ao Mercúrio Na Amazônia. Rev. bras. epidemiol. 2017, 20, 212–224. [Google Scholar] [CrossRef]
- Oliveira, R.A.A.D.; Pinto, B.D.; Rebouças, B.H.; Ciampi De Andrade, D.; Vasconcellos, A.C.S.D.; Basta, P.C. Neurological Impacts of Chronic Methylmercury Exposure in Munduruku Indigenous Adults: Somatosensory, Motor, and Cognitive Abnormalities. IJERPH 2021, 18, 10270. [Google Scholar] [CrossRef]
- Perini, J.A.; Silva, M.C.; Vasconcellos, A.C.S.D.; Viana, P.V.S.; Lima, M.O.; Jesus, I.M.; Kempton, J.W.; Oliveira, R.A.A.; Hacon, S.S.; Basta, P.C. Genetic Polymorphism of Delta Aminolevulinic Acid Dehydratase (ALAD) Gene and Symptoms of Chronic Mercury Exposure in Munduruku Indigenous Children within the Brazilian Amazon. IJERPH 2021, 18, 8746. [Google Scholar] [CrossRef]
- Vianna, A.D.S.; Câmara, V.D.M.; Barbosa, M.C.D.M.; Santos, A.D.S.E.; Asmus, C.I.R.F.; Luiz, R.R.; Jesus, I.M.D. Exposição Ao Mercúrio e Anemia Em Crianças e Adolescentes de Seis Comunidades Da Amazônia Brasileira. Ciênc. saúde coletiva 2022, 27, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Silman, A.K.; Chhabria, R.; Hafzalla, G.W.; Giffin, L.; Kucharski, K.; Myers, K.; Culquichicón, C.; Montero, S.; Lescano, A.G.; Vega, C.M.; et al. Impairment in Working Memory and Executive Function Associated with Mercury Exposure in Indigenous Populations in Upper Amazonian Peru. IJERPH 2022, 19, 10989. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.; Ortiz, E.; Feingold, B.; Berky, A.; Diringer, S.; Morales, A.; Jurado, E.; Hsu-Kim, H.; Pan, W. Spatial, Temporal, and Dietary Variables Associated with Elevated Mercury Exposure in Peruvian Riverine Communities Upstream and Downstream of Artisanal and Small-Scale Gold Mining. IJERPH 2017, 14, 1582. [Google Scholar] [CrossRef]
- Weinhouse, C.; Ortiz, E.J.; Berky, A.J.; Bullins, P.; Hare-Grogg, J.; Rogers, L.; Morales, A.-M.; Hsu-Kim, H.; Pan, W.K. Hair Mercury Level Is Associated with Anemia and Micronutrient Status in Children Living Near Artisanal and Small-Scale Gold Mining in the Peruvian Amazon. The American Journal of Tropical Medicine and Hygiene 2017, 97, 1886–1897. [Google Scholar] [CrossRef]
- Koenigsmark, F.; Weinhouse, C.; Berky, A.; Morales, A.; Ortiz, E.; Pierce, E.; Pan, W.; Hsu-Kim, H. Efficacy of Hair Total Mercury Content as a Biomarker of Methylmercury Exposure to Communities in the Area of Artisanal and Small-Scale Gold Mining in Madre de Dios, Peru. IJERPH 2021, 18, 13350. [Google Scholar] [CrossRef]
- Vasconcellos, A.C.S.D.; Barrocas, P.R.G.; Ruiz, C.M.V.; Mourão, D.D.S.; Hacon, S.D.S. Burden of Mild Mental Retardation Attributed to Prenatal Methylmercury Exposure in Amazon: Local and Regional Estimates. Ciênc. saúde coletiva 2018, 23, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- De Paula Fonseca Arrifano, G.; Del Carmen Rodriguez Martin-Doimeadios, R.; Jiménez-Moreno, M.; Augusto-Oliveira, M.; Rogério Souza-Monteiro, J.; Paraense, R.; Rodrigues Machado, C.; Farina, M.; Macchi, B.; Do Nascimento, J.L.M.; et al. Assessing Mercury Intoxication in Isolated/Remote Populations: Increased S100B mRNA in Blood in Exposed Riverine Inhabitants of the Amazon. NeuroToxicology 2018, 68, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Cerbino, M.R.; Vieira, J.C.S.; Braga, C.P.; Oliveira, G.; Padilha, I.F.; Silva, T.M.; Zara, L.F.; Silva, N.J.; Padilha, P.M. Metalloproteomics Approach to Analyze Mercury in Breast Milk and Hair Samples of Lactating Women in Communities of the Amazon Basin, Brazil. Biol Trace Elem Res 2018, 181, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.P.F.; Martín-Doimeadios, R.C.R.; Jiménez-Moreno, M.; Fernández-Trujillo, S.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Macchi, B.M.; Alvarez-Leite, J.I.; Do Nascimento, J.L.M.; Amador, M.T.; et al. Genetic Susceptibility to Neurodegeneration in Amazon: Apolipoprotein E Genotyping in Vulnerable Populations Exposed to Mercury. Front. Genet. 2018, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.J.X.; Arain, A.; Fernandez, L.E. Mercury Exposure, Risk Factors, and Perceptions among Women of Childbearing Age in an Artisanal Gold Mining Region of the Peruvian Amazon. Environmental Research 2019, 179, 108786. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.S.; Lacerda, E.M.C.B.; Rodrigues Júnior, D.; Corvelo, T.C.O.; Silveira, L.C.L.; Pinheiro, M.D.C.N.; Souza, G.S. Mercury Exposure of Children Living in Amazonian Villages: Influence of Geographical Location Where They Lived during Prenatal and Postnatal Development. An. Acad. Bras. Ciênc. 2019, 91, e20180097. [Google Scholar] [CrossRef] [PubMed]
- Santos-Lima, C.D.; Mourão, D.D.S.; Carvalho, C.F.D.; Souza-Marques, B.; Vega, C.M.; Gonçalves, R.A.; Argollo, N.; Menezes-Filho, J.A.; Abreu, N.; Hacon, S.D.S. Neuropsychological Effects of Mercury Exposure in Children and Adolescents of the Amazon Region, Brazil. NeuroToxicology 2020, 79, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Frischtak, H.; Berky, A.; Ortiz, E.J.; Morales, A.M.; Hsu-Kim, H.; Pendergast, L.L.; Pan, W.K. Elevated Hair Mercury Levels Are Associated With Neurodevelopmental Deficits in Children Living Near Artisanal and Small-Scale Gold Mining in Peru. GeoHealth 2020, 4, e2019GH000222. [Google Scholar] [CrossRef]
- Watson, L.C.; Hurtado-Gonzales, J.L.; Chin, C.J.; Persaud, J. Survey of Methylmercury Exposures and Risk Factors Among Indigenous Communities in Guyana, South America. Journal of Health and Pollution 2020, 10, 200604. [Google Scholar] [CrossRef]
- Lacerda, E.M.D.C.B.; Souza, G.D.S.; Cortes, M.I.T.; Rodrigues, A.R.; Pinheiro, M.C.N.; Silveira, L.C.D.L.; Ventura, D.F. Comparison of Visual Functions of Two Amazonian Populations: Possible Consequences of Different Mercury Exposure. Front. Neurosci. 2020, 13, 1428. [Google Scholar] [CrossRef]
- Weinhouse, C.; Gallis, J.A.; Ortiz, E.; Berky, A.J.; Morales, A.M.; Diringer, S.E.; Harrington, J.; Bullins, P.; Rogers, L.; Hare-Grogg, J.; et al. A Population-Based Mercury Exposure Assessment near an Artisanal and Small-Scale Gold Mining Site in the Peruvian Amazon. J Expo Sci Environ Epidemiol 2021, 31, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bello, T.C.S.; Buralli, R.J.; Cunha, M.P.L.; Dórea, J.G.; Diaz-Quijano, F.A.; Guimarães, J.R.D.; Marques, R.C. Mercury Exposure in Women of Reproductive Age in Rondônia State, Amazon Region, Brazil. IJERPH 2023, 20, 5225. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Araújo, A.; Arrifano, G.P.; Macchi, B.M.; Augusto-Oliveira, M.; Santos-Sacramento, L.; Rodríguez Martín-Doimeadios, R.C.; Jiménez-Moreno, M.; Martins Filho, A.J.; Alvarez-Leite, J.I.; Oriá, R.B.; et al. Hair Mercury Is Associated with Dyslipidemia and Cardiovascular Risk: An Anthropometric, Biochemical and Genetic Cross-Sectional Study of Amazonian Vulnerable Populations. Environmental Research 2023, 229, 115971. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Criado, L.; Rodríguez-González, P.; Marrugo-Negrete, J.; García Alonso, J.I.; Díez, S. Determination of Methylmercury and Inorganic Mercury in Human Hair Samples of Individuals from Colombian Gold Mining Regions by Double Spiking Isotope Dilution and GC-ICP-MS. Environmental Research 2023, 231, 115970. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.D.; Oliveira, R.A.A.D.; Vasconcellos, A.C.S.D.; Rebouças, B.H.; Pinto, B.D.; Lima, M.D.O.; Jesus, I.M.D.; Machado, D.E.; Hacon, S.S.; Basta, P.C.; et al. Chronic Mercury Exposure and GSTP1 Polymorphism in Munduruku Indigenous from Brazilian Amazon. Toxics 2023, 11, 138. [Google Scholar] [CrossRef]
- Pignoux, R.; Gourves, P.-Y.; Sow, M.; Maury-Brachet, R. Imprégnation mercurielle des femmes enceintes de Guyane (Haut Maroni) : étude et prévention. Toxicologie Analytique et Clinique 2019, 31, 37–48. [Google Scholar] [CrossRef]
- Cunha, M.; Marques, R.; Dórea, J. Influence of Maternal Fish Intake on the Anthropometric Indices of Children in the Western Amazon. Nutrients 2018, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Bastos, W.R.; Vieira, S.M.; Manzatto, Â.G.; Dórea, J.G.; Rubira, M.C.; De Souza, V.F.P.; Da Costa Junior, W.A.; Souza Bastos, M.T. Heterogeneity of Multimedia Exposures to Neurotoxic Elements (Al, As, Cd, Pb, Mn, and Hg) in Breastfed Infants from Porto Velho, Brazil. Biol Trace Elem Res 2018, 184, 7–15. [Google Scholar] [CrossRef]
- Carvalho, L.V.B.; Hacon, S.S.; Vega, C.M.; Vieira, J.A.; Larentis, A.L.; Mattos, R.C.O.C.; Valente, D.; Costa-Amaral, I.C.; Mourão, D.S.; Silva, G.P.; et al. Oxidative Stress Levels Induced by Mercury Exposure in Amazon Juvenile Populations in Brazil. International Journal of Environmental Research and Public Health 2019, 16. [Google Scholar] [CrossRef]
- Oliveira, A.T. de; Rodrigues, P. de A.; Ramos Filho, A.M.; Gomes, M.F. da S.; Liebl, A.R. da S.; de Pinho, J.V.; Aride, P.H.R.; Conte-Junior, C.A. Levels of Total Mercury and Health Risk Assessment of Consuming Freshwater Stingrays (Chondrichthyes: Potamotrygoninae) of the Brazilian Amazon. International Journal of Environmental Research and Public Health 2023, 20, 6990. [Google Scholar]
- Kumar, V.; Umesh, M.; Shanmugam, M.K.; Chakraborty, P.; Duhan, L.; Gummadi, S.N.; Pasrija, R.; Jayaraj, I.; Dasarahally Huligowda, L.K. A Retrospection on Mercury Contamination, Bioaccumulation, and Toxicity in Diverse Environments: Current Insights and Future Prospects. Sustainability 2023, 15, 13292. [Google Scholar] [CrossRef]
- Casagrande, G.C.R.; Dambros, J.; de Andrade, E.A.; Martello, F.; Sobral-Souza, T.; Moreno, M.I.C.; Battirola, L.D.; de Andrade, R.L.T. Atmospheric Mercury in Forests: Accumulation Analysis in a Gold Mining Area in the Southern Amazon, Brazil. Environmental Monitoring and Assessment 2023, 195, 477. [Google Scholar] [CrossRef] [PubMed]
- Esteban-López, M.; Arrebola, J.P.; Juliá, M.; Pärt, P.; Soto, E.; Cañas, A.; Pedraza-Díaz, S.; González-Rubio, J.; Castaño, A. Selecting the Best Non-Invasive Matrix to Measure Mercury Exposure in Human Biomonitoring Surveys. Environmental Research 2022, 204, 112394. [Google Scholar] [CrossRef] [PubMed]
- Santos Serrão De Castro, N.; De Oliveira Lima, M. Hair as a Biomarker of Long Term Mercury Exposure in Brazilian Amazon: A Systematic Review. IJERPH 2018, 15, 500. [Google Scholar] [CrossRef]
- Pestana, I.A.; Almeida, M.G.; Bastos, W.R.; Souza, C.M.M. Total Hg and Methylmercury Dynamics in a River-Floodplain System in the Western Amazon: Influence of Seasonality, Organic Matter and Physical and Chemical Parameters. Science of the Total Environment 2019, 656, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Verschoor, G. Re-Imagining Environmental Governance: Gold Dredge Mining vs Territorial Health in the Colombian Amazon. Geoforum 2020, 117, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, J.; Gasparinetti, P.; Bakker, L.B.; Lobo, F.; Nagel, G. Socioeconomic Cost of Dredge Boat Gold Mining in the Tapajós Basin, Eastern Amazon. Resources Policy 2022, 79, 103102. [Google Scholar] [CrossRef]
- Junk, W.J.; Wittmann, F.; Schöngart, J.; Piedade, M.T.F. A Classification of the Major Habitats of Amazonian Black-Water River Floodplains and a Comparison with Their White-Water Counterparts. Wetlands Ecology and Management 2015, 23, 677–693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





