Submitted:
15 February 2024
Posted:
16 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
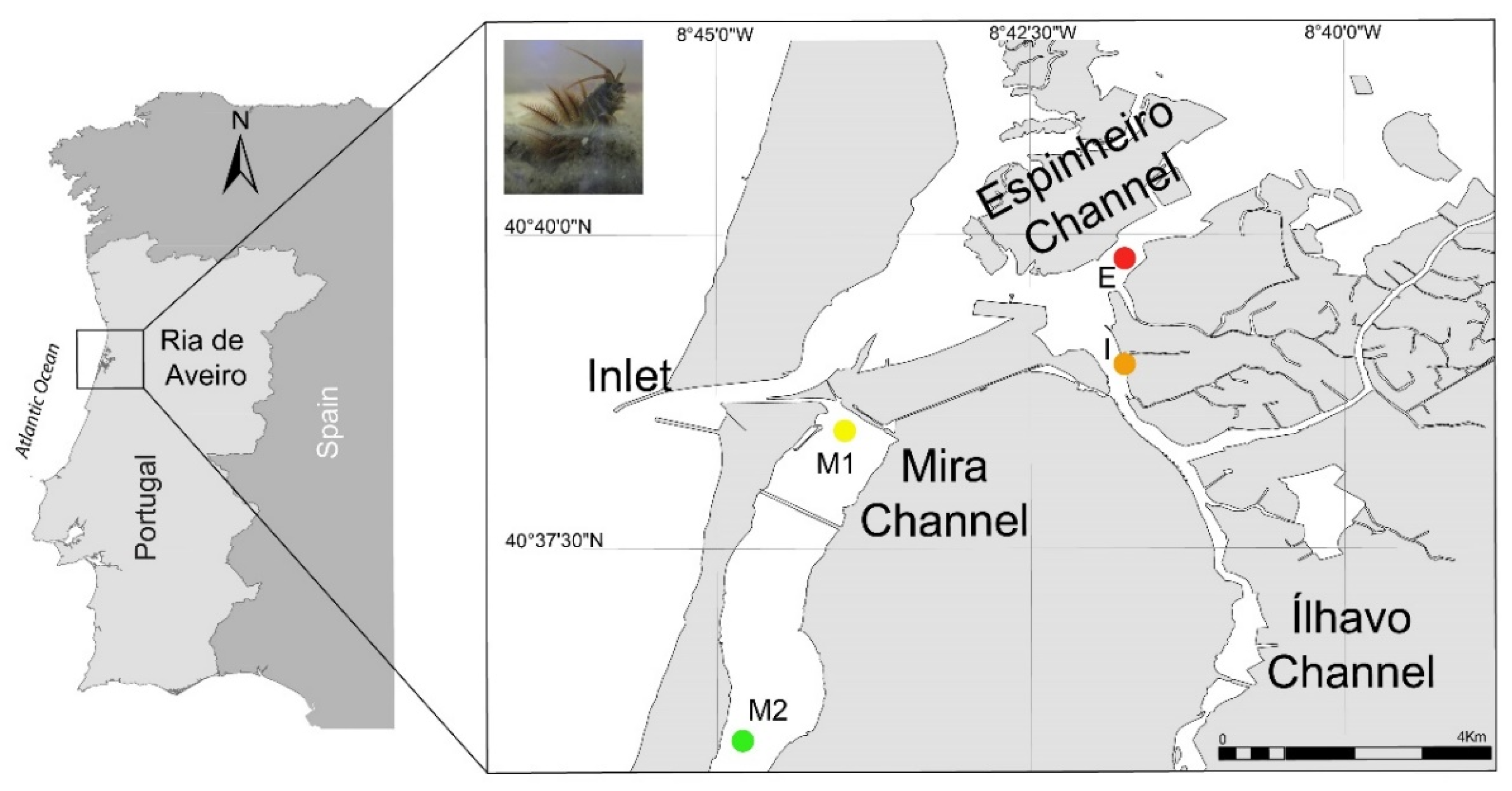
2.1. Sample collection
2.2. Fatty acid analysis
2.3. ICP-MS
2.4. Data and statistical analysis
2.4.1. Analysis of fatty acid profiles
2.4.2. Analysis of elemental fingerprint
2.4.3. Combination of fatty acid profiles and elemental fingerprint
3. Results
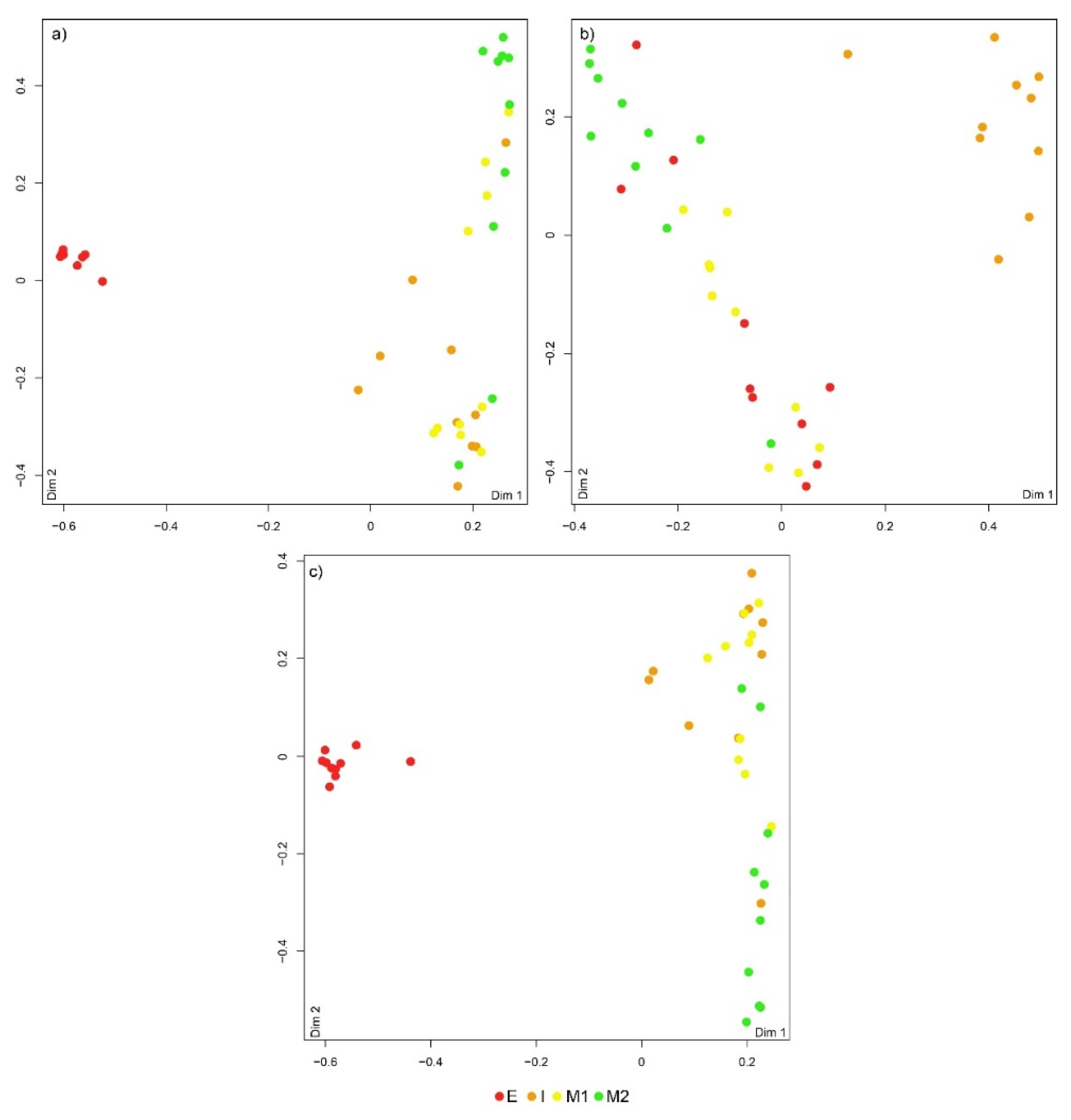
3.1. Fatty acid profiles
3.2. Elemental fingerprints
3.3. Elemental fingerprints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olive, P. Management of the exploitation of the lugworm Arenicola marina and the ragworm Nereis virens (Polychaeta) in conservation areas. Aquat. Conserv. 1993, 3, 1–24. [Google Scholar] [CrossRef]
- Cunha, T.; Hall, A.; Queiroga, H. Estimation of the Diopatra neapolitana annual harvest resulting from digging activity in Canal de Mira, Ria de Aveiro. Fish. Res. 2005, 76, 56–66. [Google Scholar] [CrossRef]
- Olive, P. Polychaeta as a world resource: a review of pattern of exploitation as sea angling baits and the potential for aquaculture based production. Mem. Mus. Natl. Hist. Nat. 1994, 162, 603–610. [Google Scholar]
- Meunpol, O.; Meejing, P.; Piyatiratitivorakul, S. Maturation diet based on fatty acid content for male Penaeus monodon (Fabricius) broodstock. Aquac. Res. 2005, 36, 1216–1225. [Google Scholar] [CrossRef]
- Marques, B.; Lillebø, A.I.; Ricardo, F.; Nunes, C.; Coimbra, M.A.; Calado, R. Adding value to ragworms (Hediste diversicolor) through the bioremediation of a super-intensive marine fish farm. Aquacult. Env. Interac. 2018, 10, 79–88. [Google Scholar] [CrossRef]
- Jerónimo, D.; Lillebø, A.I.; Santos, A.; Cremades, J.; Calado, R. Performance of polychaete assisted sand filters under contrasting nutrient loads in an integrated multi-trophic aquaculture (IMTA) system. Sci. Rep. 2020, 10, 20871. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Gentil, F.; Quintino, V.; Rodrigues, A.M. Reproductive biology of Diopatra neapolitana (Annelida, Onuphidae), an exploited natural resource in Ria de Aveiro (Northwestern Portugal). Mar. Ecol. 2012, 33, 56–65. [Google Scholar] [CrossRef]
- Pires, A.; Freitas, R.; Quintino, V.; Rodrigues, A.M. Can Diopatra neapolitana (Annelida: Onuphidae) regenerate body damage caused by bait digging or predation? Estuar. Coast. Shelf S. 2012, 110, 36–42. [Google Scholar] [CrossRef]
- Aleixo, A.; Queiroga, H.; Xenarios, S.; Lillebø, A. Catch estimates and bioecomomic analysis of bait digging: The case of the tube worm Diopatra neapolitana. Bioforsk Rapport 2014, 9, 136. [Google Scholar]
- Costa, F.E.; Sá, E.; Alves, A.S.; Cabral, S.; Castro, N.; Picard, D.; Castro, J.J.; Cancela Da Fonseca, L.; Chainho, P.; Canning-Clode, J. Anelídeos poliquetas como isco vivo: caracterização da atividade de apanha em ambientes salobros costeiros Portugueses. Entre Rios E Mares Um Património De Ambientes História E Sabers – Tomo V Da Rede BrasPor 2016, 33–43.
- Xenarios, S.; Queiroga, H.; Lillebø, A.I.; Aleixo, A. Introducing a regulatory policy framework of bait fishing in European coastal lagoons: The case of Ria de Aveiro in Portugal. Fishes 2018, 3, 2. [Google Scholar] [CrossRef]
- Olive, P.J. Polychaete aquaculture and polychaete science: a mutual synergism. In Reproductive Strategies and Developmental Patterns in Annelids; Springer: Dordrecht, The Netherlands, 1999; pp. 175–183. [Google Scholar]
- Freitas, F.; Cunha, T.; Hall, A.; Queiroga, H. Diopatra neapolitana, Importância Sócio-económica e Sustentabilidade das Capturas, no Canal de Mira, Ria de Aveiro. In Jornadas da Ria de Aveiro, Universidade de Aveiro, Portugal; 2011; pp. 60–66.
- De Carvalho, A.N.; Vaz, A.S.L.; Sérgio, T.I.B.; dos Santos, P.J.T. Sustainability of bait fishing harvesting in estuarine ecosystems–Case study in the Local Natural Reserve of Douro Estuary, Portugal. Rev. De Gestão Costeira Integr.-J. Integr. Coast. Zone Manag. 2013, 13, 157–168. [Google Scholar] [CrossRef]
- Conti, G.; Massa, F. Experienze de allavamento del polichete Diopatra neapolitana Delle Chiaje, 1841 nella Laguna di S. Gilla (Sardegna Meridionale). Biol. Mar. Mediterr. 1998, 5, 1473–1480. [Google Scholar]
- Safarik, M.; Redden, A.M.; Schreider, M.J. Density-dependent growth of the polychaete Diopatra aciculata. Sci. Mar. 2006, 70, 337–341. [Google Scholar] [CrossRef]
- Kraan, C.; Piersma, T.; Dekinga, A.; Koolhaas, A.; Van der Meer, J. Dredging for edible cockles (Cerastoderma edule) on intertidal flats: short-term consequences of fisher patch-choice decisions for target and non-target benthic fauna. ICES J. Mar. Sci. 2007, 64, 1735–1742. [Google Scholar] [CrossRef]
- Fonseca, L.; Costa, P.; Fidalgo, E. Poliquetas: sua obtenção, impactos e medidas de gestão. In Proceedings of the 14º Congresso da Associação Portuguesa para o Desenvolvimento Regional-Ambiente e Conservação da Natureza; 2008; p. 851. [Google Scholar]
- Leal, M.C.; Pimentel, T.; Ricardo, F.; Rosa, R.; Calado, R. Seafood traceability: current needs, available tools, and biotechnological challenges for origin certification. Trends Biotechnol. 2015, 33, 331–336. [Google Scholar] [CrossRef]
- Santos, A.; Ricardo, F.; Domingues, M.R.M.; Patinha, C.; Calado, R. Current trends in the traceability of geographic origin and detection of species-mislabeling in marine bivalves. Food Control 2023, 152, 109840. [Google Scholar] [CrossRef]
- Ricardo, F.; Maciel, E.; Domingues, M.R.; Calado, R. Spatio-temporal variability in the fatty acid profile of the adductor muscle of the common cockle Cerastoderma edule and its relevance for tracing geographic origin. Food Control 2017, 81, 173–180. [Google Scholar] [CrossRef]
- Ricardo, F.; Pimentel, T.; Moreira, A.S.P.; Rey, F.; Coimbra, M.A.; Rosário Domingues, M.; Domingues, P.; Costa Leal, M.; Calado, R. Potential use of fatty acid profiles of the adductor muscle of cockles (Cerastoderma edule) for traceability of collection site. Sci. Rep. 2015, 5, 11125. [Google Scholar] [CrossRef]
- Mamede, R.; Ricardo, F.; Santos, A.; Díaz, S.; Santos, S.A.O.; Bispo, R.; Domingues, M.R.M.; Calado, R. Revealing the illegal harvesting of Manila clams (Ruditapes philippinarum) using fatty acid profiles of the adductor muscle. Food Control 2020, 107368. [Google Scholar] [CrossRef]
- Nemova, N.N.; Fokina, N.N.; Nefedova, Z.A.; Ruokolainen, T.R.; Bakhmet, I.N. Modifications of gill lipid composition in littoral and cultured blue mussels Mytilus edulis L. under the influence of ambient salinity. Polar Rec. 2013, 49, 272–277. [Google Scholar] [CrossRef]
- Ricardo, F.; Mamede, R.; Bruzos, A.L.; Díaz, S.; Thébault, J.; da Silva, E.F.; Patinha, C.; Calado, R. Assessing the elemental fingerprints of cockle shells (Cerastoderma edule) to confirm their geographic origin from regional to international spatial scales. Sci. Total Environ. 2022, 814, 152304. [Google Scholar] [CrossRef]
- Ricardo, F.; Génio, L.; Costa Leal, M.; Albuquerque, R.; Queiroga, H.; Rosa, R.; Calado, R. Trace element fingerprinting of cockle (Cerastoderma edule) shells can reveal harvesting location in adjacent areas. Sci. Rep. 2015, 5, 11932. [Google Scholar] [CrossRef]
- Carson, H.S.; López-Duarte, P.C.; Cook, G.S.; Fodrie, F.J.; Becker, B.J.; DiBacco, C.; Levin, L.A. Temporal, spatial, and interspecific variation in geochemical signatures within fish otoliths, bivalve larval shells, and crustacean larvae. Mar. Ecol. Prog. Ser. 2013, 473, 133–148. [Google Scholar] [CrossRef]
- Cabral, A.E.; Ricardo, F.; Patinha, C.; Silva, E.F.d.; Correia, M.; Palma, J.; Planas, M.; Calado, R. Successful Use of Geochemical Tools to Trace the Geographic Origin of Long-Snouted Seahorse Hippocampus guttulatus Raised in Captivity. Animals 2021, 11, 1534. [Google Scholar] [CrossRef] [PubMed]
- Takesue, R.K.; Bacon, C.R.; Thompson, J.K. Influences of organic matter and calcification rate on trace elements in aragonitic estuarine bivalve shells. Geochim. Cosmochim. Ac. 2008, 72, 5431–5445. [Google Scholar] [CrossRef]
- Becker, B.J.; Fodrie, F.J.; McMillan, P.; Levin, L.A. Spatial and temporal variation in trace elemental fingerprints of mytilid mussel shells: A precursor to invertebrate larval tracking. Limnol. Oceanogr. 2004, 50, 48–61. [Google Scholar] [CrossRef]
- Sorte, C.J.; Etter, R.J.; Spackman, R.; Boyle, E.E.; Hannigan, R.E. Elemental Fingerprinting of Mussel Shells to Predict Population Sources and Redistribution Potential in the Gulf of Maine. PloS ONE 2013, 8, e80868. [Google Scholar] [CrossRef]
- Zacherl, D.C. Spatial and temporal variation in statolith and protoconch trace elements as natural tags to track larval dispersal. Mar. Ecol. Prog. Ser. 2005, 290, 145–163. [Google Scholar] [CrossRef]
- Zhang, X.; Han, D.; Chen, X.; Zhao, X.; Cheng, J.; Liu, Y. Combined use of fatty acid profile and fatty acid δ13C fingerprinting for origin traceability of scallops (Patinopecten yessoensis, Chlamys farreri, and Argopecten irradians). Food Chem. 2019, 298, 124966. [Google Scholar] [CrossRef]
- Perez, V.; Olivier, F.; Tremblay, R.; Neumeier, U.; Thébault, J.; Chauvaud, L.; Meziane, T. Trophic resources of the bivalve, Venus verrucosa, in the Chausey archipelago (Normandy, France) determined by stable isotopes and fatty acids. Aquat. Living Resour. 2013, 26, 229–239. [Google Scholar] [CrossRef]
- Chaguri, M.P.; Maulvault, A.L.; Nunes, M.L.; Santiago, D.A.; Denadai, J.C.; Fogaça, F.H.; Sant’Ana, L.S.; Ducatti, C.; Bandarra, N.; Carvalho, M.L. Different tools to trace geographic origin and seasonality of croaker (Micropogonias furnieri). LWT-Food Sci. Tech. 2015, 61, 194–200. [Google Scholar] [CrossRef]
- Matos, M.P.; Engel, M.E.; Mangrum, J.B.; Jackson, G.P. Origin determination of the Eastern oyster (Crassostrea virginica) using a combination of whole-body compound-specific isotope analysis and heavy metal analysis. Anal. Methods 2021, 13, 3493–3503. [Google Scholar] [CrossRef]
- Cabral, S.; Alves, A.S.; Castro, N.; Chainho, P.; Sá, E.; da Fonseca, L.C.; e Costa, P.F.; Castro, J.; Canning-Clode, J.; Pombo, A. Polychaete annelids as live bait in Portugal: Harvesting activity in brackish water systems. Ocean Coast. Manage. 2019, 181, 104890. [Google Scholar] [CrossRef]
- Aued-Pimentel, S.; Lago, J.H.G.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogra A 2004, 1054, 235–239. [Google Scholar] [CrossRef]
- Anderson, M.; Clarke, K.; Gorley, R. PERMANOVA+ for Primer. Guide to Software and Statistical Methods; University of Auckland and PRIMER-E Ltd: Plymouth, UK, 2008. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Found. Stat Comput Vienna Austria 2021. [Google Scholar]
- Ricardo, F.; Mamede, R.; Bispo, R.; Santos, A.; Ferreira da Silva, E.; Patinha, C.; Calado, R. Cost-efficiency improvement of bivalves shells preparation when tracing their geographic origin through ICP-MS analysis of elemental fingerprints. Food Control 2020, 118, 107383. [Google Scholar] [CrossRef]
- Kursa, M.B.; Jankowski, A.; Rudnicki, W.R. Boruta–a system for feature selection. Fundam. Informaticae 2010, 101, 271–285. [Google Scholar] [CrossRef]
- Ricardo, F.; Gonçalves, D.; Pimentel, T.; Mamede, R.; Rosário, M. Domingues, M.; Lillebø, A.I.; Calado, R. Prevalence of phylogenetic over environmental drivers on the fatty acid profiles of the adductor muscle of marine bivalves and its relevance for traceability. Ecol. Indic. 2021, 129, 108017. [Google Scholar] [CrossRef]
- da Costa, E.; Ricardo, F.; Melo, T.; Mamede, R.; Abreu, M.H.; Domingues, P.; Domingues, M.R.; Calado, R. Site-Specific Lipidomic Signatures of Sea Lettuce (Ulva spp., Chlorophyta) Hold the Potential to Trace Their Geographic Origin. Biomolecules 2020, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Mamede, R.; Ricardo, F.; Gonçalves, D.; Ferreira da Silva, E.; Patinha, C.; Calado, R. Assessing the use of surrogate species for a more cost-effective traceability of geographic origin using elemental fingerprints of bivalve shells. Ecol. Indic. 2021, 130, 108065. [Google Scholar] [CrossRef]
- Mamede, R.; Ricardo, F.; Abreu, M.H.; da Silva, E.F.; Patinha, C.; Calado, R. Spatial variability of elemental fingerprints of sea lettuce (Ulva spp.) and its potential use to trace geographic origin. Algal Res. 2021, 59, 102451. [Google Scholar] [CrossRef]
- Bennion, M.; Morrison, L.; Shelley, R.; Graham, C. Trace elemental fingerprinting of shells and soft tissues can identify the time of blue mussel (Mytilus edulis) harvesting. Food Control 2020, 121, 107515. [Google Scholar] [CrossRef]
- Bennion, M.; Morrison, L.; Brophy, D.; Carlsson, J.; Abrahantes, J.C.; Graham, C.T. Trace element fingerprinting of blue mussel (Mytilus edulis) shells and soft tissues successfully reveals harvesting locations. Sci. Total Environ. 2019, 121, 107515. [Google Scholar] [CrossRef]
- Fernandes, J.F.; Ricardo, F.; Jerónimo, D.; Santos, A.; Domingues, M.R.; Calado, R.; Madeira, D. Modulation of fatty acid profiles by global and local ocean change drivers in the ragworm Hediste diversicolor: implications for aquaculture production. Aquaculture 2021, 542, 736871. [Google Scholar] [CrossRef]
- Jerónimo, D.; Lillebø, A.I.; Maciel, E.; Domingues, M.R.M.; Cremades, J.; Calado, R. Unravelling the fatty acid profiles of different polychaete species cultured under integrated multi-trophic aquaculture (IMTA). Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nelson, M.M.; Groce, A.K.; Cary, S.C.; Coyne, K.; Gibson, J.A.; Nichols, P.D. Lipid biomarkers of deep-sea hydrothermal vent polychaetes—Alvinella pompejana, A. caudata, Paralvinella grasslei and Hesiolyra bergii. Deep Sea Res. Part I: Oceanogra. Res. 2005, 52, 2333–2352. [Google Scholar] [CrossRef]
- Olive, P.J.; Duangchinda, T.; Ashforth, E.; Craig, S.; Ward, A.C.; Davies, S.J. Net gain of long-chain polyunsaturated fatty acids (PUFA) in a lugworm Arenicola marina bioturbated mesocosm. Mar. Ecol. Prog. Ser. 2009, 387, 223–239. [Google Scholar] [CrossRef]
- Braeckman, U.; Provoost, P.; Sabbe, K.; Soetaert, K.; Middelburg, J.J.; Vincx, M.; Vanaverbeke, J. Temporal dynamics in the diet of two marine polychaetes as inferred from fatty acid biomarkers. J. Sea Res. 2012, 68, 6–19. [Google Scholar] [CrossRef]
- Dalsgaard, J.; St John, M.; Kattner, G.; Müller-Navarra, D.; Hagen, W. Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 2003, 46, 225–340. [Google Scholar]
- Madeira, D.; Fernandes, J.F.; Jerónimo, D.; Martins, P.; Ricardo, F.; Santos, A.; Domingues, M.R.; Diniz, M.S.; Calado, R. Salinity shapes the stress responses and energy reserves of marine polychaetes exposed to warming: From molecular to functional phenotypes. Sci. Total Environ. 2021, 795, 148634. [Google Scholar] [CrossRef]
- Török, Z.; Crul, T.; Maresca, B.; Schütz, G.J.; Viana, F.; Dindia, L.; Piotto, S.; Brameshuber, M.; Balogh, G.; Péter, M.; et al. Plasma membranes as heat stress sensors: From lipid-controlled molecular switches to therapeutic applications. Biochim. Biophys. Acta–Biomembr. 2014, 1838, 1594–1618. [Google Scholar] [CrossRef]
- Holmstrup, M.; Hovvang, M.H.; Slotsbo, S. Salinity of the growth medium is important for production potential and nutritional value of white worms (Enchytraeus albidus Henle). Aquac. Res. 2020, 51, 2885–2892. [Google Scholar] [CrossRef]
- Frolov, A.V.; Pankov, S.L.; Geradze, K.N.; Pankova, S.A. Influence of salinity on the biochemical composition of the rotifer Brachionus plicatilis (Muller). aspects of adaptation. Comp. Biochem. Physi. A 1991, 99, 541–550. [Google Scholar] [CrossRef]
- Lavaud, R.; Thébault, J.; Lorrain, A.; van der Geest, M.; Chauvaud, L. Senilia senilis (Linnaeus, 1758), a biogenic archive of environmental conditions on the Banc d'Arguin (Mauritania). J. Sea Res. 2013, 76, 61–72. [Google Scholar] [CrossRef]
- Albuquerque, R.; Queiroga, H.; Swearer, S.E.; Calado, R.; Leandro, S.M. Harvest locations of goose barnacles can be successfully discriminated using trace elemental signatures. Sci. Rep 2016, 6, 27787. [Google Scholar] [CrossRef] [PubMed]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Morán, P.; Cal, L.; Cobelo-García, A.; Almécija, C.; Caballero, P.; Garcia de Leaniz, C. Historical legacies of river pollution reconstructed from fish scales. Environ. Pollut. 2018, 234, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, F.; Pimentel, T.; Génio, L.; Calado, R. Spatio-temporal variability of trace elements fingerprints in cockle (Cerastoderma edule) shells and its relevance for tracing geographic origin. Sci. Rep. 2017, 7, 3475. [Google Scholar] [CrossRef] [PubMed]
- Colbath, G.K. Jaw mineralogy in eunicean polychaetes (Annelida). Micropaleontol. 1986, 186–189. [Google Scholar] [CrossRef]
- Elegbede, I.; Lawal-Are, A.; Favour, O.; Jolaosho, T.; Goussanou, A. Chemical compositions of bivalves shells: Anadara senilis, Crassostrea gasar, and Mytilus edulis and their potential for a sustainable circular economy. SN Appl. Sci. 2022, 5. [Google Scholar] [CrossRef]
- Nambiar, R.; Hauzer, H.; Gray, W.R.; Henehan, M.J.; Cotton, L.; Erez, J.; Rosenthal, Y.; Renema, W.; Müller, W.; Evans, D. Controls on potassium incorporation in foraminifera and other marine calcifying organisms. Geochim. Cosmochim. Ac. 2023, 351, 125–138. [Google Scholar] [CrossRef]
- Pires, A.; Velez, C.; Figueira, E.; Soares, A.M.V.M.; Freitas, R. Effects of sediment contamination on physiological and biochemical responses of the polychaete Diopatra neapolitana, an exploited natural resource. Mar. Pollut. Bull. 2017, 119, 119–131. [Google Scholar] [CrossRef]
- Sprovieri, M.; Feo, M.L.; Prevedello, L.; Manta, D.S.; Sammartino, S.; Tamburrino, S.; Marsella, E. Heavy metals, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in surface sediments of the Naples harbour (southern Italy). Chemosphere 2007, 67, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Iyaka, Y.A. Nickel in soils: A review of its distribution and impacts. Sci. Res. Essays 2011, 6, 6774–6777. [Google Scholar] [CrossRef]
- Lopes, J.; Dias, J.; Cardoso, A.; Silva, C. The water quality of the Ria de Aveiro lagoon, Portugal: From the observations to the implementation of a numerical model. Mar. Environ. Res. 2005, 60, 594–628. [Google Scholar] [CrossRef]
- Pereira, F.; Picado, A.; Pereira, H.; Pinheiro, J.P.; Lopes, C.L.; Dias, J.M. Impact of Extreme Wind and Freshwater Runoff on the Salinity Patterns of a Mesotidal Coastal Lagoon. J. Mar. Sci. Eng. 2023, 11, 1338. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, Y.; Zhao, X. Identification of the geographical origins of sea cucumber (Apostichopus japonicus) in northern China by using stable isotope ratios and fatty acid profiles. Food Chem. 2017, 218, 269–276. [Google Scholar] [CrossRef]
- Ortea, I.; Gallardo, J.M. Investigation of production method, geographical origin and species authentication in commercially relevant shrimps using stable isotope ratio and/or multi-element analyses combined with chemometrics: An exploratory analysis. Food Chem. 2015, 170, 145–153. [Google Scholar] [CrossRef]
- Vasconi, M.; Lopez, A.; Galimberti, C.; Moreno Rojas, J.M.; Muñoz Redondo, J.M.; Bellagamba, F.; Moretti, V.M. Authentication of farmed and wild european eel (Anguilla anguilla) by fatty acid profile and carbon and nitrogen isotopic analyses. Food Control 2019, 102, 112–121. [Google Scholar] [CrossRef]
- Thomas, F.; Jamin, E.; Wietzerbin, K.; Guérin, R.; Lees, M.; Morvan, E.; Billault, I.; Derrien, S.; Moreno Rojas, J.M.; Serra, F.; et al. Determination of Origin of Atlantic Salmon (Salmo salar): The Use of Multiprobe and Multielement Isotopic Analyses in Combination with Fatty Acid Composition to Assess Wild or Farmed Origin. J. Agric. Food Chem. 2008, 56, 989–997. [Google Scholar] [CrossRef]

| Structure (s) Fingerprint (s) | Original Location | Predicted Location | Total per location | % correct | % correct (location) | |||
| E | I | M1 | M2 | |||||
| Whole body - FA profile | E | 10 | 0 | 0 | 0 | 10 | 100 | 75 |
| I | 0 | 6 | 3 | 1 | 10 | 60 | ||
| M1 | 0 | 2 | 7 | 1 | 10 | 70 | ||
| M2 | 0 | 1 | 2 | 7 | 10 | 70 | ||
| Jaws - EF | E | 6 | 1 | 0 | 3 | 10 | 60 | 72.5 |
| I | 0 | 9 | 0 | 1 | 10 | 90 | ||
| M1 | 2 | 0 | 8 | 0 | 10 | 80 | ||
| M2 | 3 | 0 | 1 | 6 | 10 | 60 | ||
| Whole body & Jaws - FA profile & EF | E | 10 | 0 | 0 | 0 | 10 | 100 | 85 |
| I | 0 | 8 | 1 | 1 | 10 | 80 | ||
| M1 | 0 | 1 | 8 | 1 | 10 | 80 | ||
| M2 | 0 | 1 | 1 | 8 | 10 | 80 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





