Submitted:
08 February 2024
Posted:
13 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Model of Streptozotocin Diabetes
2.2 Protocol for Routine Staining with Hematoxylin-Eosin
2.3 Standard Processing Technique for Transmission Electron Microscopy (TEM)
3. Results
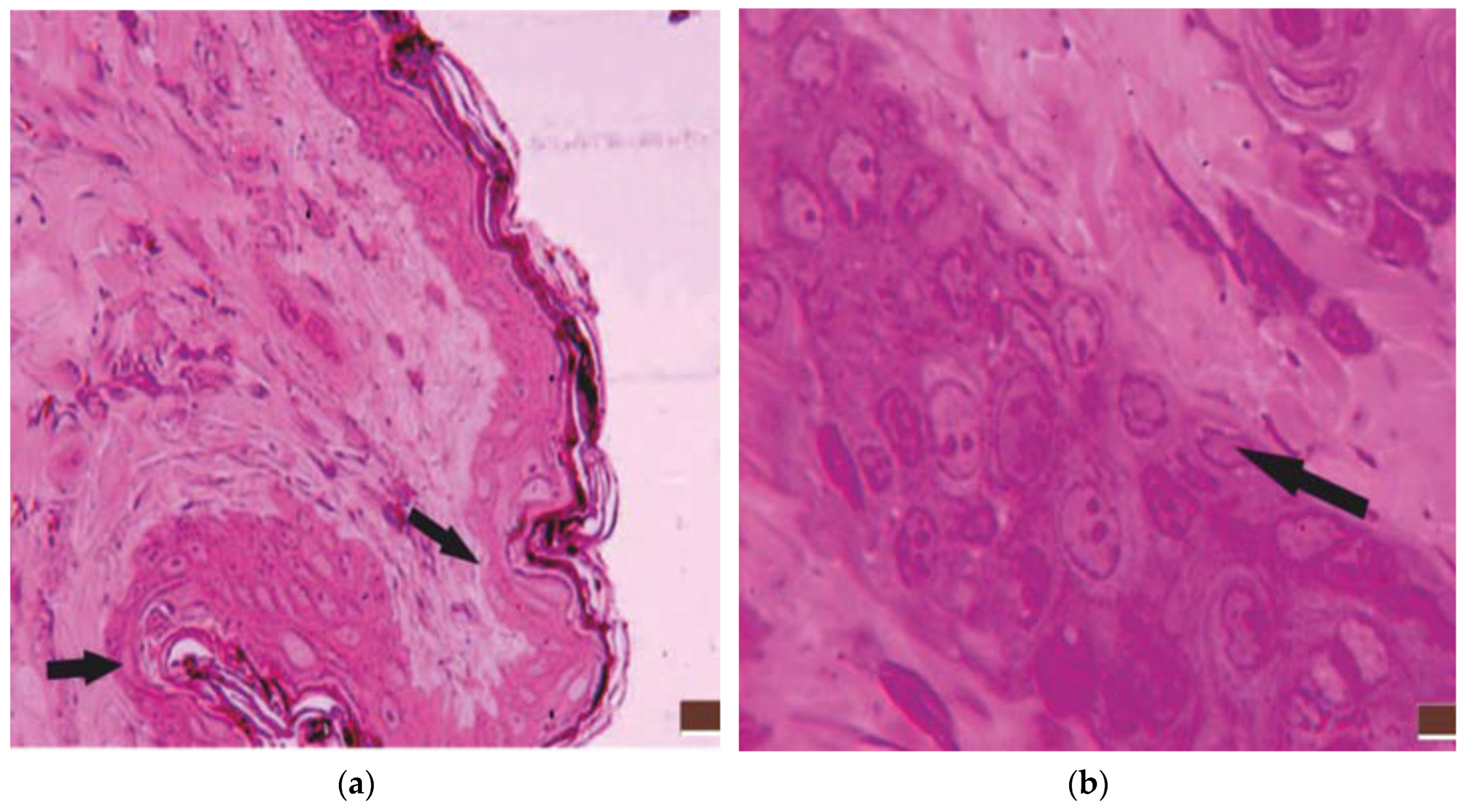
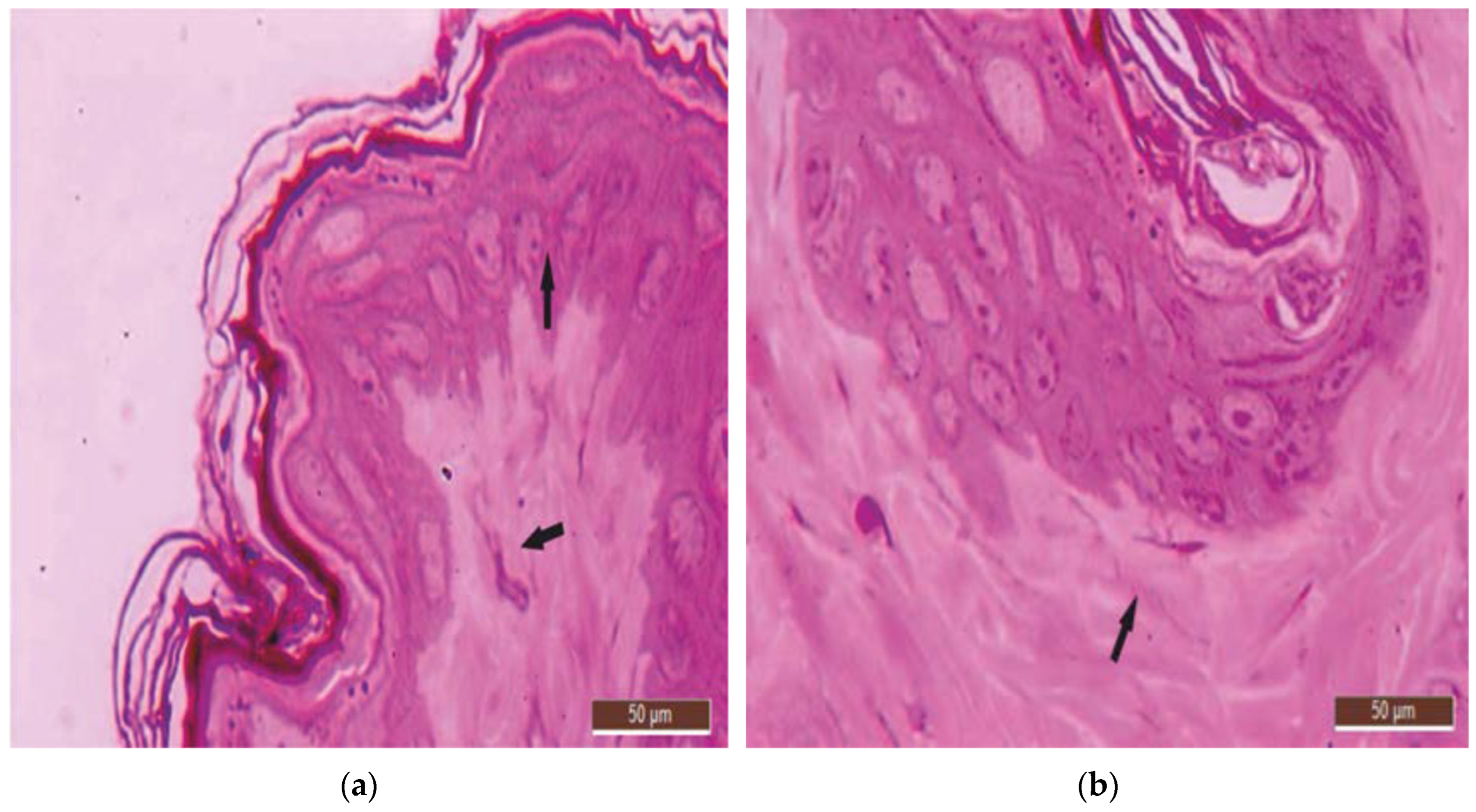
3.1. Histological Aspects
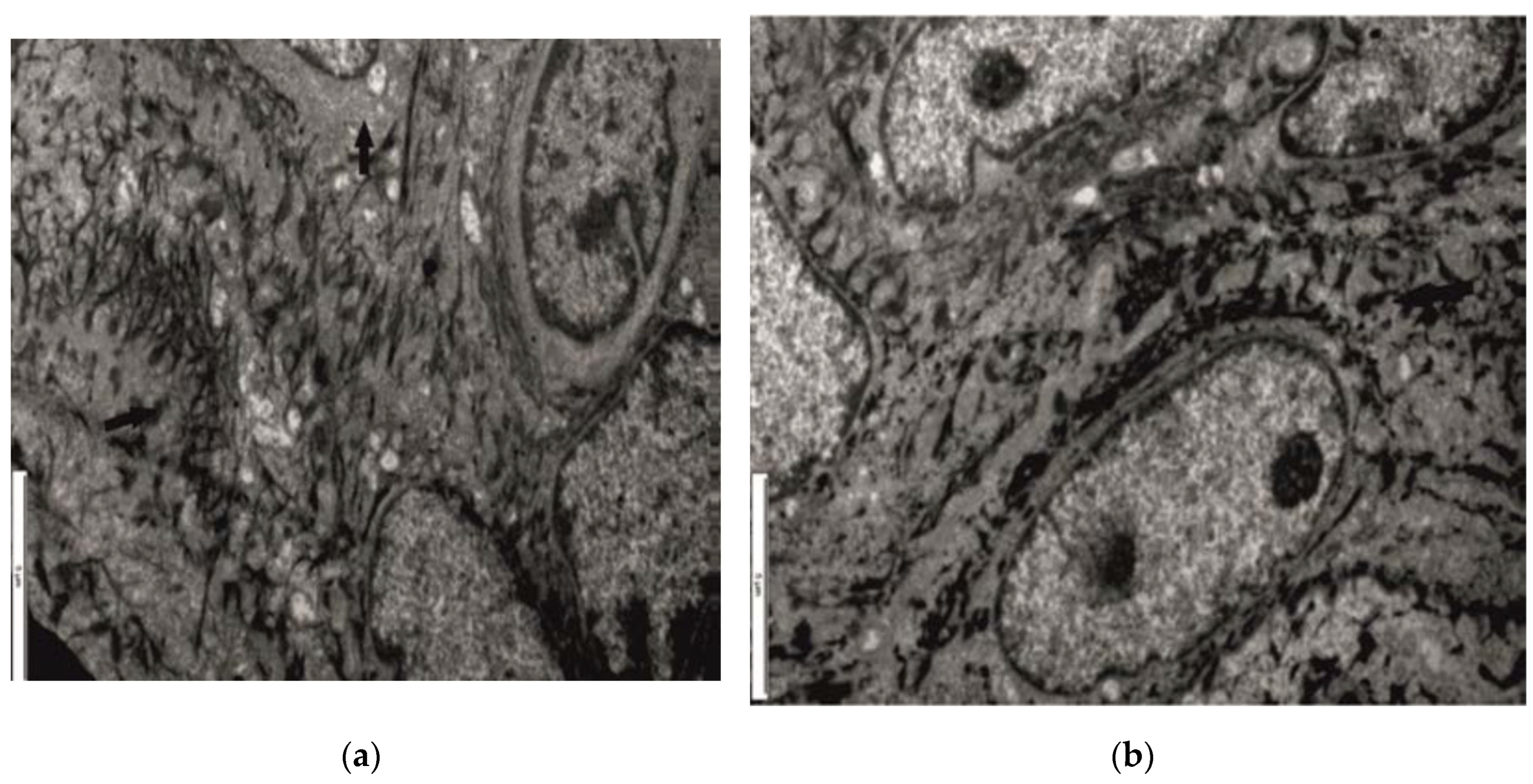
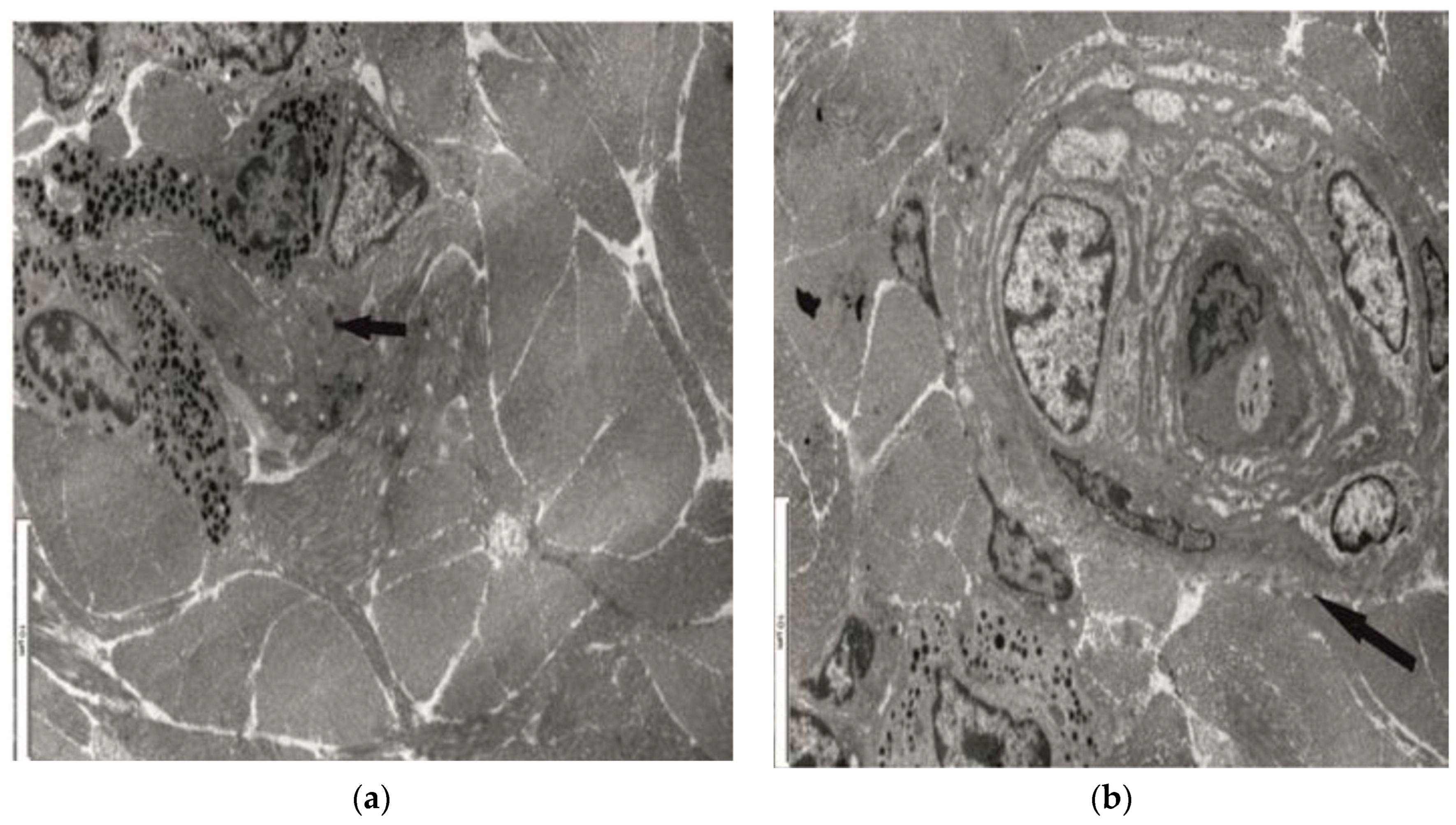
3.2. Ultrastructural Aspects
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Quondamatteo, F. Skin and diabetes mellitus: what do we know? Cell Tissue Res 2014, 355(1), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kochet, K.; Lytus, I.; Svistunov, I.; Sulaieva, O. Skin pathology in diabetes mellitus: clinical and pathophysiological correlations (Review). Georgian Med News 2017, 273, 41–46. [Google Scholar]
- Horikawa, T.; Hiramoto, K.; Goto, K.; Sekijima, H.; Ooi, K. Differences in the mechanism of type 1 and type 2 diabetes-induced skin dryness by using model mice. Int J Med Sci 2021, 18(2), 474–481. [Google Scholar] [CrossRef] [PubMed]
- Guy, R. Diagnostic devices: Managing diabetes through the skin. Nat Nanotechnol 2016, 11(6), 493–494. [Google Scholar] [CrossRef]
- Condurache Hritcu, O.M.; Botez, A.E.; Olinici, D.T.; Onofrei, P.; Stoica, L.; Grecu, V.B.; Toader, P.M.; Gheucă-Solovăstru, L.; Cotrutz, E.C. Molecular markers associated with potentially malignant oral lesions (Review). Exp Ther Med 2021, 22(2), 834. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Kimhofer, T.; Ahmad, S.; AlAma, M.N.; Mosli, H.H.; Hindawi, S.I.; Mook-Kanamori, D.O.; Šebeková, K.; Damanhouri, Z.A.; Holmes, E. Ethnicity and skin autofluorescence-based risk-engines for cardiovascular disease and diabetes mellitus. PLoS One 2017, 12(9), e0185175. [Google Scholar] [CrossRef]
- Aoki, E.; Hirashima, T.; Kumamoto, Y.; Yamamoto, Y.; Suzuki, N.; Oshima, T.; Saito, D.; Hirano, T. Clinical significance of skin autofluorescence for diabetic macroangiopathy and comparison with conventional markers of atherosclerosis: a cross-sectionaland prospective study. Diabetol Int 2022, 14(2), 145–154. [Google Scholar] [CrossRef] [PubMed]
- Škrha, J.Jr.; Horová, E.; Šoupal, J.; Valeriánová, A.; Malík, J.; Prázný, M. ; Zima T, Kalousová M, Škrha J. Skin autofluorescence corresponds to microvascular reactivity in diabetes mellitus. J Diabetes Complications 2022, 36(7), 108206. [Google Scholar] [PubMed]
- Rajaobelina, K.; Cougnard-Gregoire, A.; Delcourt, C.; Gin, H.; Barberger-Gateau, P.; Rigalleau, V. Autofluorescence of Skin Advanced Glycation End Products: Marker of Metabolic Memory in Elderly Population. J Gerontol A Biol Sci Med Sci 2015, 70(7), 841–846. [Google Scholar] [CrossRef] [PubMed]
- Pichardo-Geisinger, R. Commentary on "Diabetes Mellitus and the Skin: Recognition and Management of Cutaneous Manifestations". South Med J 2016, 109(10), 647. [Google Scholar] [CrossRef]
- Singh, A.V.; Chandrasekar, V.; Paudel, N.; Laux, P.; Luch, A.; Gemmati, D.; Tisato, V.; Prabhu, S.K.; Uddin, S.; Dakua, S.P. Integrative toxicogenomics: Advancing precision medicine and toxicology through artificial intelligence and OMICs technology. Biomedicine & Pharmacotherapy 2023, 163, 114784. [Google Scholar]
- Dremin, V.; Marcinkevics, Z.; Zherebtsov, E.; Popov, A.; Grabovskis, A.; Kronberga, H.; Geldnere, K.; Doronin, A.; Meglinski, I.; Bykov, A. Skin Complications of Diabetes Mellitus Revealed by Polarized Hyperspectral Imaging and Machine Learning. IEEE Trans Med Imaging 2021, 40(4), 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Ozdogan, C.Y.; Kenar, H.; Davun, K.E.; Yucel, D.; Doger, E.; Alagoz, S. An in vitro 3D diabetic human skin model from diabetic primary cells. Biomed Mater 2020, 16(1), 015027. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, H.; Chi, Q.; He, Y.; Mu, L.; Liu, C.; Lu, Y. Synchronized research on endothelial dysfunction and microcirculation structure in dorsal skin of rats with type 2 diabetes mellitus. Med Biol Eng Comput 2021, 59(5), 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, H.; Khamaisi, M. Skin well-being in diabetes: Role of macrophages. Cell Immunol 2020, 356, 104154. [Google Scholar] [CrossRef]
- Olinici, D.; Gheucă-Solovăstru, L.; Stoica, L.; Bădescu, L.; Onofrei, P.; Botez, E.A.; Cotrutz, C.E. The molecular mosaic of the premaignant cutaneous lesions. Rom J Morphol Embryol 2016, 57(2), 353–359. [Google Scholar]
- Fokkens, B.T.; Smit, A.J. Skin fluorescence as a clinical tool for non-invasive assessment of advanced glycation and long-term complications of diabetes. Glycoconj J 2016, 33(4), 527–35. [Google Scholar] [CrossRef]
- Nejaddehbashi, F.; Rafiee, Z.; Orazizadeh, M.; Bayati, V.; Hemmati, A.; Hashemitabar, M.; Makvandi, P. Antibacterial and antioxidant double-layered nanofibrous mat promotes wound healing in diabetic rats. Sci Rep 2023, 13(1), 1–15. [Google Scholar] [CrossRef]
- Wielicka, M.; Neubauer-Geryk, J.; Brandt-Varma, A.; Myśliwiec, M.; Bieniaszewski, L. Skin microvascular circulation is not af-437 fected by diabetes duration in young patients with non-complicated type 1 diabetes mellitus. Pediatr Endocrinol Diabetes Metab 2019, 25(4), 183–187. [Google Scholar] [CrossRef]
- Dornuf, F.; Martín-Mateos, P.; Duarte, B.; Hils, B.; Bonilla-Manrique, O.E.; Larcher, F.; Acedo, P.; Krozer, V. Classification of skin phenotypes caused by diabetes mellitus using complex scattering parameters in the millimeter-wave frequency range. Sci Rep 2017, 7(1), 5822. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Lian, T.; Wei, J.; Yue, W.; Zhang, S.; Chen, Q. Skin autofluorescence and the complexity of complications in 443 patients with type 2 diabetes mellitus: a cross-sectional study. BMC Endocr Disord 2021, 21(1), 58. [Google Scholar] [CrossRef]
- Sveen, K.A.; Dahl-Jørgensen, K.; Stensaeth, K.H.; Angel, K.; Seljeflot, I.; Sell, D.R.; Monnier, V.M.; Hanssen, K.F. Glucosepane and oxidative markers in skin collagen correlate with intima media thickness and arterial stiffness in long-term type 1 diabetes. J Diabetes Complications 2015, 29(3), 407–412. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Qin, G.; Yan, W.; Sun, T. Skin Autofluorescence Is Associated with Diabetic Peripheral Neuropathy in Chinese Patients with Type 2 Diabetes: A Cross-Sectional Study. Genet Test Mol Biomarkers 2019, 23(6), 387–392. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiao, L.; Shao, Y.; Li, M.; Gong, M.; Zhang, Y.; Tan, Z.; Wang, Y.; Yang, X.; Wang, Z.; Zhang, Y. Topical GDF11 accelerates skin 450 wound healing in both type 1 and 2 diabetic mouse models. Biochem Biophys Res Commun 2020, 529(1), 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Baik, S.H.; Oh, C.H. Quantitative measurement of desquamation and skin elasticity in diabetic patients. Skin Res 452 Technol 2020, 8(4), 250–254. [Google Scholar] [CrossRef]
- Paolillo, F.R.; Mattos, V.S.; Borghi-Silva, A.; Bagnato, V.S.; de Castro Neto, J.C. Advanced Glycation Endproducts as Biomarkers for Risk of Diabetes and Cardiovascular Diseases by Skin Autofluorescence: A Noninvasive Optical Screening. Photobiomodul Photomed Laser Surg 2019, 37(3), 168–174. [Google Scholar] [CrossRef]
- Brzezinski, P.; Chiriac, A.E.; Pinteala, T.; Foia, L.; Chiriac, A. Diabetic dermopathy ("shin spots") and diabetic bullae ("bullosis diabeticorum") at the same patient. Pak J Med Sci 2015, 31(5), 1275–6. [Google Scholar] [CrossRef]
- Adamska, A.; Pilacinski, S.; Zozulinska-Ziolkiewicz, D.; Gandecka, A.; Grzelka, A.; Konwerska, A.; Malinska, A.; Nowicki, M.; Araszkiewicz, A. An increased skin microvessel density is associated with neurovascular complications in type 1 diabetes mellitus. Diab Vasc Dis Res 2019, 16(6), 513–522. [Google Scholar] [CrossRef]
- Chehelcheraghi, F.; Chien, S.; Bayat, M. Mesenchymal stem cells improve survival in ischemic diabetic random skin flap via increased angiogenesis and VEGF expression. J Cell Biochem 2019, 120(10), 17491–17499. [Google Scholar] [CrossRef]
- Tellechea, A.; Leal, E.C.; Kafanas, A.; Auster, M.E.; Kuchibhotla, S.; Ostrovsky, Y.; Tecilazich, F.; Baltzis, D.; Zheng, Y.; Carvalho, E.; Zabolotny, J.M.; Weng, Z.; Petra, A.; Patel, A.; Panagiotidou, S.; Pradhan-Nabzdyk, L.; Theoharides, T.C.; Veves, A. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 2016, 65(7), 2006–2019. [Google Scholar] [CrossRef]
- Bönhof, G.J.; Strom, A.; Püttgen, S.; Ringel, B.; Brüggemann, J.; Bódis, K.; Müssig, K.; Szendroedi, J.; Roden, M.; Ziegler, D. Patterns 467 of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia 2017, 60(12), 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Araszkiewicz, A.; Gandecka, A.; Nowicki, M.; Uruska, A.; Malińska, A.; Kowalska, K.; Wierusz-Wysocka, B.; Zozulińska-Ziółkiewicz, D. Association between small fiber neuropathy and higher skin accumulation of advanced glycation end products in patients with type 1 diabetes. Pol Arch Med Wewn 2016, 126(11), 847–853. [Google Scholar] [CrossRef] [PubMed]
- Hoppenbrouwers, T.; Tuk, B.; Fijneman, E.M.; de Maat, M.P.; van Neck, J.W. Fibrin improves skin wound perfusion in a diabetic rat model. Thromb Res 2017, 151, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Kirsner, R.S.; Yosipovitch, G.; Hu, S.; Andriessen, A.; Hanft, J.R.; Kim, P.J.; Lavery, L.; Meneghini, L.; Ruotsi, L.C. Diabetic SkinChanges Can Benefit from Moisturizer and Cleanser Use: A Review. J Drugs Dermatol 2019, 18(12), 1211–1217. [Google Scholar]
- Virador, G.M.; de Marcos, L.; Virador, V.M. Skin Wound Healing: Refractory Wounds and Novel Solutions. Methods Mol Biol 2019, 1879, 221–241. [Google Scholar] [PubMed]
- Monnier, V.M.; Genuth, S.; Sell, D.R. The pecking order of skin Advanced Glycation Endproducts (AGEs) as long-term markers of glycemic damage and risk factors for micro- and subclinical macrovascular disease progression in Type 1 diabetes. Glycoconj J 2016, 33(4), 569–79. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, D.; Klein, R.; Klein, B.E.; Wolffenbuttel, B.H.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Atzmon, G.; Ben-Avraham, D.; Crandall, J.P.; Barzilai, N.; Bull, S.B.; Canty, A.J.; Hosseini, S.M.; Hiraki, L.T.; Maynard, J.; Sell, D.R.; Monnier, V.M.; Cleary, P.A.; Braffett, B.H.; DCCT/EDIC Research Group; Paterson, A.D. D. New Locus for Skin Intrinsic Fluorescence in Type 1Diabetes Also Associated With Blood and Skin Glycated Proteins. Diabetes 2016, 65(7), 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.A.M.; Masson-Meyers, D.S.; Caetano, G.F.; Terra, V.A.; Ovidio, P.P.; Jordão-Júnior, A.A.; Frade MAC. Skin changes in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun 2017, 490(4), 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Lykov, A.P.; Bondarenko, N.A.; Poveshchenko, O.V.; Miller, T.V.; Poveshchenko, A.F.; Surovtseva, M.A.; Bgatova, N.P.; Kone kov, VI. Prospect of Using Cell Product for the Therapy of Skin Defects in Diabetes Mellitus. Bull Exp Biol Med 2017, 164(2), 266–268. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Ouyang, Q.; Song, H.; Wang, Z.; Ou, J.; Huang, J.; Liu, L. Characteristic analysis of skin keratinocytes in patients with type 2 diabetes based on the single-cell levels. Chin Med J (Engl) 2022, 135(20), 2461–2466. [Google Scholar] [CrossRef]
- Osawa, S.; Katakami, N.; Sato, I.; Ninomiya, H.; Omori, K.; Yamamoto, Y.; Takahara, M.; Miyashita, K.; Sakamoto, F.; Kawamori, D.; Matsuoka, T.; Shimomura, I. Skin autofluorescence is associated with vascular complications in patients with type 2 diabetes. J 493 Diabetes Complications 2018, 32(9), 839–844. [Google Scholar] [CrossRef]



| Experimental diabetes mellitus (n = 36) | P1 | |
|---|---|---|
| vascular endothelium (n) |
dcapillary level (32) | 0.137 |
| metarteriolar level (26) | ||
| junctional complexes (n) | desmosomes (25) | 0.802 |
| basement membrane (23) | ||
| cellular organelles (n) | mitochondrial structure (35) | 0.201 |
| rough endoplasmic reticulum (30) | ||
| Golgi apparatus (31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





