1. Introduction
Shock in the burn patient is due to the combination of hypovolemic shock and cellular shock, characterized by specific microvascular and hemodynamic alterations [
1]. After injury, there is a significant loss of circulating plasma volume due to increased capillary permeability, which is derived from vascular injury and the release of inflammatory mediators [
2]. As a result, edema emerges in both burned and unburned tissues, followed by the depletion of intravascular volume, reduced cardiac output and increments in systemic vascular resistance [
2,
3,
4,
5,
6].
Cerebral circulation is an integral part of systemic circulation. However, the hemodynamic changes in cerebral circulation are significantly different from those in systemic circulation [
2,
7,
8]. Such a difference is largely due to the autoregulation of cerebral blood flow, which is able to remain relatively constant despite changes in systemic perfusion pressure [
8]. Burns have a significant impact on the level of intracranial pressure (ICP), which increases continuously after injury [
7,
9]. The elevated ICP was more likely to be the result of encephaledema rather than arterial carbon dioxide tension, as it remained constant post-burn [
7]. Although ICP can guide patient management in neurocritical care, it is not commonly monitored in many clinical conditions outside this setting. Provided that data of ICP can be crucial for the successful management of patients in many subcritical conditions [
10], non-invasive estimation of ICP (nICP) method would be useful to determine whether a patient’s ICP is elevated or is changing [
11,
12,
13]. Apart from many clinical applications, Transcranial colour-coded duplex (TCCD) waveform analysis has been investigated as a technique for nICP estimation, and this could represent one of its most useful applications outside the critical care setting. It is conceivable if one considers that increased ICP could affect the waveform of blood flow velocity (FV) in major cerebral vessels which have compliant walls. In addition, another nICP method can also be used: the evaluation of the optic nerve sheath diameter (ONSD) using ultrasound (US) which can detect the dilation of the optic nerve sheath, 3 mm behind the optic disk [
14,
15,
16]. A prospective study suggested TCCD has a good negative predictive value, excluding elevated ICP [
17], as a retrospective study have considered [
14].
The primary aim of the present study was to evaluate the feasibility of real-time nICP measurement [
10] in a cohort of burn patients when they accessed intensive burn care using TCCD and US ONSD.
2. Methods
2.1. Study Design
In this observational study, Authors analyzed prospectively collected data in adult (>18 years) burn patients diagnosed as severe burn that require admission to the burn intensive care unit (ICU) of A. Cardarelli Hospital, Naples, Italy, from October 2021 to December 2022. The study was conducted in accordance with the Declaration of Helsinki and the general principles of Good Clinical Practice. The Institutional Review Board of the AORN Cardarelli-Santobono approved the study to be conducted (CE 66/2019) Authors followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies (
http://www.strobe-statement.org (accessed on 20 February 2023)).
2.2. Population
The following criteria were required for adult patient enrollment: age ≥18 years, admission to ICU, burn size ≥20% Total Body Surface Area (TBSA), deep dermal burns and full thickness burns and enrollment to the study within 8 hours of injury. TCCD and ONSD data collected within 96 h from burn, were eligible for this study. Patients with a diagnosis of idiopathic intracranial hypertension or pseudotumor, with diseases resulting in increased intracranial pressure, severe burn at the level of the temporal and optic nerve ultrasound windows, with prior severe head trauma, represented limiting factors to intracranial hemodynamic assessment and exclusion criteria for TCCD and ONSD examinations. Furthermore, those patients, guardian/family member/fiduciary who did not agree to sign the consent to participate in the study were excluded.
2.3. Data Collection and Definitions
In the present study the Authors collected demographic data, medical history, the acute physiology and chronic health evaluation and burn extension on admission. A severe burn injury is defined using standard criteria, in general for adults, it is severe any burn roughly >20 percent of the TBSA or greater, excluding superficial burns (epidermal; first-degree burns) [
18] results in acute systemic responses collectively known as burn shock [
19]
The size of the burn is estimated using the “rule of Nines” method [
20]. It’s a system used to predict the chance of mortality due to burn [
21]. The proportion of patients who died (case fatality) is plotted as a function of the sum of age and the total percentage of body surface area (TBSA) burned [
22]. The mortality rate increases with the size of the burn. the population analyzed was divided by mortality risk into three categories: moderate, intermediate and high (
Table 1).
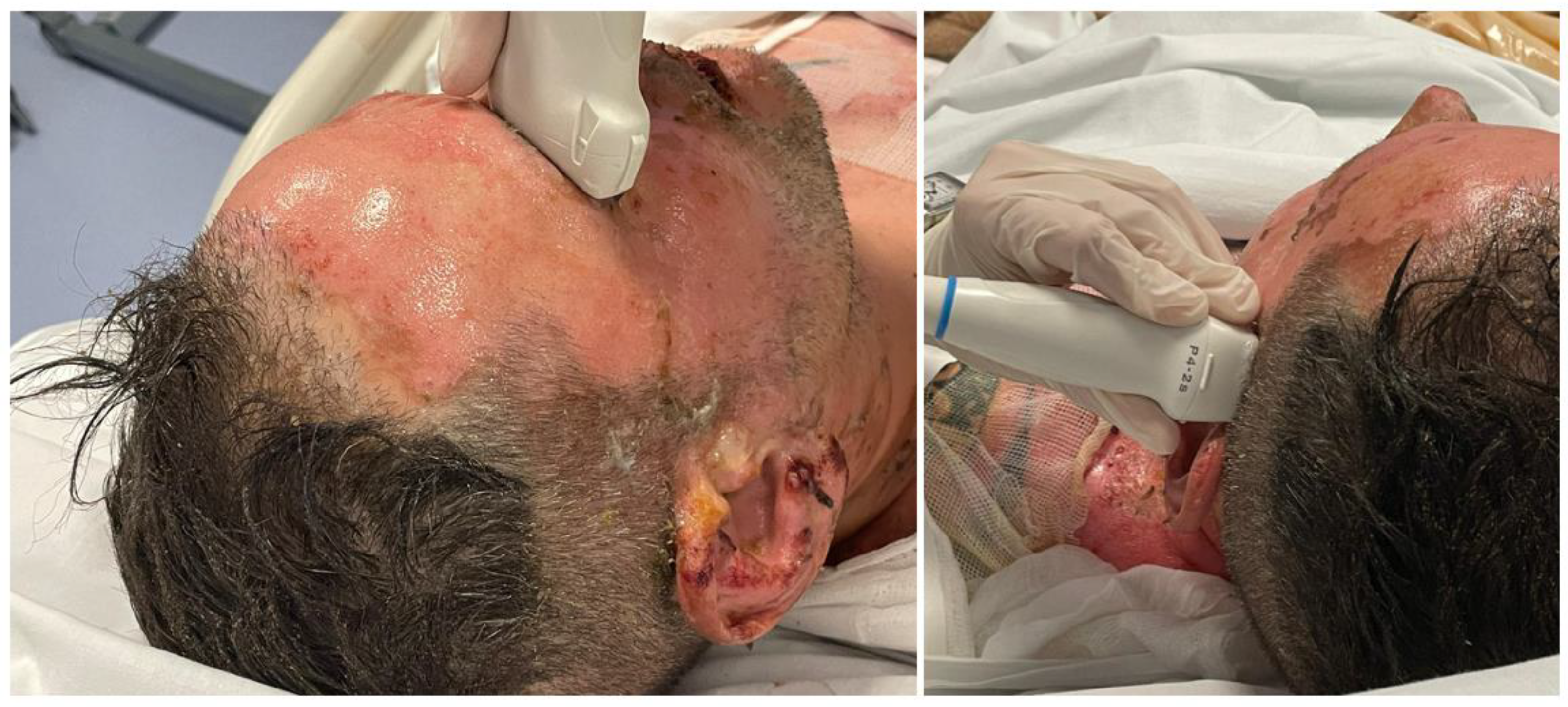
During the examination, patients were in the supine position with a head elevation of no more than 30°. Using TCCD with a Mindray ultrasound 2-MHz probe, blood FV in the middle cerebral artery (MCA) was monitored, like low diastolic cerebral blood flow velocity (FVd), mean flow velocity (FVm), and Pulsatility Index (PI) values can be observed with TCCD [
10]. The diameter of the optic nerve sheath 3 mm behind the optic disk surface, was measured using an Mindray ultrasound 7.5-MHz probe [
14]. The probe was applied with coupling gel to the closed eyelid.
Two measurements, sagittal and transverse, were taken for each optic nerve with standardized equipment settings using the digital cursor and measurement software of the ultrasound machine [
14,
23]. Data were collected exactly at time 0 (within 8 hours of the burn), 48 hours after and 96 hours after the burn.
Are considered to be pathological FVd <20 cm/sec, FVm <30 cm/sec, PI >1.4; ONSD > 5 mm is considered ICP > 20 mmHg. The combination of at least two pathological values out of the three has been considered highly suggestive of intracranial hypertension (
Figure 1).
2.4. Study Outcomes
The primary outcome of the study was to assess the occurrence of high-ICP episodes in burned patients using non-invasive evaluation (TCCD/ONSD). The secondary outcomes were the correlations of TCCD and ONSD values with burn size and mortality.
2.5. Statistical Analysis
The collected data will be analyzed descriptively with measures of centrality and variance (mean, median, standard deviation (SD) and interquartile range (IQR)), based on the distribution (normal or non-normal) of the data themselves. The ONSD values were used as averages between transverse and sagittal measurements and subsequent averages between left eye and right eye: an average ONSD value was thus obtained for each patient at each evaluation step. ONSD and TCCD parameters in the different burn groups were compared by the Mann-Whitney U test and Spearman’s rho for the non-parameter estimation of the correlation. Statistical analysis was performed using Stata statistical software version 16 (Stata Corp LLC, College Station, TX, USA).
3. Results
A total of 20 patients with burn size ≥20% TBSA, deep dermal and full thickness burns were admitted over the study period. 7 patients were female and the mean age of the patients was 56.8 years with a SD of +/- 23.78. Nine patients had TBSA between 20-30%, 7 had TBSA between 30-50%, 4 had TBSA between 50-90%. All patients receiving fluid therapy, of which 2 with extended burn between 30-50% and 4 between 50-90% were under mechanical ventilation and cardioactive medications (i.e., vasopressors and/or inotropic agents) at the time of TCCD and measurement of ONSD assessment.
3.1. TCCD FVd
The mean FVd values and SD at time 0, time 1 (48 hours) and time 2 (96 hours), distinguished by the three burn groups are described in detail in
Table 2. Only two recorded values were lower than the critical threshold < 20 cm/sec in the time 0 and 2.
FVd Flow Velocity Diastolic, SD standard deviation, IQR InterQuartile.
3.2. TCCD FVm
The mean FVm values and SD at time 0, time 1 (48 hours) and time 2 (96 hours), distinguished by the three burn groups are described in detail in
Table 3. No recorded value was lower than the critical threshold < 30 cm/sec.
3.3. TCCD PI
The mean PI values and SD at time 0, time 1 (48 hours) and time 2 (96 hours), distinguished by the three burn groups are described in detail in
Table 4. No recorded value was higher than the critical threshold > 1.4.
3.4. ONSD
The mean ONSD values and SD at time 0, time 1 (48 hours) and time 2 (96 hours), distinguished by the three burn groups are described in detail in
Table 5. No recorded value exceeded the threshold > 5 mm.
Correlations:
The FVd, FVm, PI and ONSD measurements in the 3 stages, although plausible and homogeneous, with particularly narrow SD and IQR, do not demonstrate statistical significance in non-parametric tests, There is no association between these values in the various steps and the severity of the burn.
4. Discussion
In the present study, PI and FVd and FVm were not different in TBSA percentage and patients with partial and full thickness; these parameters have always been under our cut-off values. In the same way, the mean values ONSD at time 0, time 1 and time 2 have never been more than 5mm. However, FVd in the single measurement at time 0 and 2 was found to be slightly under the cut-off values. afterwards in T1 the derivative data were again in the normal range. Obtained data on enrolled burn patients show the absence of significant alteration in cerebral hemodynamics.
These data cannot be compared because Authors did not find any other in the scientific literature, but they highlight the feasibility of the ultrasound-based method.
Burn-related brain injury remains a critical issue that should not be neglected and moreover nICP estimation could be a precious support in neuroworsing valuation algorithm.
In a report a retrospective, clinicopathologic study of 139 patients who died during treatment of a severe burn. Fifty-three percent of the patients had central nervous system complications-infections, cerebral infarcts and hemorrhages, metabolic encephalopathies, central pontine myelinolysis, and cerebral trauma. Children and adults were equally affected [
24].
It is not clear when the brain injury begins and how it can be immediately recognized and although in a specialist environment such as burns patients, computed tomography of the brain is readily available, for patients who are often complex to transport and transfer and transport due to clinical conditions. In such a scenario, methods for nICP could improve clinical management of these conditions. With present study, Authors tried to transfer knowledge applied as routine in a neurocritical care environment in a burn setting.
In addition to the irrefutable negative predictive value of TCCD, there are also promising results on ONSD. Hansen at All compared the ultrasonographic ONSD with the opening ICP measured invasively with an intraventricular catheter in order to assess the accuracy of ONSD to predict raised ICP. The results of their study suggest that an ONSD cut off of 5.5 mm is a strong predictor of ICP of > 20 mm Hg. In a similar study Kimberley et al. reported an ONSD cut off of 5.0 mm to predict an ICP > 20 mm Hg [
25].
The idea of this pilot study is to try to design a non-invasive neuromonitoring protocol in burn patients. For example, T0 describes the measurement within the first 8 hours of the occurrence, that is the hours in which a systemic inflammatory response syndrome, sepsis, develops. During the systemic inflammatory response syndrome, sepsis, severe burn injury, however, physical barriers between the systemic circulation and the cerebral parenchyma can be seriously compromised [
26]. Fluid resuscitation remains the cornerstone of early burn management. Adequate fluid administration is critical to the prevention of burn shock and other complications of thermal injury [
27]. The rationale is to match fluid resuscitation with the gradual resolution of the widespread increased vascular permeability that begins around 8-12 hours post burn [
28] To demonstrate the applicability of this monitoring, an observational case-control study with a larger series of cases will be necessary.
5. Limitations
This is a single center study, a small number of burn patients. Authors estimated hemodynamic brain, early 96 hours, thus not excluding the possibility of alterations later on during the ICU stay. Moreover, the study did not make a difference between stable systemic arterial pressure and patients with cardioactive drug regimens, respiratory parameters, ventilation settings and their modifications. Furthermore, the neurological conditions of the patients were not taken into account (patients alert, sedated, in coma): patients who are not in a spontaneous coma, hardly have even incipient intracranial hypertension.
6. Conclusions
Optimal management of the severely burned patient can only be achieved if the treating critical care personnel are intimately familiar with the systemic and cerebral pathophysiology following severe burn trauma, and adjust their treatment modalities accordingly.
The cerebral circulation dynamics can be observed with such methods as nICP changes in time domain, and tracked in real-time in the clinical setting. This is one of the advantages of TCCD and ONSD and may become particularly useful as a primary assessment tool in Centers where ICP measurements are not routinely applied, or in patients in whom ICP monitoring is unavailable or may not be clearly indicated [
10]
7. Declarations
The present manuscript complies with all instructions to Authors.
This manuscript has not been published elsewhere and is not under consideration by another journal.
Ethics Approval
CE 66/2019.
Code Availability
A specific custom code will be given by the corresponding author, RA, upon reasonable request.
Availability of Data and Material
The data supporting this study’s findings are available from the corresponding author, RA, upon reasonable request.
Conflicts of Interest/Competing Interests
The Authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
Maria Notaro, Laura Maria Beatrice Belotti, Marco Di Serafino, Adele Longobardi, Romolo Villani, Raffaele Aspide, contributed to the manuscript conception and design. Authors read and approved the final manuscript.
Funding
The Authors have not received any financial support for the research, authorship, and/or publication of this article.
Abbreviations
ICP - intracranial pressure
nICP - non-invasive ICP
TCCD - Transcranial colour-coded duplex
FV – flow velocity
ONSD - optic nerve sheath diameter
US - ultrasound
ICU - intensive care unit
STROBE - Strengthening the Reporting of Observational Studies in Epidemiology
TBSA - Total Body Surface Area
BSA - Body Surface Area
MCA - middle cerebral artery
FVd - diastolic flow velocity
FVm - mean flow velocity
PI - Pulsatily Index
SD - standard deviation
IQR - interquartile range
References
- Guilabert P, Usúa G, Martín N, et al. Fluid resuscitation management in patients with burns: update. Br J Anaesth. 2016 Sep;117(3):284-96. [CrossRef]
- Chen J, Zhang D, Zhang J, et al. Pathological changes in the brain after peripheral burns. Burns Trauma. 2023 Feb 6;11:tkac061. [CrossRef]
- Ganrot K, Jacobsson S, Rothman U. Transcapillary passage of plasma proteins in experimental burns. Acta Physiol Scand. 1974 Aug;91(4):497-501. [CrossRef]
- Vaughn L, Beckel N. Severe burn injury, burn shock, and smoke inhalation injury in small animals. Part 1: burn classification and pathophysiology. J Vet Emerg Crit Care (San Antonio). 2012 Apr;22(2):179-86. [CrossRef]
- Keck M, Herndon DH, Kamolz LP, et al. Pathophysiology of burns. Wien Med Wochenschr. 2009;159(13-14):327-36. [CrossRef]
- Latenser, B.A. Critical care of the burn patient: the first 48 hours. Crit Care Med. 2009 Oct;37(10):2819-26. [CrossRef]
- Shin C, Kinsky MP, Thomas JA, et al. Effect of cutaneous burn injury and resuscitation on the cerebral circulation in an ovine model. Burns. 1998 Feb;24(1):39-45. [CrossRef]
- Panerai, R.B. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng. 2008 Mar;8(1):42-59. [CrossRef]
- Chen J, Zhang D, Zhang J, et al. Pathological changes in the brain after peripheral burns. Burns Trauma. 2023 Feb 6;11:tkac061. [CrossRef]
- Cardim D, Robba C, Bohdanowicz M, et al. Non-invasive Monitoring of Intracranial Pressure Using Transcranial Doppler Ultrasonography: Is It Possible? Neurocrit Care. 2016 Dec;25(3):473-491. [CrossRef]
- Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003 Apr;10(4):376-81. [CrossRef]
- Rasulo FA, Bertuetti R, Robba C, et al. The accuracy of transcranial Doppler in excluding intracranial hypertension following acute brain injury: a multicenter prospective pilot study. Crit Care. 2017 Feb 27;21(1):44. [CrossRef]
- Lang EW, Lagopoulos J, Griffith J, et al. Noninvasive cerebrovascular autoregulation assessment in traumatic brain injury: validation and utility. J Neurotrauma. 2003 Jan;20(1):69-75. [CrossRef]
- Newman WD, Hollman AS, Dutton GN, et al. Measurement of optic nerve sheath diameter by ultrasound: a means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalmol. 2002 Oct;86(10):1109-13. [CrossRef]
- Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. I. Experimental study. Pediatr Radiol. 1996 Oct;26(10):701-5. [CrossRef]
- Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension II. Patient study. Pediatr Radiol. 1996 Oct;26(10):706-10. [CrossRef]
- Rasulo FA, Calza S, Robba C, et al. Transcranial Doppler as a screening test to exclude intracranial hypertension in brain-injured patients: the IMPRESSIT-2 prospective multicenter international study. Crit Care. 2022 Apr 15;26(1):110. [CrossRef]
- Elisha G Brownson, Nicole S.Gibran, Evaluation of the Burn Wound: Management Decision, Editor(s): David N. Herndon, Total Burn Care (Fifth Edition), Elsevier, 2018, ISBN9780323476614. [CrossRef]
- Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and treatment: review and advancements. Crit Care. 2015 Jun 12;19:243. [CrossRef]
- David Thom, Appraising current methods for preclinical calculation of burn size – A pre-hospital perspective, Burns, Volume 43, Issue 1, 2017,ISSN 0305-4179. [CrossRef]
- Osler, Turner MD, MSc; Glance, Laurent G. MD; Hosmer, David W. PhD. Simplified Estimates of the Probability of Death After Burn Injuries: Extending and Updating the Baux Score. The Journal of Trauma: Injury, Infection, and Critical Care 68(3):p 690-697, March 2010. |. [CrossRef]
- Bettencourt AP, Romanowski KS, Joe V, et al. Updating the Burn Center Referral Criteria: Results From the 2018 eDelphi Consensus Study. J Burn Care Res. 2020 Sep 23;41(5):1052-1062. [CrossRef]
- Aspide R, Bertolini G, Albini Riccioli L, et al. A Proposal for a New Protocol for Sonographic Assessment of the Optic Nerve Sheath Diameter: The CLOSED Protocol. Neurocrit Care. 2020 Feb;32(1):327-332. [CrossRef]
- Winkelman MD, Galloway PG. Central nervous system complications of thermal burns. A postmortem study of 139 patients. Medicine (Baltimore). 1992 Sep;71(5):271-83. [CrossRef]
- Kimberly HH, Shah S, Marill K, et al. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008 Feb;15(2):201-4. [CrossRef]
- Flierl MA, Stahel PF, Touban BM, et al. Bench-to-bedside review: Burn-induced cerebral inflammation--a neglected entity? Crit Care. 2009;13(3):215. [CrossRef]
- Klein MB, Hayden D, Elson C, et al. The association between fluid administration and outcome following major burn: a multicenter study. Ann Surg. 2007 Apr;245(4):622-8. [CrossRef]
- N, Losser MR, Lucas C, Pantet O, et al. Management of severe thermal burns in the acute phase in adults and children. Anaesth Crit Care Pain Med. 2020 Apr;39(2):253-267. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).