Submitted:
07 February 2024
Posted:
08 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- RQ1—What are the challenges and limitations associated with accurate liver segmentation in CT scans?
- RQ2 — How does the choice of the method impact the accuracy and efficiency of liver segmentation in CT scans?
- RQ3 — What are the evaluation metrics commonly used to assess the performance of AI models and traditional methods for liver segmentation in CT scans?
2. Methodology
2.1. Data Sources
2.2. Search Queries
2.3. Inclusion Criteria
2.4. Exclusion Criteria
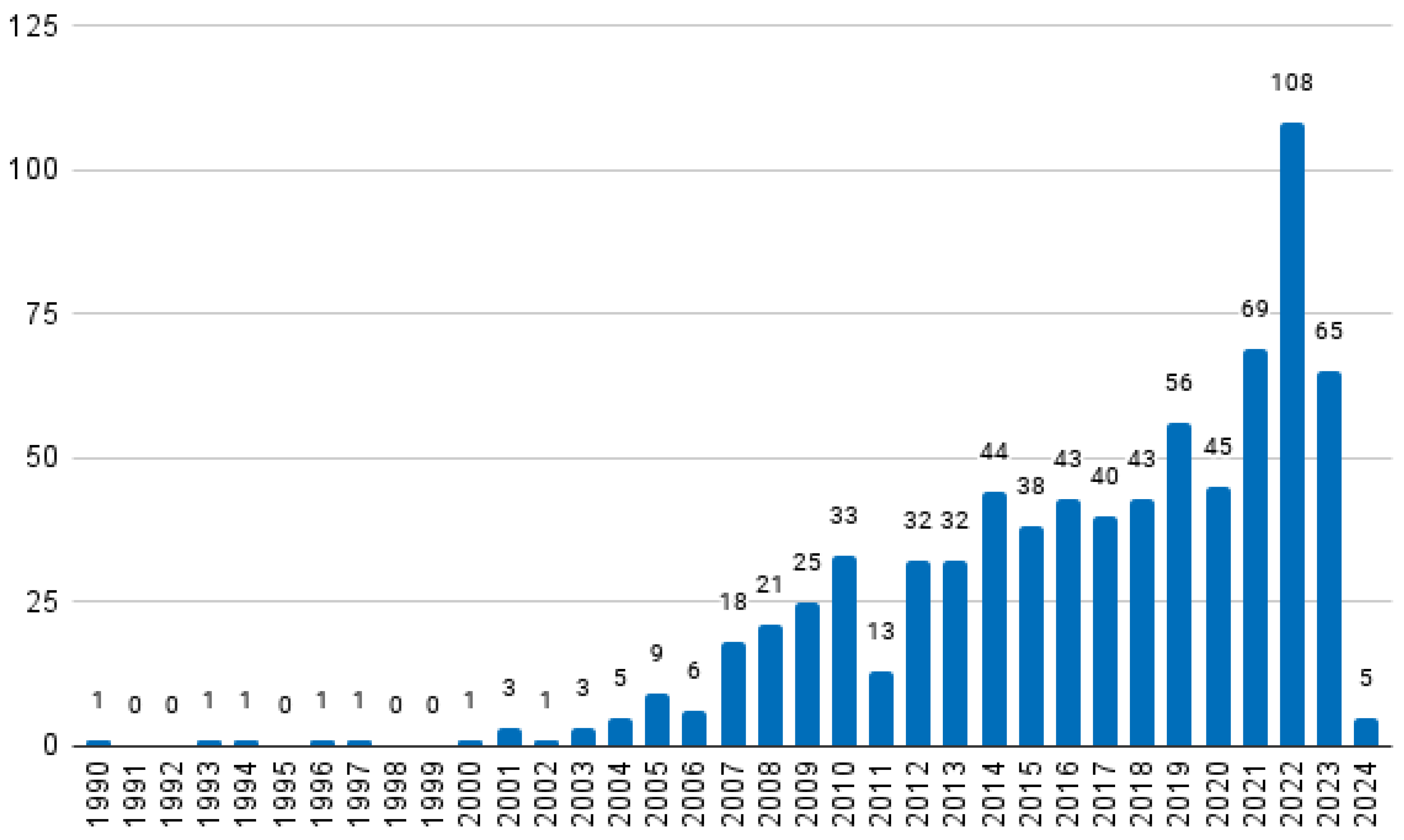
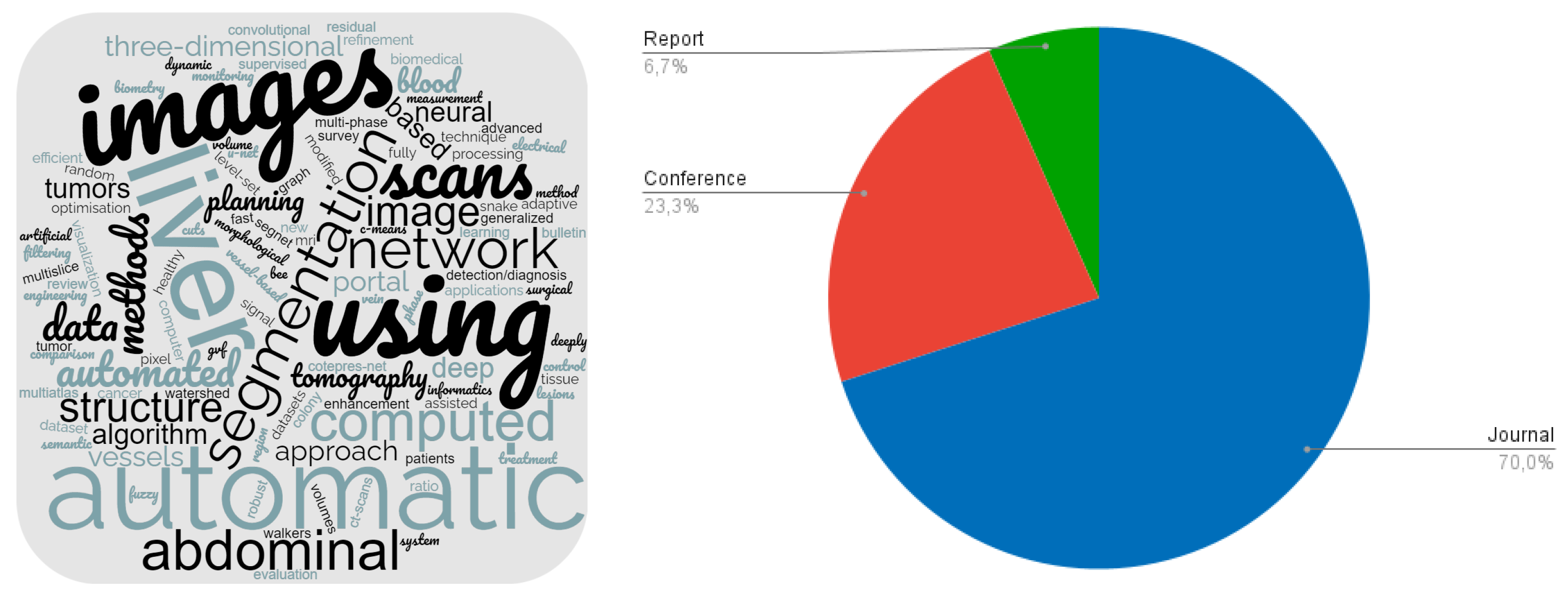
2.5. Characterisation of Selected Papers
3. Literature Review
3.1. Historical Overview
3.2. Other Review Papers
4. Findings
5. Discussion
5.1. Public Datasets Analysis
5.2. Impact of the Adoption of Neuronal Network-based Methods
5.3. Comparison between 2D and 3D Methods for Liver Segmentation
-
Importance of Choosing between 2D and 3D Methods
- –
- In medical imaging, and in particular liver segmentation, the choice between slice-based 2D and volume-based 3D segmentation methods is crucial. This decision is highly dependent on the anatomical structure of the liver. Given the complex, three-dimensional nature of the liver, 3D segmentation techniques often prove to be the most appropriate choice [21] [39]. These methods are inherently designed to understand and process the volumetric characteristics of the liver, which is a critical consideration for accurate segmentation results.
-
2D Segmentation Limitations
- –
- Although 2D slice-based segmentation is widely used, it has limitations, particularly when it comes to dealing with complex organs such as the liver. The main challenge with 2D methods is their inability to fully capture all the regions of the liver. They involve working with individual slices, which can provide a fragmented understanding of the organ structure, but this fragmentation can lead to inconsistencies and errors when these individual slices are aggregated to form a complete image [40].
-
3D Segmentation Advantages
- –
- In order to overcome the limitations of 2D segmentation, 3D segmentation has the ability to use more contextual information. Unlike 2D methods, which visualise the liver in individual slices, 3D techniques consider the organ in its integrity, as they have the ability to ensure anatomical correctness by processing the liver as a single, continuous volume, avoiding errors that can arise from the aggregation of 2D slices [14] [38]. In 2D segmentation, inconsistencies can occur when individual slices are combined, leading to inaccuracies in the representation of liver anatomy. The holistic view provided by the 3D segmentation, results in more accurate segmentation, as it takes into account the spatial relationships and continuity between the different sections of the liver. The inclusion of this additional contextual information can potentially lead to segmentation results, especially in complex cases where the shape and size of the liver can vary considerably.
5.4. Exploring Research Questions
-
RQ1—What are the challenges and limitations associated with accurate liver segmentation in CT scans?
- –
- Challenges and limitations associated with accurate liver segmentation in CT images include under-segmentation, over-segmentation, low contrast, poor boundary detection and background segmentation due to noise. In addition, liver segmentation in CT scans is further challenged by the presence of artefacts, such as partial volumes, noise, and low sharpness and contrast between organs, making it difficult to identify the boundaries between different tissues.
-
RQ2 — How does the choice of the method impact the accuracy and efficiency of liver segmentation in CT scans?
- –
- The choice of the method has a significant impact on the accuracy and efficiency of liver segmentation in CT scans. Traditional techniques such as image processing and region-growing approaches have shown varying degrees of sensitivity and specificity, with some challenges in dealing with large injuries. In contrast, newer methods such as FCN, DBN-DNN, and techniques like ResU-Net and SegNet showed higher accuracy, with some reaching up the highest accuracy levels. Notably, advances in methods have also led to significant improvements in processing times, with strategies such as using GPU over CPU leading to significant time reductions, indicating a trend towards more efficient and accurate liver segmentation techniques.
-
RQ3 — What are the evaluation metrics commonly used to assess the performance of AI models and traditional methods for liver segmentation in CT scans?
- –
- Some of the key metrics used to measure the outcome of segmentation techniques include Dice Similarity Coefficient (DSC), accuracy, precision, sensitivity, specificity, and segmentation speed. There is not much consistency in the metrics presented by the various studies except for DSC.
6. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Francque, S. The Liver and the Cardiovascular System: Two of a Kind? Journal of the American Heart Association 2021, 10, e020286. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Current Biology 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Mendes, B.; Domingues, I.; Silva, A.; Santos, J. Prostate Cancer Aggressiveness Prediction Using CT Images. Life 2021, 11, 1164. [Google Scholar] [CrossRef]
- Pereira, G.; Domingues, I.; Martins, P.; Abreu, P.H.; Duarte, H.; Santos, J. Registration of CT with PET: A Comparison of Intensity-Based Approaches. In International Workshop on Combinatorial Image Analysis (IWCIA); Springer, 2018; pp. 134–149. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, K.; Deng, R.; Li, S.; Duan, S. Deep Learning-Based CT Imaging for the Diagnosis of Liver Tumor. Computational Intelligence and Neuroscience 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Vernuccio, F.; Cannella, R.; Bartolotta, T.V.; Galia, M.; Tang, A.; Brancatelli, G. Advances in liver US, CT, and MRI: moving toward the future. European Radiology Experimental 2021, 5, 52. [Google Scholar] [CrossRef]
- Domingues, I.; Cardoso, J.S. Using Bayesian surprise to detect calcifications in mammogram images. 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2014, pp. 1091–1094. [CrossRef]
- Mendes, B.; Domingues, I.; Santos, J. Multi-class Semantic Segmentation for Prostate Cancer Radiotherapy Treatment Optimization. International Conference on Mathematical Analysis and Applications in Science and Engineering (ICMA2SC), 2022.
- Bechar, M.E.A.; Settouti, N.; Domingues, I. Deep Learning vs. Super Pixel Classification for Breast Masses Segmentation. In Deep Learning for Biomedical Applications; CRC Press, 2021; pp. 121–156. [Google Scholar]
- Oliveira, A.C.; Domingues, I.; Duarte, H.; Santos, J.; Abreu, P.H. Going Back to Basics on Volumetric Segmentation of the Lungs in CT: A Fully Image Processing Based Technique. In Iberian Conference on Pattern Recognition and Image Analysis (IbPRIA); Springer, 2019; Vol. 11868 LNCS, pp. 322–334. [Google Scholar]
- Carbone, I.; Martins, P.; Teixeira, A.; Silva, A. A Vocal Tract Segmentation and Analysis over a European Portuguese MRI Database. Revista do Departamento de Electrónica e Telecomunicações, Universidade de Aveiro 2008, 4, 1050–1053. [Google Scholar]
- Zhou, L.Q.; Wang, J.Y.; Yu, S.Y.; Wu, G.G.; Wei, Q.; Deng, Y.B.; Wu, X.L.; Cui, X.W.; Dietrich, C.F. Artificial intelligence in medical imaging of the liver. World Journal of Gastroenterology 2019, 25, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Chlebus, G.; Schenk, A.; Moltz, J.H.; van Ginneken, B.; Hahn, H.K.; Meine, H. Automatic liver tumor segmentation in CT with fully convolutional neural networks and object-based postprocessing. Scientific Reports 2018, 8, 15497. [Google Scholar] [CrossRef] [PubMed]
- Christ, P.F.; Ettlinger, F.; Grün, F.; Elshaera, M.E.A.; Lipkova, J.; Schlecht, S.; Ahmaddy, F.; Tatavarty, S.; Bickel, M.; Bilic, P.; Rempfler, M.; Hofmann, F.; Anastasi, M.D.; Ahmadi, S.A.; Kaissis, G.; Holch, J.; Sommer, W.; Braren, R.; Heinemann, V.; Menze, B. Automatic Liver and Tumor Segmentation of CT and MRI Volumes using Cascaded Fully Convolutional Neural Networks. arXiv 1702.05970 2017, [1702.05970].
- Ansari, M.Y.; Abdalla, A.; Ansari, M.Y.; Ansari, M.I.; Malluhi, B.; Mohanty, S.; Mishra, S.; Singh, S.S.; Abinahed, J.; Al-Ansari, A.; Balakrishnan, S.; Dakua, S.P. Practical utility of liver segmentation methods in clinical surgeries and interventions. BMC Medical Imaging 2022, 22, 97. [Google Scholar] [CrossRef]
- Le, D.C.; Chinnasarn, K.; Chansangrat, J.; Keeratibharat, N.; Horkaew, P. Semi-automatic liver segmentation based on probabilistic models and anatomical constraints. Scientific Reports 2021, 11, 6106. [Google Scholar] [CrossRef] [PubMed]
- Halevi, G.; Moed, H.; Bar-Ilan, J. Suitability of Google Scholar as a source of scientific information and as a source of data for scientific evaluation—Review of the Literature. Journal of Informetrics 2017, 11, 823–834. [Google Scholar] [CrossRef]
- Sakshi.; Kukreja, V. Image Segmentation Techniques: Statistical, Comprehensive, Semi-Automated Analysis and an Application Perspective Analysis of Mathematical Expressions. Archives of Computational Methods in Engineering 2023, 30, 457–495. [CrossRef]
- Bae, K.T.; Giger, M.L.; Chen, C.T.; Kahn Jr, C.E. Automatic segmentation of liver structure in CT images. Medical physics 1993, 20, 71–78. [Google Scholar] [CrossRef]
- Gao, L.; Heath, D.G.; Kuszyk, B.S.; Fishman, E.K. Automatic liver segmentation technique for three-dimensional visualization of CT data. Radiology 1996, 201, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Soler, L.; Malandain, G.; Montagnat, J.; Delingette, H.; Ayache, N.; Clément, J.M.; Roy, C.; Russier, Y.; Tassetti, V.; Marescaux, J. Automatic Segmentation of Portal Vein in CT-Scans of the Liver. World Congress on Medical Physics and Biomedical Engineering (MPBE), 1997, p. 788.
- Yoo, S.W.; Cho, J.S.; Noh, S.M.; Shin, K.S.; Park, J.W. Advanced Liver Segmentation by Using Pixel Ratio in Abdominal CT Image. IEEK Conference. The Institute of Electronics and Information Engineers, 2000, pp. 39–42.
- Pan, S.; Dawant, B.M. Automatic 3D segmentation of the liver from abdominal CT images: a level-set approach. Medical Imaging: Image Processing. SPIE, 2001, Vol. 4322, pp. 128–138. [CrossRef]
- Saitoh, T.; Tamura, Y.; Kaneko, T. Automatic segmentation of liver region through blood vessels on multi-phase CT. International Conference on Pattern Recognition. IEEE, 2002, Vol. 1, pp. 735–738. [CrossRef]
- Masumoto, J.; Hori, M.; Sato, Y.; Murakami, T.; Johkoh, T.; Nakamura, H.; Tamura, S. Automated liver segmentation using multislice CT images. Systems and Computers in Japan 2003, 34, 71–82. [Google Scholar] [CrossRef]
- Lim, S.J.; Jeong, Y.Y.; Lee, C.W.; Ho, Y.S. Automatic segmentation of the liver in CT images using the watershed algorithm based on morphological filtering. Medical Imaging: Image Processing. SPIE, 2004, Vol. 5370, pp. 1658–1666. [CrossRef]
- Liu, F.; Zhao, B.; Kijewski, P.K.; Wang, L.; Schwartz, L.H. Liver segmentation for CT images using GVF snake. Medical physics 2005, 32, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Jeong, Y.Y.; Ho, Y.S. Automatic liver segmentation for volume measurement in CT Images. Journal of Visual Communication and Image Representation 2006, 17, 860–875. [Google Scholar] [CrossRef]
- Beichel, R.; Bauer, C.; Bornik, A.; Sorantin, E.; Bischof, H. Liver segmentation in CT data: A segmentation refinement approach. 3D Segmentation in The Clinic: A Grand Challenge, 2007, pp. 235–245.
- Massoptier, L.; Casciaro, S. A new fully automatic and robust algorithm for fast segmentation of liver tissue and tumors from CT scans. European radiology 2008, 18, 1658–1665. [Google Scholar] [CrossRef]
- Heimann, T.; Van Ginneken, B.; Styner, M.A.; Arzhaeva, Y.; Aurich, V.; Bauer, C.; Beck, A.; Becker, C.; Beichel, R.; Bekes, G.; others. Comparison and evaluation of methods for liver segmentation from CT datasets. IEEE transactions on medical imaging 2009, 28, 1251–1265. [CrossRef]
- Akram, M.U.; Khanum, A.; Iqbal, K. An automated system for liver CT enhancement and segmentation. ICGST Journal of Graphics, Vision and Image Processing (ICGST-GVIP) 2010, 10, 17–22. [Google Scholar]
- Oliveira, D.A.; Feitosa, R.Q.; Correia, M.M. Segmentation of liver, its vessels and lesions from CT images for surgical planning. Biomedical engineering online 2011, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Linguraru, M.G.; Richbourg, W.J.; Jianfei Liu.; Watt, J.M.; Pamulapati, V.; Shijun Wang.; Summers, R.M. Tumor Burden Analysis on Computed Tomography by Automated Liver and Tumor Segmentation. IEEE Transactions on Medical Imaging 2012, 31, 1965–1976. [CrossRef]
- Li, X.; Luo, S.; Li, J. Liver Segmentation from CT Image Using Fuzzy Clustering and Level Set. Journal of Signal and Information Processing 2013, 04, 36–42. [Google Scholar] [CrossRef]
- Platero, C.; Tobar, M.C.; others. A multiatlas segmentation using graph cuts with applications to liver segmentation in CT scans. Computational and mathematical methods in medicine 2014, 2014. [CrossRef]
- Mostafa, A.; Fouad, A.; Abd Elfattah, M.; Hassanien, A.E.; Hefny, H.; Zhu, S.Y.; Schaefer, G. CT liver segmentation using artificial bee colony optimisation. Procedia Computer Science 2015, 60, 1622–1630. [Google Scholar] [CrossRef]
- Dou, Q.; Chen, H.; Jin, Y.; Yu, L.; Qin, J.; Heng, P.A. 3D deeply supervised network for automatic liver segmentation from CT volumes. 19th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI). Springer, 2016, pp. 149–157. [CrossRef]
- Hiraman, A. Liver segmentation using 3D CT scans. PhD thesis, University of Kwazulu-Natal, 2018.
- Wang, K.; Mamidipalli, A.; Retson, T.; Bahrami, N.; Hasenstab, K.; Blansit, K.; Bass, E.; Delgado, T.; Cunha, G.; Middleton, M.S.; others. Automated CT and MRI liver segmentation and biometry using a generalized convolutional neural network. Radiology: Artificial Intelligence 2019, 1, 180022. [CrossRef]
- Almotairi, S.; Kareem, G.; Aouf, M.; Almutairi, B.; Salem, M.A.M. Liver tumor segmentation in CT scans using modified SegNet. Sensors 2020, 20, 1516. [Google Scholar] [CrossRef]
- Soler, L.; Hostettler, A.; Agnus, V.; Charnoz, A.; Fasquel, J.; Moreau, J.; Osswald, A.; Bouhadjar, M.; Marescaux, J. 3D Image Reconstruction for Comparison of Algorithm Database: A Patient Specific Anatomical and Medical Image Database. Tech. rep, IRCAD, Strasbourg, France, 2010.
- Ayalew, Y.A.; Fante, K.A.; Mohammed, M.A. Modified U-Net for liver cancer segmentation from computed tomography images with a new class balancing method. BMC Biomedical Engineering 2021, 3, 4. [Google Scholar] [CrossRef]
- LiTS Challenge Dataset. https://competitions.codalab.org/competitions/17094. Accessed: Nov. 27, 2023.
- Scicluna, D. Automatic segmentation of healthy liver in abdominal computed tomography scans. Master’s thesis, University of Malta, 2022.
- Kavur, A.E.; Gezer, N.S.; Barış, M.; Aslan, S.; Conze, P.H.; Groza, V.; Pham, D.D.; Chatterjee, S.; Ernst, P.; Özkan, S.; Baydar, B.; Lachinov, D.; Han, S.; Pauli, J.; Isensee, F.; Perkonigg, M.; Sathish, R.; Rajan, R.; Sheet, D.; Dovletov, G.; Speck, O.; Nürnberger, A.; Maier-Hein, K.H.; Bozdağı Akar, G.; Ünal, G.; Dicle, O.; Selver, M.A. CHAOS Challenge - combined (CT-MR) healthy abdominal organ segmentation. Medical Image Analysis 2021, 69, 101950. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, K.A.; Omran, L.N.; El Seddawy, A.I.B. Automatic liver segmentation in computed tomography scans using deep semantic segmentation. Bulletin of Electrical Engineering and Informatics 2023, 12, 250–256. [Google Scholar] [CrossRef]
- Shao, J.; Luan, S.; Ding, Y.; Xue, X.; Zhu, B.; Wei, W. Attention Connect Network for Liver Tumor Segmentation from CT and MRI Images. Technology in Cancer Research & Treatment 2024, 23, 15330338231219366. [Google Scholar] [CrossRef]
- Maurício, J.; Domingues, I.; Bernardino, J. Comparing Vision Transformers and Convolutional Neural Networks for Image Classification: A Literature Review. Applied Sciences 2023, 13. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Selvathi, D. Survey on segmentation of liver from CT images. IEEE international conference on advanced communication control and computing technologies (ICACCCT), 2012, pp. 234–238. [CrossRef]
- Mharib, A.M.; Ramli, A.R.; Mashohor, S.; Mahmood, R.B. Survey on liver CT image segmentation methods. Artificial Intelligence Review 2012, 37, 83–95. [Google Scholar] [CrossRef]
- Khan, F. Automated segmentation of CT liver images: a review. Journal of Communications Technology, Electronics and Computer Science 2018, 19, 5–9. [Google Scholar]
- Moghbel, M.; Mashohor, S.; Mahmud, R.; Saripan, M.I.B. Review of liver segmentation and computer assisted detection/diagnosis methods in computed tomography. Artificial Intelligence Review 2018, 50, 497–537. [Google Scholar] [CrossRef]
- Vanmore, S.V.; Chougule, S.R. Survey on automatic liver segmentation techniques from abdominal CT images. International Conference on Intelligent Computing and Control Systems (ICCS). IEEE, 2019, pp. 1030–1035. [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Advances in neural information processing systems 2012, 25. [Google Scholar] [CrossRef]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; Berg, A.C.; Fei-Fei, L. ImageNet Large Scale Visual Recognition Challenge. International Journal of Computer Vision (IJCV) 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. CoRR 2015, abs/1505.04597, [1505.04597].
- Al-Saeed, Y.; Gab-Allah, W.; Elmogy, M. Hepatic tumors diagnosis system based on fuzzy c-means using computed tomography images. Research Square Preprint 2022. [Google Scholar] [CrossRef]
- MICCAI-Sliver07. https://sliver07.grand-challenge.org/. Accessed: Nov. 27, 2023.
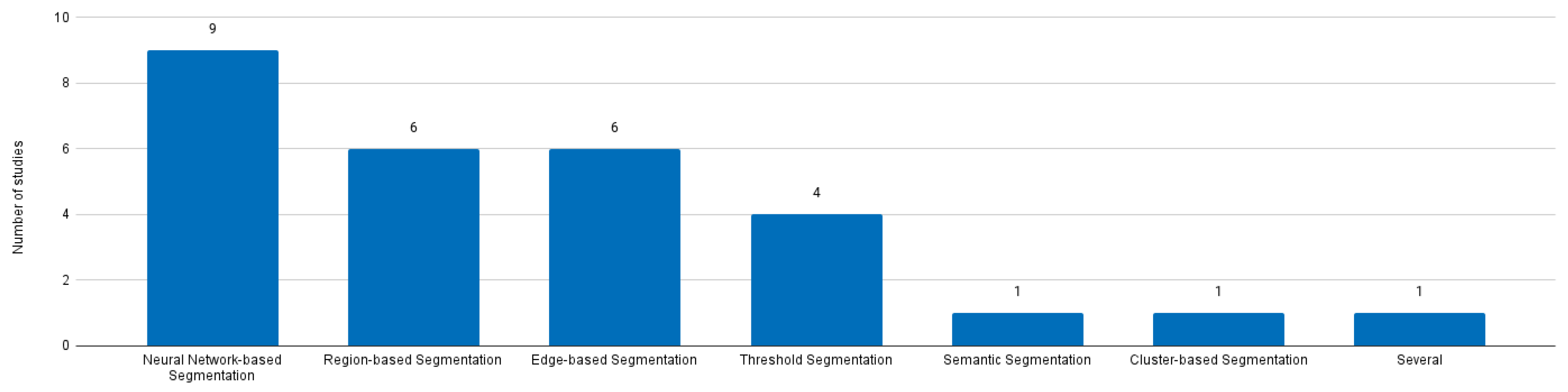
| Authors | Year | Segmentation Category | Method | Autom. Level | Dim. | Database | Results |
| Bae et al. | 1993 | Threshold | Gray-level Thresholding | Semi | 2D | Private | 0.985 DSC with mean percent error within 10%. |
| Gao et al. | 1996 | Edge | Parametrically deformable contour model | Fully | 3D | Private | 13.2% of the results required user modifications. |
| Soler et al. | 1997 | Region | Deformable models | Fully | 3D | Private | Claimed to be comparable to manual segmentations. |
| Yoo et al. | 2000 | Threshold | Threshold | Fully | 2D | Private | 3.41% error. |
| Pan and Dawant | 2001 | Edge | Level sets | Fully | Both | Private | [0.874,0.963] average similarities. |
| Saitoh et al. | 2002 | Threshold | Threshold | Fully | 3D | Private | ∼20 minutes computation time. |
| Masumoto et al. | 2003 | Region | Differences between time-phase images | Fully | 3D | Private | 67% Volume ratio average; 32% in the worst cases. |
| Lim et al. | 2004 | Region | Watershed | Fully | 2D | Private | Only qualitative. |
| Liu et al. | 2005 | Edge | GVF snake | Semi | 2D | Private | 5.3% median value of the difference ratios. |
| Lim et al. | 2006 | Semantic | Labeling-based search | Fully | 2D | Private | 96% average correctness; 3% average error rate. |
| Beichel et al. | 2007 | Region | Graph cuts | Semi | 3D | Private | 5.2% average overlap error. |
| Massoptier and Casciaro | 2008 | Edge | Active contour | Fully | 3D | Private | 94.2% mean DSC. |
| Heimann et al. | 2009 | Several | Majority Voting | Both | Both | Private | 5% Overlap error; -0.7 Volume difference; 0.8 Average Distance; 1.7 RMS distance; 19.1 Max Distance. |
| Akram et al. | 2010 | Threshold | Global Threshold | Fully | 3D | Private | 0.96 Average accuracy; 0.0017 std; 96% Accurately Segmented; 4% Poorly Segmented. |
| Oliveira et al. | 2011 | Edge | Level sets | Semi | 2D | SLiver07 | 82.05 overall score. |
| Linguraru et al. | 2012 | Region | Graph cuts | Fully | 3D | Private; SLiver07 | 2.2 VOE. |
| Li et al. | 2013 | Edge | Fuzzy clust. and level set | Fully | 2D | Private | 0.9986 average accuracy; 0.9989 average specificity. |
| Platero et al. | 2014 | Region | Graph cuts | Semi | 3D | SLiver07 | 76.3 maximum score; 0.973 DSC. |
| Mostafa et al. | 2015 | Cluster | ABC optimization | Fully | 2D | Private | 93.73% accuracy; 84.82% average SI. |
| Dou et al. | 2016 | NN | 3D DSN | Fully | 3D | SLiver07 | 5.42% VOE; 0.79mm ASD. |
| Christ et al. | 2017 | NN | CFCN | Fully | 2D | 3Dircadb | 94.3% mean DSC. |
| Hiraman | 2018 | NN | CNN | Fully | 2D | SLiver07 | 12.07% average VOE; -1.96% RVD; 2.25mm ASD; 2.60mm RMSD; 43.01mm MSSD. |
| Wang et al. | 2019 | NN | CNN | Fully | 3D | Private | 0.94 ± 0.06 DSC. |
| Almotairi et al. | 2020 | NN | SegNet | Fully | 3D | 3D-IRCADb01 | 94.57% overall accuracy. |
| Ayalew et al. | 2021 | NN | U-Net | Fully | 2D | 3D-IRCADb01; LiTS | 0.9612 DSC. |
| Scicluna | 2022 | NN | UNet; VGG16UNetC | Fully | 2D | CHAOS | 85.84 mean score; 97.85 DSC; 80.33 RAVD; 94.80 ASD; 70.38 MSSD. |
| Ezzat et al. | 2023 | NN | CNN | Fully | 2D | Private | 98.80% accuracy. |
| Shao et al. | 2024 | NN | AC-Net | Fully | 3D | LiTS; Private | 0.90 DSC; 0.82 JC; 0.92 recall; 0.89 precision; 11.96 HD; 4.59 ASD. |
| Dataset | Date | Format | Number of subjects | Slices per subject | Resolution |
| MICCAI-SLiver07 [59] | 2007 | RAW | 30 | 74 to 260 | 512x512 |
| 3D-IRCADb01 [42] | 2010 | DICOM | 20 | 74 to 260 | 512x512 |
| LiTS17 [44] | 2017 | RAW | 200 | 42 to 1024 | Variable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





