Submitted:
07 February 2024
Posted:
08 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
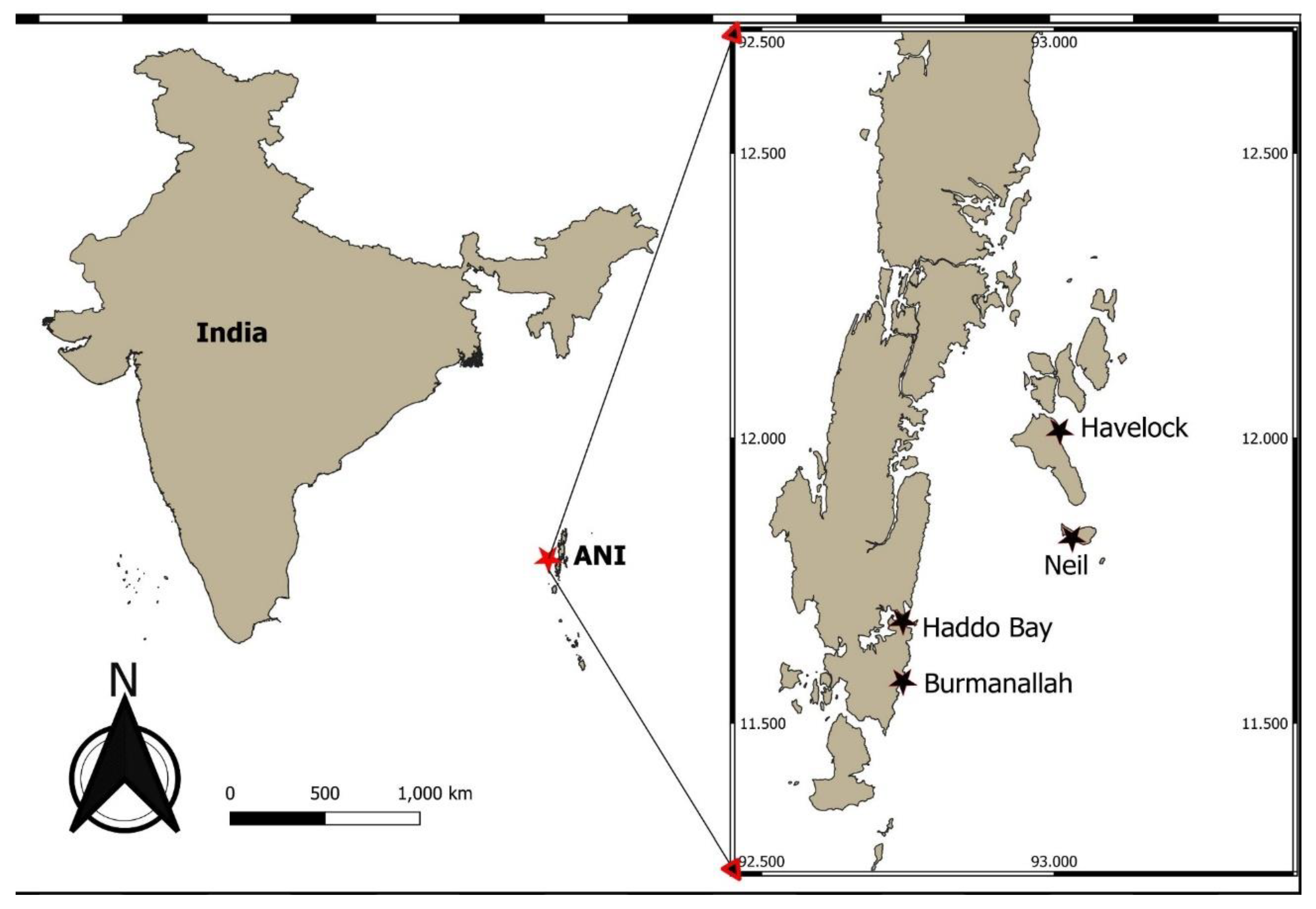
2.1. Study Site
2.2. Water and Sediment Sampling and Analysis
| Class | Value | Sediment Quality |
| 0 | Igeo <0 | Uncontaminated |
| 1 | 0 < Igeo <1 | Uncontaminated to moderate contaminated |
| 2 | 1<Igeo < 2 | Moderately contaminated |
| 3 | 2 <Igeo <3 | Moderately to heavily contaminated |
| 4 | 3 <Igeo <4 | Heavily contaminated |
| 5 | 4 <Igeo <5 | Heavily to extremely contaminated |
| 6 | 5 <Igeo <6 | Extremely contaminated |
2.3. Seagrass Sampling and Analysis
2.4. Analytical Methods for Water, Sediment and Seagrass
2.4. Statistics
3. Results
3.1. Variation in Abiotic Parameters in Water and Sediment
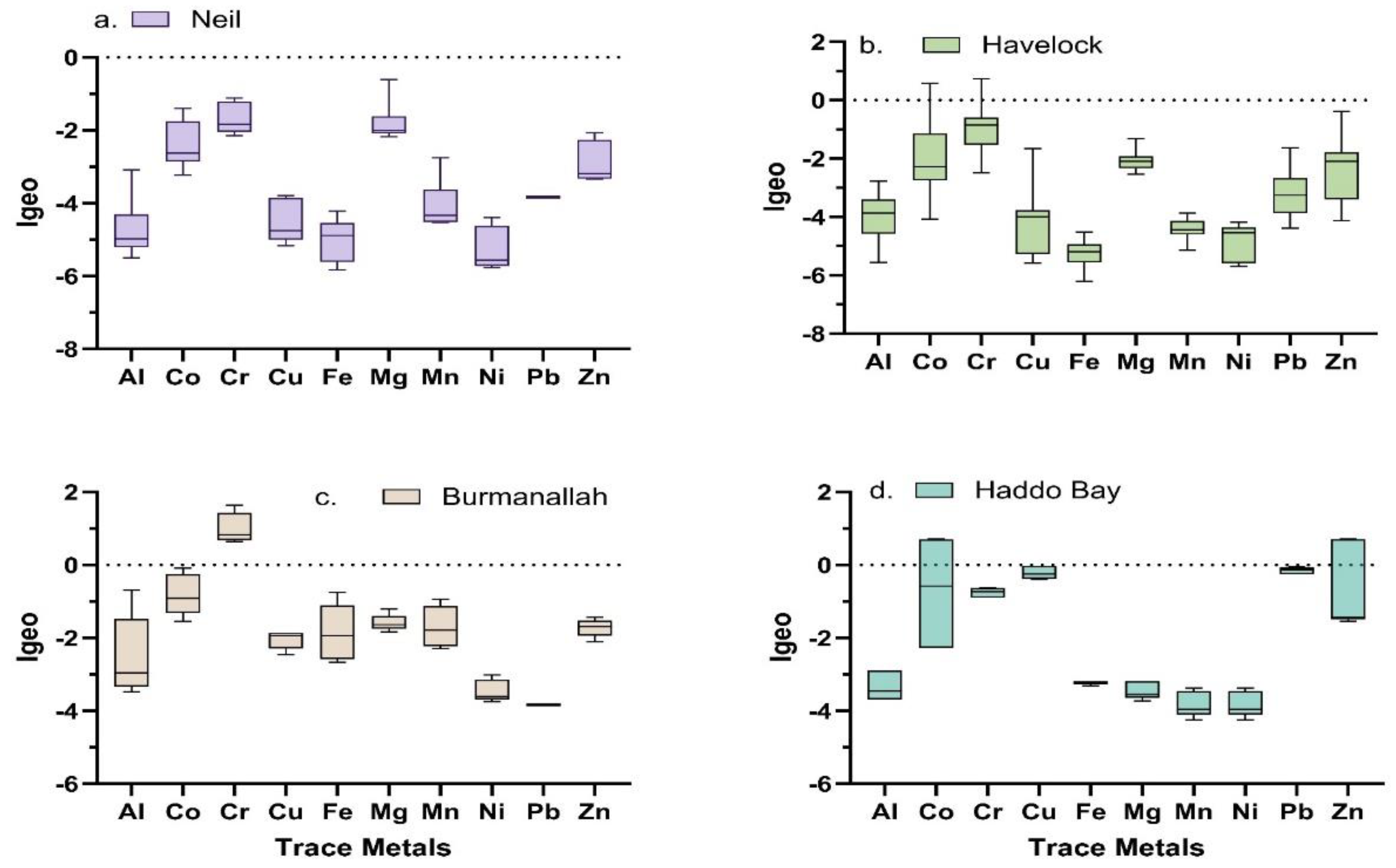
3.2. Trace Metal Concentrations in Water, Sediment and Seagrass Biomass
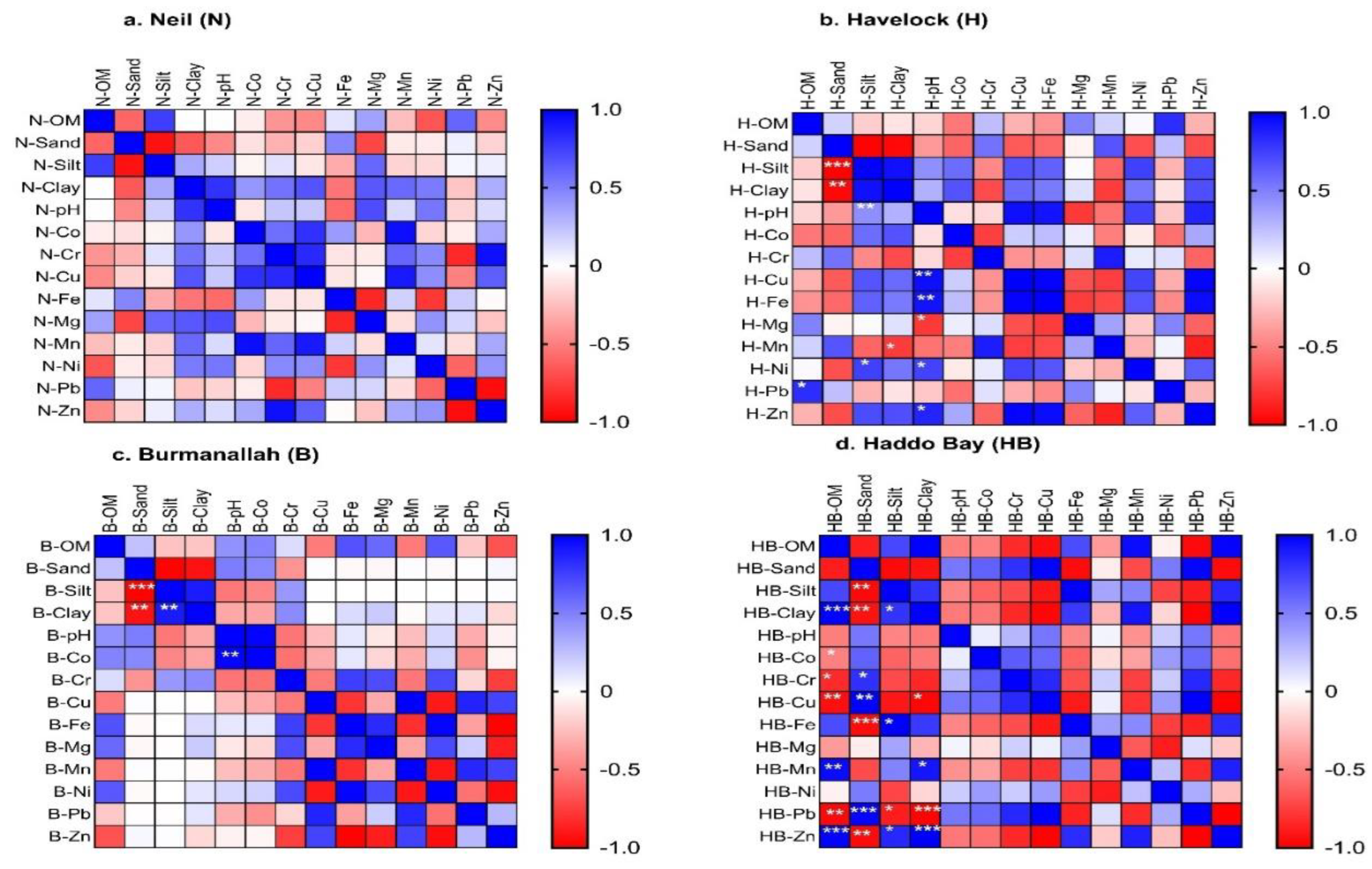
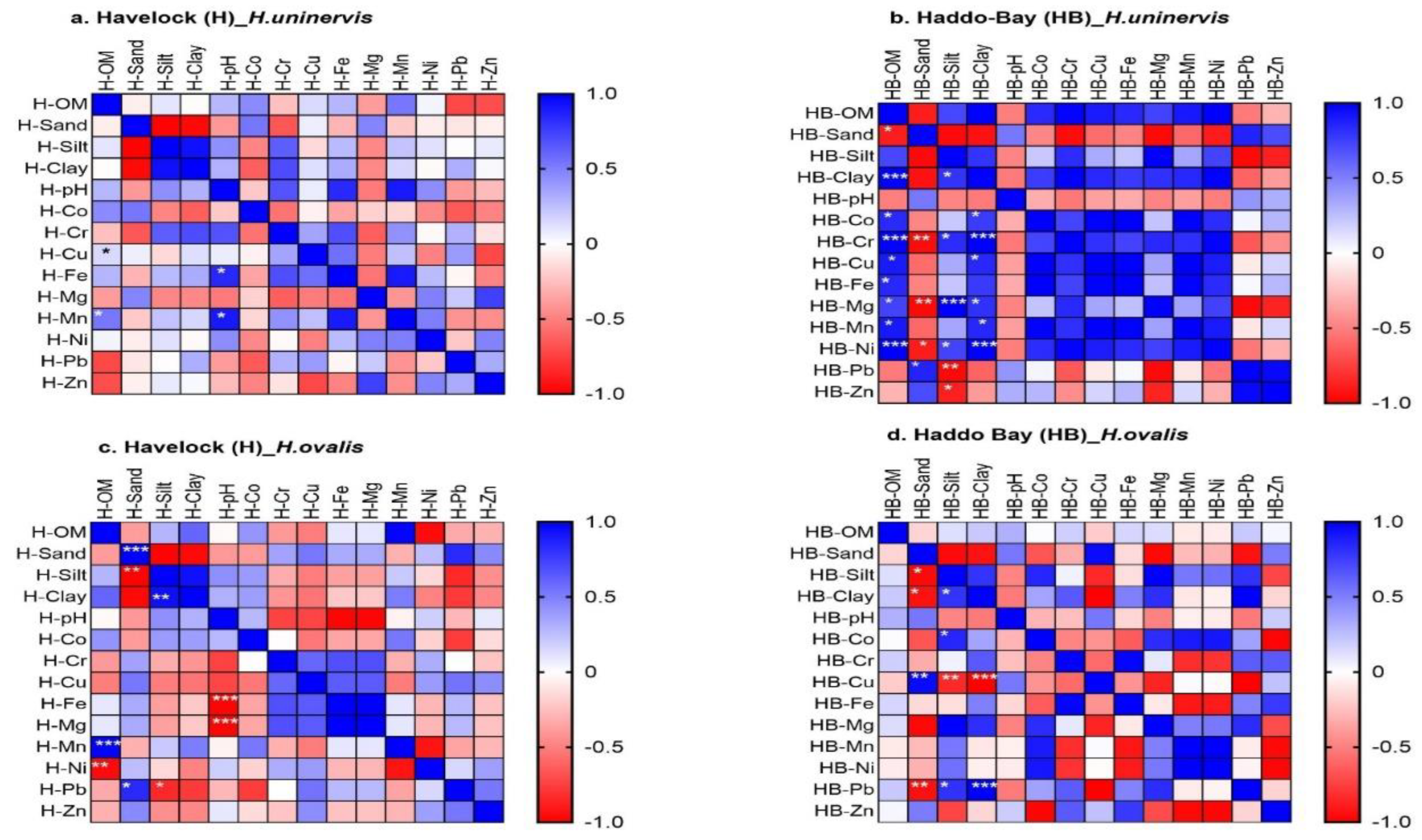
3.3. Correlation between Surface Water and Sediment Abiotic Parameters on Trace Metals in Seagrass Sediment
3.3.1. Correlations between Seagrass Species at Havelock Island
3.3.2. Correlations between Seagrass Species at Burmanallah
3.3.3. Correlations between Seagrass Species at Haddo Bay
4. Discussion
4.1. Effect of Sediment Traits (Organic Matter Content) on Metal Accumulation in Seagrass Sediment
4.2. Species-Specific Accumulation
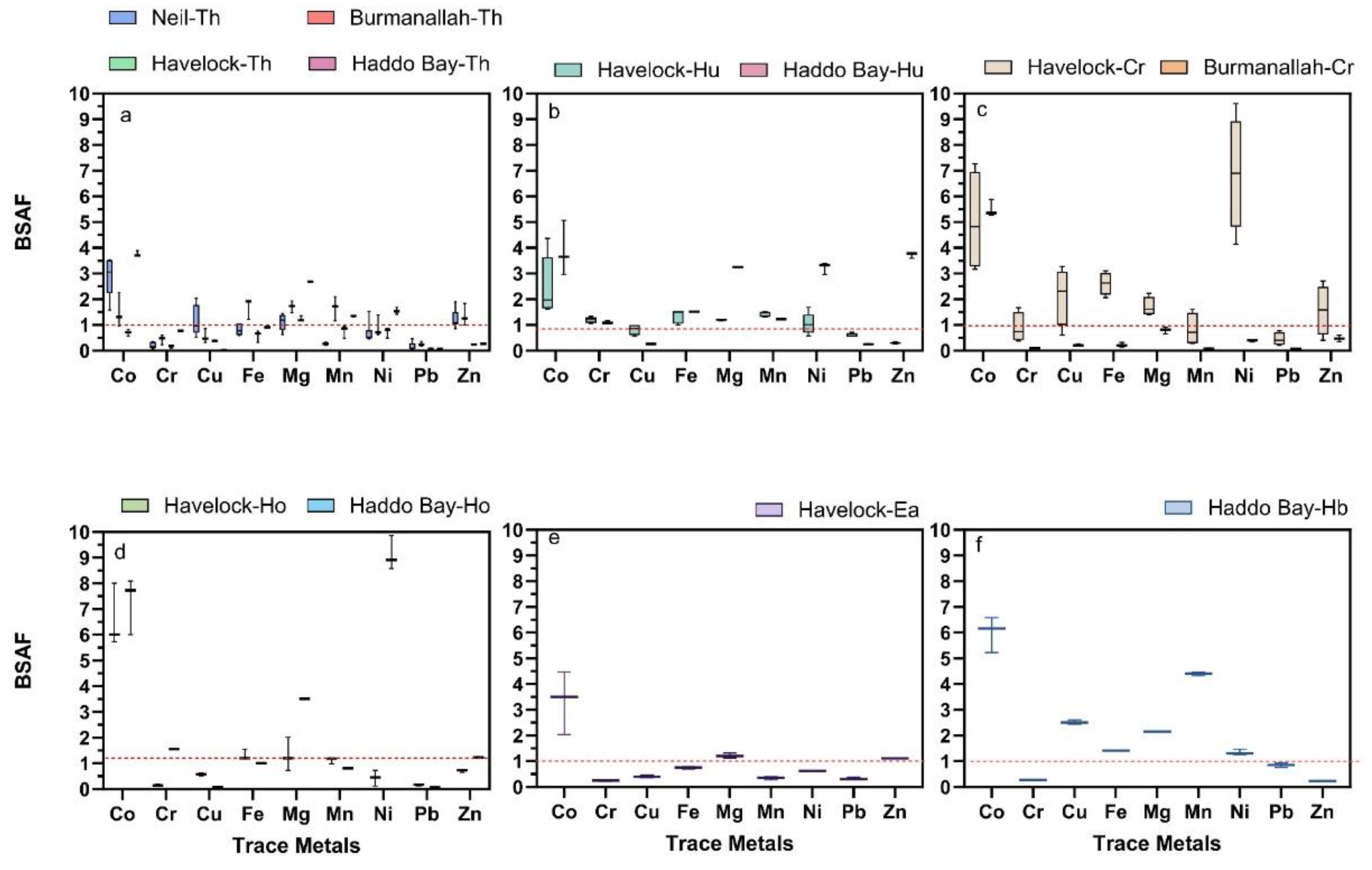
4.3. Bioindicator Potential
4.3. Toxic Effects of Trace Metals on Seagrasses
5. Conclusion
Supplementary Materials
Funding
Data availability
Acknowledgements
Declaration of competing interest
References
- Aljahdali, M.O.; Alhassan, A.B. Heavy metal accumulation and anti-oxidative feedback as a biomarker in Seagrass Cymodocea Serrulata. Sustainability (Switzerland) 2020, 12(7). [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. The efficiency of trace element uptake by seagrass Cymodocea serrulata in Rabigh lagoon, Red Sea. Environmental Science and Pollution Research 2022, 29(10), 14948–14960. [Google Scholar] [CrossRef]
- Ambo-rappe, R.; Lajus, D.L.; Schreider, M.J. Heavy metal impact on growth and leaf asymmetry of seagrass, Halophila ovalis. Environmental Chemistry 2011, 3(June), 149–159. [Google Scholar]
- Arisekar, U.; Jeya Shakila, R.; Shalini, R.; Jeyasekaran, G.; Sivaraman, B.; Surya, T. Heavy metal concentrations in the macroalgae, seagrasses, mangroves, and crabs collected from the Tuticorin coast (Hare Island), Gulf of Mannar, South India. Marine Pollution Bulletin 2021, 163(November 2020), 111971. [Google Scholar] [CrossRef]
- Arulkumar, A.; Nigariga, P.; Paramasivam, S.; Rajaram, R. Metals accumulation in edible marine algae collected from Thondi coast of Palk Bay, Southeastern India. Chemosphere 2019, 221, 856–862. [Google Scholar] [CrossRef]
- Avelar, M.; Bonilla-Heredia, B.; Merino-Ibarra, M.; Herrera-Silveira, J.A.; Ramirez, J.; Rosas, H.; Valdespino, J.; Carricart-Ganivet, J.P.; Martínez, A. Iron, cadmium, and chromium in seagrass (Thalassia testudinum) from a coastal nature reserve in karstic Yucatán. Environmental Monitoring and Assessment 2013, 185(9), 7591–7603. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecological Monographs 2011, 81(2), 169–193. [Google Scholar] [CrossRef]
- Basallote, M.D.; De Orte, M.R.; DelValls, T.Á.; Riba, I. Studying the Effect of CO2 -Induced Acidification on Sediment Toxicity Using Acute Amphipod Toxicity Test. Environmental Science & Technology 2014, 48(15), 8864–8872. [Google Scholar] [CrossRef]
- Sahu, B.K.; Goswami, P.; Begum, M.; Jha, D.K.; Vinithkumar, N.V.; Dharani, G. A comparative investigation of physicochemical and biological variables of Aerial & Port Blair Bays, Andaman Islands with focus on the anthropogenic influence. Indian Journal of Geo-Marine Sciences 2023, 52(02), 79–90. [Google Scholar] [CrossRef]
- Bayyana, S.; Pawar, S.; Gole, S.; Dudhat, S.; Pande, A.; Mitra, D.; Johnson, J.A.; Sivakumar, K. Detection and mapping of seagrass meadows at Ritchie’s archipelago using Sentinel 2A satellite imagery. Current Science 2020, 118(8), 1275–1282. [Google Scholar] [CrossRef]
- Bonanno, G.; Borg, J.A. Comparative analysis of trace element accumulation in seagrasses Posidonia oceanica and Cymodocea nodosa: Biomonitoring applications and legislative issues. Marine Pollution Bulletin 2018, 128(December 2017), 24–31. [Google Scholar] [CrossRef]
- Bonanno, G.; Borg, J.A. Comparative analysis of trace element accumulation in seagrasses Posidonia oceanica and Cymodocea nodosa: Biomonitoring applications and legislative issues. Marine Pollution Bulletin 2018, 128(January), 24–31. [Google Scholar] [CrossRef]
- Bonanno, G.; Orlando-Bonaca, M. Trace elements in Mediterranean seagrasses and macroalgae. A review. Science of the Total Environment 2018, 618, 1152–1159. [Google Scholar] [CrossRef]
- Bonanno, G.; Raccuia, S.A. Comparative assessment of trace element accumulation and bioindication in seagrasses Posidonia oceanica, Cymodocea nodosa and Halophila stipulacea. Marine Pollution Bulletin 2018, 131(February), 260–266. [Google Scholar] [CrossRef]
- Coclet, C.; Garnier, C.; D’Onofrio, S.; Durrieu, G.; Pasero, E.; Le Poupon, C.; Omanović, D.; Mullot, J.U.; Misson, B.; Briand, J.F. Trace Metal Contamination Impacts Predicted Functions More Than Structure of Marine Prokaryotic Biofilm Communities in an Anthropized Coastal Area. Frontiers in Microbiology 2021, 12(February), 1–16. [Google Scholar] [CrossRef]
- De Boer, W.F. Seagrass-sediment interactions, positive feedbacks and critical thresholds for occurrence: A review. Hydrobiologia 2007, 591(1), 5–24. [Google Scholar] [CrossRef]
- de los Santos, C.B.; Arenas, F.; Neuparth, T.; Santos, M.M. Interaction of short-term copper pollution and ocean acidification in seagrass ecosystems: Toxicity, bioconcentration and dietary transfer. Marine Pollution Bulletin 2019, 142(July 2018), 155–163. [Google Scholar] [CrossRef]
- Dilipan, E.; Nobi, E.P.; Change, C.; Thangaradjou, T. Spatial variability in productivity and biomass of seagrasses in the Andaman group of Islands, India. Ecology 2020, 26(4), 1693–1701. [Google Scholar]
- Dong, Y.; Rosenbaum, R.K.; Hauschild, M.Z. Assessment of Metal Toxicity in Marine Ecosystems: Comparative Toxicity Potentials for Nine Cationic Metals in Coastal Seawater. Environmental Science and Technology 2016, 50(1), 269–278. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Krause-Jensen, D. Export from seagrass meadows contributes to marine carbon sequestration. Frontiers in Marine Science 2017, 4(JAN), 1–7. [Google Scholar] [CrossRef]
- (EPA), E.P.A. (2009). Occurrence of Contaminants of Emerging Concern in Wastewater From Nine Publi c ly Owned Treatment Works August 2009. Epa, August.
- Esteban, N.; Unsworth, R.K.F.; Gourlay, J.B.Q.; Hays, G.C. The discovery of deep-water seagrass meadows in a pristine Indian Ocean wilderness revealed by tracking green turtles. Marine Pollution Bulletin 2018, 134(April 2017), 99–105. [Google Scholar] [CrossRef]
- Gao, W.; Du, Y.; Gao, S.; Ingels, J.; Wang, D. Heavy metal accumulation reflecting natural sedimentary processes and anthropogenic activities in two contrasting coastal wetland ecosystems, eastern China. Journal of Soils and Sediments 2016, 16(3), 1093–1108. [Google Scholar] [CrossRef]
- Geevarghese, G.A.; Akhil, B.; Magesh, G.; Krishnan, P.; Purvaja, R.; Ramesh, R. A comprehensive geospatial assessment of seagrass distribution in India. Ocean and Coastal Management 2018, 159(May 2017), 16–25. [Google Scholar] [CrossRef]
- Gole, S.; Mohammed, P.I.; Apte, D.; Marimuthu, N. Holothurian spatial variability and substratum preference in the intertidal habitats of the Andaman Sea. Regional Studies in Marine Science 2022, 56, 102633. [Google Scholar] [CrossRef]
- Gole, S.; Prajapati, S.; Prabakaran, N.; Das, H.; Kuppusamy, S.; Johnson, J.A. Spatial diversity and habitat characteristics of seagrass meadows with management recommendations in the Andaman and Nicobar Islands, India. Frontiers in Marine Science 2023, 10(December), 1–17. [Google Scholar] [CrossRef]
- Gopi, S.; Arulkumar, A.; Ganeshkumar, A.; Rajaram, R.; Miranda, J.M.; Paramasivam, S. Heavy metals accumulation in seagrasses collected from Palk Bay, South-eastern India. Marine Pollution Bulletin 2020, 157(June), 111305. [Google Scholar] [CrossRef]
- Gopinath, A.; Muraleedharan, N.S.; Chandramohanakumar, N.; Jayalakshmi, K.V. Statistical significance of biomonitoring of marine algae for trace metal levels in a coral environment. Environmental Forensics 2011, 12(1), 98–105. [Google Scholar] [CrossRef]
- Govers, L. L., Lamers, L. P. M., Bouma, T. J., Eygensteyn, J., de Brouwer, J. H. F., Hendriks, A. J., Huijbers, C. M., & van Katwijk, M. M. (2014). Seagrasses as indicators for coastal trace metal pollution: a global meta-analysis serving as a benchmark, and a Caribbean case study. Environmental Pollution (Barking, Essex : 1987), 195, 210–217. [CrossRef]
- Govindasamy, C.; Azariah, J. Seasonal variation of heavy metals in coastal water of the Coromandel coast, Bay of Bengal, India. Indian Journal of Marine Sciences 1999, 28(3), 249–256. [Google Scholar]
- Gu, R.; Lin, H.; Zhou, Y.; Song, X.; Xu, S.; Yue, S.; Zhang, Y.; Xu, S.; Zhang, X. Programmed responses of different life-stages of the seagrass Ruppia sinensis to copper and cadmium exposure. Journal of Hazardous Materials 2021, 403(February 2020), 123875. [Google Scholar] [CrossRef]
- Haviland, K.A.; Howarth, R.W.; Marino, R.; Hayn, M. Variation in sediment and seagrass characteristics reflect multiple stressors along a nitrogen-enrichment gradient in a New England lagoon. Limnology and Oceanography 2022, 67(3), 660–672. [Google Scholar] [CrossRef]
- Ho, N.A.J.; Ooi, J.L.S.; Affendi, Y.A.; Chong, V.C. Influence of habitat complexity on fish density and species richness in structurally simple forereef seagrass meadows. Botanica Marina 2018, 61(6), 547–557. [Google Scholar] [CrossRef]
- Howard, J.; Hoyt, S.; Isensee, K.; Pidgeon, E.; Telszewski, M. Coastal Blue Carbon. National Wetlands Newsletter 2014, 36(1), 5–7. [Google Scholar]
- Hu, W.; Zhang, D.; Chen, B.; Liu, X.; Ye, X.; Jiang, Q.; Zheng, X.; Du, J.; Chen, S. Mapping the seagrass conservation and restoration priorities : Coupling habitat suitability and anthropogenic pressures. Ecological Indicators 2021, 129, 107960. [Google Scholar] [CrossRef]
- Immaculate, TT, L., & Patterson, J. (2018). Macro and micro nutrients of seagrass species from Gulf of Mannar, India. MOJ Food Processing & Technology, 6(4), 391–398. [CrossRef]
- Jagtap, T.G. Metal Distribution in Halophila beccarii (Aschers) and sourrounding environment along the central west coast of India.pdf. Oceanography 1983, 16(4), 429–434. [Google Scholar]
- Jagtap,TG and Untawale, A. (1984). Chemical composition of marine macrophytes and their surrounding water and sediments, from Minicoy, Lakshadweep. Indian Journal of Marine Sciences, 13(September), 123–125.
- Jayaprakash, M.; Gopal, V.; Anandasabari, K.; Kalaivanan, R.; Sujitha, S.B.; Jonathan, M.P. Enrichment and toxicity of trace metals in near-shore bottom sediments of Cuddalore, SE coast of India. Environmental Earth Sciences 2016, 75(19), 1303. [Google Scholar] [CrossRef]
- Jiang, R.; Wu, Y.; Huang, X.; Liu, S.; Chen, Q. Heavy metal enrichment characteristics and risk assessment of typical fishes in tropical seagrass beds. South China Fisheries Science 2023, 19(1), 48–57. [Google Scholar] [CrossRef]
- Joseph, L.; Singh, P.; Singh, A.A.; Raj, K.; Maharaj, A. Implications of Seagrass Ecosystem Degradation on Marine Resources and People’s Livelihood: A Case Study from Komave Village, Fiji. Asian Journal of Fisheries and Aquatic Research 2018, February, 1–13. [Google Scholar] [CrossRef]
- Lee, G.; Suonan, Z.; Kim, S.H.; Hwang, D.-W.; Lee, K.-S. Heavy metal accumulation and phytoremediation potential by transplants of the seagrass Zostera marina in the polluted bay systems. Marine Pollution Bulletin 2019, 149(July), 110509. [Google Scholar] [CrossRef]
- Lee, H.; Morrison, C.; Doriean, N.J.C.; Welsh, D.T.; Bennett, W.W. Metals in coastal seagrass habitats: A systematic quantitative literature review. Critical Reviews in Environmental Science and Technology 2023, 53(17), 1568–1585. [Google Scholar] [CrossRef]
- Lei, L.I.; Xiaoping, H.; Borthakur, D.; Hui, N.I. Photosynthetic activity and antioxidative response of seagrass T halassia hemprichii to trace metal stress. Acta Oceanologica Sinica 2012, 31(3), 98–108. [Google Scholar] [CrossRef]
- Lewis, M.A.; Richard, D. Nonnutrient anthropogenic chemicals in seagrass ecosystems: Fate and effects. Environmental Toxicology and Chemistry 2009, 28(3), 644–661. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, X. Three tropical seagrasses as potential bio-indicators to trace metals in Xincun Bay, Hainan Island, South China. Chinese Journal of Oceanology and Limnology 2012, 30(2), 212–224. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Zhou, R.; Zheng, X.; Pan, K.; Qiu, G.; Wu, Z.; Chen, S.; Wang, D. A review of metal contamination in seagrasses with an emphasis on metal kinetics and detoxification. Journal of Hazardous Materials 2023, 454(April), 131500. [Google Scholar] [CrossRef] [PubMed]
- Libin Baby, Gireesh Kumar TR, Remyakumari KR, J. V., & Sankar TV and Chandramohanakumar N. (2017). Comparison of hydrographic and sediment characteristics of seagrass meadows of Gulf of Mannar and Palk Bay, South West Coast of India. International Journal of Fisheries and Aquatic Studies, 5(2), 80–84. http://www.fisheriesjournal.com/archives/2017/vol5issue2/PartB/4-6-91-582.pdf.
- Lin, H.; Sun, T.; Zhou, Y.; Zhang, X. Anti-oxidative feedback and biomarkers in the intertidal seagrass Zostera japonica induced by exposure to copper, lead and cadmium. Marine Pollution Bulletin 2016, 109(1), 325–333. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, J.; Lu, X.; Su, C.; Zhang, Y.; Wang, C.; Cao, X.; Li, Q.; Su, J.; Ittekkot, V.; Garbutt, R.A.; Bush, S.; Fletcher, S.; Wagey, T.; Kachur, A.; Sweijd, N. Major threats of pollution and climate change to global coastal ecosystems and enhanced management for sustainability. Environmental Pollution 2018, 239, 670–680. [Google Scholar] [CrossRef] [PubMed]
- M. Stankovic, R. Ambo-Rappe, F. Carly, et al. (2021). Quantification ofblue carbon in seagrass ecosystems ofSoutheast Asia and their potential for climate change mitigation. Science of The Total Environment, 146858. [CrossRef]
- Malea, P., & Kevrekidis, T. (2013). Trace element ( Al , As , B , Ba , Cr , Mo , Ni , Se , Sr , Tl , U and V ) distribution and seasonality in compartments of the seagrass Cymodocea nodosa. Science of the Total Environment, The, 463–464, 611–623. [CrossRef]
- McKenzie, L.; Yoshida, R.; Aini, J.; Andréfouët, S.; Colin, P.; Cullen-Unsworth, L.; Hughes, A.; Payri, C.; Rota, M.; Shaw, C.; Tsuda, R.; Vuki, V.; Unsworth, R. Seagrass ecosystem contributions to people’s quality of life in the Pacific Island Countries and Territories. Marine Pollution Bulletin 2021, 167. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Apte, D. Ecological connectivity with mangroves influences tropical seagrass population longevity and meadow traits within an island ecosystem. Marine Ecology Progress Series 2020, 644, 47–63. [Google Scholar] [CrossRef]
- Mishra, A.K.; Acharya, P.; Apte, D.; Farooq, S.H. Seagrass ecosystem adjacent to mangroves store higher amount of organic carbon of Andaman and Nicobar Islands, Andaman Sea. Marine Pollution Bulletin 2023, 193(June), 115135. [Google Scholar] [CrossRef]
- Mishra, A.K.; Cabaço, S.; de los Santos, C.B.; Apostolaki, E.T.; Vizzini, S.; Santos, R. Long-term effects of elevated CO2 on the population dynamics of the seagrass Cymodocea nodosa: Evidence from volcanic seeps. Marine Pollution Bulletin 2021, 162(July 2020), 111824. [Google Scholar] [CrossRef]
- Mishra, A.K.; Farooq, S.H. Trace metal accumulation in seagrass and saltmarsh ecosystems of India: comparative assessment and bioindicator potential. Marine Pollution Bulletin 2022, 174, 113251. [Google Scholar] [CrossRef]
- Mishra, A.K.; Farooq, S.H. Trace metal accumulation in seagrass and saltmarsh ecosystems of India: comparative assessment and bioindicator potential. Marine Pollution Bulletin 2022, 174, 113251. [Google Scholar] [CrossRef]
- Mishra, A.K.; Farooq, S.H. Sediment organic matter content drives bivalve density in tropical oligotrophic seagrass ecosystem. Indian Journal of Geo-Marine Sciences 2023, 52(02), 91–100. [Google Scholar] [CrossRef]
- Mishra, A. K., Khadanga, M. K., Patro, S., Apte, D., & Farooq, S. H. (2021). Population structure of a newly recorded ( Halodule uninervis ) and native seagrass ( Halophila ovalis ) species from an intertidal creek ecosystem. Lakes & Reservoirs: Science, Policy and Management for Sustainable Use, 26(3), 1–12. [CrossRef]
- Mishra, A.K.; Kumar, M. Andaman mangrove sediments : source of nutrients and sink of heavy metals. Indian Journal of Geo Marine Sciences 2020, 49(January), 156–166. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mohanraju, R. Epiphytic Bacterial Communities in Seagrass Meadows of Oligotrophic Waters of Andaman Sea. OALib 2018, 05(03), 1–12. [Google Scholar] [CrossRef]
- Mishra, A.K.; Narayana, S.; Apte, D. Loss of Dugong Grass [Halophila Ovalis (R. Brown)] Population Structure Due to Habitat Disturbance in an Island Ecosystem. Indian Journal of Geo-Marine Sciences 2021, 50(02), 115–121. http://nopr.niscair.res.in/handle/123456789/56320. [CrossRef]
- Mishra, A. K., Sahoo, R., Samantaray, S. S., & Apte, D. (2022). Seagrass Ecosystems of India as Bioindicators of Trace Elements. In S. P. Madhav S., Nazneen S. (Ed.), Coastal Ecosystems (1st ed., pp. 45–65). Springer Nature. [CrossRef]
- 65. Mishra, A. K., Santos, R., & Hall -Spencer, J. M. (2020). Elevated trace elements in sediments and seagrasses at CO2 seeps. Marine Environmental Research, 153(September 2019), 104810. [CrossRef]
- Mishra, A. Kumar., & Apte, Deepak. (2021). The current status of Halophila beccarii: An ecologically significant, yet vulnerable seagrass of India. Ocean & Coastal Management, 200(August 2020), 105484. [CrossRef]
- Naik, S., Pradhan, U., Karthikeyan, P., Bandyopadhyay, D., Sahoo, R. K., Panda, U. S., Mishra, P., & Murthy, M. V. R. (2023). Ecological risk assessment of heavy metals in the coastal sediment in the South-western Bay of Bengal. Frontiers in Marine Science, 10(October). [CrossRef]
- Nazneen, S.; Mishra, A.K.; Raju, N.J.; Mehmood, G. Coastal macrophytes as bioindicators of trace metals in the Asia’s largest lagoon ecosystem. Marine Pollution Bulletin 2022, 178, 113576. [Google Scholar] [CrossRef] [PubMed]
- Nikalje, G.C.; Suprasanna, P. Coping with metal toxicity – cues from halophytes. Frontiers in Plant Science 2018, 9(June), 1–11. [Google Scholar] [CrossRef]
- Nishitha, D.; Amrish, V.N.; Arun, K.; Warrier, A.K.; Udayashankar, H.N.; Balakrishna, K. Study of trace metal contamination and ecological risk assessment in the sediments of a tropical river estuary, Southwestern India. Environmental Monitoring and Assessment 2022, 194(2), 94. [Google Scholar] [CrossRef] [PubMed]
- Nobi, E.P.; Dilipan, E.; Thangaradjou, T.; Sivakumar, K.; Kannan, L. Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Estuarine, Coastal and Shelf Science 2010, 87(2), 253–264. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Koch, E.W.; Barbier, E.B.; Creed, J.C. Seagrass Ecosystem Services and Their Variability across Genera and Geographical Regions. PLoS ONE 2016, October. [Google Scholar] [CrossRef]
- Olivé, I.; Silva, J.; Lauritano, C.; Costa, M.M.; Ruocco, M.; Procaccini, G.; Santos, R. Linking gene expression to productivity to unravel long- and short-term responses of seagrasses exposed to CO2 in volcanic vents. Scientific Reports 2017, 7(February), 42278. [Google Scholar] [CrossRef] [PubMed]
- Oreska, M.P.J.; Wilkinson, G.M.; McGlathery, K.J.; Bost, M.; McKee, B.A. Non-seagrass carbon contributions to seagrass sediment blue carbon. Limnology and Oceanography 2018, 63, S3–S18. [Google Scholar] [CrossRef]
- Pasumpon, N.; Vasudevan, S. Seasonal variation of heavy metals in seagrasses along Thondi coast, Palk Bay, India. Environmental Science and Pollution Research 2021, 28(21), 26849–26857. [Google Scholar] [CrossRef]
- Pradhan, U.K.; Wu, Y.; Shirodkar, P.V.; Zhang, J.; Zhang, G. Sources and distribution of organic matter in thirty five tropical estuaries along the west coast of India-a preliminary assessment. Estuarine, Coastal and Shelf Science 2014, 151, 21–33. [Google Scholar] [CrossRef]
- Prange, J. a., & Dennison, W. C. (2000). Physiological responses of five seagrass species to trace metals. Marine Pollution Bulletin, 41(7–12), 327–336. [CrossRef]
- Ragupathi Raja Kannan, R.; Arumugam, R.; Anantharaman, P. Chemometric studies of multielemental composition of few seagrasses from Gulf of Mannar, India. Biological Trace Element Research 2011, 143(2), 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, P.S. Trace metal bioaccumulation: Models, metabolic availability and toxicity. Environment International 2007, 33(4), 576–582. [Google Scholar] [CrossRef] [PubMed]
- Renzi, M.; Romeo, T.; Guerranti, C.; Perra, G.; Italiano, F.; Focardi, S.E.; Esposito, V.; Andaloro, F. Temporal trends and matrix-dependent behaviors of trace elements closed to a geothermal hot-spot source (Aeolian Archipelago, Italy). Procedia Earth and Planetary Science 2011, 4(1), 10–28. [Google Scholar] [CrossRef]
- Sachithanandam, V.; Bonthu, S.; Mageswaran, T.; Singh, K.S.; Vimala, J.; Sridhar, R.; Purvaja, R.; Ramesh, R. Effect of hydrodynamic conditions on seagrass ecosystems during Cyclone Lehar in the South Andaman Islands, India. Ecohydrology and Hydrobiology 2022, 22(4), 640–659. [Google Scholar] [CrossRef]
- Sachithanandam, V., Parthasarathy, P., Sai Elangovan, S., Kasilingam, K., Dhivya, P., Mageswaran, T., & Mohan, P. M. (2020a). A baseline study on trace metals concentration and its ecological risk assessment from the coast of South Andaman Island, India. Regional Studies in Marine Science, 36, 101242. [CrossRef]
- Sachithanandam, V.; Parthasarathy, P.; Sai Elangovan, S.; Kasilingam, K.; Dhivya, P.; Mageswaran, T.; Mohan, P.M. A baseline study on trace metals concentration and its ecological risk assessment from the coast of South Andaman Island, India. Regional Studies in Marine Science 2020, 36, 101242. [Google Scholar] [CrossRef]
- Sadanandan, H.; Dharmalingam, S.N.; Sridharan, M.; Agarwal, N.; Anbuselvan, N. Assessment of Trace Metal Pollution in Surface Sediments from Southwestern Part of Bay of Bengal, India. Journal of the Geological Society of India 2023, 99(3), 383–389. [Google Scholar] [CrossRef]
- Sahu, B.K.; Begum, M.; Khadanga, M.K.; Jha, D.K.; Vinithkumar, N.V.; Kirubagaran, R. Evaluation of significant sources influencing the variation of physico-chemical parameters in Port Blair Bay, South Andaman, India by using multivariate statistics. Marine Pollution Bulletin 2013, 66(1–2), 246–251. [Google Scholar] [CrossRef]
- Sanz-Lázaro, C.; Malea, P.; Apostolaki, E.T.; Kalantzi, I.; Marín, a.; Karakassis, I. The role of the seagrass Posidonia oceanica in the cycling of trace elements. Biogeosciences 2012, 9(7), 2497–2507. [Google Scholar] [CrossRef]
- Savurirajan, M.; Lakra, R.K.; Equbal, J.; Ganesh, K.S.T. A note on morphometric, shoot density and biomass of Thalassia hemprichii from the South Andaman coast, Andaman and Nicobar Islands, India. Indian Journal of Geo-Marine Sciences 2018, 47(6), 1222–1227. [Google Scholar]
- Schneider, L., Maher, W. A., Potts, J., Taylor, A. M., Batley, G. E., Krikowa, F., Adamack, A., Chariton, A. A., & Gruber, B. (2018a). Trophic transfer of metals in a seagrass food web: Bioaccumulation of essential and non-essential metals. Marine Pollution Bulletin, 131(December 2017), 468–480. 20 December. [CrossRef]
- Schneider, L., Maher, W. A., Potts, J., Taylor, A. M., Batley, G. E., Krikowa, F., Adamack, A., Chariton, A. A., & Gruber, B. (2018b). Trophic transfer of metals in a seagrass food web: Bioaccumulation of essential and non-essential metals. Marine Pollution Bulletin, 131(December 2017), 468–480. 20 December. [CrossRef]
- Short, F., Carruthers, T., Dennison, W., & Waycott, M. (2007). Global seagrass distribution and diversity: A bioregional model. Journal of Experimental Marine Biology and Ecology, 350(1–2), 3–20. [CrossRef]
- Stockbridge, J.; Jones, A.R.; Gillanders, B.M. A meta-analysis of multiple stressors on seagrasses in the context of marine spatial cumulative impacts assessment. Scientific Reports 2020, 10(1), 1–11. [Google Scholar] [CrossRef]
- Stockdale, A.; Tipping, E.; Lofts, S.; Mortimer, R.J.G. Effect of Ocean Acidification on Organic and Inorganic Speciation of Trace Metals. Environmental Science and Technology 2016, 50(4), 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Suheryanto, S., & Ismarti, I. (2018). Bio-concentration factors of copper (Cu) and lead (Pb) in seagrass and some fish from coast Batam, Riau Islands, Indonesia. Journal of Physics: Conference Series, 1095(1), 0–5. [CrossRef]
- Sungur, A.; Özcan, H. Chemometric and geochemical study of the heavy metal accumulation in the soils of a salt marsh area (Kavak Delta, NW Turkey). Journal of Soils and Sediments 2015, 15(2), 323–331. [Google Scholar] [CrossRef]
- Thangaradjou, T.; Nobi, E.P.; Dilipan, E.; Sivakumar, K.; Susila, S. Heavy metal enrichment in seagrasses of Andaman Islands and its implication to the health of the coastal ecosystem. Indian Journal of Marine Sciences 2010, 39(March), 85–91. [Google Scholar]
- Thangaradjou, T.; Raja, S.; Subhashini, P.; Nobi, E.P.; Dilipan, E. Heavy metal enrichment in the seagrasses of Lakshadweep group of islands - A multivariate statistical analysis. Environmental Monitoring and Assessment 2013, 185(1), 673–685. [Google Scholar] [CrossRef]
- Thangaradjou, T.; Subhashini, P.; Raja, S.; Dilipan, E.; Nobi, E.P. Evidences for heavy metal contamination in surface sediments of seagrass ecosystem of Lakshadweep archipelago, India. Environmental Earth Sciences 2014, 71(3), 1135–1146. [Google Scholar] [CrossRef]
- Thorne-Bazarra, T.; Lozano-Bilbao, E.; Hardisson, A.; González-Weller, D.; Rubio, C.; Paz, S.; Gutiérrez, Á.J. Seagrass meadows serve as buffers for metal concentrations in the fish species Sparisoma cretense in the Canary Islands (Atlantic EC, Spain). Regional Studies in Marine Science 2023, 67, 103192. [Google Scholar] [CrossRef]
- Tupan, C.I.; Azrianingsih, R. Accumulation and deposition of lead heavy metal in the tissues of roots, rhizomes and leaves of seagrass Thalassia hemprichii (Monocotyledoneae, Hydrocharitaceae). AACL Bioflux 2016, 9(3), 580–589. [Google Scholar]
- Turekian, K.K., and Wedepohl,K.H., T. (1961). Distribution of the Elements in Some Major Units of the Earth’s Crust. Geological Society of America Bulletin, 72(February), 175–192. [CrossRef]
- Unsworth, Richard K F., C.-U. (2022). The planetary role of seagrass conservation. Science, August, 0–6. [CrossRef]
- Vieira, V.M.N.C.S.; Lobo-Arteaga, J.; Santos, R.; Leitão-Silva, D.; Veronez, A.; Neves, J.M.; Nogueira, M.; Creed, J.C.; Bertelli, C.M.; Samper-Villarreal, J.; Pettersen, M.R.S. Seagrasses benefit from mild anthropogenic nutrient additions. Frontiers in Marine Science 2022, 9(November), 1–14. [Google Scholar] [CrossRef]
- VishnuRadhan, R.; Thresyamma, D.D.; Sarma, K.; George, G.; Shirodkar, P.; Vethamony, P. Influence of natural and anthropogenic factors on the water quality of the coastal waters around the South Andaman in the Bay of Bengal. Natural Hazards 2015, 78(1), 309–331. [Google Scholar] [CrossRef]
- Vizzini, S.; Costa, V.; Tramati, C.; Gianguzza, P.; Mazzola, A. Trophic transfer of trace elements in an isotopically constructed food chain from a semi-enclosed marine coastal area (Stagnone di Marsala, Sicily, Mediterranean). Archives of Environmental Contamination and Toxicology 2013, 65(4), 642–653. [Google Scholar] [CrossRef]
- Ward, T. J. (1984). Role of acute metal toxicity in structuring seagrass fauna near a lead smelter. 17, 117–124.
- Wilkinson, A.; Ariel, E.; van de Merwe, J.; Brodie, J. Trace element concentrations in forage seagrass species of Chelonia mydas along the Great Barrier Reef. PLOS ONE 2022, 17(6), e0269806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L., Ni, Z., Cui, L., Li, J., He, J., Jiang, Z., & Huang, X. (2021a). Heavy metal accumulation and ecological risk on four seagrass species in South China. Marine Pollution Bulletin, 173(PB), 113153. [CrossRef]
- Zhang, L., Ni, Z., Cui, L., Li, J., He, J., Jiang, Z., & Huang, X. (2021b). Heavy metal accumulation and ecological risk on four seagrass species in South China. Marine Pollution Bulletin, 173(PB), 113153. [CrossRef]
- Zhang, L., Ni, Z., Cui, L., Li, J., He, J., Jiang, Z., & Huang, X. (2021c). Heavy metal accumulation and ecological risk on four seagrass species in South China. Marine Pollution Bulletin, 173(PB), 113153. [CrossRef]
- zu Ermgassen, P. S. E., DeAngelis, B., Gair, J. R., Ermgassen, S. zu, Baker, R., Daniels, A., MacDonald, T. C., Meckley, K., Powers, S., Ribera, M., Rozas, L. P., & Grabowski, J. H. (2021). Estimating and Applying Fish and Invertebrate Density and Production Enhancement from Seagrass, Salt Marsh Edge, and Oyster Reef Nursery Habitats in the Gulf of Mexico. Estuaries and Coasts, 1588–1603. [CrossRef]


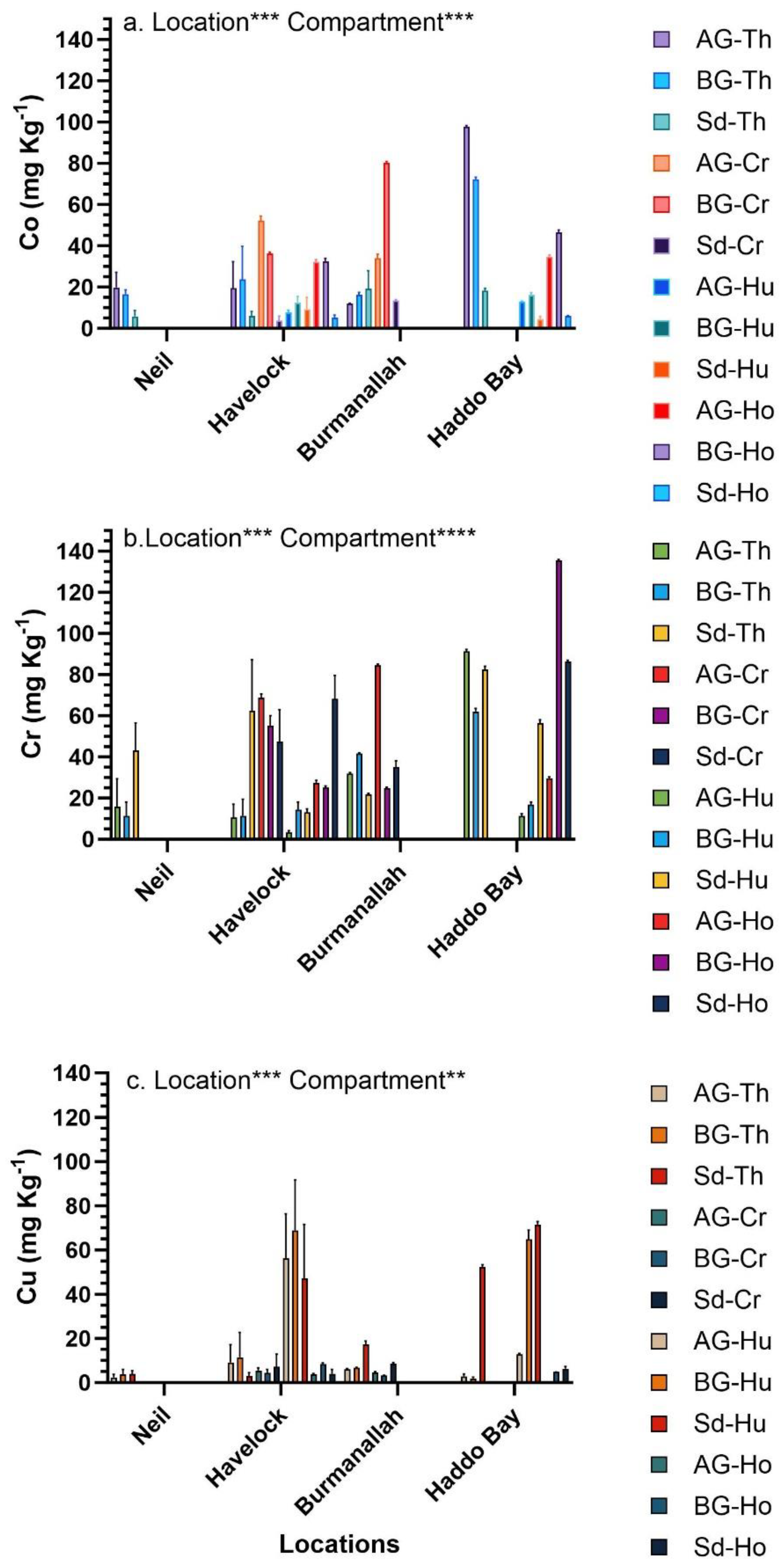
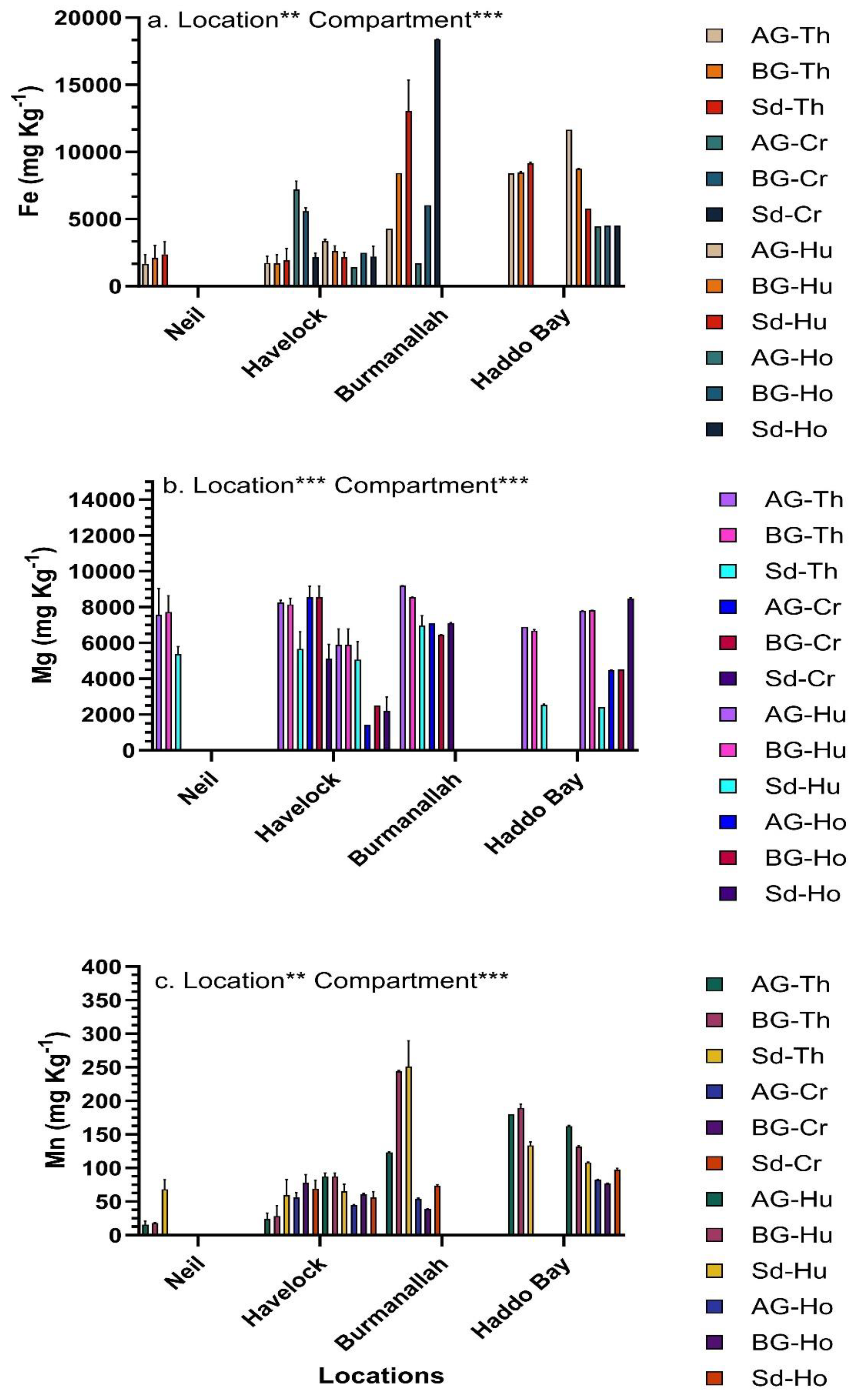
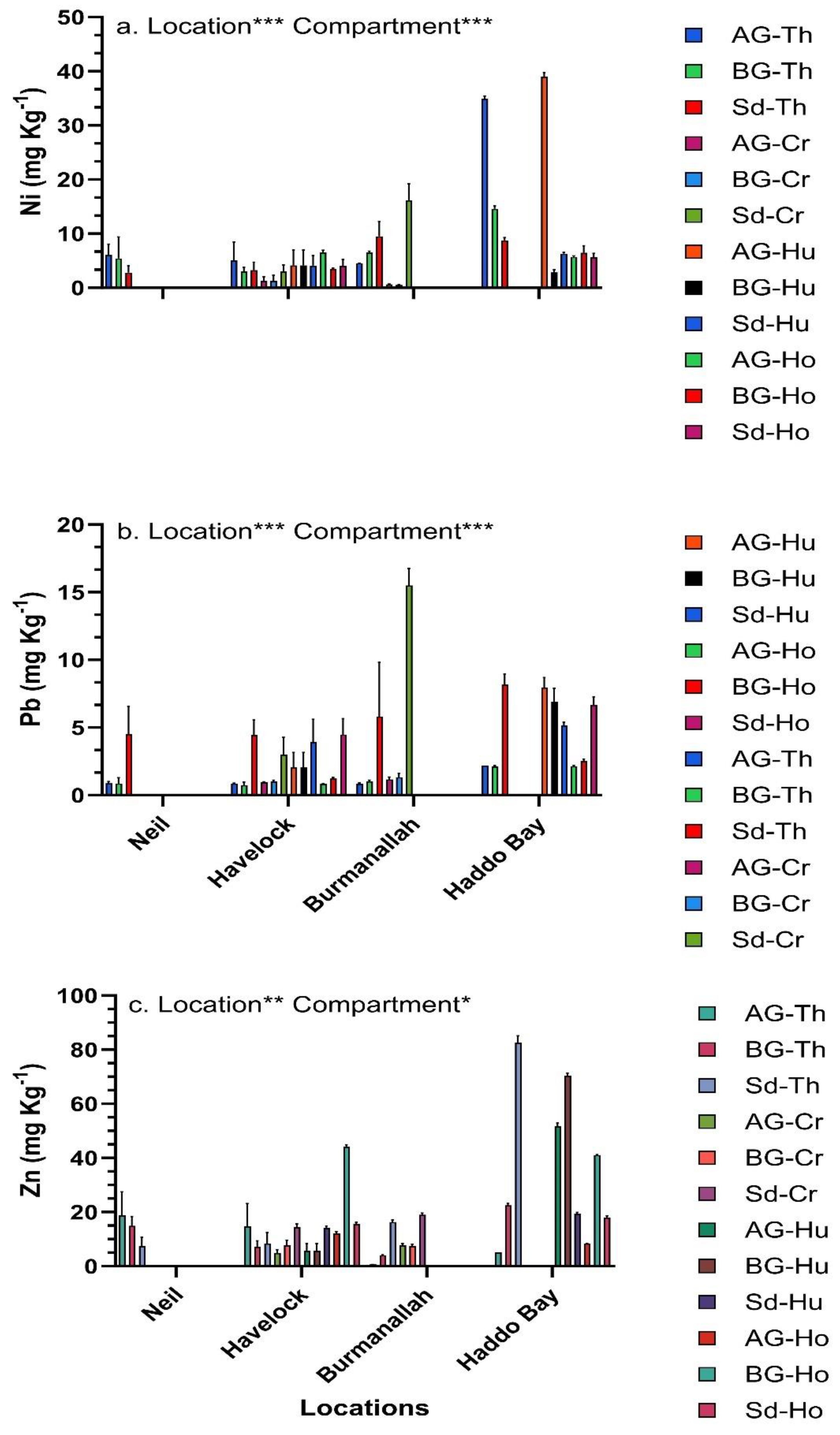
| Plant biomass | Sediment | |||||
|---|---|---|---|---|---|---|
| Trace metals | Certified value for ERM-CD281 (mg/Kg) | Recovered value (mg/Kg) | Percentage recovery (%) | Certified value for Hiss-1 (mg/Kg) | Recovered value (mg/Kg) | Percentage recovery (%) |
| Co | - | - | - | 0.65 | 0.60 | 93.77 |
| Cr | 24.8 | 23.01 | 92.79 | 30 | 27.16 | 90.54 |
| Cu | 10.2 | 9.71 | 95.26 | 2.29 | 2.11 | 92.55 |
| Fe | 180 | 172.85 | 96.03 | 2460 | 2342.90 | 95.24 |
| Mg | 1600 | 1441 | 90.10 | |||
| Mn | 82 | 74.17 | 90.46 | 66.10 | 61.60 | 93.20 |
| Ni | 15.2 | 14.33 | 94.28 | 2.16 | 2.02 | 93.84 |
| Pb | 1.67 | 1.57 | 94.38 | 3.13 | 3.06 | 97.91 |
| Zn | 30.5 | 30.06 | 98.57 | 4.94 | 4.89 | 99 |
| Variables | Locations | One-way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| Neil | Havelock | Burmanallah | Haddo Bay | DF | MS | F (DFn, DFd) |
P value | |
| pH | 8.10 ± 0.01 | 8.14 ± 0.05 | 8.14 ± 0.08 | 8.00 ± 0.08 | 3 | 0.025 | F (3,20) = 10.50 | = 0.002 |
| Temp.°C | 33.20 ± 1.30 | 34 ± 1.22 | 31.00 ±0.89 | 30 ± 0.46 | 3 | 17.87 | F (3,16) = 16.96 | <0.001 |
| Salinity (‰) | 32.67 ± 0.57 | 32 ± 1.01 | 33 ± 0.01 | 32.33 ± 1.15 | 3 | 0.55 | F (3,8) = 0.83 | = 0.512 |
| OM (%) | 38.17 ± 4.32 | 27.52 ± 7.86 | 38.70 ±5.07 | 25.44 ± 3.01 | 3 | 634.0 | F (3,66) = 18.21 | <0.0001 |
| Grain size (Sand%) | 43.70 ± 15.13 | 45.23 ± 9.50 | 33.89 ± 9.98 | 37.94 ± 13.63 | 3 | 202.8 | F (3,24) = 0.26 | = 0.85 |
| Grain size (Silt%) | 46.88 ± 12.11 | 39.33 ± 2.09 | 51.34 ± 3.16 | 49.60 ±9.37 | 3 | 232.2 | F (3,25) = 0.51 | =0.67 |
| Grain size (Clay%) | 9.41 ± 5.89 | 15.44 ± 8.46 | 14.76 ± 8.94 | 12.85 ± 6.25 | 3 | 65.28 | F (3,24) = 1.01 | =0.36 |
| Metals | Seagrass ecosystems | This study | References | ||
|---|---|---|---|---|---|
| Water (µg L-1) |
Sediment (mg kg-1) |
Water (µg L-1) |
Sediment (mg kg-1) |
||
| Co | - | 0.16–100 | BDL | 3.72 – 18.35 | Nobi et al., 2010; Thangaradjou et al., 2014; Sachithanandam et al., 2020 |
| Cr | 0.26-2.03 | 2.32–887 | BDL | 13.09 – 86.92 | Baby et al., 2017; Govindaswamy et al., 2011; Thangaradjou et al., 2014; Nobi et al., 2010; Jagtap and Untawale, 1984; Sachithanandam et al., 2020 |
| Cu | 0.11-1.02 | 1.58–130 | BDL | 2.97 – 71.44 | Baby et al., 2017; Govindaswamy et al., 2011; Thangaradjou et al., 2014; Nobi et al., 2010; Jagtap,1983; Jagtap and Untawale, 1984; Kumaresan et al., 1998 Sachithanandam et al., 2020 |
| Fe | 0.12-7.04 | 16.5–75500 | BDL | 1864 – 18400.99 | Jagtap, 1983; Jagtap and Untawale, 1984; Govindaswamy et al., 2011; Thangaradjou et al., 2014; Baby et al., 2017; Nobi et al., 2010; Kumarsen et al., 1998; Sachithanandam et al., 2020 |
| Mg | 0.16-18338 | 42–6204 | 25.49–26.34 | 2206.07 –8474.93 | Jagtap,1983; Jagtap and Untawale, 1984; Baby et al., 2017; Nobi et al., 2010 |
| Mn | 0.35-0.89 | 4–940 | BDL | 56.23 – 251.24 | Jagtap,1983; Govindaswamy et al., 2011; Thangaradjou et al., 2014; Baby et al., 2017; Nobi et al., 2010; Kumarsen et al., 1998; Sachithanandam et al., 2020; |
| Ni | 0.19-0.56 | 0.64–607 | BDL | 2.83 – 16.18 | Jagtap,1983; Jagtap and Untawale, 1984; Govindaswamy et al., 2011; Baby et al., 2017; Thangaradjou et al., 2014; Nobi et al., 2010; Sachithanandam et al., 2020 |
| Pb | 0.01-0.12 | 0.54–29 | BDL | 2.25 – 15.48 | Jagtap and Untawale, 1984; Baby et al., 2017; Sachithanandam et al., 2020 |
| Zn | 0.1-11.61 | 2–127.2 | BDL | 7.49 – 25.93 | Jagtap and Untawale, 1984; Govindaswamy et al., 2011; Baby et al., 2017; Thangaradjou et al., 2014; Nobi et al., 2010; Kumarsen et al., 1998; Sachithanandam et al., 2020 |
| Seagrass | Seagrass tissues; This study | References | |||
|---|---|---|---|---|---|
| Min (mg kg-1) | Max (mg kg-1) | Min (mg kg-1) | Max (mg kg-1) | ||
| Species | S. isoetifolium (LK) | H. uninervis (LK) | H. uninervis (H) | T. hemprichii (HB) | Nobi et al., 2010; Kannan et al., 2011; Thangaradjou et al., 2013; Arisekar et al., 2021 |
| Co | 0.16 | 11.21 | 7.74 | 97.85 | |
| Species | S. isoetifolium (GOM) | Seagrass (ANI) | H. uninervis (N) | H. ovalis (HB) | Nobi et al., 2010; Kannan et al., 2011; Thangaradjou et al., 2013; Immaculate et al., 2018; Arisekar et al., 2021; Pasumpon and Vasudevan, 2021 |
| Cr | 0.1 | 138.2 | 3.28 | 135.56 | |
| Species | S. isoetifolium (PB) | Seagrass (ANI) | H. ovalis (HB) | H. uninervis (HV) | Jagtap, 1983; Mathevan, 1990; Kannan et al., 1992; Nobi et al., 2010; Kannan et al., 2011; Govindaswamy et al., 2011; Gopinath et al., 2011; Sudharsan et al., 2012; Thangaradjou et al., 2013; Immaculate et al., 2018; Gopi et al., 2020; Arisekar et al., 2021; Pasumpon and Vasudevan, 2021 |
| Cu | 0.05 | 86.75 | 0.28 | 68.85 | |
| Species | S. isoetifolium (PB) | H. beccarii (GO) | E. acoroides (HV) | H. uninervis (HB) | Jagtap, 1983; Mathevan, 1990; Kannan et al., 1992; Nobi et al., 2010; Kannan et al., 2011; Govindaswamy et al., 2011; Gopinath et al., 2011; Sudharsan et al., 2012; Thangaradjou et al., 2013; Immaculate et al., 2018; Arisekar et al., 2021 |
| Fe | 0.22 | 32562 | 543 | 11655.49 | |
| Species | C. rotundata (GOM) | S. isoetifolium (LK) | H. ovalis (HV) | T. hemprichii (B) | Jagtap, 1983; Nobi et al., 2010; Thangaradjou et al., 2010; Kannan et al., 2011; Thangaradjou et al., 2013; Immaculate et al., 2018 |
| Mg | 91.54 | 80,050 | 1428.36 | 9190.79 | |
| Species | C. serrulata (PB) | H. ovalis (PB) | T. hemprichii (N) | T. hemprichii (B) | Jagtap, 1983; Mathevan, 1990; Kannan et al., 1992; Nobi et al., 2010; Govindaswamy et al., 2011; Kannan et al., 2011; Sudharsan et al., 2012; Thangaradjou et al., 2013; Arisekar et al., 2021; Pasumpon and Vasudevan, 2021 |
| Mn | 0.24 | 2250 | 15.41 | 244.10 | |
| Species | S. isoetifolium (PB) | H. decipens (LK) | C. rotundata (B) | H. uninervis (HB) | Jagtap, 1983; Nobi et al., 2010; Kannan et al., 2011; Thangaradjou et al., 2013; Sudharsan et al., 2012; Immaculate et al., 2018 |
| Ni | 0.1 | 19.49 | 0.54 | 39 | |
| Species | S. isoetifolium (LK) | H. uninervis (LK) | E. acoroides (HV) | H. uninervis (HB) | Nobi et al., 2010; Kannan et al., 2011; Sudharsan et al., 2012; Thangaradjou et al., 2013; Immaculate et al., 2018; Gopi et al., 2020; Arisekar et al., 2021; Pasumpon and Vasudevan, 2021 |
| Pb | 0.1 | 23.12 | 0.47 | 7.95 | |
| Species | S. isoetifolium (PB) | H. pinifolia (PB) | T. hemprichii (B) | H. beccarii (HB) | Mathevan, 1990; Kannan et al., 1992; Nobi et al., 2010; Kannan et al., 2011; Gopinath et al., 2011; Sudharsan et al., 2012; Govindaswamy et al., 2012; Thangaradjou et al., 2013; Immaculate et al., 2018; Gopi et al., 2020; Arisekar et al., 2021; Pasumpon and Vasudevan, 2021 |
| Zn | 0.15 | 69.17 | 0.60 | 70.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





