1. Introduction
The prevalent prevalence of obesity, coupled with its profound influence on the demographic composition of the global population, has experienced a notable and concerning escalation over the last four decades. The most recent national prevalence figures for the years 2015-2019, following Chinese criteria, indicate rates of 3.6% for obesity in children under 6 years, 7.9% for obesity in children and adolescents aged 6-17 years, and 16.4% for obesity in adults (≥18 years) 1. The study revealed a connection between obesity and the aging process. Further research is imperative to comprehend the morphological and molecular alterations associated with age in adipose tissue (AT), aiming to address and combat age-related metabolic diseases.
Aging adipose depots exhibit heightened infiltration of inflammatory cells, enlarged lipid droplets, and an increased prevalence of senescent cells 2. These age-related changes in AT result in a reduced basal metabolic rate, impaired insulin responsiveness, elevated ectopic deposition of lipids, and consequent lipotoxicity. Emerging evidence suggests that exercise is a highly effective intervention in alleviating obesity and plays a significant role in individual metabolism, as evidenced by its impact on the morphology and function of adipose depots 3-8. Moreover, circulating factors induced by exercise, known as exerkines, are involved in the metabolism of AT in response to aging 6,9-11. The goal of this review is to offer a comprehensive overview of the benefits of regular exercise in counteracting age-related declines in AT function. This includes addressing issues such as adipose expansion, decreased vascularity and mitochondrial function, fibrosis, inflammatory cells infiltration. The relevance of regular exercise in mitigating metabolic disorders associated with aging AT will also be discussed.
2. Morphological Changes in Aged Adipose Tissue
AT, an extraordinary flexible and heterogeneous organ, plays a crucial role in regulating immune responses, body temperature, energy balance, insulin sensitivity, and overall physiological functions
12. AT exhibits an extraordinary capacity to adapt to a range of internal and external signals, owing to its high degree of plasticity
12. Nevertheless, a newfound understanding of the cellular and functional remodeling of white adipose tissue (WAT) and brown adipose tissue (BAT) during aging has surfaced in recent years. Adipose plasticity becomes compromised with age, as indicated by heightened visceral adiposity, reduced lipolysis and thermogenesis, and an inability to maintain body temperature during cold stress
13,14. Current endeavors focus on investigating the potential underlying mechanisms behind age-related alterations in AT, including hypertrophy, adipogenesis, hypoxia, angiogenesis, fibrosis, inflammation, mitochondrial biogenesis and function
15 (
Figure 1).
2.1. Hypertrophy and Adipogenesis Declines
AT exhibits a significant degree of plasticity and plays a role in influencing metabolism during both health and aging in response to various physiological stimuli. These stimuli include obesity, diabetes, fasting, fatty liver, cardiometabolic disease, cold exposure, local hyperthermia, and prolonged exercise 12,15. With advancing age, the plasticity of adipose tissue becomes compromised 16, affecting the ability of preadipocytes to self-renew and the replication of adipocyte progenitors in the stromovascular fraction (SVF) 17. Adipocytes undergo expansion as body weight increases with age. Hypertrophic adipocytes exhibit reduced expression of fat identity genes, compromising their ability to store excess lipid and releasing inflammatory adipokines that exacerbate the adipose tissue microenvironment 18. Excessive enlargement of WAT and inadequate angiogenesis result in cellular hypoxia, triggering a pro-inflammatory response. This cascade effect diminishes adipogenesis, promotes fibrosis, and hampers metabolic flexibility and thermogenesis in aging and age-related diseases 19-21. BAT, characterized by multilocular fat droplets and abundant mitochondria, serves as a thermogenic energy-expending tissue. It regulates body temperature through the mediation of mitochondrial uncoupling protein 1 (UCP1) in response to aging and age-related diseases 22,23. The activation of brown or beige adipocytes contributes to alleviating metabolic disorders 24.
With advancing age, the decline in adipogenic potential can be associated with cellular senescence, as indicated by elevated markers of senescence in WAT depots, such as p16Ink4a and senescence-associated beta-galactosidase activity 25. The activation of the senescent pathway may compromise adipogenesis. Adipose-derived stem cells from older donors exhibited heightened expression of p16Ink4a, which significantly contributes to reduced cellular differentiation 26. However, cellular senescence, among other aging-related processes, influences the endocrine function of AT. Functional WAT releases various factors that contribute to maintaining energy homeostasis, such as leptin, resistin, chemerin, and adiponectin. Furthermore, the secretion of these adipokines is affected by the aging process.
2.2. Hypoxia and Angiogenesis Disorder
The excessive enlargement of WAT and inadequate angiogenesis create a hypoxic environment in cells in response to obesity. This condition leads to a pro-inflammatory response and disorder in angiogenesis. With aging and obesity, the reduced availability of oxygen can trigger cellular hypoxia and inflammation, contributing to local and systemic metabolic dysfunction. Hypoxia-inducible factors (HIFs) play a role in various cellular functions, including glucose utilization, angiogenesis, apoptosis, extracellular matrix (ECM) remodeling, recruitment of macrophages, and fibrosis 27,28. The hypertrophic growth associated with aging results in reduced oxygen diffusion, exacerbated by insufficient compensation from the vasculature. Despite the absence of angiogenesis, HIF-1α seems to be upregulated in aged AT. However, the instability of the HIF-1α protein can pose a challenge to quantification 29,30. Furthermore, HIF-1α plays a role in mitochondrial biogenesis and function in aged AT. Mitochondrial complex IV (CIV) activity and assembly are already suppressed in white adipocytes of middle-aged mice, involving a HIF1α-dependent decline of essential CIV components, such as COX5B 29.
2.3. Fibrosis
Fibrosis has been recognized as a hallmark of dysfunctional AT in aging and obesity. It is a common pathological consequence of ECM dysregulation and arises from an imbalance between the synthesis and degradation of ECM fibrillar components 30. However, the excessive deposition of collagen in AT triggers persistent and chronic inflammation, ultimately disrupting AT homeostasis and exacerbating metabolic dysfunction in aging and obesity 31,32. Importantly, AT fibrosis is linked to insulin resistance in individuals with obesity 33,34. The regulation of AT fibrosis involves hypoxia, which induces the transcription of ECM components and alters cellular redox status to impact collagen crosslinking enzymes such as lysyl oxidase 34. Furthermore, unresolved inflammation is frequently linked to the progression of fibrosis in various pathological conditions 35. Mechanistically, the activation of macrophage toll-like receptor 4 (TLR4) recruits macrophage-inducible C-type lectin, stimulating pathways involved in ECM production and degradation, as well as fibroblast proliferation and differentiation 36. Additionally, the accumulation of fibrosis in subcutaneous WAT is associated with resistance to weight loss one year after bariatric surgery 37. BAT can selectively release various cytokines to counteract fibrosis when transplanted into WAT, achieved by upregulating lipogenesis and fatty acid metabolism 38.
2.4. Inflammation
Adipose tissue exhibits an enrichment of proinflammatory macrophages in response to both obesity and aging 39. During the aging process, visceral adiposity is frequently linked to changes in AT leukocytes, inflammation, and metabolic dysfunction. In contrast to obesity, the accumulation of inflammatory factors with age is not dependent on macrophage abundance, as evidenced by the lack of increase in the number of macrophages with age. Indeed, aging regulates macrophage polarization by activating TLR4 signaling and influencing transcript levels of inflammatory IL-6 and monocyte chemoattractant protein 1 (MCP-1). Aging is additionally linked to an expansion of resident immune cells in AT, including B and T cells, which exhibit distinct transcriptional profiles compared to age-related splenic B and T cells 40,41. Studies have demonstrated that mice lacking fat-resident regulatory T cells are safeguarded against age-related insulin resistance, although they remain vulnerable to insulin resistance and metabolic diseases associated with obesity 41. Furthermore, inhibiting NLRP3-dependent B cell accumulation can reverse metabolic impairment in aged AT 40.
3. Therapeutic Approaches to Enhance Aging Adipose Tissue
3.1. Cold Exposure
Environmental cold exposure triggers the formation of mitochondria-rich and thermogenic beige adipocytes in WAT, a process known as browning
42,43. (
Figure 2) It has been reported that cold exposure is a remarkably potent stimulus for enhancing insulin sensitivity, glucose and lipid metabolism. This occurs through the reduction of large lipid droplet accumulation, clearance of serum triacylglycerol, promotion of FFA oxidation, and the delivery of long-chain fatty acids. These actions contribute to increased expression of UCP1, improvement of mitochondrial biogenesis and function, and enhancement of browning in white adipocytes within WAT
42,44,45. BAT is characterized by its capacity to dissipate energy as heat through the action of UCP1, which is activated by the sympathetic nervous system (SNS) during activities such as exercise or exposure to cold
7,46,47. Nevertheless, triggering the senescence pathway in young beige progenitors induces premature cellular senescence and hinders their potential to form cold-induced beige adipocytes. On the contrary, genetically or pharmacologically reversing cellular aging through the p38/MAPK-p16
Ink4a pathway in aged mouse or human beige progenitor cells rejuvenates cold-induced beiging
48.
3.2. Local Hyperthermia Therapy
The earlier researches have demonstrated that cold exposure or activation of adrenergic signaling can be a beneficial method for promoting the generation of beige adipose tissue
24,49. Conversely, these treatments have limited applications due to associated cardiovascular risks
50-53. Recent studies have highlighted that local hyperthermia therapy could offer promising scientific benefits and serve as a potential therapeutic approach for aging-related diseases
54,55. The underlying molecular mechanism behind these positive outcomes of hyperthermia therapy involves the expression of heat shock protein 72 (HSP72), a classic stress-responsive protein that plays a role in stabilizing intracellular proteins. This mechanism is supported by evidence demonstrating enhanced glucose tolerance and insulin resistance, improved mitochondrial function, and a reduction in lipid accumulation
56. Recent studies have suggested that local hyperthermia therapy stimulates thermogenesis, enhances fat metabolism, and boosts the activation of beige adipose tissue through the activation of the HSF1-A2B1 transcriptional axis
57. Heat shock factor 1 (HSF1) plays a regulatory role in modulating the levels of PGC-1α both transcriptionally and post-transcriptionally in response to obesity and aging, contributing to the maintenance of cellular homeostasis. Additionally, non-lethal hyperthermia-induced perturbations upregulate HSF1 and result in mitohormesis, yielding beneficial outcomes in the context of aging
58-61. (
Figure 3)
3.3. Regular Exercise
Epidemiological studies unequivocally demonstrate that physical inactivity is a significant contributor to abdominal adiposity. Nevertheless, regular exercise has long been recognized as a therapeutic approach for managing obesity and diabetes, leading to a reduction in abdominal adiposity and mitigating metabolic syndrome. Serving as a valuable strategy in primary care and community health, regular exercise proves beneficial in addressing aging and age-related diseases. The enduring enhancement in glucose clearance induced by long-term exercise training persists for a considerable duration.
In summary, regular exercise plays a crucial role in counteracting the development of obesity and diabetes stimulated by aging 62. The research indicates that engaging in physical activity can lead to a reduction in food intake, low-grade inflammation, and lipogenesis, thereby alleviating insulin resistance in response to both obesity and aging 63. In elderly individuals who engage in prolonged endurance exercise, there is an observed increase in macrophage content and mitochondrial respiration in adipose tissue 64. A 12-month exercise program revealed that prolonged exercise training may signify a certain degree of remodeling in adipose tissue among older patients with coronary artery disease and diabetes 65. Furthermore, both aerobic and resistance exercise not only decrease the mass of epicardial adipose tissue in individuals with abdominal obesity but also mitigate obesity-induced cardiac fat accumulation 66. Nevertheless, the precise mechanism by which exercise ameliorates metabolic disorders induced by aging and obesity remains not fully identified.
4. The Potential Role of Regular Exercise in Aged Adipose Tissue
Regular exercise and physical activity have been shown to induce significant alterations in the morphology and function of AT, particularly in response to metabolic diseases. These changes include an increase in fat browning, a reduction in adipocyte hypertrophy, and improvements in glucose and lipid metabolism in AT
67-70. Moreover, regular exercise not only triggers a phenotypic transformation of AT, shifting it from primarily storing energy as white adipocytes to thermogenic beige adipocytes, especially in the context of obesity and diabetes. Additionally, it enhances processes such as FFA oxidation, insulin sensitivity, alleviation of oxidative stress, as well as the promotion of mitochondrial biogenesis and function
9,71 (
Figure 4).
4.1. White Adipose Tissue
WAT, being a highly prevalent form of AT, is distributed throughout nearly every region of the body 72. Nevertheless, the functional decline of AT in the context of obesity and diabetes is involved in a reduction in AT plasticity. This is evident in the significant decrease in AT metabolism and alterations in phenotype to meet the demands of the organism 12. The maladaptive remodeling of AT, marked by heightened fibrosis proliferation and a pro-inflammatory response, is triggered by a breakdown in angiogenesis and local hypoxia 73,74. As a result, adipose tissue becomes insulin resistant, inflamed, fibrotic, and dysfunctional, particularly in the context of aging.
Numerous studies have demonstrated that exercise has a profound impact on systemic metabolism by adapting to various tissues, including the heart 75-77, liver 78, skeletal muscle 79,80, and AT 6,62,81-84. AT depots, which play crucial roles in metabolism, are implicated in mitochondrial biogenesis, glucose metabolism, and FFA oxidation and uptake in response to exercise. These depots include inguinal WAT, perigonadal WAT, and interscapular BAT 4. Routine aerobic exercise brings about a significant reduction in WAT and a substantial increase in BAT in both mice and humans. This effect is achieved through the stimulation of various growth factors and cytokines, fostering the proliferation and differentiation of brown preadipocytes 85,86. In WAT, regular exercise leads to a considerable decrease in adipocyte size 87, an increase in mitochondrial biogenesis 88-90, regulation of adipokine secretion 91,92, and an overall enhancement of whole-body metabolic health 93. Long-term exercise training induce adaptability in WAT, as indicated by elevated FFA oxidation and a reduction in the impact of inflammation, achieved through the regulation of pro/anti-inflammatory gene expression and the infiltration of macrophages 94. Furthermore, exercise training contributes to the improvement of mitochondrial biogenesis and thermogenesis by facilitating the transformation of white adipocytes into beige adipocytes in WAT, counteracting the effects of aging and obesity 94.
4.2. Brown Adipose Tissue
BAT, a specialized heat-generating organ rich in mitochondria, is crucial for maintaining body temperature in cold conditions 95. Mitochondrial biogenesis and function in BAT play a pivotal role in thermoregulation and metabolic processes. Regular exercise has been shown to enhance UCP1 content, mitochondrial respiration and activity, and upregulate genes associated with mitochondrial biogenesis in BAT 4. Consistent physical activity significantly reduces fat mass and body weight gain, enhances energy expenditure, and elevates UCP1 expression in BAT by activating the AMP-activated protein kinase (AMPK) signaling pathway 96. UCP1, responsible for dissipating the proton motive force as heat, augments the energy metabolism of mitochondria in BAT, contributing to adaptive non-shivering thermogenesis (NST) 95. The presence and function of BAT are reported to be diminished by metabolic diseases 97 and aging 98-101. Nonetheless, functional BAT has been shown to reduce oxidative stress, alleviate pathological cardiac hypertrophy, and enhance cardiac function by promoting the release of exerkines such as FGF-21 and IL-6 102,103.
Exercise training or physical activity in young sedentary adults enhances BAT volume, playing a significant role in regulating glucose metabolism in an intensity-dependent manner. This study demonstrates that the BAT response becomes stronger with increasing exercise intensity 7. Furthermore, exercise training induces alterations in lipid metabolism in AT by modifying the lipidomes of both WAT and BAT. This is evident in the reduction of specific molecular species of phosphatidic acid (PA), phosphatidylcholines (PC), phosphatidylethanolamines (PE), and phosphatidylserines (PS) in WAT, and the increase in specific molecular species of PC and PE in BAT. There is also a decrease in the majority of triacylglycerols (TAGs) in both WAT and BAT 3. Additionally, physical activity or exercise training enhances mitochondrial activity, glucose uptake, insulin sensitivity, and thermogenesis in BAT 6,70,82,104-107. Cardiolipin (CL), a mitochondrial phospholipid, is essential for mitochondrial metabolism and structural integrity 108-112. Moreover, CL serves as a key effector in the thermogenic programs of brown and beige adipocytes and is involved in insulin sensitivity in AT 113. Conversely, the depletion of CL in brown and beige adipocytes impairs thermogenesis and glucose metabolism, resulting in reduced insulin sensitivity 113.
4.3. Beige Adipose Tissue
In addition to BAT, cells within WAT undergo adaptive thermogenesis in response to cold exposure or prolonged exercise training, and are referred to as beige adipocytes. The development of beige adipocytes is regulated by factors such as PR domain containing 16 (PRDM16), peroxisome proliferator-activated receptor gamma (PPARγ), and CCAAT-enhancer-binding proteins (C/EBP) 114. Beige cells represent an inducible profile of thermogenic adipocytes that can be activated by various stimuli, enhancing their capacity for fuel oxidation and thermogenesis. These stimuli include exercise, cold exposure, local hyperthermia therapy, and β-adrenergic intervention 57,114-116. The research has shown that sustained physical activity and exercise induce the beiging of WAT by modulating the secretion of brain-derived neurotrophic factor (BDNF), irisin, PGC-1α, interleukin-6 (IL-6), and meteorin-like protein (Metrnl) 93,117,118. Moreover, exercise activates signaling pathways associated with beiging in WAT, including the Wnt/β-catenin signaling pathway-a novel pathway crucial for driving the adipocyte population required for beiging. Additionally, exercise influences PGC-1α-related pathways, which mediate mitochondrial biogenesis and function 119.
Regrettably, aging results in a reduction in the mass of BAT in adult humans 120-122, and it diminishes cold and exercise-induced beiging in aged mice. This is evidenced by a decrease in the expression of transcriptional markers associated with beige adipocytes 48,114,123,124. The number of senescent cells increases while the differentiation of beige adipocytes decreases in aged mice and middle-aged humans. This is indicated by elevated transcriptional factors of senescence in WAT, including p16Ink4a, p21, and insulin-like growth factor binding protein 5 (IGFBP5). Furthermore, this phenomenon leads to an increase in glucose content and mitophagy, coupled with an incapacity to regulate the adaptation of body temperature in response to cold exposure. These findings demonstrate that cellular senescence plays a pivotal role in the age-induced decline of beige adipocyte generation 48,125-127. The study revealed that sustained stimulation of β-adrenergic agonists induces beiging in middle-aged mice 14,128. Various factors act as transcriptional regulators influencing differentiation in adipose tissues in response to aging.
5. Effect of Exercise-induced Adipokine in Aged Adipose Tissue
Aging induces structural, compositional, and functional changes in AT, characterized by reduced adipogenesis, alterations in the immune cell profile, and increased inflammation
129. As the largest endocrine gland, AT releases various cytokines that regulate metabolic responses, encompassing pre-production, adipogenesis, glucose and lipid homeostasis, inflammation, and several other physiological functions
130. Aging exerts a negative regulatory impact on the secretion of adipokines, as evidenced by an increase in proinflammatory adipokines (e.g., leptin, resistin, chemerin, retinol binding protein 4, lipocalin 2, CCL2, IL-1β, IL-6, IL-12, IL-18, and TNF-α)
131-136, coupled with a decline in anti-inflammatory mediators (e.g., adiponectin, vaspin, secreted-frizzled-related protein 5, omentin-1, and C1q/TNF-related proteins)
137-140 (
Table 1). Nevertheless, regular exercise can enhance the secretion of adipokines and mitigate the morphology and function of AT in response to metabolic diseases. This includes promoting fat browning, reducing adipocyte hypertrophy, improving FFA oxidation, insulin resistance, and enhancing mitochondrial homeostasis in aging AT
67-70.
Adipokines such as adiponectin and spexin, which decrease with aging in AT, play a crucial role in insulin resistance and are associated with the onset of diabetes and other metabolic disorders 62,141,142. Aging adipose tissue impacts the secretion of adipokines, promoting a chronic state of low-grade systemic inflammation 137. The exerkine IL-6, when exposed to acute inflammatory stress, is significantly increased with aging in AT. The age-dependent secretion of IL-6 is regulated by the autocrine/paracrine action of IL-1β in aged AT 143. BAT, fulfilling endocrine functions, also releases hormones known as batokines, which play a role in regulating energy balance, glucose uptake, lipid metabolism, and thermogenesis 144-146. Batokines are exercise-related humoral factors originating from BAT, exerting local autocrine or paracrine effects. These factors include peptides, metabolites, lipids, or microRNAs 10. Multiple studies demonstrate that exercise training or physical activity induces the differentiation of white adipocytes into functionally equivalent brown adipocytes, enhancing BAT function. Additionally, brown adipose tissue plays a role in mediating exercise performance 93,144,145. Nevertheless, exercise training enhances energy metabolism in response to cold exposure, as demonstrated by the promotion of mitochondrial biogenesis, reduction in oxidative stress, and increased exercise capacity 82. Moreover, the study reveals that small extracellular vesicles secreted from BAT not only promote metabolism within BAT but also regulate cardiomyocyte survival and participate in the response to exercise and myocardial ischemia/reperfusion injury. This is evidenced by the suppression of the proapoptotic MAPK pathway 6.
6. Conclusions
Aging of adipose tissue is linked to alterations in structure, composition, and function, encompassing changes in adipokine secretion, reduced adipogenesis, shifts in immune cell profile, heightened cellular senescence, increased insulin resistance, elevated inflammation, and enhanced fibrosis. As the largest endocrine gland, adipose tissue releases a variety of cytokines that regulate metabolic responses. Adipokines released through regular exercise play potential roles in mitigating metabolic diseases, improving glucose and lipid metabolism, reducing inflammation and fibrosis, and promoting fat browning and thermogenesis in adipose tissue. In this review, we delve into the molecular and cellular mechanisms that underlie the aging process of adipose tissue. Furthermore, the purpose of this review is to provide a comprehensive overview of the benefits of regular exercise in addressing the age-related decline in adipose tissue function. The relevance of regular exercise in mitigating metabolic disorders associated with aging adipose tissue will be explored.
Author Contributions
Literature search, D.J. and H.Z.; writing—original draft preparation, D.J.; writing—review and editing, T.L. and R.W. ; supervision, R.W. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This work supported in part by a grant from the National Natural Science Foundation of China (31971097, 32271226), the National Key R&D Program of China (2020YFA0803800), the Shanghai “Science and Technology Innovation Action Plan” Social Development Science and Technology Reach Project (22dz1204600), Shanghai Municipal Science and Technology Committee of Shanghai outstanding academic leaders plan (21XD1403200), The research project of Shanghai University of Sport (2023STD023), China.
Conflicts of Interest
No conflict of interest statement.
References
- Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373-392. [CrossRef]
- Tabula Muris C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. 2020;583(7817):590-595. [CrossRef]
- May FJ, Baer LA, Lehnig AC, et al. Lipidomic Adaptations in White and Brown Adipose Tissue in Response to Exercise Demonstrate Molecular Species-Specific Remodeling. Cell Rep. 2017;18(6):1558-1572. [CrossRef]
- Lehnig AC, Dewal RS, Baer LA, et al. Exercise Training Induces Depot-Specific Adaptations to White and Brown Adipose Tissue. iScience. 2019;11:425-439. [CrossRef]
- Sepa-Kishi DM, Ceddia RB. Exercise-Mediated Effects on White and Brown Adipose Tissue Plasticity and Metabolism. Exerc Sport Sci Rev. 2016;44(1):37-44. [CrossRef]
- Zhao H, Chen X, Hu G, et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circ Res. 2022;130(10):1490-1506. [CrossRef]
- Martinez-Tellez B, Sanchez-Delgado G, Acosta FM, et al. No evidence of brown adipose tissue activation after 24 weeks of supervised exercise training in young sedentary adults in the ACTIBATE randomized controlled trial. Nat Commun. 2022;13(1):5259. [CrossRef]
- Peres Valgas da Silva C, Hernandez-Saavedra D, White JD, Stanford KI. Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology (Basel). 2019;8(1). [CrossRef]
- Aldiss P, Betts J, Sale C, Pope M, Budge H, Symonds ME. Exercise-induced 'browning' of adipose tissues. Metabolism. 2018;81:63-70. [CrossRef]
- Chow LS, Gerszten RE, Taylor JM, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18(5):273-289. [CrossRef]
- Montanari T, Poscic N, Colitti M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: a review. Obes Rev. 2017;18(5):495-513. [CrossRef]
- Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. 2022;185(3):419-446. [CrossRef]
- Scambi I, Peroni D, Nodari A, et al. The transcriptional profile of adipose-derived stromal cells (ASC) mirrors the whitening of adipose tissue with age. Eur J Cell Biol. 2022;101(2):151206. [CrossRef]
- Rogers NH, Landa A, Park S, Smith RG. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell. 2012;11(6):1074-1083. [CrossRef]
- Von Bank H, Kirsh C, Simcox J. Aging adipose: Depot location dictates age-associated expansion and dysfunction. Ageing Res Rev. 2021;67:101259. [CrossRef]
- Guillermier C, Fazeli PK, Kim S, et al. Imaging mass spectrometry demonstrates age-related decline in human adipose plasticity. JCI Insight. 2017;2(5):e90349. [CrossRef]
- Kim SM, Lun M, Wang M, et al. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014;20(6):1049-1058. [CrossRef]
- Shen H, Huang X, Zhao Y, et al. The Hippo pathway links adipocyte plasticity to adipose tissue fibrosis. Nat Commun. 2022;13(1):6030. [CrossRef]
- Yuan F, Jiang H, Yin H, et al. Activation of GCN2/ATF4 signals in amygdalar PKC-delta neurons promotes WAT browning under leucine deprivation. Nat Commun. 2020;11(1):2847. [CrossRef]
- Sato H, Taketomi Y, Miki Y, Murase R, Yamamoto K, Murakami M. Secreted Phospholipase PLA2G2D Contributes to Metabolic Health by Mobilizing omega3 Polyunsaturated Fatty Acids in WAT. Cell Rep. 2020;31(5):107579. [CrossRef]
- Ma QX, Zhu WY, Lu XC, et al. BCAA-BCKA axis regulates WAT browning through acetylation of PRDM16. Nat Metab. 2022;4(1):106-122. [CrossRef]
- Friedrichs V, Toussaint C, Schafer A, et al. Landscape and age dynamics of immune cells in the Egyptian rousette bat. Cell Rep. 2022;40(10):111305. [CrossRef]
- He Y, Zhang R, Yu L, et al. PPARgamma Acetylation in Adipocytes Exacerbates BAT Whitening and Worsens Age-Associated Metabolic Dysfunction. Cells. 2023;12(10). [CrossRef]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252-1263. [CrossRef]
- Liu Z, Wu KKL, Jiang X, Xu A, Cheng KKY. The role of adipose tissue senescence in obesity- and ageing-related metabolic disorders. Clin Sci (Lond). 2020;134(2):315-330. [CrossRef]
- Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112(46):E6301-6310. [CrossRef]
- Zhang J, Sharma D, Dinabandhu A, et al. Targeting hypoxia-inducible factors with 32-134D safely and effectively treats diabetic eye disease in mice. J Clin Invest. 2023;133(13). [CrossRef]
- Liang Y, Ruan W, Jiang Y, Smalling R, Yuan X, Eltzschig HK. Interplay of hypoxia-inducible factors and oxygen therapy in cardiovascular medicine. Nat Rev Cardiol. 2023;20(11):723-737. [CrossRef]
- Soro-Arnaiz I, Li QOY, Torres-Capelli M, et al. Role of Mitochondrial Complex IV in Age-Dependent Obesity. Cell Rep. 2016;16(11):2991-3002. [CrossRef]
- Zoico E, Policastro G, Rizzatti V, et al. Mechanisms of adipose tissue extracellular matrix alterations in an in vitro model of adipocytes hypoxia and aging. Mech Ageing Dev. 2020;192:111374. [CrossRef]
- Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470-477. [CrossRef]
- Sun K, Park J, Gupta OT, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. [CrossRef]
- Starling S. Unravelling adipose tissue fibrosis in obesity. Nat Rev Endocrinol. 2022;18(7):393. [CrossRef]
- Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Invest. 2019;129(10):3978-3989. [CrossRef]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028-1040. [CrossRef]
- Tanaka M, Ikeda K, Suganami T, et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat Commun. 2014;5:4982. [CrossRef]
- Bel Lassen P, Charlotte F, Liu Y, et al. The FAT Score, a Fibrosis Score of Adipose Tissue: Predicting Weight-Loss Outcome After Gastric Bypass. J Clin Endocrinol Metab. 2017;102(7):2443-2453. [CrossRef]
- Zhang Q, Liang Z, Zhang Y, et al. Brown adipose tissue transplantation improves skin fibrosis in localized scleroderma. FASEB J. 2023;37(12):e23315. [CrossRef]
- Amano SU, Cohen JL, Vangala P, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19(1):162-171. [CrossRef]
- Camell CD, Gunther P, Lee A, et al. Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab. 2019;30(6):1024-1039 e1026. [CrossRef]
- Bapat SP, Myoung Suh J, Fang S, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528(7580):137-141. [CrossRef]
- Blondin DP, Tingelstad HC, Noll C, et al. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat Commun. 2017;8:14146. [CrossRef]
- Berry DC, Jiang Y, Graff JM. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun. 2016;7:10184. [CrossRef]
- Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200-205. [CrossRef]
- Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab. 2011;13(3):238-240. [CrossRef]
- M UD, Raiko J, Saari T, et al. Human brown adipose tissue [(15)O]O2 PET imaging in the presence and absence of cold stimulus. Eur J Nucl Med Mol Imaging. 2016;43(10):1878-1886. [CrossRef]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500-1508. [CrossRef]
- Berry DC, Jiang Y, Arpke RW, et al. Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans. Cell Metab. 2017;25(1):166-181. [CrossRef]
- Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53(4):619-629. [CrossRef]
- Bhadada SV, Patel BM, Mehta AA, Goyal RK. beta(3) Receptors: Role in Cardiometabolic Disorders. Ther Adv Endocrinol Metab. 2011;2(2):65-79. [CrossRef]
- Larsen TM, Toubro S, van Baak MA, et al. Effect of a 28-d treatment with L-796568, a novel beta(3)-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76(4):780-788. [CrossRef]
- Redman LM, de Jonge L, Fang X, et al. Lack of an effect of a novel beta3-adrenoceptor agonist, TAK-677, on energy metabolism in obese individuals: a double-blind, placebo-controlled randomized study. J Clin Endocrinol Metab. 2007;92(2):527-531. [CrossRef]
- Vasconcelos J, Freire E, Almendra R, Silva GL, Santana P. The impact of winter cold weather on acute myocardial infarctions in Portugal. Environ Pollut. 2013;183:14-18. [CrossRef]
- Brunt VE, Eymann TM, Francisco MA, Howard MJ, Minson CT. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J Appl Physiol (1985). 2016;121(3):716-723. [CrossRef]
- Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med. 2015;175(4):542-548. [CrossRef]
- Chung J, Nguyen AK, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105(5):1739-1744. [CrossRef]
- Li Y, Wang D, Ping X, et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell. 2022;185(6):949-966 e919. [CrossRef]
- Labbadia J, Brielmann RM, Neto MF, Lin YF, Haynes CM, Morimoto RI. Mitochondrial Stress Restores the Heat Shock Response and Prevents Proteostasis Collapse during Aging. Cell Rep. 2017;21(6):1481-1494. [CrossRef]
- Tharp KM, Higuchi-Sanabria R, Timblin GA, et al. Adhesion-mediated mechanosignaling forces mitohormesis. Cell Metab. 2021;33(7):1322-1341 e1313. [CrossRef]
- Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19(5):757-766. [CrossRef]
- Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med. 2014;20(7):709-711. [CrossRef]
- Fang P, Ge R, She Y, et al. Adipose tissue spexin in physical exercise and age-associated diseases. Ageing Res Rev. 2022;73:101509. [CrossRef]
- Fang P, She Y, Yu M, Min W, Shang W, Zhang Z. Adipose-Muscle crosstalk in age-related metabolic disorders: The emerging roles of adipo-myokines. Ageing Res Rev. 2023;84:101829. [CrossRef]
- Sahl RE, Patsi I, Hansen MT, et al. Prolonged endurance exercise increases macrophage content and mitochondrial respiration in adipose tissue in trained men. J Clin Endocrinol Metab. 2023. [CrossRef]
- Zaidi H, Byrkjeland R, Njerve IU, et al. Effects of exercise training on markers of adipose tissue remodeling in patients with coronary artery disease and type 2 diabetes mellitus: sub study of the randomized controlled EXCADI trial. Diabetol Metab Syndr. 2019;11:109. [CrossRef]
- Christensen RH, Wedell-Neergaard AS, Lehrskov LL, et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses From a Randomized Clinical Trial. JAMA Cardiol. 2019;4(8):778-787. [CrossRef]
- Joseph LC, Morrow JP. Paracardial fat and vitamin A: a mechanism for regulating exercise performance. J Clin Invest. 2021;131(4). [CrossRef]
- Brenmoehl J, Ohde D, Walz C, Langhammer M, Schultz J, Hoeflich A. Analysis of Activity-Dependent Energy Metabolism in Mice Reveals Regulation of Mitochondrial Fission and Fusion mRNA by Voluntary Physical Exercise in Subcutaneous Fat from Male Marathon Mice (DUhTP). Cells. 2020;9(12). [CrossRef]
- Yuan Y, Xu P, Jiang Q, et al. Exercise-induced alpha-ketoglutaric acid stimulates muscle hypertrophy and fat loss through OXGR1-dependent adrenal activation. EMBO J. 2020;39(7):e103304. [CrossRef]
- Xiong Y, Wu Z, Zhang B, et al. Fndc5 loss-of-function attenuates exercise-induced browning of white adipose tissue in mice. FASEB J. 2019;33(5):5876-5886. [CrossRef]
- Nigro P, Middelbeek RJW, Alves CRR, et al. Exercise Training Promotes Sex-Specific Adaptations in Mouse Inguinal White Adipose Tissue. Diabetes. 2021;70(6):1250-1264. [CrossRef]
- Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018;27(1):68-83. [CrossRef]
- Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127(1):74-82. [CrossRef]
- Hepler C, Gupta RK. The expanding problem of adipose depot remodeling and postnatal adipocyte progenitor recruitment. Mol Cell Endocrinol. 2017;445:95-108. [CrossRef]
- Jakobsson E. Exercise and heart disease. Science. 1977;195(4281):822. [CrossRef]
- Sarma S, MacNamara JP, Balmain BN, et al. Challenging the Hemodynamic Hypothesis in Heart Failure With Preserved Ejection Fraction: Is Exercise Capacity Limited by Elevated Pulmonary Capillary Wedge Pressure? Circulation. 2023;147(5):378-387. [CrossRef]
- Sachdev V, Sharma K, Keteyian SJ, et al. Supervised Exercise Training for Chronic Heart Failure With Preserved Ejection Fraction: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation. 2023;147(16):e699-e715. [CrossRef]
- Fudim M, Sobotka PA, Dunlap ME. Extracardiac Abnormalities of Preload Reserve: Mechanisms Underlying Exercise Limitation in Heart Failure with Preserved Ejection Fraction, Autonomic Dysfunction, and Liver Disease. Circ Heart Fail. 2021;14(1):e007308. [CrossRef]
- Tucker WJ, Kitzman DW. Defining the Specific Skeletal Muscle Adaptations Responsible for Exercise Training Improvements in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2022;15(10):e010003. [CrossRef]
- Peter JB, Jeffress RN, Lamb DR. Exercise: effects on hexokinase activity in red and white skeletal muscle. Science. 1968;160(3824):200-201. [CrossRef]
- Nigro P, Vamvini M, Yang J, et al. Exercise training remodels inguinal white adipose tissue through adaptations in innervation, vascularization, and the extracellular matrix. Cell Rep. 2023;42(4):112392. [CrossRef]
- Vatner DE, Oydanich M, Zhang J, Campbell SC, Vatner SF. Exercise enhancement by RGS14 disruption is mediated by brown adipose tissue. Aging Cell. 2023;22(4):e13791. [CrossRef]
- Yang J, Vamvini M, Nigro P, et al. Single-cell dissection of the obesity-exercise axis in adipose-muscle tissues implies a critical role for mesenchymal stem cells. Cell Metab. 2022;34(10):1578-1593 e1576. [CrossRef]
- Engin B, Willis SA, Malaikah S, et al. The effect of exercise training on adipose tissue insulin sensitivity: A systematic review and meta-analysis. Obes Rev. 2022;23(7):e13445. [CrossRef]
- Lehnig AC, Stanford KI. Exercise-induced adaptations to white and brown adipose tissue. J Exp Biol. 2018;221(Pt Suppl 1). [CrossRef]
- Severinsen MCK, Scheele C, Pedersen BK. Exercise and browning of white adipose tissue - a translational perspective. Curr Opin Pharmacol. 2020;52:18-24. [CrossRef]
- Stanford KI, Middelbeek RJ, Townsend KL, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64(6):2002-2014. [CrossRef]
- Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes. 2015;64(7):2361-2368. [CrossRef]
- Golbidi S, Laher I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res. 2014;2014:726861. [CrossRef]
- Trevellin E, Scorzeto M, Olivieri M, et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes. 2014;63(8):2800-2811. [CrossRef]
- Kanaley JA, Fenicchia LM, Miller CS, et al. Resting leptin responses to acute and chronic resistance training in type 2 diabetic men and women. Int J Obes Relat Metab Disord. 2001;25(10):1474-1480. [CrossRef]
- Zachwieja JJ, Hendry SL, Smith SR, Harris RB. Voluntary wheel running decreases adipose tissue mass and expression of leptin mRNA in Osborne-Mendel rats. Diabetes. 1997;46(7):1159-1166. [CrossRef]
- Dewal RS, Stanford KI. Effects of exercise on brown and beige adipocytes. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(1):71-78. [CrossRef]
- Felix-Soriano E, Sainz N, Gil-Iturbe E, et al. Differential remodeling of subcutaneous white and interscapular brown adipose tissue by long-term exercise training in aged obese female mice. J Physiol Biochem. 2023;79(2):451-465. [CrossRef]
- Oelkrug R, Polymeropoulos ET, Jastroch M. Brown adipose tissue: physiological function and evolutionary significance. J Comp Physiol B. 2015;185(6):587-606. [CrossRef]
- Kim HJ, Kim YJ, Seong JK. AMP-activated protein kinase activation in skeletal muscle modulates exercise-induced uncoupled protein 1 expression in brown adipocyte in mouse model. J Physiol. 2022;600(10):2359-2376. [CrossRef]
- Khalagi K, Ansarifar A, Fahimfar N, et al. Cardio-metabolic and socio-demographic risk factors associated with dependency in basic and instrumental activities of daily living among older Iranian adults: Bushehr elderly health program. BMC Geriatr. 2021;21(1):172. [CrossRef]
- Rossato M. Aging and brown adipose tissue activity decline in human: does the brain extinguish the fire? Aging Clin Exp Res. 2016;28(3):579-581. [CrossRef]
- Enerback S. Human brown adipose tissue. Cell Metab. 2010;11(4):248-252. [CrossRef]
- Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11(4):268-272. [CrossRef]
- Harb E, Kheder O, Poopalasingam G, Rashid R, Srinivasan A, Izzi-Engbeaya C. Brown adipose tissue and regulation of human body weight. Diabetes Metab Res Rev. 2023;39(1):e3594. [CrossRef]
- Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15(9):507-524. [CrossRef]
- Neeland IJ, Ross R, Despres JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715-725. [CrossRef]
- Zhu Y, Qi Z, Ding S. Exercise-Induced Adipose Tissue Thermogenesis and Browning: How to Explain the Conflicting Findings? Int J Mol Sci. 2022;23(21). [CrossRef]
- Sanchez-Delgado G, Martinez-Tellez B, Olza J, Aguilera CM, Gil A, Ruiz JR. Role of Exercise in the Activation of Brown Adipose Tissue. Ann Nutr Metab. 2015;67(1):21-32. [CrossRef]
- Maalouf GE, El Khoury D. Exercise-Induced Irisin, the Fat Browning Myokine, as a Potential Anticancer Agent. J Obes. 2019;2019:6561726. [CrossRef]
- Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-468. [CrossRef]
- Shi Y. Emerging roles of cardiolipin remodeling in mitochondrial dysfunction associated with diabetes, obesity, and cardiovascular diseases. J Biomed Res. 2010;24(1):6-15. [CrossRef]
- Jia D, Zhang J, Nie J, et al. Cardiolipin Remodeling by ALCAT1 Links Hypoxia to Coronary Artery Disease by Promoting Mitochondrial Dysfunction. Mol Ther. 2021. [CrossRef]
- Song C, Zhang J, Qi S, et al. Cardiolipin remodeling by ALCAT1 links mitochondrial dysfunction to Parkinson's diseases. Aging Cell. 2019;18(3):e12941. [CrossRef]
- Li J, Liu X, Wang H, Zhang W, Chan DC, Shi Y. Lysocardiolipin acyltransferase 1 (ALCAT1) controls mitochondrial DNA fidelity and biogenesis through modulation of MFN2 expression. Proc Natl Acad Sci U S A. 2012;109(18):6975-6980. [CrossRef]
- Han X, Yang J, Cheng H, Yang K, Abendschein DR, Gross RW. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry. 2005;44(50):16684-16694. [CrossRef]
- Sustarsic EG, Ma T, Lynes MD, et al. Cardiolipin Synthesis in Brown and Beige Fat Mitochondria Is Essential for Systemic Energy Homeostasis. Cell Metab. 2018;28(1):159-174 e111. [CrossRef]
- Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366-376. [CrossRef]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285(10):7153-7164. [CrossRef]
- Ishibashi J, Seale P. Medicine. Beige can be slimming. Science. 2010;328(5982):1113-1114. [CrossRef]
- Luo Z, Ma L, Zhao Z, et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res. 2012;22(3):551-564. [CrossRef]
- Ringseis R, Mooren FC, Keller J, et al. Regular endurance exercise improves the diminished hepatic carnitine status in mice fed a high-fat diet. Mol Nutr Food Res. 2011;55 Suppl 2:S193-202. [CrossRef]
- Li H, Zhang X, Huang C, et al. FGF2 disruption enhances thermogenesis in brown and beige fat to protect against adiposity and hepatic steatosis. Mol Metab. 2021;54:101358. [CrossRef]
- Cypess AM, White AP, Vernochet C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19(5):635-639. [CrossRef]
- Jespersen NZ, Larsen TJ, Peijs L, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17(5):798-805. [CrossRef]
- Shinoda K, Luijten IH, Hasegawa Y, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015;21(4):389-394. [CrossRef]
- Kwon I, Talib NF, Zhu J, Yang HI, Kim KS. Effects of aging-induced obesity on the transcriptional expression of adipogenesis and thermogenic activity in the gonadal white adipose, brown adipose, and skeletal muscle tissues. Phys Act Nutr. 2023;27(2):39-49. [CrossRef]
- Singh R, Barrios A, Dirakvand G, Pervin S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells. 2021;10(11). [CrossRef]
- Fu W, Liu Y, Sun C, Yin H. Transient p53 inhibition sensitizes aged white adipose tissue for beige adipocyte recruitment by blocking mitophagy. FASEB J. 2019;33(1):844-856. [CrossRef]
- Khanh VC, Zulkifli AF, Tokunaga C, Yamashita T, Hiramatsu Y, Ohneda O. Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of Sirtuin 1. Biochem Biophys Res Commun. 2018;500(3):682-690. [CrossRef]
- Duteil D, Tosic M, Willmann D, Georgiadi A, Kanouni T, Schule R. Lsd1 prevents age-programed loss of beige adipocytes. Proc Natl Acad Sci U S A. 2017;114(20):5265-5270. [CrossRef]
- Jiang Y, Berry DC, Graff JM. Distinct cellular and molecular mechanisms for beta3 adrenergic receptor-induced beige adipocyte formation. Elife. 2017;6. [CrossRef]
- Tarantini S, Subramanian M, Butcher JT, et al. Revisiting adipose thermogenesis for delaying aging and age-related diseases: Opportunities and challenges. Ageing Res Rev. 2023;87:101912. [CrossRef]
- Clemente-Suarez VJ, Redondo-Florez L, Beltran-Velasco AI, et al. The Role of Adipokines in Health and Disease. Biomedicines. 2023;11(5). [CrossRef]
- Wijetunge S, Ratnayake R, Kotakadeniya H, et al. Association between serum and adipose tissue resistin with dysglycemia in South Asian women. Nutr Diabetes. 2019;9(1):5. [CrossRef]
- Roszkowska-Gancarz M, Jonas M, Owczarz M, et al. Age-related changes of leptin and leptin receptor variants in healthy elderly and long-lived adults. Geriatr Gerontol Int. 2015;15(3):365-371. [CrossRef]
- Gencer B, Auer R, de Rekeneire N, et al. Association between resistin levels and cardiovascular disease events in older adults: The health, aging and body composition study. Atherosclerosis. 2016;245:181-186. [CrossRef]
- Zhang Y, Shen WJ, Qiu S, et al. Chemerin regulates formation and function of brown adipose tissue: Ablation results in increased insulin resistance with high fat challenge and aging. FASEB J. 2021;35(7):e21687. [CrossRef]
- Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014;19(3):512-526. [CrossRef]
- Su H, Guo H, Qiu X, et al. Lipocalin 2 regulates mitochondrial phospholipidome remodeling, dynamics, and function in brown adipose tissue in male mice. Nat Commun. 2023;14(1):6729. [CrossRef]
- Mancuso P, Bouchard B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front Endocrinol (Lausanne). 2019;10:137. [CrossRef]
- Weiner J, Rohde K, Krause K, et al. Brown adipose tissue (BAT) specific vaspin expression is increased after obesogenic diets and cold exposure and linked to acute changes in DNA-methylation. Mol Metab. 2017;6(6):482-493. [CrossRef]
- Ouchi N, Higuchi A, Ohashi K, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329(5990):454-457. [CrossRef]
- Koutaki D, Michos A, Bacopoulou F, Charmandari E. The Emerging Role of Sfrp5 and Wnt5a in the Pathogenesis of Obesity: Implications for a Healthy Diet and Lifestyle. Nutrients. 2021;13(7). [CrossRef]
- Fang P, Guo W, Ju M, et al. Exercise training rescues adipose tissue spexin expression and secretion in diet-induced obese mice. Physiol Behav. 2022;256:113958. [CrossRef]
- Miller KN, Burhans MS, Clark JP, et al. Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell. 2017;16(3):497-507. [CrossRef]
- Starr ME, Saito M, Evers BM, Saito H. Age-Associated Increase in Cytokine Production During Systemic Inflammation-II: The Role of IL-1beta in Age-Dependent IL-6 Upregulation in Adipose Tissue. J Gerontol A Biol Sci Med Sci. 2015;70(12):1508-1515. [CrossRef]
- Hua L, Zhuo Y, Jiang D, et al. Identification of hepatic fibroblast growth factor 21 as a mediator in 17beta-estradiol-induced white adipose tissue browning. FASEB J. 2018;32(10):5602-5611. [CrossRef]
- Cuevas-Ramos D, Mehta R, Aguilar-Salinas CA. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front Physiol. 2019;10:37. [CrossRef]
- Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. 2012;92(1):157-191. [CrossRef]
- Kobayashi M, Uta S, Otsubo M, et al. Srebp-1c/Fgf21/Pgc-1alpha Axis Regulated by Leptin Signaling in Adipocytes-Possible Mechanism of Caloric Restriction-Associated Metabolic Remodeling of White Adipose Tissue. Nutrients. 2020;12(7). [CrossRef]
- Graff EC, Fang H, Wanders D, Judd RL. The Absence of Adiponectin Alters Niacin's Effects on Adipose Tissue Inflammation in Mice. Nutrients. 2020;12(8). [CrossRef]
- Shanaki M, Shabani P, Goudarzi A, Omidifar A, Bashash D, Emamgholipour S. The C1q/TNF-related proteins (CTRPs) in pathogenesis of obesity-related metabolic disorders: Focus on type 2 diabetes and cardiovascular diseases. Life Sci. 2020;256:117913. [CrossRef]
- Xu F, Li FX, Lin X, et al. Adipose tissue-derived omentin-1 attenuates arterial calcification via AMPK/Akt signaling pathway. Aging (Albany NY). 2019;11(20):8760-8776. [CrossRef]
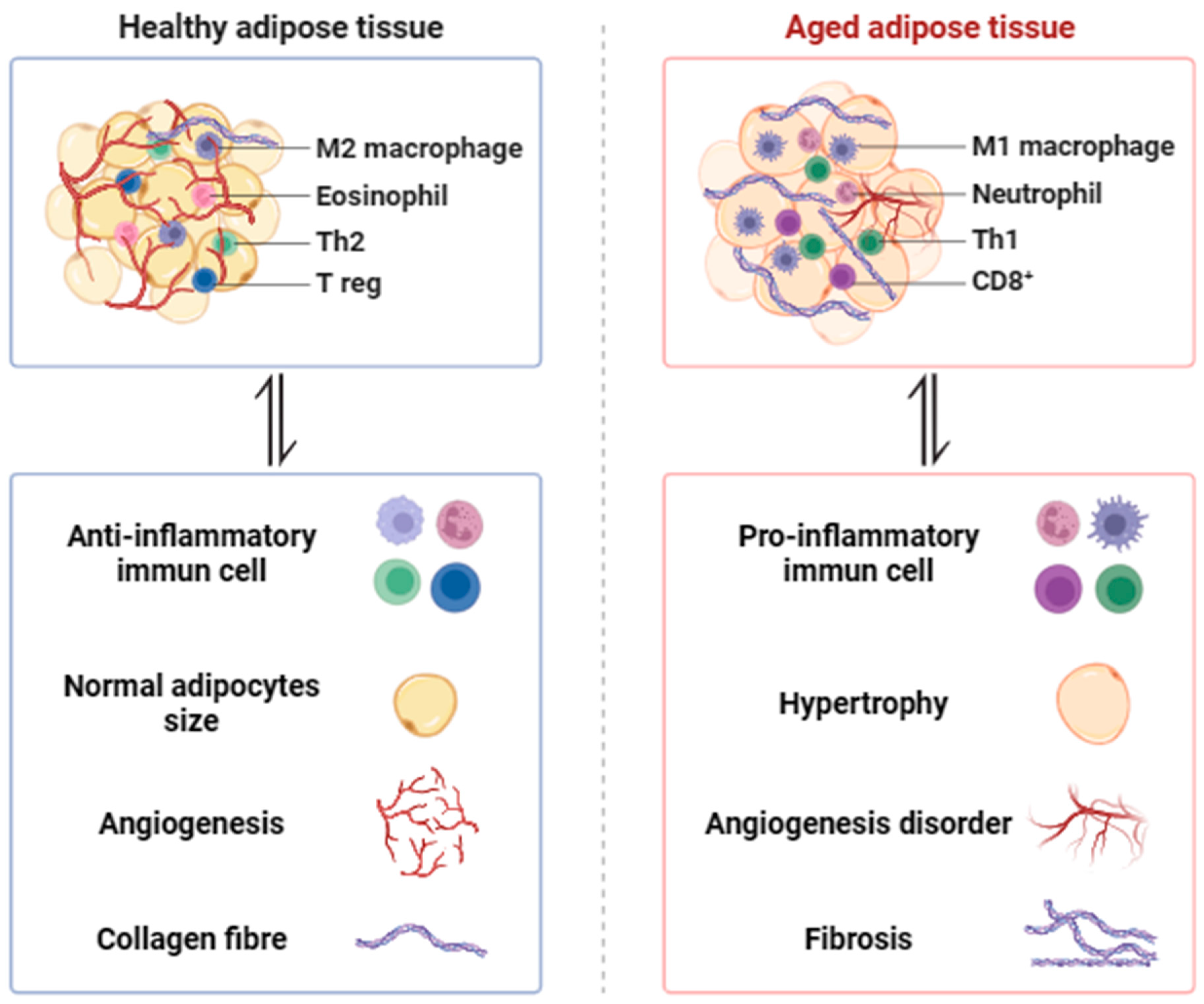
Figure 1.
The morphological changes in aging adipose tissue. Adipose plasticity becomes compromised with age, leading to adipocyte hypertrophy, a decline in adipogenesis, decreased angiogenesis, increased fibrosis, pro-inflammatory macrophage infiltration (M1 macrophage, Neutrophil, Th1, CD8+), and decreased anti-inflammatory macrophage infiltration (M2 macrophage, Eosinophil, Th2, T reg).
Figure 1.
The morphological changes in aging adipose tissue. Adipose plasticity becomes compromised with age, leading to adipocyte hypertrophy, a decline in adipogenesis, decreased angiogenesis, increased fibrosis, pro-inflammatory macrophage infiltration (M1 macrophage, Neutrophil, Th1, CD8+), and decreased anti-inflammatory macrophage infiltration (M2 macrophage, Eosinophil, Th2, T reg).
Figure 2.
Cold exposure enhances adipocyte browning in response to obesity but not in age-related obesity. Cold exposure is implicated in the prevention and management of obesity, as evidenced by the increased expression of UCP1, reduced accumulation of large lipid droplets, enhanced mitochondrial biogenesis and function, promotion of FFA oxidation, increased insulin sensitivity, and improvement of white adipocyte browning and thermogenesis in WAT. Nevertheless, the potential to form cold-induced beige adipocytes decline with age. In contrast, reversing cellular aging through the p38/MAPK-p16Ink4a pathway rejuvenates cold-induced beiging. HFD, high fat diet.
Figure 2.
Cold exposure enhances adipocyte browning in response to obesity but not in age-related obesity. Cold exposure is implicated in the prevention and management of obesity, as evidenced by the increased expression of UCP1, reduced accumulation of large lipid droplets, enhanced mitochondrial biogenesis and function, promotion of FFA oxidation, increased insulin sensitivity, and improvement of white adipocyte browning and thermogenesis in WAT. Nevertheless, the potential to form cold-induced beige adipocytes decline with age. In contrast, reversing cellular aging through the p38/MAPK-p16Ink4a pathway rejuvenates cold-induced beiging. HFD, high fat diet.
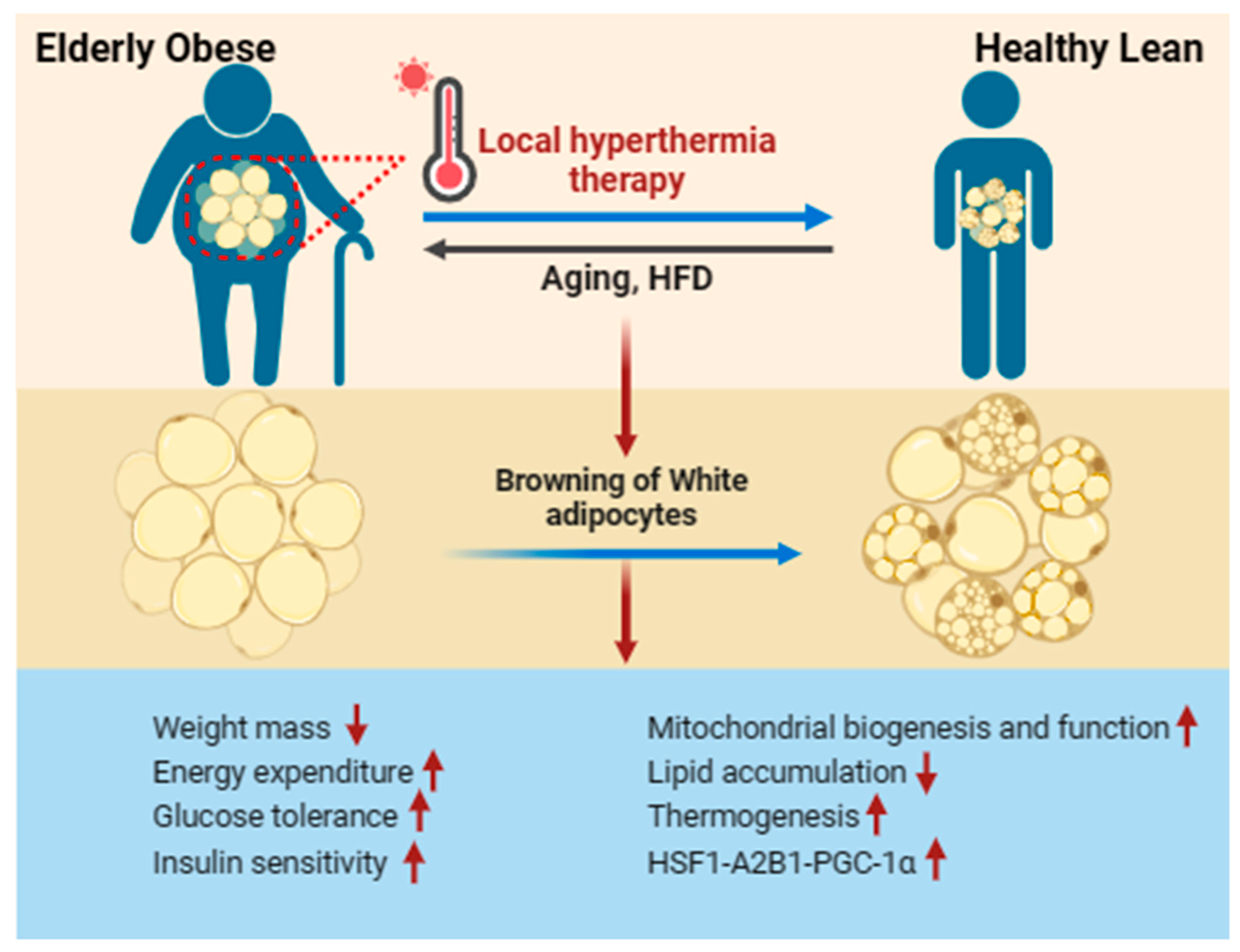
Figure 3.
Local hyperthermia therapy improves WAT browning in aging-induced obese. Local hyperthermia therapy stimulates the activation and production of beige adipocytes in individuals with obesity, enhancing metabolic performance. This includes reductions in lipid accumulation and body mass, improvements in diabetic neuropathic symptoms, enhanced glucose tolerance, increased insulin sensitivity, and the promotion of mitochondrial biogenesis and thermogenesis. These effects are achieved through the activation of the HSF1-A2BA-PGC-1α pathway.
Figure 3.
Local hyperthermia therapy improves WAT browning in aging-induced obese. Local hyperthermia therapy stimulates the activation and production of beige adipocytes in individuals with obesity, enhancing metabolic performance. This includes reductions in lipid accumulation and body mass, improvements in diabetic neuropathic symptoms, enhanced glucose tolerance, increased insulin sensitivity, and the promotion of mitochondrial biogenesis and thermogenesis. These effects are achieved through the activation of the HSF1-A2BA-PGC-1α pathway.
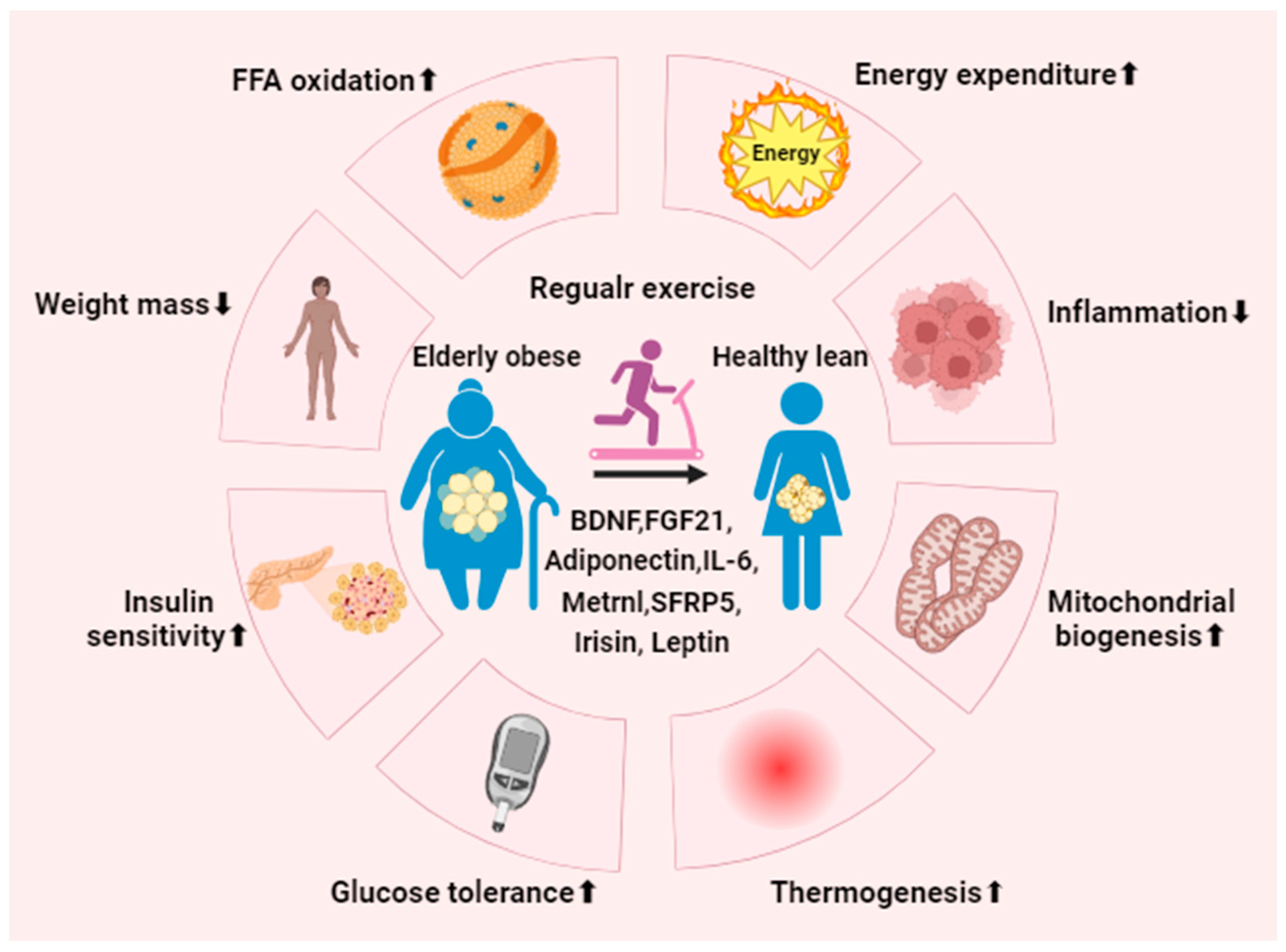
Figure 4.
Regular exercise mitigates metabolic syndrome. Consistent physical activity promotes the activation and generation of beige adipocytes in individuals with obesity by regulating associated exerkines and adipokines. Ultimately, this process enhances metabolic performance, leading to notable benefits such as decreased inflammation and body mass, heightened energy expenditure, improved glucose tolerance, increased insulin sensitivity, and the stimulation of mitochondrial biogenesis and UCP1-dependent thermogenesis. BDNF, brain-derived neurotrophic factor; FGF21, fibroblast growth factor 21; Metrnl, meteorin-like protein; SFRP5, secreted-frizzled-related protein 5.
Figure 4.
Regular exercise mitigates metabolic syndrome. Consistent physical activity promotes the activation and generation of beige adipocytes in individuals with obesity by regulating associated exerkines and adipokines. Ultimately, this process enhances metabolic performance, leading to notable benefits such as decreased inflammation and body mass, heightened energy expenditure, improved glucose tolerance, increased insulin sensitivity, and the stimulation of mitochondrial biogenesis and UCP1-dependent thermogenesis. BDNF, brain-derived neurotrophic factor; FGF21, fibroblast growth factor 21; Metrnl, meteorin-like protein; SFRP5, secreted-frizzled-related protein 5.
Table 1.
The impact of adipokine in aged AT.
Table 1.
The impact of adipokine in aged AT.
| Adipokines |
Main mechanism |
Main biological action |
Target |
Refs |
| Leptin |
Srebp-1c/FGF21/
PGC-1α |
Regulates FA biosynthesis and mitochondrial biogenesis |
AT |
Kobayashi, M., et al.147
|
| Resistin |
CRP/IL-6/TNF-α |
Associates with aging-related cardiovascular disease |
Heart |
Gencer, B., et al.133
|
| Chemerin |
PRDM16/CPT1/
DIO2 |
Regulates formation and function of BAT |
BAT |
Zhang, Y., et al.134
|
| RBP4 |
JNK/TNF/IL-1β |
Causes insulin resistance and inflammation by activating innate immunity |
AT |
Moraes-Vieira, P. M., et al.135
|
| LCN2 |
mTORC1/ERK |
Regulates mitochondrial bioenergetics |
BAT |
Su, H., et al.136
|
| IL-6 |
IL-1β/TNF-α |
Impact age-associated inflammatory diseases |
AT |
Starr, M. E., et al.143
|
| Adiponectin |
ARG1/TNF |
Mediates the anti-inflammatory effects of niacin |
AT |
Graff, E. C., et al.148
|
| Vaspin |
ANGPTL4/DNA methylation |
Reduces inflammation and activists BAT |
BAT |
Weiner, J., et al.138
|
| SFRP5 |
JNK/Wnt |
Regulates inflammation and obesity-related complication |
AT |
Koutaki, D., et al.140
|
| CTRPs |
AMPK/Akt, ERK |
Mitigates heart failure by improving inflammation |
Heart |
Shanaki, M., et al.149
|
| Omentin-1 |
AMPK/Akt |
Improves cardiovascular disease by mitigating inflammation |
Heart |
Xu, F., et al.150
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).