1. Introduction
Cracking or splitting of fruit is a common symptom of fruit surface fractures and is encountered in many fruit crops, and it has been described as “ the physical failure of the fruit skin” due to the internal growth is not in harmony with the external environment, and occurs in the form of fractures in the cuticle or peel that universally do not penetrate the flesh (Li et al., 2014; Seo et al., 2022; Santos et al., 2023). As a common and severe physiological disorder, cracking has an adverse and common bearing on both natural beauty and yield performance. And cracking reduces the fruit marketability as which causes unfavorable effects in fruit quality, such as poor appearance and severe nutrient loss, decreased shelf life and increased susceptibility to infections by certain fungi or bacteria, and other pathogens, resulting in unquantifiable losses in the fresh market. Thus, the cracked fruits can only be used in processing industries before they are infected by fungi (Peet, 1992; Simon, 2006; Khadivi-Khub, 2015; Lara et al., 2019; Butani et al., 2019; Schumann et al., 2019). In spite of fruit cracking or splitting has been investigated since the 1930s, and many fruit breeders are also working hard to incorporated crack resistance into these fruit crops to enhance their crack resistance, very slight advance have been made in understanding the physiology and genetics of fruit cracking. In turn, this also makes it particularly difficult to recommend effective measures to prevent fruit from cracking. Therefore, it is significant to better understand the underlying mechanism of fruit cracking in different fruits for reasonably controlling this physiological disorder.
Fruit cracking or splitting has challenged the scientific community for decades, the first systematic study and reports about sweet cherry (Prunus avium L.) cracking began more than 90 years ago (Verner & Blodgett, 1931), and since then, a large number of studies with the fundamental aim to classify the CR (cracking-resistant) and the CS (cracking-susceptible) sweet cherry cultivars based on their susceptibility to cracking have been following (Christensen et al., 1972; Measham et al., 2009; Cristián et al., 2013; Correia et al., 2018). Furthermore, researches have reported that many plant crops, such as litchi (Litchi chinensis Sonn.) (Sharma et al., 1986; Munish et al., 2003), tomato (Lycopersicon esculentum Mill.) (Batal et al., 1970), persimmon (Diospyros kaki Thunb.) (Yamada et al., 1988), peach (Prunus Persica L.) (Caroline et al., 2007; Gibert et al., 2010), grape (Vitis vinifera L.) (Considine et al., 1982), apple (Malus domestica) (Opara et al.,1996; Andrews et al., 1999), sweet orange (Citrus sinensis) (Agusti et al., 2002), pomegranate (Punica granatum L.) (Hoda & Khalil, 2013), fresh fig (Ficus carica L.) (Michelle et al., 2013), pear (Pyrus spp.) (Choi et al., 2002; Kwon et al., 2016), jujube (Ziziphus jujuba Mill.) (Burhan, et al., 2018), watermelon (Citrullus lanatus) (Liao et al., 2020), as well as strawberry (Fragaria×ananassa Duch.) (Hurtado & Knoche, 2023), are liable to crack or split, and the cracking rate is often about 30% in several species, some varieties as high as 60-80%, serious years up to 90% or more, even extremely, which leading to seriously compromise quality and crop profitability, considerable economic loss and agricultural resources waste, and the loss caused by fruit cracking reaches 30%~60%, even more (Simon, 2006; Khadivi-Khub, 2015; Correia et al., 2018; Butani et al., 2019).
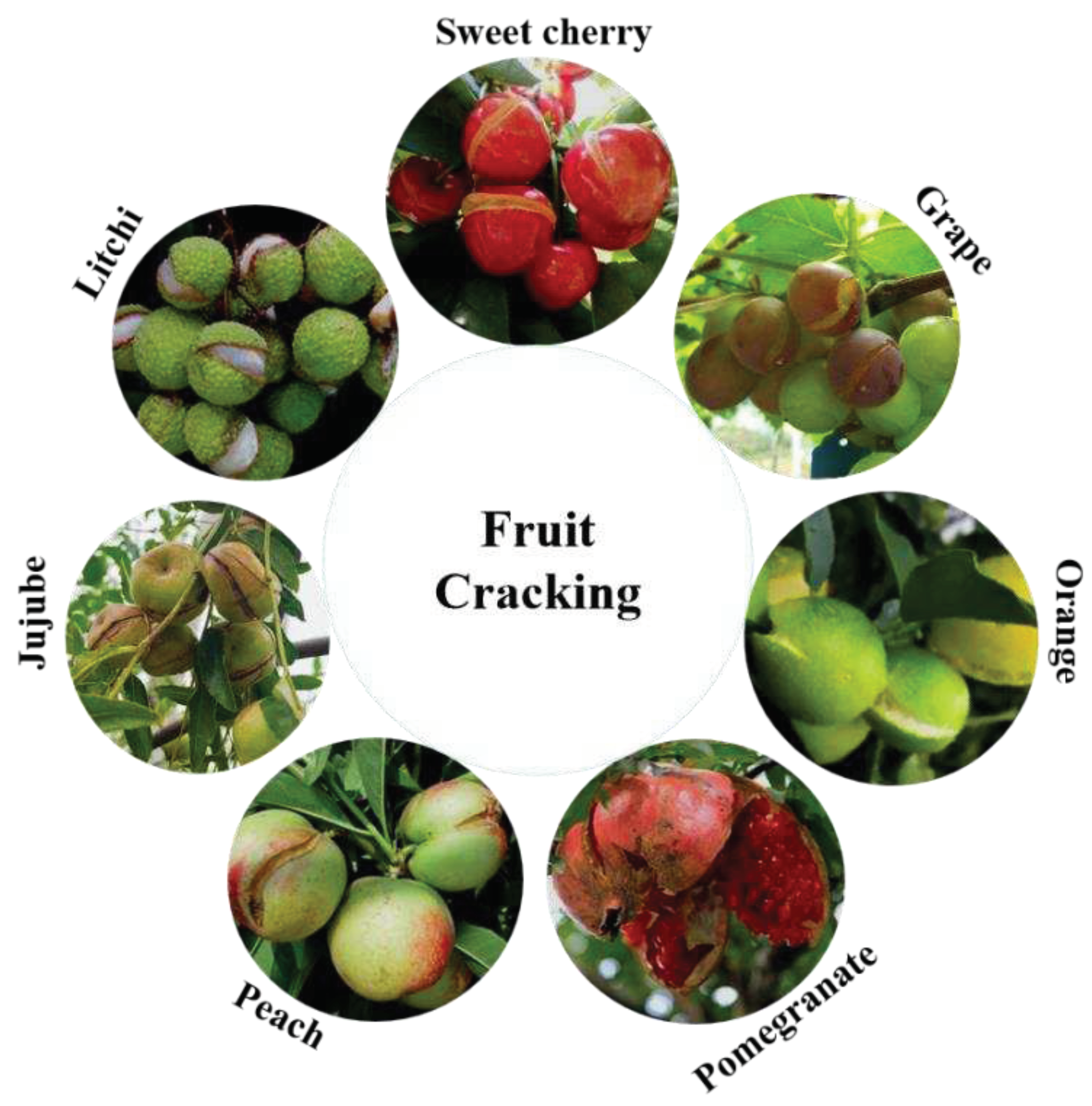
Figure 1.
Cracking in several different fruit species. Seven common fruits that are liable to crack, including sweet cherry, grape, orange, pomegranate, peach, jujube and litchi, were represented. Among them, sweet cherry (
https://baijiahao.baidu.com/s?id=1668282007129830555&wfr=spider&for=pc), grape (
https://doc.wendoc.com/bf22b19f9b77f69ababc170ed6133bbfb099a5290.html), orange (
https://www.sohu.com/a/381112523_99993524), pomegranate (
https://www.cnhnb.com/xt/article-1274.html), peach (
https://www.sohu.com/picture/397690934), and litchi (
https://v.youku.com/v_show/id_XMjgzNjI1NDY1Ng==.html?).
Figure 1.
Cracking in several different fruit species. Seven common fruits that are liable to crack, including sweet cherry, grape, orange, pomegranate, peach, jujube and litchi, were represented. Among them, sweet cherry (
https://baijiahao.baidu.com/s?id=1668282007129830555&wfr=spider&for=pc), grape (
https://doc.wendoc.com/bf22b19f9b77f69ababc170ed6133bbfb099a5290.html), orange (
https://www.sohu.com/a/381112523_99993524), pomegranate (
https://www.cnhnb.com/xt/article-1274.html), peach (
https://www.sohu.com/picture/397690934), and litchi (
https://v.youku.com/v_show/id_XMjgzNjI1NDY1Ng==.html?).
It is well known that damage from cracking causes huge yield losses in several fruit crops worldwide, while the underlying mechanisms of fruit cracking are comprehensive and quite complex. Researchers have reported that the high occurrence of fruit cracking can be influenced by several factors, such as genetic characteristics, stressful external environmental condition, orchard management, fruit growth rate, postharvest storage factors, physiological, biochemical, anatomical, and plant hormones (Khadivi-Khub, 2015; Rehman et al., 2015; Knoche & Lang, 2017; Correia et al., 2018). Noteworthily, under the same environmental conditions (light, temperature, wind, rainfall, etc), orchard management (nutrition, irrigation, sun-shade, mineral, growth regulator, etc), different fruits cultivars show differences in cracking susceptibility. There are some relevant influence factors have been observed between susceptibility of fruit cracking and some fruit traits, including size, shape, hardness, growth rate, water content, peel characteristics (anatomy and strength of the fruit skin, stomata in fruit skin, cuticular properties, osmotic concentration, cuticle) of the fruits (Knoche & Peschel, 2006; Brüggenwirth and Knoche, 2017; Giné-Bordonaba et al., 2017; Seo et al., 2022), water capacity of the fruit pulp, and the genes expression related to growth stage of the fruits. Besides, the regulation of phytohormone and plant growth regulator (PGRs) are very vital factors of fruit cracking, due to the growth and development of fruits can not be separated from the regulation of phytohormone. Homeostasis of endogenous phytohormone plays an vital role in the normal growth of the pericarp, and its imbalance may cause fruit cracking (Rai et al., 2002; Yilmaz & Ozguven, 2006; Marboh et al., 2017; Singh et al., 2020). PGRs have similar functions to the endogenous phytohormone are well known, and they can control plant growth and development, and also have a certain effect on fruit cracking. Although foliar sprays with PGRs could be an important orchard management, little is known on the effectiveness or the response of fruits to PGRs, and molecular details and physiological roles of each of them in mediating fruits cracking are arevagued.
Generally speaking, previous studies indicated that fruit cracking or splitting is a quantitative trait and is closely related to the content of endogenous phytohormone and the exogenous PGRs treatments, and also medicated by a large amount of genes that involved in phytohormone metabolic pathway (Yilmaz & Ozguven, 2006; Cao et al., 2014; de Freitas et al., 2011; Knoche & Lang, 2017; Wang et al., 2019a,b; 2021b; Hou et al., 2022). Remarkably, during the past decades, functions of phytohormone in fruit cracking determinant have been unraveled mainly through investigating the effect of endogenous phytohormone (ABA, GA, Auxin, cytokinin, etc) content in the pulp or pericarp of fruits and exogenous PGRs treatment, and commonly used PGRs are GA3, GA4+7, ABA, brassinolide (BRs), naphthalene acetic acid (NAA), JA (jasmonic acid), cytokinin (CTK), and so on. Among them, the growth-promoting plant hormone GA and growth-inhibiting plant hormones ABA antagonistically regulate numerous developmental processes and also play pivotal roles in modulating fruit cracking during the process of fruit growth, development, ripening and postharvest storage. Previous studies have shown that normal fruits contains higher level of GA and lower level of ABA, and an imbalance between the two phytohormone of GA and ABA in fruit peels can lead to cracking in different fruit crops (Sharma and Dhillon, 2002; Li et al., 2014; Yilmaz and Ozguven, 2006). Here, progress in studies on ABA and GA that regulate fruit cracking is reviewed to provide a reference for prevention and control of fruit cracking. As such, understanding the underlying function mechanism of antagonistic ABA and GA medicating fruit cracking would better for governing cracking or splitting of different fruit crops properly.
2. Progress in Biological Function of GA and ABA
2.1. Biological Function of GA
GA are diterpenoid phytohormone that have multiple biological functions act throughout the life cycle of plants. As a growth-promoting plant hormone, GA is crucial for many aspects of plant growth and development, including influencing seed germination, stem elongation, flower induction, anther development, and seed and pericarp growth, especially in promoting cell elongation. Meanwhile, GA can also promote plant root growth, preserve flower and fruit, promote peel development, increase fruit weight and increase yield. These effects also give GA the effect of reducing fruit cracking rate, the specific mechanism is mainly: delays fruit maturity; increases peel elasticity; increases the deposition of stratum corneum components to increase the elasticity of stratum corneum; decreases the activity of PME (Pectin methylesterase) and PG (Polygalacturonase) to delay the softening of fruit, increases the plasticity of cell wall and maintains the hardness of fruit (Demirsoy et al., 2000; Byers et al., 1990; Andrews et al., 1995; Usenik et al., 2005; Sekse et al., 2005). Notably, it is well known that GA is widely used in many horticultural crops to improve fruit set, increase fruit size and firmness (Proebsting et al., 1973; Facteau et al., 1985; Choi et al., 2002; Kappel & MacDonald 2002; Clayton et al., 2006), and make pomegranate (Sepahi, 1986; Hoda and Khalil, 2013), litchi (Sharma & Dhillon, 1986) and sweet cherry (Cline & Trought, 2007) fruit more resistant to cracking. And some studies have suggested that the foliar spraying of the PGRs that function similar to active GA could help to improve quality and reduce cracking, and application of exogenous GA can reduce fruit cracking (Munish et al., 2003) because of the increased GA levels in fruits. But so far, to our knowledge, evidence that GA can decrease cracking or splitting of fruit is contradictory. Altogether, it is important to further determine whether GA can enhance fruit crack resistance well.
2.2. Biological Function of ABA
ABA is a major growth-inhibiting phytohormone with sesquiterpene structure, which is common and widely existing in many plant species, regulating a broad range of plant traits and is especially singificant for adaptation to adverse environmental conditions. Studies have shown that ABA plays important roles in regulating various processes of the plant growth and development, and it can significantly inhibit the growth and development of most plant crops through promoting the rapid senescence and fall off of flowers, leaves, fruits and other organs of most plants, and ABA can also stop the growth of seeds and induce the seeds quickly enter a dormant state especially. It is well known that ABA involved in fruit cuticle integrity, controls the response of plants to environmental stress, and also plays significant roles in the process of fruit adaptation to abiotic stresses and the fruit cracking control. Changed levels of ABA have been related to cracking or splitting in many fruit crops. For example, the accumulation of ABA in aril is necessary for the growth and development of the aril, but a higher content of ABA could induce fruit cracking in litchi (Marboh et al., 2017). Besides, several researches have reported that ABA affects fruit development probably by closing leaf stomata, reducing water loss, and promoting water inflow into the fruit thereby increasing internal fruit pressure, thus, resulting in an increased cracking rate of tomato fruit (de Freitas et al., 2011; Jiang et al., 2019)。
3. Progress of GA and ABA in Fruit Cracking Control
3.1. GA in Fruit Cracking Control
3.1.1. Effect of Endogenous GA Content on Fruit Cracking
Changed levels of endogenous GA have long been associated with fruit cracking in many plant species (Cao et al., 2014; Suran et al., 2016; Burhan et al., 2018; Joshi et al., 2018). As early as 1986, Sharma and Dhillon investigated the relationship between the endogenous GA level and cracking of litchi fruit, and observed that GA content in the seed and pericarp of litchi is higher in cracked fruits than in non-cracked ‘Dehradun’ litchi. Then, through comparing the content difference of endogenous phytohormones in cracked and uncracked jujube fruits, Cao et al. (2014) found that the content of GA3 in the exocarp of the CS cultivars of jujube were significantly higher than those of the CR jujube varieties. Analogously, the research of Wang et al. (2020c) in jujube cracking further reported that the GA3 content was significantly higher in the exocarp of the cracked parts than the uncracked parts of CS cultivar ‘Fucuimi’ jujube. Based on these findings, it is speculated that there might be a positive relationship between fruit cracking and the GA level. Inversely, Yang et al. (2009) showed that the endogenous GAs level in cracked fruit was significantly lower than that in normal jujube fruit. In addition, it was also observed that the fruit cracking index was negatively correlated with the concentration of endogenous GA in the researches of jujube cracking (Burhan et al., 2018). Nevertheless, Yilmaz and Ozguven (2006) estimated the contents of exogenous phytohormone in the fruit peel of pomegranate cultivars, while no significant differences were detected between the content of GA3 in cracked and uncracked pomegranate fruit peels. Taken altogether, these above results suggested that the content of endogenous GA in the fruits is closely related to cracking or splitting of several fruit species, but the effect of endogenic GAs on fruit cracking might exist some differences in different species or among different cultivars of the same species, while influence differences of GA among fruits cultivars have not been identified well, which will need further study.
3.1.2. Effect of Exogenous GA Treatment on Fruit Cracking
Many researchers investigating the roles of phytohormone GA in cracking or splitting of fruit by using exogenous GA analogs treatments. Remarkably, exogenous PGRs, especially exogenous GA
3 is the most widely used exogenous PGRs in agriculture, forestry and horticulture, and medicates fruit cracking in several fruit crops (
Table 1). For instance, it has been reported that exogenous GA
3 can reduce the cracking rate of litchi (Srivastava et al., 1969), and the ratio of cracked ‘Dehradun’ litchi was effectively reduced by spraying with GA
3 compared with those of the control (Sharma et al., 1984; Munish et al., 2003). Notably, application of GA
3 (20 mg /L) had no significant effect on cracking, while treated with a mixture of GA
3 (20 mg /L) and 2,4-D (20 mg /L) could significantly reduce fruit cracking of ‘Nova’ mandarin (Almela et al., 1994; Agusti et al., 2002). Josan et al. (1998) reported that GA
3 treatments could increase the contents of IAA and GAs, but reduced the ABA levels in the peel and pulp of ‘Baramasi’ lemon fruits, and reduced the ratio of cracked fruit compared to the control. And Maotani et al. (1990) reported that GA tape contain contains 6% GA (GA
3:GA
4=9:1) treatment could reduce fruit cracking of the ‘Kosui’ and ‘Niitaka’ Japanese pear fruit.
In recent years, exogenous GA
3 have consistently been used for controlling fruit cracking in various horticultural crops, and studies have shown that exogenous GAs treatment was available for reducing the fruit cracking rate of several fruit crops (
Table 1). For example, in the researches of cracking control of jujube fruits, it was observed that exogenous GA
3 (15 mg/L) treatment at 3 and 2 weeks before the commercial harvest date, lowered the fruit cracking rate of the jujube cultivar ‘Lizao’ (Burhan et al., 2018). It is also reported that cracking of ‘Fucuimi’ fruit that spraying treatment with the GA
3 (15 mg/L) also lowered compared to the control (Song, 2022). Similarly, exogenous GA
3 treatment decreased the rain-induced cracking rate of ‘Justyna’, ‘Tamara’ and ‘Regina’ cherry varieties by 9-11% (Suran et al., 2016). Spraying the mixture of GA
3 (40 ppm), calcium hydroxide, zinc sulfate and boron (50 ppm) minimized the incidence of cracking on pomegranate young fruits (Yilmaz & Ozguven, 2006). Meanwhile, GA
3 treatment also caused reduction in splitting of pomegranate varieties ‘Hicaz’, ‘Silifke Aşısı’ (Yilmaz & Ozguven, 2009), ‘Manfalouty’ (Hoda and Khalil, 2013). More recently, a further 2-year study of pomegranate fruit cracking control, has also uncovered that foliar spraying with exogenous GA
3 (75 or 150 mg /L) in July could substantially decrease the cracking rate (Drogoudi & Pantelidis, 2022). Multiple treatment of ‘Pink Lady’ apple fruit with GA
4+7 and BA at early phenological stages(50-65 DAFB, Days after full-blossom), results in increased epidermal cell density and reduced calyx-end cracking disorder until fruit mature and harvest (210 DAFB), implying exogenous PGRs have a long-term effect of the treated plants (Joshi et al., 2018; Ginzberg & Stern, 2019). On the contrary, GA
3 sprays increased cracking of cherry (Proebsting et al. 1973; Rasmussen and Grauslund 1983), Cline and Trought (2007) also demonstrated that pre-harvest single or repeated foliar applications of GA
3 (10 or 40 ppm) in CR variety ‘Binga’ and CS variety ‘Sam’, increased both fruit firmness and fruit cracking in sweet cherry. While, several studies showed thatsingle or multiple GA
3 treatment on the cultivars ‘Merton Premier’, ‘Bing’, ‘Dawson’ and ‘Sweetheart’ had no influence on cracking of sweet cherry (Facteau, 1984; Horvitz et al., 2003). Interestingly, Agusti et al. (2002) found that GA
3 influences fruit cracking might be based on the application time, application of GA
3 at flowering increased cracking of citrus fruits, but decreased fruit cracking shortly after the end of the June drop. Given the above findings, it is suggested that the exogenous GA applying is closely related to cracking or splitting control, and likely contributes to restrain cracking of several fruit specie effectively, while the effect of exogenous GA on fruit cracking also might be related to species, variety of fruit and the application time of exogenous GA.
3.1.3. GA Metabolism Pathway Genes in Fruit Cracking Control
It is well known that both the endogenous GA level and exogenous GA treatment play important roles in fruit cracking control. Hadjipieri et al. (2021) showed that exogenous hormones can change the morphology of the epidermis and cuticle, which can be used to improve the physical properties of the exocarp and thus reduce the occurrence of fruit cracking, control of fruit quality overall. And applications of exogenous GA3 decreased cracking of fruits likely because this treatment could increase the deposition of cuticular material in the epidermis and makes it more elastic (Knoche & Peschel, 2007). In recent years, to further clarify the molecular mechanism of GA regulation on fruit cracking, the genes related to fruit cracking and involved in GA metabolism pathway were successfully investigated.
Previous studies have studied the metabolism and signaling pathways of GA widely. In higher plants, GA biosynthesis is catalyzed by 6 key enzymes, including ent-copalyl diphosphate synthase (CPS), ent-kaurene synthase (KS), ent-kaurene oxidase (KO), ent-kaurenoic acid oxidase (KAO), GA20-oxidase (GA20ox), GA3-oxidase (GA3ox); the deactivation of active GAs is catalyzed by GA2-oxidase (GA2ox) (Yamaguchi, 2008); and the GA signaling pathway contains three crucial components, including the GA receptor 1 (GID1, GA INSENSITIVE DWARF1), the F-box protein (GID2/SLY1), as well as the DELLA protein, a repressor of GA signaling (Sun, 2011). The changes of gene expression level of GA pathway are directly affecting the active GA content and its biological function. For example, previous studies have shown that GA2ox negatively regulates the GA levels, and upregulated either GA2ox7 or GA2ox8 can reduce GA levels (Schomburg et al., 2003). The GA levels is positively correlated with the expression of GID1 gene, and over-expressing of GID1 could rescue the dwarf phenotype of mutants sly1 and gid2 through altering GA levels (Tohru et al., 2008).
To identify candidate genes and further investigate the molecular mechanism of GA controlled fruit cracking, high-throughput RNA sequencing (RNA-Seq) was first used for
de novo assembly and characterization of the transcriptome of cracked pericarp of litchi, the transcriptional levels of genes involved in GA metabolic and signaling pathways were analyzed (Li et al., 2014). Furthermore, it was found that five genes involved in GA pathway, including two GA biosynthesis genes (
LcKSs), two GA deactivation genes (
LcGA2oxs), and one GA receptor gene (
LcGID1) were differentially expressed genes (DEGs) in cracked fruits compared to uncracked litchi fruits (
Table 2). Notably, of these DEGs, two
LcKS genes, encodes the enzyme responsible for catalysis of the second step in the GA biosynthesis pathway, and one
LcGID1 were monitored and found to be downregulated more than 2-fold in peels of cracked fruits compared to the normal litchi fruits (Li et al., 2014). Conversely, two
LcGA2ox genes, responsible for deactivation of active GAs through 2
β-hydroxylation, were upregulated more than 2-fold in peels of cracked fruits than that in normal litchi fruits (Li et al., 2014). This further implied that endogenous GA were highly accumulated in normal litchi fruits. Moreover, two GA-regulated protein gene (
GPRs,
Lc.1.532 and
Lc.1.534) (
Table 2) related to GA were found to be downregulated in the pericarp of CS cultivar ‘Nuomici’ litchi, leading to differences in fruit development and pericarp mechanical strength. Meanwhile one GA receptor gene
LcGID1c (
Lc.8.678) (
Table 2) was also downregulated in aril of ‘Nuomici’ (Wang et al., 2021b). During the fruit development process of apple, the
MdGID1b (
Table 2), which mediates GA perception in fruit ovules by interacting with DELLAs, is markedly upregulated, and
MdGID1b is also significantly upregulated in the skin of mature apple fruits treated with the GA
4+7 and BA compared with the control, and cause increased epidermal cell density and prevent the cracking initiation of apple fruits (Joshi et al., 2018). Further, it was found that the expression of
MdGID1b was also significantly upregulated in the peel of mature fruit following the GA
4+7 and BA treatment (Joshi et al., 2018). In jujube, Hou et al.(2022) found GA biosynthesis gene
ZjGA20ox1 (
gene19292) and GA deactivation (
ZjGA2ox,
gene243) were gradually downregulated in the the cracked and uncracked fruit samples of CS variety ‘Jinsixiaozao’ and non-cracking CS variety ‘Muzao’. Taken altogether, these above results suggested that the changes in transcript level of genes involved in GA pathways were closely related to the fruit cracking, especially for
KS,
GA20ox,
GA2ox,
GID1 and
GPRs, are likely the key GA pathway-related genes controlling fruit cracking, but the underlying molecular mechanisms of these genes regulating cracking need to be further studied.
3.2. Abscisic Acid in Fruit Cracking Control
3.2.1. Effect of Endogenous ABA Content on Fruit Cracking
Previous studies have shown that the content of endogenous ABA is closely related to cracking in various fruits, and the higher ABA levels in pericarp is easy to induce fruit cracking or splitting. For example, Sharma and Dhillon (1998) reported that the ABA contents in the aril and peel of cracked fruit was higher than that of uncracked litchi fruits. The ABA content and fruit cracking ratio was higher in control fruits in lemon, compare to the GA3 and NAA treatments (Josan et al., 1998). The endogenous ABA concentration in the peels of cracked fruits was generally higher than that in the peels of uncracked pomegranate fruits (Yilmaz & Ozguven., 2006). In litchi, a balance among pericarp strength and aril expanding pressure can be responsible by litchi cracking, which can occur due an unbalance of plant hormone metabolisms, and the content of ABA in the CS variety ‘Baitangying’ is higher compared with the CR variety ‘Feizixiao’ pericarps (Wang et al., 2019b; Wang et al., 2021b). Marboh et al. (2017) observed that the accumulation of ABA in aril is necessary for the growth and development of the aril, but a higher content of ABA can induce litchi fruit cracking. Moreover, fruit cracking or splitting in ‘Muzafarpur’ litchi is directly and closely correlated with higher ABA levels in the pericarps of the fruits (Khadivi-Khub, 2015). Romero & Lafuente. (2020) reported that ABA deficiency altered the metabolism and morphology of the waxy layer, leading to increased permeability of the cuticle during the sweet orange fruit ripening, which might induce the occurence of fruit cracking. Meanwhile, in the study of ABA content in fruit cracking control of jujube, it was found that the high concentration of ABA promotes fruit senescence and accelerates the softening of pericarp tissue during the growth and ripening process of jujube fruit, leading to the occurrence of fruit cracking, and the split index of jujube was positively correlated with the level of endogenous ABA. Yang et al. (2009) illustrated that the endogenous ABA level in cracked jujube fruit was significantly higher than that in the corresponding part of normal fruit by comparing the difference of endogenous hormones in cracked and uncracked jujube fruits. During the high cracking period of jujube, it was shown that the amount of ABA content in the exocarp of a CS cultivar was higher than that of a CR cultivar (Cao et al., 2014). And then, Wang et al. (2020) revealed that the content of endogenous ABA was high in the mesocarp of the cracked parts of jujube cultivar ‘Fucuimi’. Recently, Liu et al. (2023) reported that the amount of endogenous ABA in the exocarp of CS jujube was remarkably higher than that in CR jujube individuals, and the ABA content in the exocarp was significantly higher than that in the mesocarp throughout the fruit development of jujube. the ABA content in the exocarp was significantly higher than that in the mesocarp throughout fruit development. Based on these studies, it is suggested that fruit cracking may be positively correlated with the content of endogenous ABA, which is consistent with the findings on the relationship between ABA and fruit cracking in litchi and pomegranate. Therefore, it is reasonable to speculate that ABA is one of the key factors that contributes to the occurrence of fruit cracking or splitting. On the contrary, in the study of grape, it was reported that the decrease of endogenous ABA content could reduced the activity of the PME and PG enzymes, and delay the degradation of pectin, increase the amount of exocarp protopectin, enhance the mechanical properties of exocarp, and thus improve fruit crack resistance of grape berry (Yu et al., 2020). In conclusion, all of these results indicated that the content of endogenous phytohormone ABA in the fruits is closely related to cracking or splitting of many fruit species, while the effect of endogenous ABA on fruit cracking might be differences in different fruit species.
3.2.2. Effect of Exogenous ABA Treatment on Fruit Cracking
Recently, to investigate the roles of phytohormone in fruit cracking, the exogenous plant growth regulators were usually carried out in fruit cracking control. Effect of exogenous hormone ABA treatment on fruit cracking has also been extensively and successfully studied and verified, and it is observed that exogenous ABA treatment inducing cracking or splitting in several fruit species. Li et al. (1992) reported that application of exogenous ABA through spraying treatment could increase the expansion rate and fruit cracking rate of litchi. Treating plants with ABA increases water movement into the fruits and promotes enlargement of the fruits, and ABA treatment also increases the tendency of fruit to crack. For example, studies have shown that ABA treatment could increase the rate of fruit cracking in jujube (Li et al., 2016) and tomato fruits (de Freitas et al., 2011; Jiang et al., 2019). The research of Gutiérrez et al. (2021) indicated that exogenous ABA treatment before the fruits were harvested could increase the content of cell wall and cuticle wax components at maturity and improves the crack resistance of sweet cherries, meaning that ABA induce fruit cracking might mainly by regulating the cell wall metabolic pathway. It has also been shown that spraying treatment with the ABA (50 mg/L) had the best anti-cracking effect on ‘Fucuimi’, which decreased cracking by 39% than that of the control(Song, 2022), and the fruit cracking index of ‘Pingshunbenzao’ jujube that treated with exogenous ABA solution (50 mg/L) at the white-ripening stage was significantly increased, compared to the control that treated with sterile ultrapure water (Liu et al., 2023), indicating that the hormones ABA have a certain regulatory effect on the fruit cracking of jujube (Liu et al., 2023). At the same time, ABA was induced after water absorption in sweet cherry fruits, and the expression level of genes related to ethylene synthesis was increased, which was because ABA stimulated the production of ethylene in sweet cherry (Ren et al., 2011), and then the cell wall was degraded under the action of ethylene, and finally the fruit was cracked (Giné-Bordonaba et al., 2017). And take it altogether, the obove findings suggest treating fruits with growth-regulating agents to manipulate the growth cycle and reduce susceptibility to growth-induced cracking, and the exogenous ABA can play either a dominant or supportive role in modulating cracking or splitting in development of several fruit species.
3.2.3. ABA Metabolism Pathway Genes in Fruit Cracking Control
At different stages of fruit cracking, the contents of different form of ABA in fruit were different, which is related to the biosynthesis, metabolism, transport, and regulation of ABA. Many studies have reported that changes in endogenous ABA levels and exogenous PGRs ABA treatment can play either a dominant or supportive role in modulating fruit ripening and fruit cracking, and influences fruit quality traits during fruit growth and development (Li et al.,1992; de Freitas et al., 2011; Kou et al., 2021; Liu et al., 2023; Gutiérrez et al., 2021), however, compared with the mechanism of ABA controlling fruit ripening, our understanding of the underlying molecular cues of ABA medicating fruit cracking is still at the beginning stage. Studies have shown that ABA may induce fruit cracking mainly through regulating cell wall metabolism, and then affect fruit yield, quality and economic benefits. Thus, elucidating the molecular mechanism of ABA-mediated fruit cracking has considerable potential to improve our understanding of both fruit cracking during ripening and to develop new fruit traits and varieties, especially the cracking resistant fruit varieties.
Comprehensive knowledge of the genes involved in ABA biosynthesis, metabolism, transport, signal transduction pathways, and regulation of ABA revealed that ABA is synthesized in the plastid and cytoplasm and is derived from zeaxanthin (a plant pigment) (Nambara & Marion-Pol, 2005), and some enzymes, such as ZEP (Zeaxanthin epoxidase), NCED (9-cis-epoxycarotenoid dioxygenase) and AO (aldehyde oxidase) may play key roles in regulating ABA biosynthesis in higher plants. Among them, ZEP can produce the precursor of ABA biosynthesis via catalyzing carotenoids, and convert zeaxanthin to violaxanthin (Seo et al., 2002; Schwartz et al., 2003). While, the deactivation of active ABA is catalyzed by a key enzymes: ABA-8’-hydroxylase, encoded by CYP707A gene (a member of the cytochrome P450 gene family) (Okamoto et al., 2006), and ABA can be reversibly inactivated by glucosylation. ABA-glucosylester (ABA-GE) is a physiologically inactive storage and transfer form, and ABA is conjugated with glucose by ABA glycosyltransferase (GT), forming ABA-GE (Xu et al., 2002), while β-glucosidase (β-Glu) can convert ABA-GE to ABA (Schroeder & Nambara, 2006; Kushiro et al., 2004). In the ABA signaling pathway, ABI3 (ABA INSENSITIVE 3) is a central regulator in ABA signaling and is unstable in vivo, and it interacts with and can by polyubiquitinated by AIP2 in vivo; and there are three major components, including ABA receptor protein (PYR/PYL/RCAR), ABA signaling negative regulator PP2C (a type 2C protein phosphatase, including ABI1/PP2C), is a negative regulator of ABA signaling pathway (Irigoyen et al., 2014; Rodriguez et al.,2014). ABI5/DPBF1 belong to AREB/ABF/bZIP (ABF) subfamily genes, is a transcription activator of ABRE-dependent ABA signaling (Gosti et al., 1999; Finkelstein et al., 2000; Fujita et al., 2005).
To better elucidate the molecular mechanism of ABA regulation of fruit cracking, many researchers in related fields began to screen and study the genes related to ABA metabolism pathway that may be involved in the regulation of fruit cracking. For example, high-throughput RNA-Seq was first used for
de novo assembly and characterization of the comparative transcriptomic analyses of uncracked and cracked pericarp of litchi (Li et al., 2014). Amony of the DEGs, there are 21 genes that involved in ABA metabolism in the pericarp of cracked and uncracked litchi fruits, including two
LcCYP707A, nine
LcGT, six
Lcβ-Glu, two
LcPP2C, one
LcABI1, one
LcABI5) (
Table 2). And among them, two
LcCYP707A genes were found to be upregulated 2-fold and 5-fold in uncracked litchi compared to cracked fruits, respectively. Kushiro et al. (2004) found that increase of
CYP707A2 expression level results in decrease of ABA content, and the ABA content was 6-fold higher in
cyp707a2 mutants than wild type plants. Two
LcCYP707A genes were found to be downregulated in cracked litchi fruits, suggesting that ABA may accumulate in cracked litchi fruit. ABA is conjugated with glucose by GT, forming the physiologically inactive ABA-GE;
AtBG1(a
β-Glu) contributes to increase ABA level, and loss of
AtBG1 causes decreased ABA content; and PP2C acts as a negative regulator of ABA signaling (Xu et al., 2002), Li et al. (2014) found that nine
LcGT genes were upregulated between 2-fold and 17-fold, and six
Lcβ-Glu genes were downregulated in between 2-fold and 12-fold uncracked fruits compared to the cracked fruits of litchi, implying that the ABA content is lower in uncracked litchi fruit. Two
LcPP2C and one
LcABI1 genes were upregulated 2-fold to 9-fold, and one
LcABI5 gene was downregulated at least 2-fold in uncracked fruits compared to cracked litchi fruits (
Table 2), which indicated that cracking of litchi is related to the genes involved in ABA signaling. Besides, based on the expression levels of
LcCYP707A and
LcPP2C in the three groups of samples, it was speculated that the ABA contents were higher in the cracked CS variety ‘Baitangying’ sample compared with the CR variety ‘Feizixiao’ pericarps (Wang et al., 2019a). In summary, upregulated expression of
Lcβ-Glu and
LcABI5, and downregulated of
LcCYP707A,
LcGT,
LcPP2C and
LcABI1 genes in pericarp, which could then lead to higher ABA level in pericarp and eventually to fruit cracking in litchi.
Recent years, it was found that changes in genes expression and metabolites can explain the cracking susceptibility (
Table 2), for example, an upregulation of
LcCYP707A (
c42183_g1_i1) gene involved in ABA metabolisms was found in CR litichi cultivar ‘Feizixiao’ (Wang et al., 2019b). Besides, observed that one
LcZEP (
Lc.0.938), and genes encoding 1 GT family family protein(
LcGT,
Lc.12.1389), 4 β-Glu (
Lc.0.11,
Lc.0.3431,
Lc.0.157,
Lc.0.3975), and 1 GSK (
Lc.0.1659) involved in ABA siginaling was higher expressed in CS cultivar ‘Nuomici’ than that in CR cultivar ‘Huaizhi’ aril, implying that the increased of ABA content and these osmotic regulation substances might be higher in the ‘Nuomici’ aril than that in the CR cultivar ‘Huaizhi’ aril (Wang et al., 2021b), and then inducing cracking of litichi. In the study of Hou et al. (2022), the cracked and uncracked fruits of CS cultivars ‘Cuizaohong’ and ‘Jinsixiaozao’, and uncracked fruits of the CR cultivar ‘Muzao’ were selected for comparative transcriptome analyses, the expression levels of two ABA related genes (
Table 2), 1
ZjNCED1 (
gene30271) and 1 Zj
RBOHPD (
gene16443), were upregulated in the cracked samples of ‘Cuizaohong’ and ‘Jinsixiaozao’ compared to the cracked samples, respectively. And five ABA metabolism genes, including 2
ZjNCED1 (
gene30271 and
gene1854), 1
ZjZEP (
gene18925), 1
ZjPP2C (
gene7093) and 1
ZjSAPK1 (
gene17084) (
Table 2), were gradually downregulated in the the cracked compared with uncracked fruit samples of ‘Jinsixiaozao’ and non-cracking ‘Muzao’. Beside, it was found that
NAC058 (
Table 2), a gene involved in ABA signaling, is upregulated during apple fruit development, and may prevent the cracking initiation (Joshi et al., 2018). In sweet cherry, the transcriptional level of genes related to ABA metabolism or signaling, such as,
PaPYL1/4/8/9/12,
PaABA1,
PaABI1.2,
PaAREB3,
PaHVA22 (
Table 2) were downregulated in the skin and flesh of CR cultivar ‘Regina’ compared to the CS cultivar ‘Early Bigi’ (Michailidis et al., 2020), while
PaAAO3,
PaABAH1,
PaABI1.1/1.3/1.4/1.5/5,
PaABF2/3,
PaABO5,
PaFCA,
PaCALB4/7 and
PaABA4 (
Table 2), were upregulated in the skin and flesh of CR variety ‘Regina’ compared to the CS variety ‘Early Bigi’ (Michailidis et al., 2020). Thus, the above genes may be the significant ABA metabolism and signaling pathway genes that involved in mediating fruit cracking, and the roles of the genes regulated or related to ABA need to be further studied and considered while developing cracking-resistant fruit varieties through breeding programs.
4. Prospects
Several fleshy fruits species are highly affected by fruit cracking or splitting, a common and severe physiological disorder that severely compromises the fruit appearance and diminishes fruit quality, shelf life, and market value, and enhances susceptibility to microbial infections, and as well as causes immense economical losses to the fruit producers. Cracking or splitting can not be attributed to a single influencing factor, and it is difficult to study fruit cracking in vitro under controlled conditions due to lack of the experimental methods to induce cracking. Although fruit cracking has been investigated for nearly 100 years, and there are many researches have been carried out to investigate the possible reasons and control methods of cracking in numerous fruit crops, the detailed physiology and mechanisms of this phenomena are poorly understood, and it remains a huge challenge to the fruit producers and researchers in related fields, and this extremely challenging research difficulty has become a research hotspot for scholars at home and abroad, attracting more and more people to engage in the study of fruit cracking.
It is well known that fruit cracking is the result of the split of the fruit peel surface and the outer flesh around the calyx during cell enlargement (Choi et al., 2020), which is a very complex physiological disorder process, and closely associated with the homeostasis of endogenous phytohormone. In recent years, advancements in several fleshy fruit genome research have promoted the emergence and development of the functional genomics, and pose significant opportunities and challenges to the explore, analyze and clarify the underlying genetic mechanisms governing fruit cracking. And with the help of multi-omics-based biological research technologies, including genetics, epigenetics, transcriptomics, proteomics, metabolomics, molecular biology, physiological and biochemical methods, we have witnessed remarkable progress on fruit cracking, and opened news perspectives to understand how this physiological disorder occurs at molecular level. And the genetic component has been discovered and makes fruit cracking an attractive field for researchers who work with molecular breeding of fruit crops, and breeders have incorporated the molecules into practical breeding strategies, including gene editing, transgenic approaches, and progressive breeding, which holds the potential for achieving molecular-design breeding and efficient genetic enhancement of fruit crops, and developed crack-resistant cultivars in many fruit species, while only minor advances have been made in understanding physiology and molecular mechanisms of fruit splitting and cracking in any of these fruit crops. In turn, this has made it difficult to recommend reasonable and effective preventive measures for fruit cracking.
It has been preliminarily demonstrated that the growth-promoting plant hormone GA and growth-inhibiting plant hormone ABA play important regulatory roles in governing fruit cracking. And fruit cracking is directly correlated with the higher levels of GA and ABA in the fruits (Menzel, 1984). During the process of fruit cracking, the expression levels of many genes is precisely regulated by endogenous phytohormone GA and ABA to cope with the transition of fruit development and cracking state and the change of environmental conditions (Li et al., 2014; Lin et al., 2015; Joshi et al., 2018; Wang et al., 2021a). And recently publications have suggested GA and ABA related and regulated genes specific transcripts play a crucial role in cracking development, namely GA pathway related genes, including KS, GA2ox, GA20ox, GID1, GPR, and ABA pathway related genes, including ABI , ABF , ABO5, FCA, ABI1, ABI5, b-Glu, GT, CYP707A, PP2C, ZEP, NAC058, and so on (Li et al., 2014; Wang et al., 2021b; Wang et al., 2019a,b; Joshi et al., 2018; Michailidis et al., 2020; Hou et al., 2022), they may be the major candidate genes that regulate fruit cracking, and they can be further applied in molecular breeding efforts to produce improved crack-resistance that meet the demands of modern a fruit crops production.
ABA is a stress responsive phytohormone that inhibits seed germination and seedling growth to adapt to unfavourable environmental conditions while GA is a major growth promoting phytohormone that promotes seed germination, seedling growth, flowering and leaf expansion (Cutler et al., 2010; Weiner et al., 2010; Golldack et al., 2013). In previous studies, it has reported that GA and ABA have antagonistic effects on many aspects of regulating plant growth and development processes, and responses to biotic or abiotic stresses in higher plants, especially seed dormancy or germination, which mainly depends on the balance of the two hormone signals of GA and ABA (Brady & Mccourt, 2003). Previous studies have reported that the main components of ABA and GA signaling pathway and have shown that DELLA proteins represent a regulatory hub that mediates the repression of ABA on GA signaling in plants (Vanstraelen & Benkova, et al., 2012; Nonogaki et al., 2014; Piskurewicz et al., 2008; Gubleret al., 2002; Oh et al., 2007). Besides, there are studies observed that ABA can antagonize GA-promoted degradation of DELLA proteins (Achard et al., 2006), and the results presented in the study of Lin et al. (2015) suggested that the SnRK2s-APC/CTE regulatory module represents a new signaling hub mediating the antagonistic action of GA on ABA signaling in higher plants. GA can reduce ABA signaling by promoting the interaction between TE and OsPYL/RCARs by reducing SnRK2 activity, and causing subsequent proteasomal degradation of OsPYL/RCARs. When the environmental conditions became more favourable, the GA pathway may be activated to promote the degradation of ABA core signaling components (ABA receptors and SnRK2s) and DELLA proteins (Ueguchi-Tanaka et al., 2005), which would allow plants to resume normal growth and development. Antagonistic action of GA and ABA thus may serve as a ‘rheostat’ to fine tune plant growth and development in response to the fluctuating environments. Considering these facts, it is notable that the employment of multiple E3 ligases for proteasomal degradation of the ABA receptor proteins possibly enables the plants to more effectively respond to different developmental or external signals and adds additional complexity of ABA signaling regulation in higher plants. Thus, identification and functional studies of other unknown E3 ligases will lead to a better understanding of GA and ABA signaling mechanism and the crosstalk with other signaling pathways in regulating fruit cracking.
With the completion of several fruit genome sequencing and the identification of more GA and ABA mutants, there are more and more studies on the key genes of ABA and GA regulating fruit cracking. Even so, the molecular mechanisms involved in cracking are based on correlations as direct proof of concept based on mutations or reverse genetics are still missing, and the regulatory mechanism underlying the antagonism of GA and ABA signaling pathway in fruit cracking control remains largely unknown. It is still unclear whether GA and ABA have antagonistic effects on the regulation of fruit cracking? And how do phytohormone GA and ABA control fruit cracking through regulatory networks? These details further need more genetic analysis and molecular identification to clarify. Yeast one-hybrid (Y1H) and yeast two-hybrid (Y2H) techniques were used to isolate and identify the transcription factors that combine GA response element (GARE) and ABA response element (ABRE) in the signaling pathway of ABA - and GA-regulated plant growth and development, and and then study their functions and interactions, which can promote the study progress of fruit cracking mechanism, and provide new ideas and research methods for fruit cracking resistance breeding.
Author Contributions
The authors confirm contribution to the paper as follows: collection and organization of references, writing this manuscript: M.Z., Y.L., Z.C., Z.Z., A.W. and G.Z.; structure design and manuscript modification and editing of this manuscript: G.Z., S.Z., F.L., and H.Y. All authors reviewed and approved the final version of the manuscript.
Data Availability Statement
All data generated in this study are included in the previous published article.
Acknowledgments
This work is supported by the Fundamental Research Funds for the Central Non-profit Research Institution of CAF (CAFYBB2023MB032).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Achard, P.; Cheng, H.; De Grauwe, L.; Decat, J.; Schoutteten, H.; Moritz, T.; Van Der Straeten, D.; Peng, J.; Harberd, N.P. Integration of Plant Responses to Environmentally Activated Phytohormonal Signals. Science 2006, 311, 91–94. [CrossRef]
- Agusti M, Martinez-Fuentes A, Mesejo C. (2002) Citrus fruit quality. Physiological basis and techniques of improvement. Agrocienica 6:1-16.
- Almela V, Zaragoza S, Primomillo E, Agusti M. (1994) Hormonal control of splitting of Nova mandarin fruit. J Hortic Sci 69:969-973.
- Andrews PK, Collier ML, Fahy D, Evans RB. (1999) Gala stem-end splitting and internal ring cracking. Good Fruit Grow 50:20-23.
- Andrews, P.K.; Li, S. Cell wall hydrolytic enzyme activity during development of nonclimacteric sweet cherry (Prunus aviumL.) fruit. J. Hortic. Sci. 1995, 70, 561–567. [CrossRef]
- Balbontín, Cristian,Gutiérrez, Camilo, Wolff M ,et al. Effect of abscisic acid and methyl jasmonate preharvest applications on fruit quality and cracking tolerance of sweet cherry[J].Chilean journal of agricultural research, 2018, 78(3):438.
- Batal KM, Weigle JL, Foley DC. 1970. Relation of stress-strain properties of tomato skin to cracking of tomato fruit. HortScience 5(4):223-224.
- Brüggenwirth, M.; Knoche, M. Cell wall swelling, fracture mode, and the mechanical properties of cherry fruit skins are closely related. Planta 2016, 245, 765–777. [CrossRef]
- Butani, A., Purohit, H., Solanki, R., Mishra, P., and Dadhaniya, D. (2019). A chronic problem of fruit cracking in fruit crops: A review. Acta Sci. Agric. 3 (4), 270-274.
- Byers RE, Carbaugh DH, Presley CN. ‘Stayman’ fruit cracking as affected by surfactants, plant growth regulators, and other chemicals. J Amer Soc Hort Sci, 1990, 115(3): 405-411.
- CAO Yibo, LI Changjiang, SUN Fan, et al. (2014) Comparison of the Endogenous Hormones Content and the Activities of Enzymes Related to Cell-wall Metabolism Between Jujube Cultivars Suscepti ble and Resistant to Fruit Cracking. Acta Horticulturae Sinica, 41(1):139-148. in Chinese.
- Caroline, G.; Chadoeuf, J.; Gilles, V.; Michel, G.; Francxoise, L. Cuticular cracking on nectarine fruit surface: Spatial distribution and development in relation to irrigation and thinning. J. Am. Soc. Hortic. Sci. 2007, 132, 583-591.
- Choi, C.; Wiersma, P.; Toivonen, P.; Kappel, F. Fruit growth, firmness and cell wall hydrolytic enzyme activity during development of sweet cherry fruit treated with gibberellic acid (GA3). J. Hortic. Sci. Biotechnol. 2002, 77, 615–621. [CrossRef]
- Choi, J.H.; Lee, B.; Gu, M.; Lee, U.Y.; Kim, M.S.; Jung, S.K.; Choi, H.S. Course of Fruit Cracking in ‘Whansan’ Pears. Hortic. Environ. Biotechnol. 2020, 61, 51-59.
- Christensen J Vittrup. Cracking in Cherries: III. Determination of Cracking Susceptibility, Acta Agric. Scand. 1972;22(2):128-136.
- Clayton, M., Biasi, W. V., Agar, I. T., Southwick, S. M. and Mitcham, E. J. 2006. Sensory quality of ‘Bing’ sweet cherries following preharvest treatment with hydrogen cyanamide, calcium ammonium nitrate, or gibberellic acid. Hort Science 41: 745-748.
- Cline, J.A.; Trought, M. Effect of gibberellic acid on fruit cracking and quality of Bing and Sam sweet cherries. Can. J. Plant Sci. 2007, 87, 545–550. [CrossRef]
- Considine, J. Physical Aspects of Fruit Growth: Cuticular Fracture and Fracture Patterns in Relation to Fruit Structure inVitis Vinifera. J. Hortic. Sci. 1982, 57, 79–91. [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [CrossRef]
- Cristián, B.; Héctor, A.; Richard, M.; Gerardo, T.; et al. (2013) Cracking in sweet cherries: A comprehensive review from a physiological, molecular, and genomic perspective. J. Chil. J. Agric. Res. 73, 66-72.
- Cutler S, Rodriguez P, Finkelstein R, et al. Abscisic Acid: Emergence of a Core Signaling Network[J].Annual review of plant biology. 2010:61.
- de Freitas, S.T.; Shackel, K.A.; Mitcham, E.J. Abscisic acid triggers whole-plant and fruit-specific mechanisms to increase fruit calcium uptake and prevent blossom end rot development in tomato fruit. J. Exp. Bot. 2011, 62, 2645–2656. [CrossRef]
- Demirsoy L, Bilgener S. The effect of chemical applications on cuticular and epidermal properties of some sweet cherry cultivars with respect to fruit cracking susceptibility. Turkish J Agric For, 2000, 24(5): 541-550.
- Drogoudi, P.; E Pantelidis, G. Comparative effects of gibberellin A3, glycine betaine, and Si, Ca, and K fertilizers on physiological disorders and yield of pomegranate cv. Wonderful. J. Sci. Food Agric. 2021, 102, 259–267. [CrossRef]
- Facteau, T. J. (1984) Sweet cherry trials, Blenheim, New Zealand. Marlborough Research Centre Internal Report. 14 pp.
- Facteau, T.J.; Rowe, K.E.; Chestnut, N.E. Firmness of Sweet Cherry Fruit following Multiple Applications of Gibberellic Acid. J. Am. Soc. Hortic. Sci. 1985, 110, 775–777. [CrossRef]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis Abscisic Acid Response Gene ABI5 Encodes a Basic Leucine Zipper Transcription Factor. Plant Cell 2000, 12, 599. [CrossRef]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 Is a Transcription Activator of Novel ABRE-Dependent ABA Signaling That Enhances Drought Stress Tolerance inArabidopsis. Plant Cell 2005, 17, 3470–3488. [CrossRef]
- Gibert, C.; Génard, M.; Vercambre, G.; Lescourret, F. Quantification and modelling of the stomatal, cuticular and crack components of peach fruit surface conductance. Funct. Plant Biol. 2010, 37, 264–274. [CrossRef]
- Giné-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguiló-Aguayo, I.; López, M.L.; Larrigaudière, C. Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [CrossRef]
- Ginzberg, I.; Stern, R.A. (2019) Control of Fruit Cracking by Shaping Skin Traits-Apple as a Model. Crit. Rev. Plant Sci. 38, 401-410.
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Gibberellins and abscisic acid signal crosstalk: living and developing under unfavorable conditions. Plant Cell Rep. 2013, 32, 1007–1016. [CrossRef]
- Gosti, F.; Beaudoin, N.; Serizet, C.; Webb, A.A.R.; Vartanian, N.; Giraudat, J. ABI1 Protein Phosphatase 2C Is a Negative Regulator of Abscisic Acid Signaling. Plant Cell 1999, 11, 1897. [CrossRef]
- Gubler, F.; Chandler, P.M.; White, R.G.; Llewellyn, D.J.; Jacobsen, J.V. Gibberellin Signaling in Barley Aleurone Cells. Control of SLN1 and GAMYB Expression. Plant Physiol. 2002, 129, 191–200. [CrossRef]
- Gutiérrez, C.; Figueroa, C.R.; Turner, A.; Munné-Bosch, S.; Muñoz, P.; Schreiber, L.; Zeisler, V.; Marín, J.C.; Balbontín, C. Abscisic acid applied to sweet cherry at fruit set increases amounts of cell wall and cuticular wax components at the ripe stage. Sci. Hortic. 2021, 283, 110097. [CrossRef]
- Hadjipieri, M.; Georgiadou, E.; Drogoudi, P.; Fotopoulos, V.; Manganaris, G. The efficacy of acetylsalicylic acid, spermidine and calcium preharvest foliar spray applications on yield efficiency, incidence of physiological disorders and shelf-life performance of loquat fruit. Sci. Hortic. 2021, 289, 110439. [CrossRef]
- Hoda A., Khalil S.H.A. Cracking and fruit quality of pomegranate (Punica granatum L.) as affected by pre-harvest sprays of some growth regulators and mineral nutrients. J. Hortic. Sci. Ornam. Plants. 2013;5:71-76.
- Horvitz, S.; López Godoy, C.; Camelo, A.F.L.; Yommi, A. C. Godoy, C. Application of Gibberellic Acid to ‘Sweetheart’ Sweet Cherries: Effects on Fruit Quality at Harvest and during Cold Storage. Acta Hortic. 2003, 311–316. [CrossRef]
- Hou, L.; Li, M.; Zhang, C.; Liu, N.; Liu, X.; Bo, W.; Pang, X.; Li, Y. Comparative Transcriptomic Analyses of Different Jujube Cultivars Reveal the Co-Regulation of Multiple Pathways during Fruit Cracking. Genes 2022, 13, 105. [CrossRef]
- Hurtado, G.; Knoche, M. Necked strawberries are especially susceptible to cracking. PeerJ 2023, 11, e15402. [CrossRef]
- Irigoyen, M.L.; Iniesto, E.; Rodriguez, L.; Puga, M.I.; Yanagawa, Y.; Pick, E.; Strickland, E.; Paz-Ares, J.; Wei, N.; De Jaeger, G.; et al. Correction to: Targeted Degradation of Abscisic Acid Receptors Is Mediated by the Ubiquitin Ligase Substrate Adaptor DDA1 in Arabidopsis. Plant Cell 2022, 34, 2807–2808. [CrossRef]
- Jiang, F.; Lopez, A.; Jeon, S.; de Freitas, S.T.; Yu, Q.; Wu, Z.; Labavitch, J.M.; Tian, S.; Powell, A.L.T.; Mitcham, E. Disassembly of the fruit cell wall by the ripening-associated polygalacturonase and expansin influences tomato cracking. Hortic. Res. 2019, 6, 1–15. [CrossRef]
- Josan JS, Sandhu AS, Zora S (1998) Effect of plant growth regulators sparys on the endogenous level of phytohormones and splitting of lemon fruit. Recent Hortic 4:19-21.
- Joshi, M.; Baghel, R.S.; Fogelman, E.; Stern, R.A.; Ginzberg, I. Identification of candidate genes mediating apple fruit-cracking resistance following the application of gibberellic acids 4 + 7 and the cytokinin 6-benzyladenine. Plant Physiol. Biochem. 2018, 127, 436–445. [CrossRef]
- Kappel, F. and MacDonald, R. A. (2002) Gibberellic acid increases fruit firmness, fruit size, and delays maturity of ‘Sweetheart’ sweet cherry. J. Am. Pomol. Soc. 56: 219-222.
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant. 2014, 37, 1–14. [CrossRef]
- Knoche, M.; Lang, A. Ongoing Growth Challenges Fruit Skin Integrity. Crit. Rev. Plant Sci. 2017, 36, 190–215. [CrossRef]
- Knoche, M.; Peschel, S. Water on the Surface Aggravates Microscopic Cracking of the Sweet Cherry Fruit Cuticle. J. Am. Soc. Hortic. Sci. 2006, 131, 192–200. [CrossRef]
- Kou, X.; Yang, S.; Chai, L.; Wu, C.; Zhou, J.; Liu, Y.; Xue, Z. Abscisic acid and fruit ripening: Multifaceted analysis of the effect of abscisic acid on fleshy fruit ripening. Sci. Hortic. 2021, 281, 109999. [CrossRef]
- Kushiro, T.; Okamoto, M.; Nakabayashi, K.; Yamagishi, K.; Kitamura, S.; Asami, T.; Hirai, N.; Koshiba, T.; Kamiya, Y.; Nambara, E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J. 2004, 23, 1647–1656. [CrossRef]
- Kwon, Y.; Han, H.-H.; Park, H.-S. The characteristics of cork and hypodermis tissues and cracking in Asian pear (Pyrus pyrifolia cv. Mansoo). Sci. Hortic. 2016, 199, 224–228. [CrossRef]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf Life Potential and the Fruit Cuticle: The Unexpected Player. Front. Plant Sci. 2019, 10, 770. [CrossRef]
- Li JG, Huang HB, Yuan RC, et al. (1992) Litchi fruit cracking in relation to fruit growth and water-uptake kinetics. J South China Agric Univ,13(4): 129-135.
- Li, W.-C.; Wu, J.-Y.; Zhang, H.-N.; Shi, S.-Y.; Liu, L.-Q.; Shu, B.; Liang, Q.-Z.; Xie, J.-H.; Wei, Y.-Z. De Novo Assembly and Characterization of Pericarp Transcriptome and Identification of Candidate Genes Mediating Fruit Cracking in Litchi chinensis Sonn.. Int. J. Mol. Sci. 2014, 15, 17667–17685. [CrossRef]
- Li, J.; Chen, J. Citrus Fruit-Cracking: Causes and Occurrence. Hortic. Plant J. 2017, 3, 255–260. [CrossRef]
- Liao, N.; Hu, Z.; Li, Y.; Hao, J.; Chen, S.; Xue, Q.; Ma, Y.; Zhang, K.; Mahmoud, A.; Ali, A.; et al. Ethylene-responsive factor 4 is associated with the desirable rind hardness trait conferring cracking resistance in fresh fruits of watermelon. Plant Biotechnol. J. 2019, 18, 1066–1077. [CrossRef]
- Lin Q, Wu F, Sheng P, et al. (2015) The SnRK2-APC/CTE regulatory module mediates the antagonistic action of gibberellic acid and abscisic acid pathways[J]. Nature Communications, 6:7981.
- Liu, N.; Zhao, H.; Hou, L.; Zhang, C.; Bo, W.; Pang, X.; Li, Y. HPLC-MS/MS-based and transcriptome analysis reveal the effects of ABA and MeJA on jujube (Ziziphus jujuba Mill.) cracking. Food Chem. 2023, 421, 136155. [CrossRef]
- Maotani, T.; Suzuki, A.; Tanaka, K.; Kimura, K.; et al. (1990) Control of Fruit Cracking of Japanese Pear ‘Kosui’ and ‘Niitaka’ Using Gibberellin Tape. J. Jpn. Soc. Hortic. Sci.58, 859-863.
- Marboh, E.S.; Singh, S.K.; Pandey, S.; Nath, V.; Gupta, A.K.; Pongener, A. Fruit cracking in litchi (Litchi chinensis): An overview. Indian J. Agric. Sci. 2017, 87, 03–11–03–11. [CrossRef]
- Measham PF, Bound SA, Gracie AJ, Wilson SJ. (2009) Incidence and type of cracking in sweet cherry (Prunus avium L.) are affected by genotype and season. Crop Pasture Sci.60(10):1002.
- Menzel, C. The pattern and control of reproductive development in lychee: A review. Sci. Hortic. 1984, 22, 333–345. [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Karamanoli, K.; Lazaridou, A.; Martens, S.; Molassiotis, A. Sweet cherry fruit cracking: follow-up testing methods and cultivar-metabolic screening. Plant Methods 2020, 16, 1–14. [CrossRef]
- Michelle, K.; Bruce, L.; Ken, S.; Carlos, H.C. (2013) Fruit skin side cracking and ostiole-end splitting shorten postharvest life in fresh figs (Ficus carica L.), but are reduced by deficit irrigation. Postharvest Biol. Technol. 85, 154-161.
- Maotani, T.; Suzuki, A.; Tanaka, K.; Kimura, K.; Sugiura, T.; Kumamoto, O.; Nishimura, T.; Oshima, K.; Masada, T. (1990 ) Control of Fruit Cracking of Japanese Pear ‘Kosui’ and ‘Niitaka’ Using Gibberellin Tape. J. Jpn. Soc. Hortic. Sci. 58, 859-863.
- Munish A, Kahlon P S and Mahajan B V C ( 2003) Effect of exogenous application of growth regulators on fruit drop, cracking and quality of litchi (Litchi chinensis Sonn.) CV. Dehradun. Agricultural Science Digest, 23: 191-194.
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [CrossRef]
- Nonogaki, H. (2014) Seed dormancy and germination-emerging mechanisms and new hypotheses. Front Plant Sci. 5, 233 .
- Oh, E. et al. (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell, 19, 1192-1208.
- Okamoto, M.; Kuwahara, A.; Seo, M.; Kushiro, T.; Asami, T.; Hirai, N.; Kamiya, Y.; Koshiba, T.; Nambara, E. CYP707A1 and CYP707A2, Which Encode Abscisic Acid 8′-Hydroxylases, Are Indispensable for Proper Control of Seed Dormancy and Germination in Arabidopsis. Plant Physiol. 2006, 141, 97–107. [CrossRef]
- Opara, L.U.; Hodson, A.D.; Studman, S.P. (2000) Stem-end splitting and internal ring-cracking of ‘Gala’ apples as influenced by orchard management practices. J. Hortic. Sci. Biotechnol. 75, 465-469.
- Burhan O , Erdinc B , Erdal A ,et al. (2018) Cracking and quality attributes of jujube fruits as affected by covering and pre-harvest Parka and GA 3 treatments [J].Scientia Horticulturae, 240:65-71.
- Peet, M. M. (1992) Fruit cracking in tomato. HortTechnology, 2, 216-223.
- Piskurewicz, U.; Jikumaru, Y.; Kinoshita, N.; Nambara, E.; Kamiya, Y.; Lopez-Molina, L. The Gibberellic Acid Signaling Repressor RGL2 Inhibits Arabidopsis Seed Germination by Stimulating Abscisic Acid Synthesis and ABI5 Activity. Plant Cell 2008, 20, 2729–2745. [CrossRef]
- Proebsting, E. L., Carter, G. H. and Mills, H. H. (1973) Quality improvement in canned ‘Rainier’ cherries (P. avium L.). with gibberellic acid. J. Am. Soc. Hortic. Sci. 98: 334-336.
- Rai, M., P. Dey, V. Nath, and B. Das (2002) Litchi production technology. Horti. And Agroforestry Res. Prog., Ranchi 21p.
- Rehman, M. U., Rather, G. H., Dar, N. A., Mir, M. M., et al. (2015) Causes and prevention of cherry cracking: A review, in Crop production and global environmental issues. Ed. K. R. Hakeem, 543-552.
- Ren, J.; Chen, P.; Dai, S.; Li, P.; Li, Q.; Ji, K.; Wang, Y.; Leng, P. Role of abscisic acid and ethylene in sweet cherry fruit maturation: molecular aspects. New Zealand J. Crop. Hortic. Sci. 2011, 39, 161–174. [CrossRef]
- Rodriguez, L.; Gonzalez-Guzman, M.; Diaz, M.; Rodrigues, A.; Izquierdo-Garcia, A.C.; Peirats-Llobet, M.; Fernandez, M.A.; Antoni, R.; Fernandez, D.; Marquez, J.A.; et al. C2-Domain Abscisic Acid-Related Proteins Mediate the Interaction of PYR/PYL/RCAR Abscisic Acid Receptors with the Plasma Membrane and Regulate Abscisic Acid Sensitivity in Arabidopsis. Plant Cell 2014, 26, 4802–4820. [CrossRef]
- Romero, P.; Lafuente, M.T. Abscisic Acid Deficiency Alters Epicuticular Wax Metabolism and Morphology That Leads to Increased Cuticle Permeability During Sweet Orange (Citrus sinensis) Fruit Ripening. Front. Plant Sci. 2020, 11. [CrossRef]
- Santos, M.; Egea-Cortines, M.; Gonçalves, B.; Matos, M. Molecular mechanisms involved in fruit cracking: A review. Front. Plant Sci. 2023, 14, 1130857. [CrossRef]
- Schomburg, F.M.; Bizzell, C.M.; Lee, D.J.; Zeevaart, J.A.D.; Amasino, R.M. Overexpression of a Novel Class of Gibberellin 2-Oxidases Decreases Gibberellin Levels and Creates Dwarf Plants. Plant Cell 2002, 15, 151–163. [CrossRef]
- Schroeder, J.I.; Nambara, E. A Quick Release Mechanism for Abscisic Acid. Cell 2006, 126, 1023–1025. [CrossRef]
- Schumann, C., Winkler, A., Brüggenwirth, M., Köpcke, K., and Knoche, M. (2019) Crack initiation and propagation in sweet cherry skin: A simple chain reaction causes the crack to ‘run’. PloS One 14 (7), e0219794.
- Schwartz, S.H., Qin, X., and Zeevaart, J.A.D. (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol. 131: 1591-1601.
- Sekse L, Bjerke KL, Vangdal E. (2005) Fruit cracking in sweet cherries—An integrated approach. Acta Hortic, 667: 471-474.
- Seo, H.-J., Sawant, S. S., & Song, J. (2022) Fruit cracking in pears: Its cause and management-A review. Agronomy, 12(10).
- Seo, M.; Koshiba, T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002, 7, 41–48. [CrossRef]
- Sepahi A . After effect of gibberellic acid on pomegranate trees[J]. 1986.
- Sharma S.B., Dhillon B.S. (1986) Endogenous level of gibberellins in relation to fruit cracking in litchi (Litchi chinensis Sonn.) J. Res. Punjab Agric. Univ. 23:432-434.
- Sharma, S.B.; Dhillon, B.S. (1984) Effect of zinc sulphate and growth regulators on the growth of litchi fruit. Prog. Hortic. 16, 19-22.
- Sharma, K.K., and W.S. Dhillon. (2002) Evaluation of evergreen varieties of pomegranate under Punjab conditions. Agric. Sci. Digest. 22:42-44.
- Simon, G. (2006) Review on rain induced fruit cracking of sweet cherries (Prunus avium L.), its causes and the possibilities of prevention. International Journal of Horticultural Science 12:27-35.
- Singh, A.; Shukla, A.K.; Meghwal, P.R. (2020) Fruit Cracking in Pomegranate: Extent, Cause, and Management—A Review. Int. J. Fruit Sci. 20, S1234-S1253.
- Srivastava, R.P.; Singh, L. Effect of growth substances on the quality of litchi. Hort. Sci. 1969, 1, 1-6.
- Sun, Q.; Greve, L.C.; Labavitch, J.M. Polysaccharide Compositions of Intervessel Pit Membranes Contribute to Pierce’s Disease Resistance of Grapevines. Plant Physiol. 2011, 155, 1976–1987. [CrossRef]
- Suran, P.; Vávra, R.; Zelený, L. Effectiveness of potential products to reduce rain cracking of cherry fruit. Acta Hortic. 2016, 183–186. [CrossRef]
- Tohru, A.; Kohji, M.; Sun, T.P.; Camille, M. (2008) Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor gibberellin insensitive dwarf1. Plant Cell, 20, 2447-2459.
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.-Y.; Hsing, Y.-I.C.; Kitano, H.; Yamaguchi, I.; et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [CrossRef]
- Usenik, V.; Kastelec, D.; Štampar, F. Physicochemical changes of sweet cherry fruits related to application of gibberellic acid. Food Chem. 2005, 90, 663–671. [CrossRef]
- Vanstraelen, M.; Benková, E. Hormonal Interactions in the Regulation of Plant Development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [CrossRef]
- Verner L & Blodgett EC. Physiological studies of the cracking of sweet cherries., Univ. Idaho Agr. Expt. Sta. Bul. 1931;184:1-15.
- Wang, J., Gao, X., Ma, Z., Chen, J., and Liu, Y. (2019a) Analysis of the molecular basis of fruit cracking susceptibility in Litchi chinensis cv. baitangying by transcriptome and quantitative proteome profiling. J. Plant Physiol. 234-235, 106-116.
- Wang, H.C.; Wei, B.W.; Gao, F.F. (2000) Studies on the relation among fruit skin structure, cell division and fruit cracking in litchi (Litchi chinensis Sonn.). J. South China Agri. Univ. 21, 10-13.
- Wang, J. G., Gao, X. M., Ma, Z. L., Chen, J., Liu, Y. N., and Shi, W. Q. (2019b) Metabolomic and transcriptomic profiling of three types of litchi pericarps reveals that changes in the hormone balance constitute the molecular basis of the fruit cracking susceptibility of Litchi chinensis cv. baitangying. Mol. Biol. Rep. 46 (5), 5295-5308.
- Wang, J., Gao, Q., Wang, Z., & Lin, M. (2020) Relationship between cell metabolism enzyme activity, carbohydrate, endogenous hormones and fruit cracking. Xinjiang Agricultural Sciences, 57(9), 1689-1696. in Chinese.
- Wang, J., Wu, X. F., Tang, Y., Li, J. G., and Zhao, M. L. (2021b) RNA-seq provides new insights into the molecular events involved in “ Ball-skin versus bladder effect ” on fruit cracking in litchi. Int. J. Mol. Sci. 22 (1), 454.
- Wang, Y.; Guo, L.; Zhao, X.; Zhao, Y.; Hao, Z.; Luo, H.; Yuan, Z. Advances in Mechanisms and Omics Pertaining to Fruit Cracking in Horticultural Plants. Agronomy 2021, 11, 1045. [CrossRef]
- Weiner, J.J.; Peterson, F.C.; Volkman, B.F.; Cutler, S.R. Structural and functional insights into core ABA signaling. Curr. Opin. Plant Biol. 2010, 13, 495–502. [CrossRef]
- Xu, Z.J.; Masatoshi, N.; Yoshihito, S.; Isomaro, Y. (2002) Cloning and characterization of the abscisic acidspecific glucosyltransferase gene from adzuki bean seedlings. Plant Physiol. 129, 1285-1295.
- Yamada M, Ikeda I, Yamane H, Hirabayashi T (1988) Inheritance of fruit cracking at the calyx end and stylar end in Japanese persimmon. J Jpn Soc Hortic Sci 57:8-16.
- Yamaguchi, S. Gibberellin Metabolism and its Regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [CrossRef]
- Yang JQ, Wang BM, Wang XY. (2009) Advance of fruit cracking of jujube[J]. Shanxi Agricultural Science,37(03):86-89.
- Yilmaz, C.; Ozguven, A. Hormone physiology of preharvest fruit cracking in pomegranate (Punica granatum L.). Acta Hortic. 2006, 545–550. [CrossRef]
- Yılmaz, C.; Özgüven, A. The effects of some plant nutrients, gibberellic acid and pinolene treatments on the yield, fruit quality and cracking in pomegranate. Acta Hortic. 2009, 205–212. [CrossRef]
- Yu Jun, Zhu Mingtao, Wang Meijun, Tang Wanying, Wu Sheng, Zhang Kai, et al. (2020) Effect of nordihydroguaiaretic acid on grape berry cracking. Sci. Hortic. (Amsterdam). 261:108979.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).