1. Introduction
Staphylococcus aureus is a gram-positive coccus, catalase positive, coagulase positive and PYR-positive bacteria that is recognized as one of the major infectious disease-causing pathogens in humans. Antimicrobials such as β-lactams are generally used for chemotherapy of these diseases; however, the emergence of methicillin-resistant
S. aureus (MRSA), which is virtually resistant to all antimicrobials from this class, has posed serious problems [
1]. Currently, it is estimated that 11,000 people die each year from MRSA-related infections in the USA, representing nearly half of all fatalities caused by multidrug-resistant (MDR) pathogens in the country [
2]. Furthermore, estimates indicate that MRSA kills more Americans each year than HIV/AIDS, Parkinson’s disease, tuberculosis, emphysema, and homicide combined [
3]. In cases of MRSA infections, few pharmacological options are clinically available, such as vancomycin, mupirocin and daptomycin, which are drugs that have a slow bactericidal effect, significant adverse reactions and are expensive [
4]. Thus, the development of new effective and safe therapeutic agents against MRSA-induced infections is imperative to control the public health emergency related to this superbug [
5].
In this scenario, antimicrobial peptides (AMPs) highlight as a promising source of new antimicrobials against MDR pathogens, especially against MRSA [
6,
7]. The AMPs are components of innate immunity found in various species, from bacteria and fungi to animals and plants [
8,
9]. There are eight AMPs approved for clinical use by the Food and Drug Administration (FDA) (
i.e., gramicidin, colistin, polymyxin B, daptomycin, vancomycin, oritavancin, dalbavancin and telavancin), all of them are considered “last line” antimicrobials and active even against MDR specimens [
10]. These agents are known to act on the cell membrane of bacterial pathogens stimulating the lysis and release of cytoplasmic contents. Furthermore, they can cross the membrane inhibiting different metabolic pathways in bacterial cell and can modulate the antibacterial immune response [
11,
12,
13]. AMPs have a series of advantages over conventional therapies such as a rapid bactericidal effect, low potential for inducing resistance, absence of the formation of active residues that can contaminate the environment and action on MDR isolates [
14,
15].
One of the main sources of AMPs in nature are animal toxins. In fact, numerous compounds with antimicrobial activity have been isolated from the venom of spiders, scorpions, snakes, frogs, bees, wasps, ants and other animals, highlighting this biological matrix as a potential source for the search for new antimicrobials against MRSA. Herein, our group has isolated a peptide containing 25 amino acid residues (H-IWLTALKFLGKNLGKHLAKQQLAKL-NH
2) from the venom of the Brazilian spider
Lycosa erythrognatha, popularly known as the “
aranha-lobo”, “
aranha-de-jardim”, or “
tarantula”, which has demonstrated good antimicrobial activity. In vitro studies shown that the LyeTx I peptide proved to be active against
Escherichia coli (MIC: 1.41-7.81 μM),
Pseudomonas aeruginosa (MIC: 2.82 μM),
Acinetobacter baumannii (MIC: 0.70 μM),
Staphylococcus aureus (MIC: 0.70-3.79 μM),
Staphylococcus epidermidis (MIC: 0.70 μM),
Pichia kudriavzevii (formerly
Candida krusei) (MIC: 14.23-26.30 μM),
Cryptococcus gattii (MIC: 2.23 μM) and
Cryptococcus neoformans (MIC: 7.11-13.20 μM) [
16]. However, peptides that have many amino acid residues end up having a restriction on their availability since the synthesis process of this molecules is laborious and expensive, with the costs being directly proportional to the size of the primary sequence. Thus, in order to obtain a smaller molecule while retaining antimicrobial activity, LyeTx I was minimized from the
C-terminal region by sequentially removing amino acid residues up to the alanine at position 15 [
17]. Additionally, this shortened derivative was changed by insertion of a lysine residue at position 5, resulting in a new peptide named LyeTx I mnΔK that contains only 16 amino acid residues (H-IWLTKALKFLGKNLGK-NH
2). This compound, in addition to presenting a spectrum of activity very similar to that of LyeTx I [
17], was able to act on both, planktonic cells and biofilms of
Candida spp. [
18] and carbapenem-resistant
Acinetobacter baumannii (CRAB) [
18]. Moreover, intranasal instillation of LyeTx I mnΔK (1-10 mg/Kg) was able to reduce the pulmonary bacterial load of animals with CRAB-induced lower airway infection, indicating its potential therapeutic use against superbug infections [
19].
Considering the clinical relevance of MRSA infections and the limited therapeutic arsenal available against this pathogen, and taking into account the good antibacterial activity obtained in vitro and in vivo with LyeTx I mnΔK against MDR bacteria, the use of this compound to treat MRSA infections is promising. In this study, we investigated the therapeutic potential of the LyeTx I mnΔK for non-surgical wounds caused by MRSA in mice, and in addition, we employed biophysical studies to evaluate the membrane binding properties of this peptide, in the presence of S. aureus mimetic membranes.
3. Discussion
Several studies indicate that the spread of infections caused by MRSA is a major public health challenge worldwide [
27]. In this sense, the World Health Organization (WHO) categorized this superbug as a high priority microorganism for the search and development of new antimicrobials [
27], and AMPs stand out as relevant agents in this scenario. Here, we showed that a synthetic peptide inspired by a compound isolated from the venom of the
L. erythrognatha spider, called LyeTx I mnΔK, has good activity against planktonic cells and biofilms of MRSA, in addition to being useful in the treatment of wounds caused by this superbug.
The peptide LyeTx I mnΔK showed remarkable antibacterial activity against the MRSA isolates tested, having a pronounced bactericidal action at low concentrations and with rapid maximum effect. In the treatment of infections, it is important that the antimicrobial agent acts quickly and can completely eradicate the microorganism to prevent the development of microbial resistance and complications of infection [
19,
21]. In this regard, the predominantly bactericidal activity of peptide has shown promise, especially in highly resistant microorganisms such as MRSA. Despite the rapid bactericidal effect of LyeTx I mnΔK, vancomycin exhibits a very slow antibacterial activity, which may compromise its efficacy. Clinical use of vancomycin often leads to therapeutic failure due to its limited bactericidal capacity [
28], which makes LyeTx I mnΔK advantageous because of its efficient microbicidal property.
In addition to its potential to acquire antimicrobial resistance genes,
S. aureus exhibits another virulence factors as its ability to form biofilms. Bacterial biofilms are clusters of bacteria that are attached to a surface and/or to each other and embedded in a self-produced exopolymer matrix that protect them from adverse conditions such as sanitizers and antimicrobials agents [
29]. When MRSA-formed biofilms are established in wounds, they exhibit resistance to destruction by conventional antimicrobials, which involves a high risk of inflammation and/or purulence [
30]. Therefore, the activity of the LyeTx I mnΔK peptide was evaluated against MRSA biofilms and the results revealed that this compound has a promising anti-biofilm effect. These results were similar to those observed by Lima et al. (2021) who showed that LyeTx I mnΔK has the ability to disrupt mature biofilms and inhibit the formation of this bacterial structure in carbapenem-resistant
Acinetobacter baumannii cells [
19]. It is worth noting that the effect of the peptide on mature biofilms formed by MRSA was superior to that observed by vancomycin. Conventional antimicrobials have a low ability to act on biofilms because, in addition to the exopolymeric matrix that physically protects bacterial cells from interaction with these compounds, the microorganisms within the biofilm are in a stationary phase of growth [
31]. As all available antibacterial agents have activity only against cells in the logarithmic growth phase, bacteria within the biofilm are naturally insensitive to the action of these drugs [
32,
33]. Therefore, the present result shows that the LyeTx I mnΔK peptide, different from vancomycin, can act in both phases of microbial growth,
i.e., logarithmic and stationary.
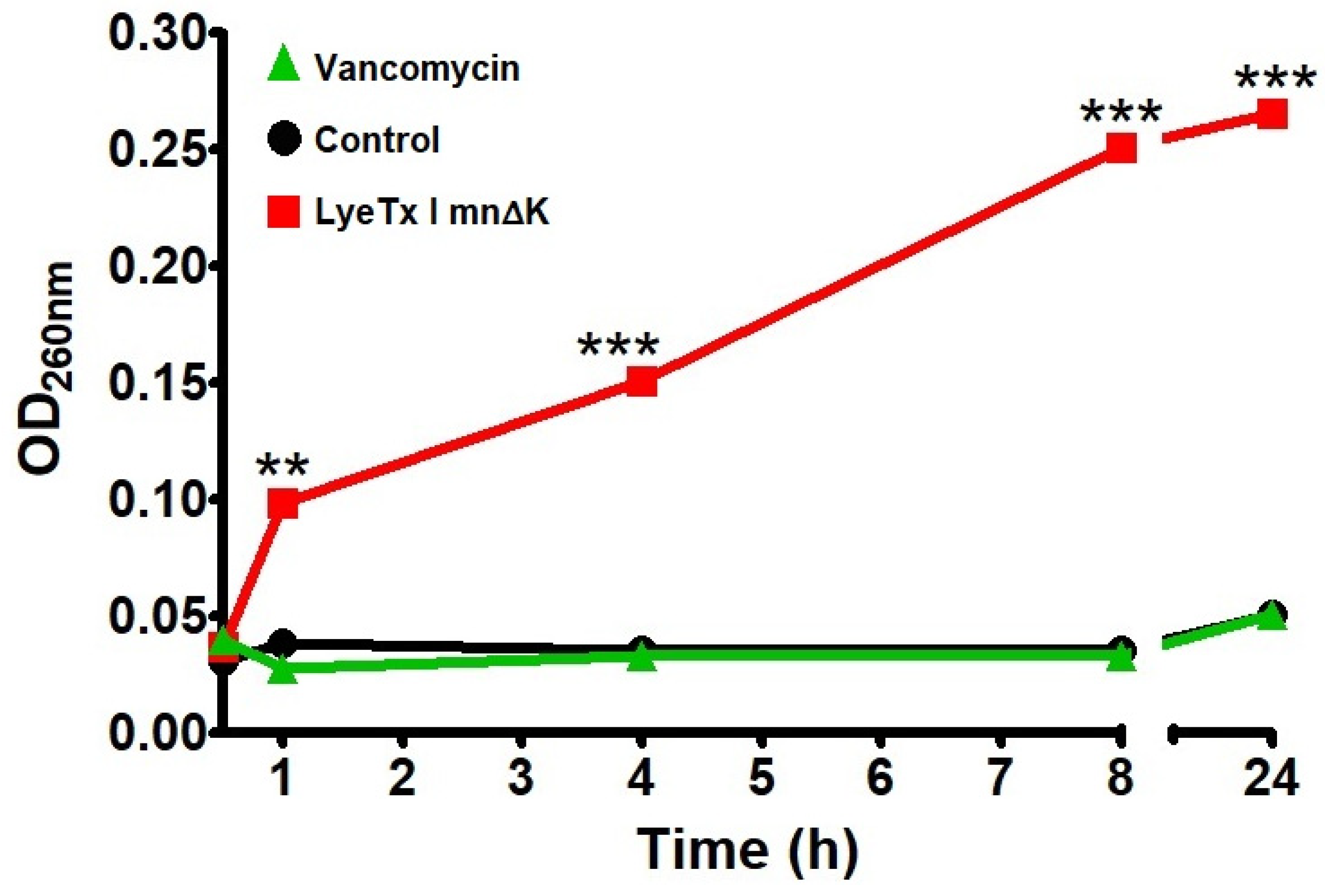
A rapid bactericidal effect and the activity on bacterial biofilms is many times associated with the ability of compound to damage the bacterial cell membrane, which can result in lysis. In fact, the LyeTx I mnΔK peptide significantly increases the loss of genetic material from MRSA cells as indicated by the release of 260-nm-absorbing intracellular compounds, showing that this AMP damages the bacterial plasma membrane. The characteristic of AMPs to cause membrane damage, associated with a rapid and instantaneous bactericidal action, has been highlighted by several authors such as Brogden (2005) [
11] and Avelar (2015) [
34]. In addition to being associated with a rapid antimicrobial effect, the action of LyeTx I mnΔK on the MRSA membrane may come with other advantages such as the reduced potential for inducing resistance and low doses required to clinical use. In this context, it is widely known that compounds that act on plasma membrane have a low capacity to induce antimicrobial resistance because membrane redesign by bacteria would be a “costly” solution for most microbial species [
35,
36]. Moreover, due to the low MIC values related to these antimicrobial agents, the concentrations normally required for clinical use are low, which reduces the possibility of adverse events related to this medication [
10]. Vancomycin, in turn, did not alter the release of materials with absorption at 260 nm, which suggests a discrete effect on the MRSA membrane. This glycopeptide acts by binding to the end of the cross-links that form the bacterial wall (specifically, to the D-Ala-D-Ala residues) [
37], therefore, it does not interfere with the bacterial lipid membrane, which is confirmed by our results.
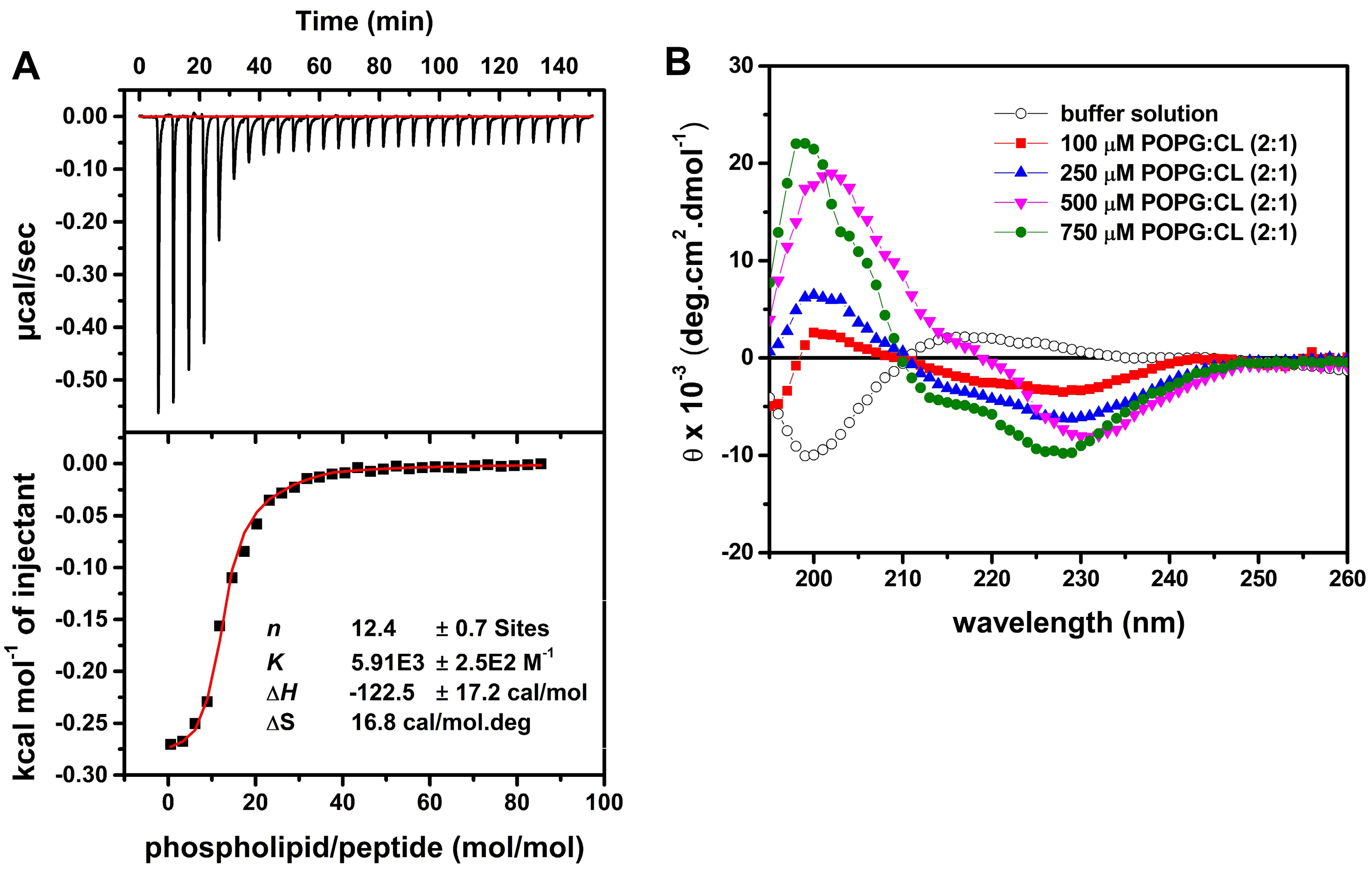
In order to comprehend better the mechanism of action of LyeTx I mn∆K on MRSA membranes, we studied the peptide-membrane interaction in the presence of POPG:CL (2:1, mol/mol). This phospholipid composition was selected as it represnets the predominant constituents of
S. aureus membranes [
38,
39,
40]. Initially, the thermodynamic studies regarding the membrane interactions of LyeTx ImnΔK with POPG:CL LUVs indicated that the peptide presents from moderate to high affinity to negatively charged vesicles at 35 ◦C. Although, the negative enthalpy values indicate exothermic binding of the peptides to the anionic vesicles and the predominance of attractive Coulomb forces, the binding process for the peptide is mainly driven by entropic factors, since |TΔS| >> |ΔH|. This interaction should occur by partial peptide incorporation in the bilayers interface, as it has been shown the increase of the vesicle size observed by the DLS experiments [
41]. Interestingly, above this critical concentration the PDI values ramp up (>0,3) and two size populations were noted. This suggests that the peptide adsorption to the membranes is followed by vesicle aggregation at high peptide concentration (>20 μM) [
42]. Notably, the peptide concentration dependence is also observed in the ζ-potencial measurements. Initially, the interaction of the positively charged peptide with the negatively charged bilayers results in a linear increase of ΔP, reaching a plateau between 15 – 20 μM peptide.
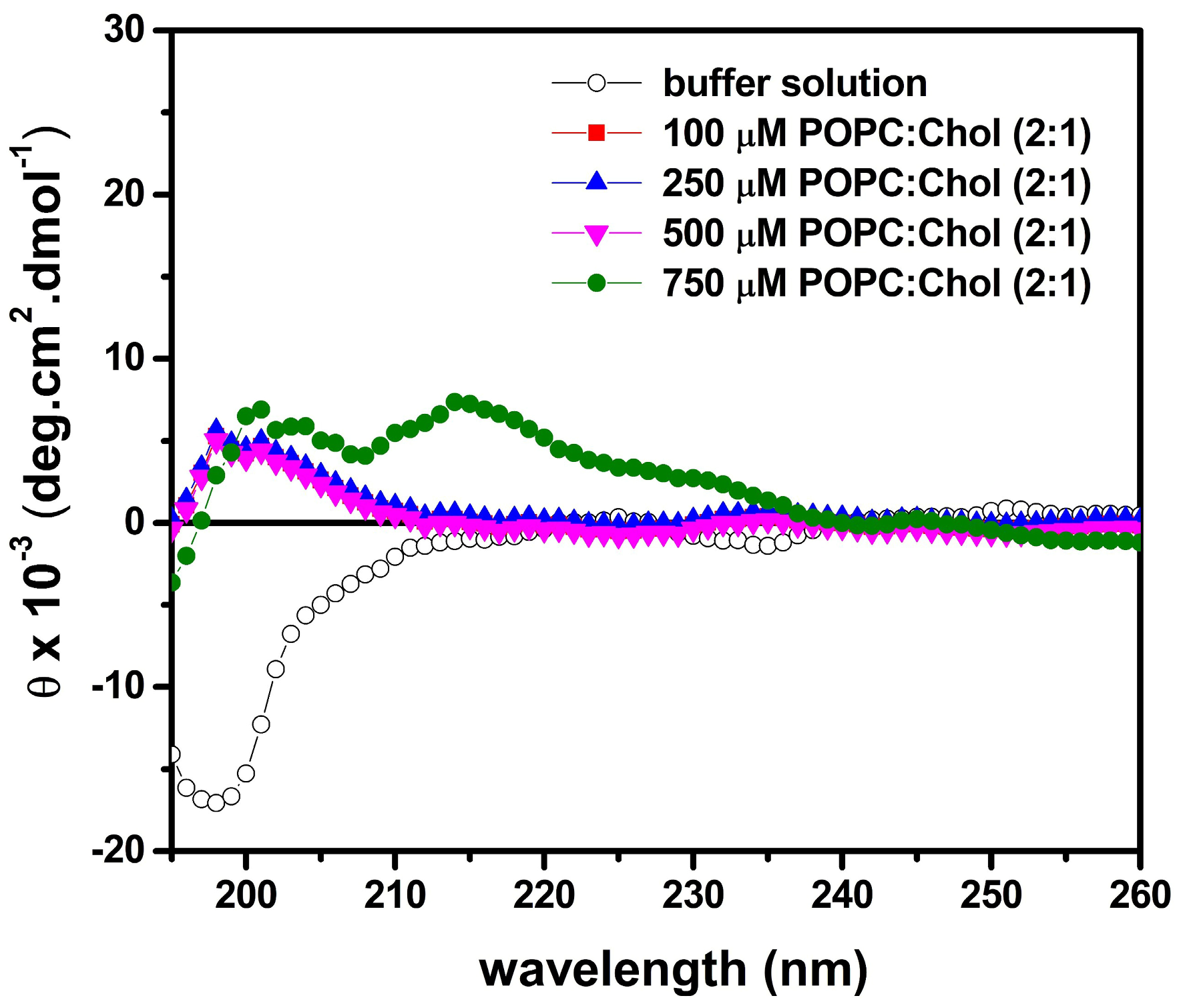
Regarding the secondary structure of the peptide, our results show
α-helical structures when in contact with membrane surface of POPG:CL [
43]. LyeTx I mn∆K presents a significant affinity to both phospholipid vesicles, with an association constant (5,9 x 10
3 M
-1) that falls within the range of 10
3 – 10
4 M
-1 [
41,
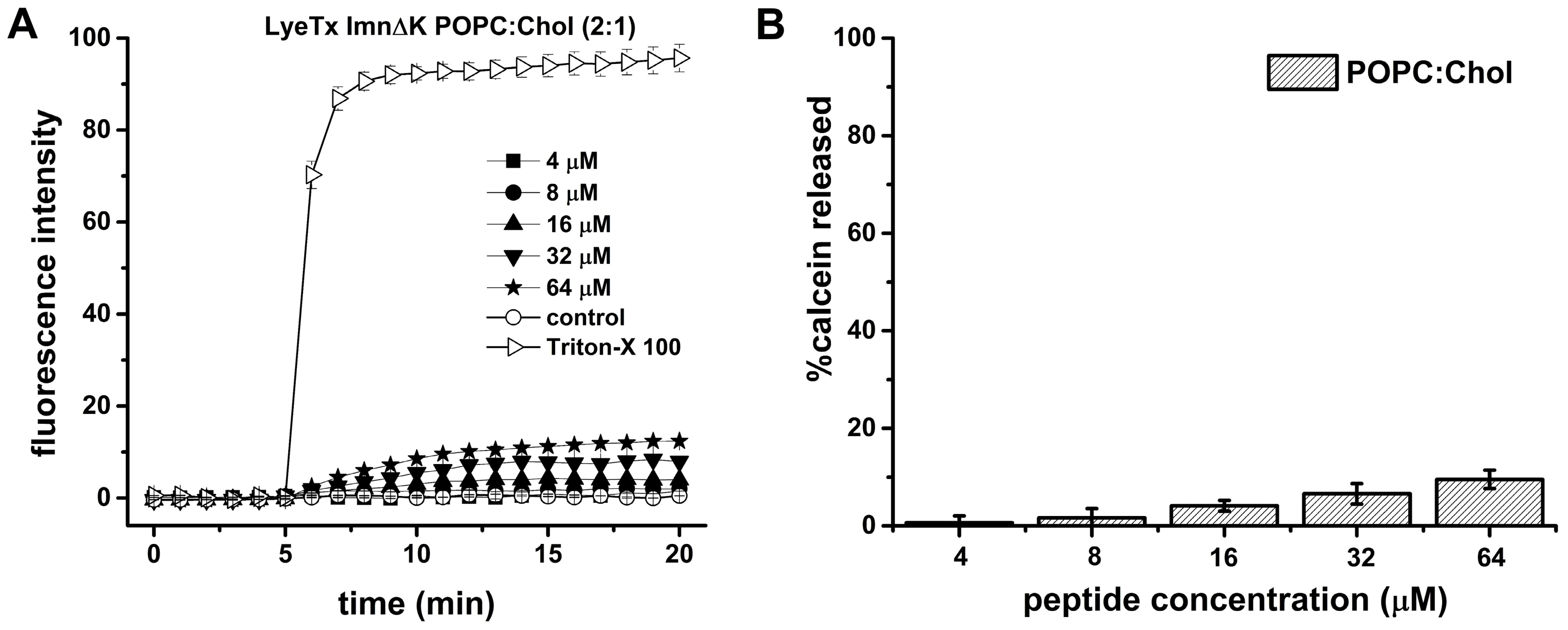
44]. However, in the presence of POPC:Chol (2:1) lipid bilayers, mimicking eukaryotic membranes, the LyeTx I mn∆K spectra reveal no defined conformation. These results suggest that shortened form presents lower affinity to the mimetics of eukaryotic membranes. The same can be observed with the calcein release, which the LyeTx I mn∆K reached a level of less than 20% in vesicles that simulate mammalian cells compared to membranes related to the envelope of
S. aureus. Corroborating these findings, previous studies have shown that the LyeTx I mnΔK peptide has low toxicity against eukariotic cells. The high concentration required to kill 50% (CC
50) of Lund human mesencephalic (LUHMES; CC
50 15.02 µM) [
17] and african green monkey kidney epithelium (Vero; CC
50 55.31±5.0 µM) [
19] cells in culture indicate that this peptide preferentially binds to bacterial cells. Furthermore, LyeTx I mnΔK has low hemolytic activity, with a cytotoxic concentration for 50% of erythrocytes ranging from 44.67 µM to 77.07 µM [
17,
19].
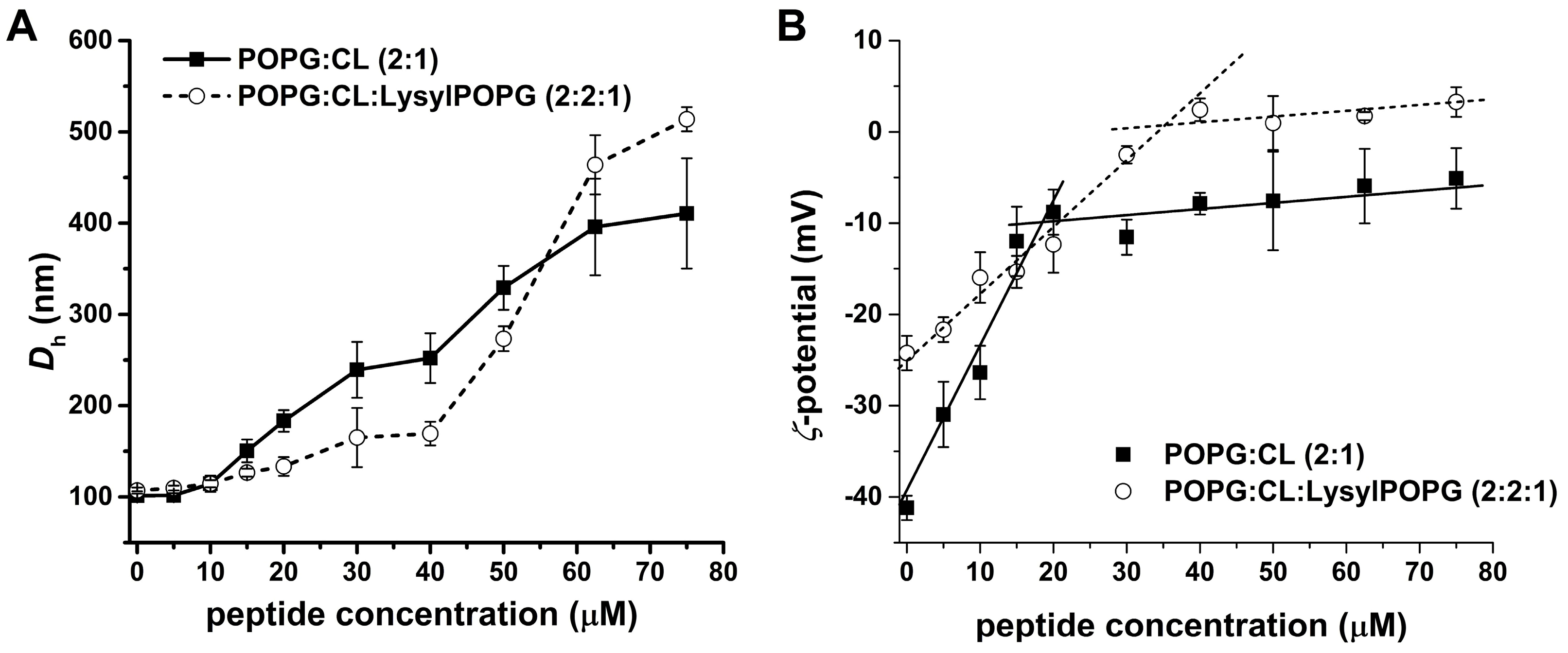
Lysyl phosphatidylglycerol (LysylPOPG) is commonly found in the multidrug-resistant
Staphylococcus aureus such as MRSA by modification of POPG. This process is driven by multiple peptide resistance factors (MprF), which modifies the phopholipids with positively charged lysine residues in the cytoplasmic membrane leaflet and translocate the LysylPOPG to the outer cytoplasmic membrane [
45]. This modification has shown resistance to cationic antimicrobial peptides such as colistin, nisin, human-β-defensin 3, and polymyxin B.
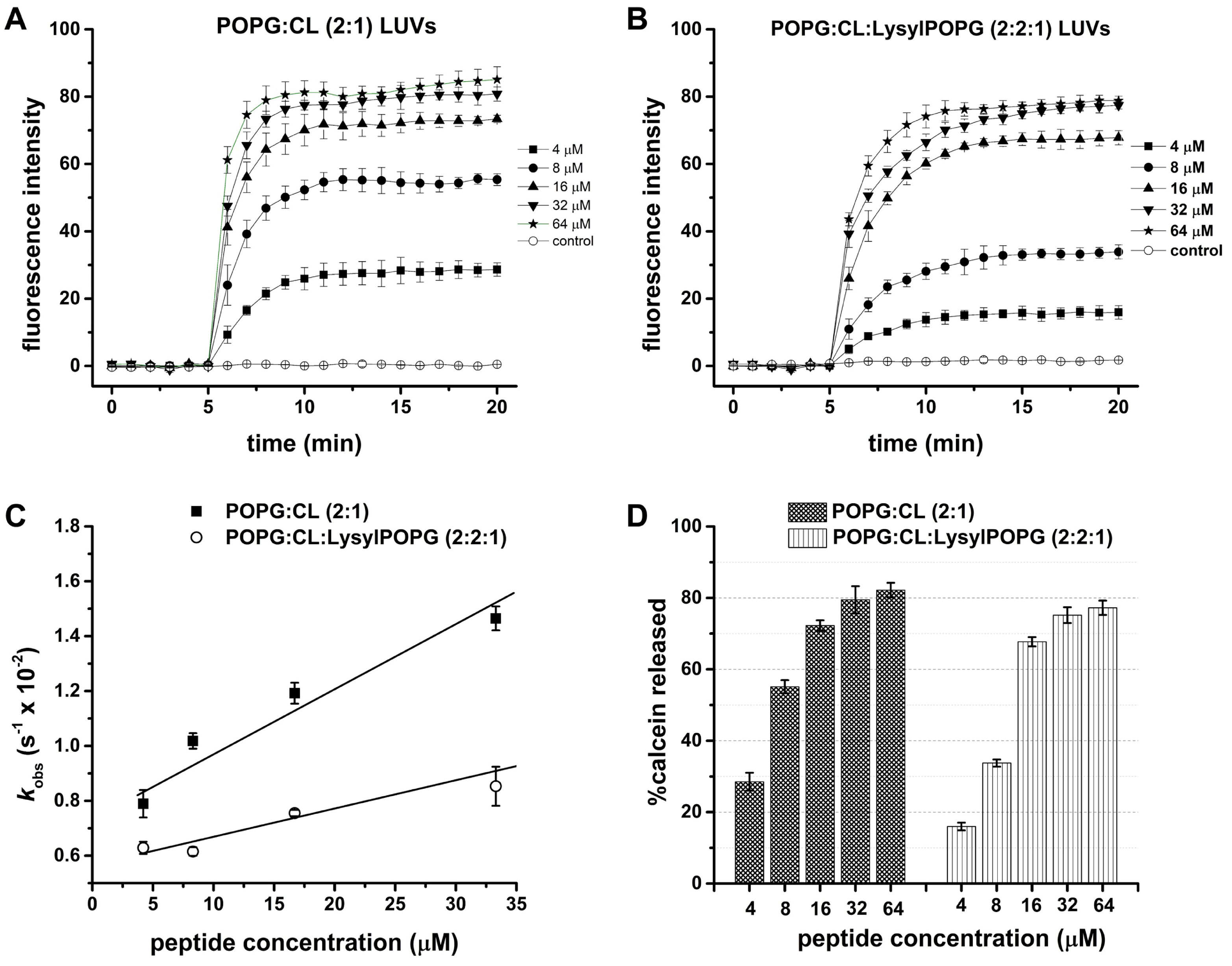
Therefore, to explore the effect of the addition of LysylPOPG lipid in membrane permeabilization ability of the LyeTx I mn∆K, we evaluate the D
h, ζP and calcein release from LUVs containing LysylPOPG. Although the addition of LysylPOPG decreases the kinetics (k
obs) of lytic activity for LyeTx I mn∆K, this lipid does not affect the maximum release of calcein caused by the peptide. The effect the peptide was also virtually equivalent to the changes of ζP of the LUVs in the absence and in the presence of LysylPOPG (
ζP ~ − 30 mV for POPG:CL and -25 mV for POPG:CL:LysylPOPG). The polydispersity index (PDI) of the suspensions increased, suggesting that peptide-membrane interaction affects the originally homogeneous vesicles resulting in a deformed lipid-peptide complex. Interestingly, two populations can be observed with increasing of peptide concentration, one centered around 100 nm and the other around 1000 nm (Figure S1). These findings suggest a fusion process as compared to aggregation which results in the polydispersity of the size population [
46,
47]. Similarly, the effect of the peptide in the D
h of both LUVs was comparable, resulting in vesicle agglutination. These findings are in accordance with the antimicrobial assays, which have demonstrated high activity of LyeTx I mn∆K even against the MRSA USA300 strains (MIC
50 and CBM
50 of 8 µM and 32 µM, respectively). Thus the presence of LysylPOPG which constitutes part of the adaptation of MRSA to antibiotic treatments is surpassed by LyeTx I mn∆K, indicating its effectiveness even in challenging conditions.
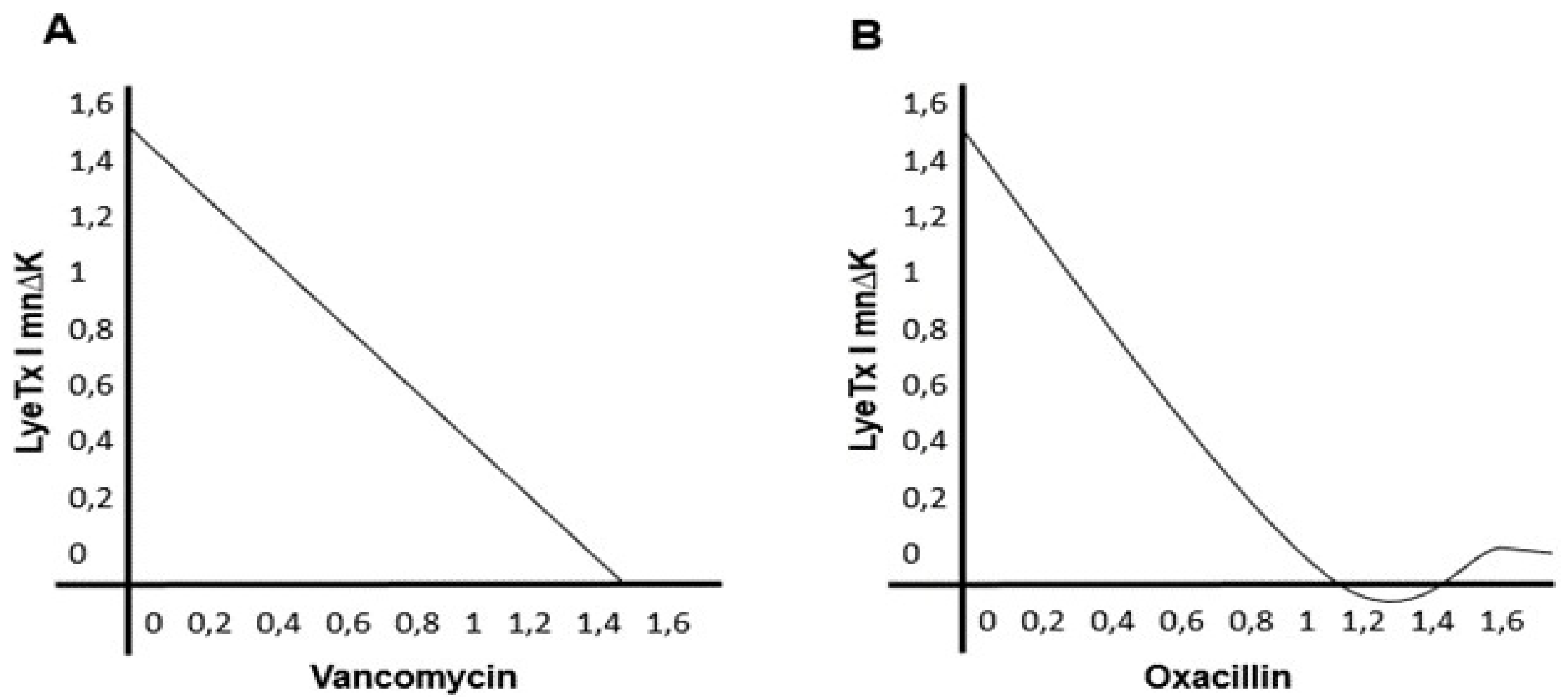
One of the most commonly used strategies for treating infections caused by MDR bacteria, such as MRSA, is the combination of different antimicrobial agents [
48]. This therapeutic protocol has several advantages such as rapid antibacterial effect, lower possibility of therapeutic failure due to selection of resistant bacteria, reduced concentration of each antimicrobial used in the combination and lower risk of serious adverse reactions associated with high doses of the drug [
49]. LyeTx I mn∆K is indifferent to the activity of the antimicrobial’s oxacillin and vancomycin. This indicates that both compounds can be combined to broaden the spectrum of antimicrobial action, thus helping to treat polymicrobial infections [
36]. Furthermore, prior exposure to LyeTx I mn∆K reduces the active concentrations of these two antimicrobials, which may generate a benefit in reducing the dose of these agents with a consequent lower frequency of adverse reactions [
49].
Clinical manifestations of MRSA range of auto-limited superficial infection to life-challenging bacteremia, however the wounds are still most commonly problem caused by this pathogen [
50]. MRSA-infected wounds are difficult to heal, purulent, painful and treatment is restricted to a few options such as vancomycin, mupirocin and clindamycin [
51]. Here, we show that a formulated gel containing LyeTx I mnΔK was effective in treating MRSA-induced non-surgical wounds in mice, reducing bacterial load in a non-dose-dependent manner. Interestingly, this effect was superior to that of the reference drug (vancomycin). Due to their amphoteric nature, AMPs are able to interact better with the corneal extract of the epidermis. This characteristic facilitates absorption through the skin, allowing them to reach deeper layers of the dermis, including infection sites. Thus, the LyeTx I mnΔK highlighted as a promising alternative for the treatment of cutaneous infections by MRSA. On the other hand, vancomycin, being a hydrophilic molecule [
52], faces limitations in penetrating through the skin. This characteristic hinders its access to deeper layers of the dermis, compromising its effectiveness in treating deep skin infections.
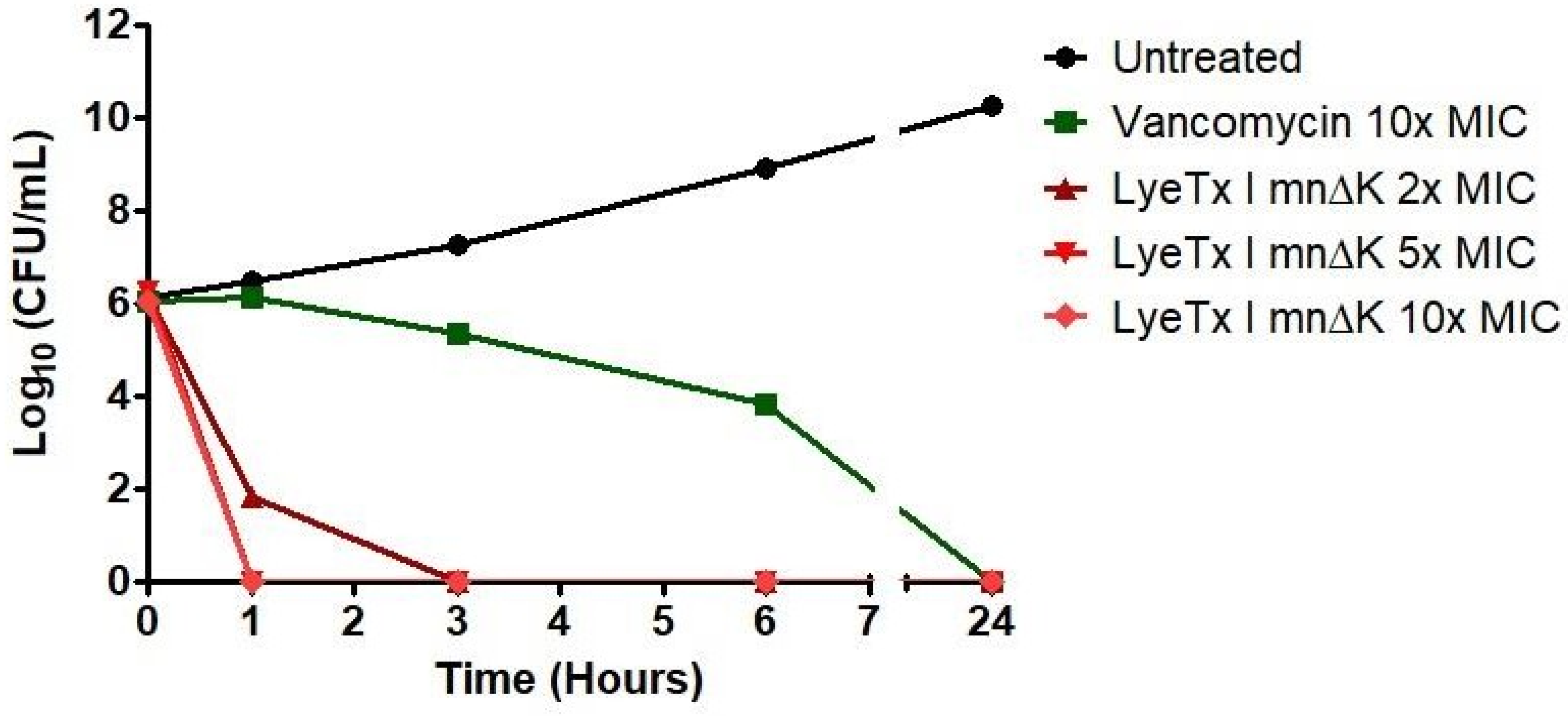
Figure 1.
Time-kill curves of LyeTx I mn∆K and vancomycin against a suspension of methicillin-resistant Staphylococcus aureus (MRSA) on logarithmic growth phase (106 CFU/mL). MIC: Minimum inhibitory concentration.
Figure 1.
Time-kill curves of LyeTx I mn∆K and vancomycin against a suspension of methicillin-resistant Staphylococcus aureus (MRSA) on logarithmic growth phase (106 CFU/mL). MIC: Minimum inhibitory concentration.
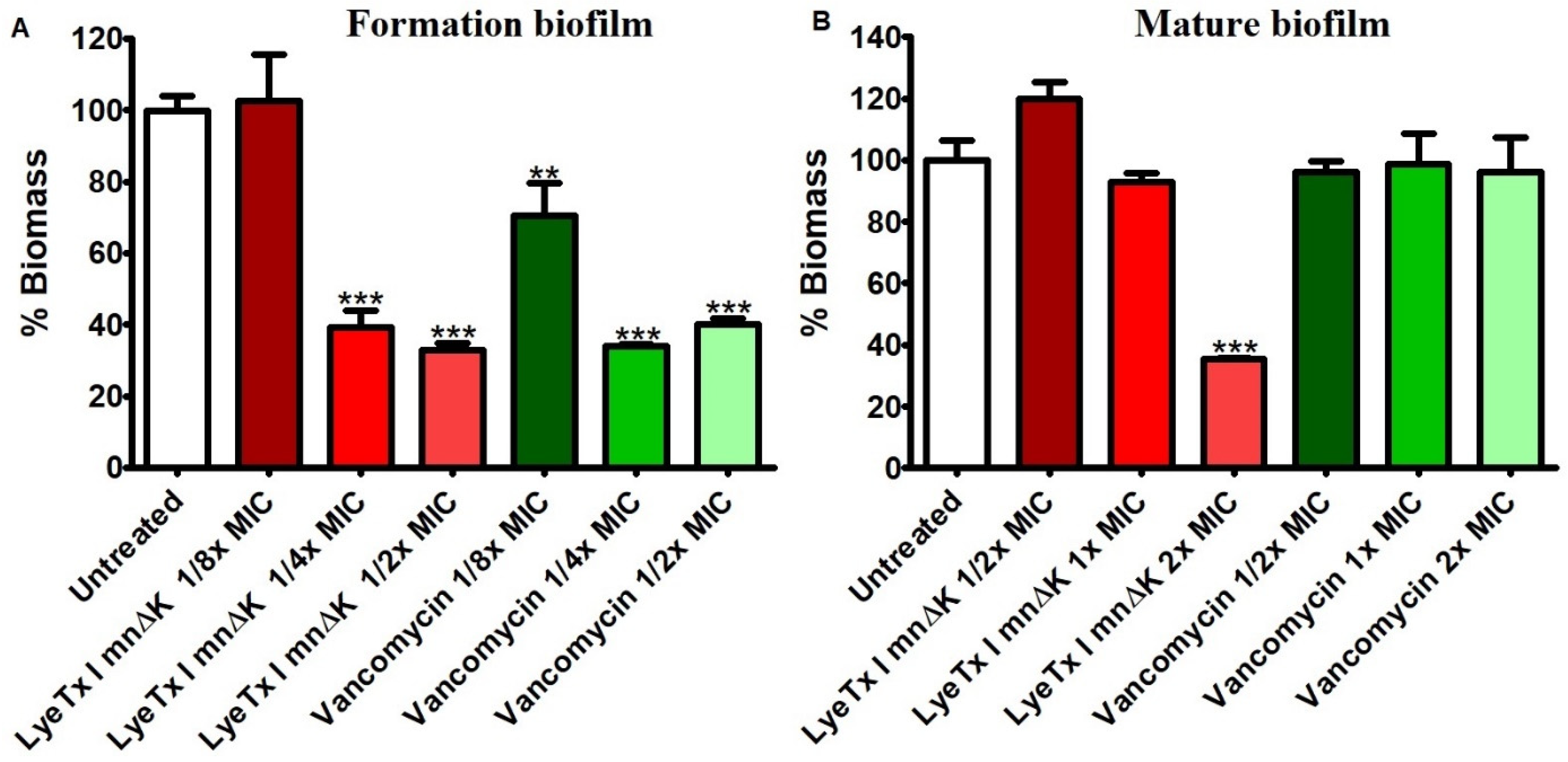
Figure 2.
Evaluation of the effect of different concentrations of LyeTx I mnΔK and the control (vancomycin) on the formation of biofilm and pre-formed biofilm of methicillin-resistant Staphylococcus aureus (MRSA). (A) Reduction of mature biofilm; (B) prevention of the formation of biofilm. Two asterisks (**) indicate statistically significant difference compared to the control, with p-value <0.01. Three asterisks (***) indicate statistically significant difference compared to the control, with p-value < 0.0001. The results were analyzed by one-way ANOVA with Dunnett’s post-test.
Figure 2.
Evaluation of the effect of different concentrations of LyeTx I mnΔK and the control (vancomycin) on the formation of biofilm and pre-formed biofilm of methicillin-resistant Staphylococcus aureus (MRSA). (A) Reduction of mature biofilm; (B) prevention of the formation of biofilm. Two asterisks (**) indicate statistically significant difference compared to the control, with p-value <0.01. Three asterisks (***) indicate statistically significant difference compared to the control, with p-value < 0.0001. The results were analyzed by one-way ANOVA with Dunnett’s post-test.
Figure 3.
Isobolograms of combination of peptide LyeTx I mn∆K with vancomycin (A) and oxacillin (B) against methicillin-resistant Staphylococcus aureus (MRSA).
Figure 3.
Isobolograms of combination of peptide LyeTx I mn∆K with vancomycin (A) and oxacillin (B) against methicillin-resistant Staphylococcus aureus (MRSA).
Figure 4.
Release of 260-nm-absorbing intracellular material over time for methicillin-resistant Staphylococcus aureus (MRSA) suspension treated with LyeTx I mn∆K (80 µM) or vancomycin (10 µM) at 10x MIC. Two asterisks (**) indicate statistically significant difference compared to the control, with p-value <0.01. Three asterisks (***) indicate statistically significant difference compared to the control, with p-value <0.0001. Results were analyzed by One-way ANOVA with Dunnett’s post-test.
Figure 4.
Release of 260-nm-absorbing intracellular material over time for methicillin-resistant Staphylococcus aureus (MRSA) suspension treated with LyeTx I mn∆K (80 µM) or vancomycin (10 µM) at 10x MIC. Two asterisks (**) indicate statistically significant difference compared to the control, with p-value <0.01. Three asterisks (***) indicate statistically significant difference compared to the control, with p-value <0.0001. Results were analyzed by One-way ANOVA with Dunnett’s post-test.
Figure 5.
Calorimetric titration isotherms obtained from the titration of a solution of 25 μM LyeTx I mn∆K with 20 mM POPG:CL (2:1, mol/mol) unilamellar phospholipid vesicles (LUVs) (A). In the upper panel the heat flow graphs for each LUVs injection as a function of time. The lower panel shows the enthalpy as a function of the phospholipid/peptide molar ratio (the red curve represents the non-linear fitting by eq. (1)). Circular dichroism (CD) spectra of 50 µM LyeTx I mn∆K in the presence of POPG:CL (2:1) (B). CD spectra of the peptides in 10 mM Tris, 50 mM NaCl, pH 7.4 are presented by black opened circles, and after addition of different phospholipid concentration: 100 µM by red filled squares, 250 µM by blue filled upper triangles, 500 µM by magenta filled down triangles and 750 µM by filled green circles.
Figure 5.
Calorimetric titration isotherms obtained from the titration of a solution of 25 μM LyeTx I mn∆K with 20 mM POPG:CL (2:1, mol/mol) unilamellar phospholipid vesicles (LUVs) (A). In the upper panel the heat flow graphs for each LUVs injection as a function of time. The lower panel shows the enthalpy as a function of the phospholipid/peptide molar ratio (the red curve represents the non-linear fitting by eq. (1)). Circular dichroism (CD) spectra of 50 µM LyeTx I mn∆K in the presence of POPG:CL (2:1) (B). CD spectra of the peptides in 10 mM Tris, 50 mM NaCl, pH 7.4 are presented by black opened circles, and after addition of different phospholipid concentration: 100 µM by red filled squares, 250 µM by blue filled upper triangles, 500 µM by magenta filled down triangles and 750 µM by filled green circles.
Figure 6.
Circular dichroism (CD) spectra of 50 µM LyeTx I mn∆K in the presence of POPC:Chol (2:1). The CD spectra of the peptide in 10 mM Tris, 50 mM NaCl, pH 7.4 are presented by black open circles, and after addition of different phospholipid concentrations: 100 µM by red filled squares, 250 µM by blue filled upper triangles, 500 µM by magenta filled down triangles and 750 µM by filled green circles.
Figure 6.
Circular dichroism (CD) spectra of 50 µM LyeTx I mn∆K in the presence of POPC:Chol (2:1). The CD spectra of the peptide in 10 mM Tris, 50 mM NaCl, pH 7.4 are presented by black open circles, and after addition of different phospholipid concentrations: 100 µM by red filled squares, 250 µM by blue filled upper triangles, 500 µM by magenta filled down triangles and 750 µM by filled green circles.
Figure 7.
Effect of LyeTx I mn∆K on phospholipid membranes as assessed by calcein release from POPC:Chol (2:1) at 100 µM lipid concentration. (A) Time dependence of membrane permeability with LyeTx I mn∆K. (B) Percentage of calcein release as a function of peptide concentration (positive control - 2% Triton X-100, considered as 100%). Data are representative of three independent experiments.
Figure 7.
Effect of LyeTx I mn∆K on phospholipid membranes as assessed by calcein release from POPC:Chol (2:1) at 100 µM lipid concentration. (A) Time dependence of membrane permeability with LyeTx I mn∆K. (B) Percentage of calcein release as a function of peptide concentration (positive control - 2% Triton X-100, considered as 100%). Data are representative of three independent experiments.
Figure 8.
Effect of LyeTx I mn∆K on the size and charge of 200 µM POPG:CL (2:1 mol/mol) and 200 µM POPG:CL:LysylPOPG LUVs (2:2:1 mol/mol/mol). (A) Changes in the hydrodynamic diameter (Dh) and (B) in the zeta potential as a function of the peptide concentration. Data are representative of three independent experiments.
Figure 8.
Effect of LyeTx I mn∆K on the size and charge of 200 µM POPG:CL (2:1 mol/mol) and 200 µM POPG:CL:LysylPOPG LUVs (2:2:1 mol/mol/mol). (A) Changes in the hydrodynamic diameter (Dh) and (B) in the zeta potential as a function of the peptide concentration. Data are representative of three independent experiments.
Figure 9.
Effect of [LyeTx I mn∆K] on phospholipid membranes as assessed by calcein release from POPG:CL (2:1) and POPG:CL:LysylPOPG LUVs (2:2:1), both at 100 µM of lipid concentration. Time dependence of membrane permeability with LyeTx I mn∆K (a) POPG:CL (2:1); (b) POPG:CL:LysylPOPG LUVs (2:2:1); (c) observed rate constants, kobs, of CF release vs. peptide concentration and (d) percentage of calcein release compared to what was observed after addition of 2% Triton X-100 (positive control, considered as 100%). Data are representative of three independent experiments.
Figure 9.
Effect of [LyeTx I mn∆K] on phospholipid membranes as assessed by calcein release from POPG:CL (2:1) and POPG:CL:LysylPOPG LUVs (2:2:1), both at 100 µM of lipid concentration. Time dependence of membrane permeability with LyeTx I mn∆K (a) POPG:CL (2:1); (b) POPG:CL:LysylPOPG LUVs (2:2:1); (c) observed rate constants, kobs, of CF release vs. peptide concentration and (d) percentage of calcein release compared to what was observed after addition of 2% Triton X-100 (positive control, considered as 100%). Data are representative of three independent experiments.
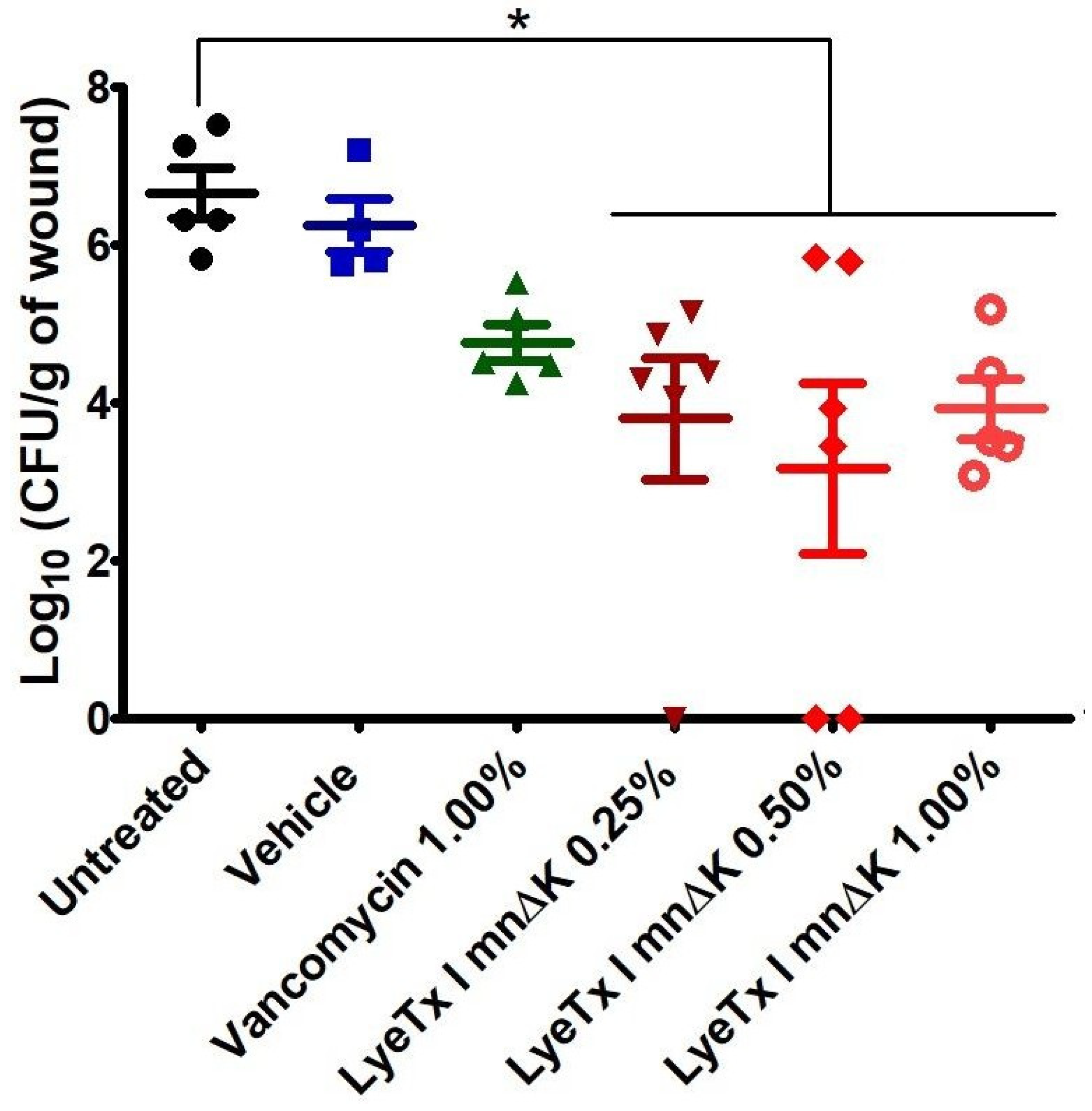
Figure 10.
Bacterial load (Log10CFU/g of wound) after the topical treatment of non-surgical methicillin-resistant Staphylococcus aureus (MRSA)-infected wounds with gel contained LyeTx I mnΔK (0.25%, 0.50%, or 1.00%), vancomycin 1%, gel ointment or saline (control) (n = 5). Mice were intradermally injected with 107 CFU of highly virulent MRSA USA300. Followed 48 h after injection, the mice developed an open wound/abscess at the local site of injection and were treated twice daily for 3 days. An asterisk (*) indicates a statistically significant difference compared to the control with p-value <0.05. All results were analyzed by one-way ANOVA with Dunnett’s post-test.
Figure 10.
Bacterial load (Log10CFU/g of wound) after the topical treatment of non-surgical methicillin-resistant Staphylococcus aureus (MRSA)-infected wounds with gel contained LyeTx I mnΔK (0.25%, 0.50%, or 1.00%), vancomycin 1%, gel ointment or saline (control) (n = 5). Mice were intradermally injected with 107 CFU of highly virulent MRSA USA300. Followed 48 h after injection, the mice developed an open wound/abscess at the local site of injection and were treated twice daily for 3 days. An asterisk (*) indicates a statistically significant difference compared to the control with p-value <0.05. All results were analyzed by one-way ANOVA with Dunnett’s post-test.
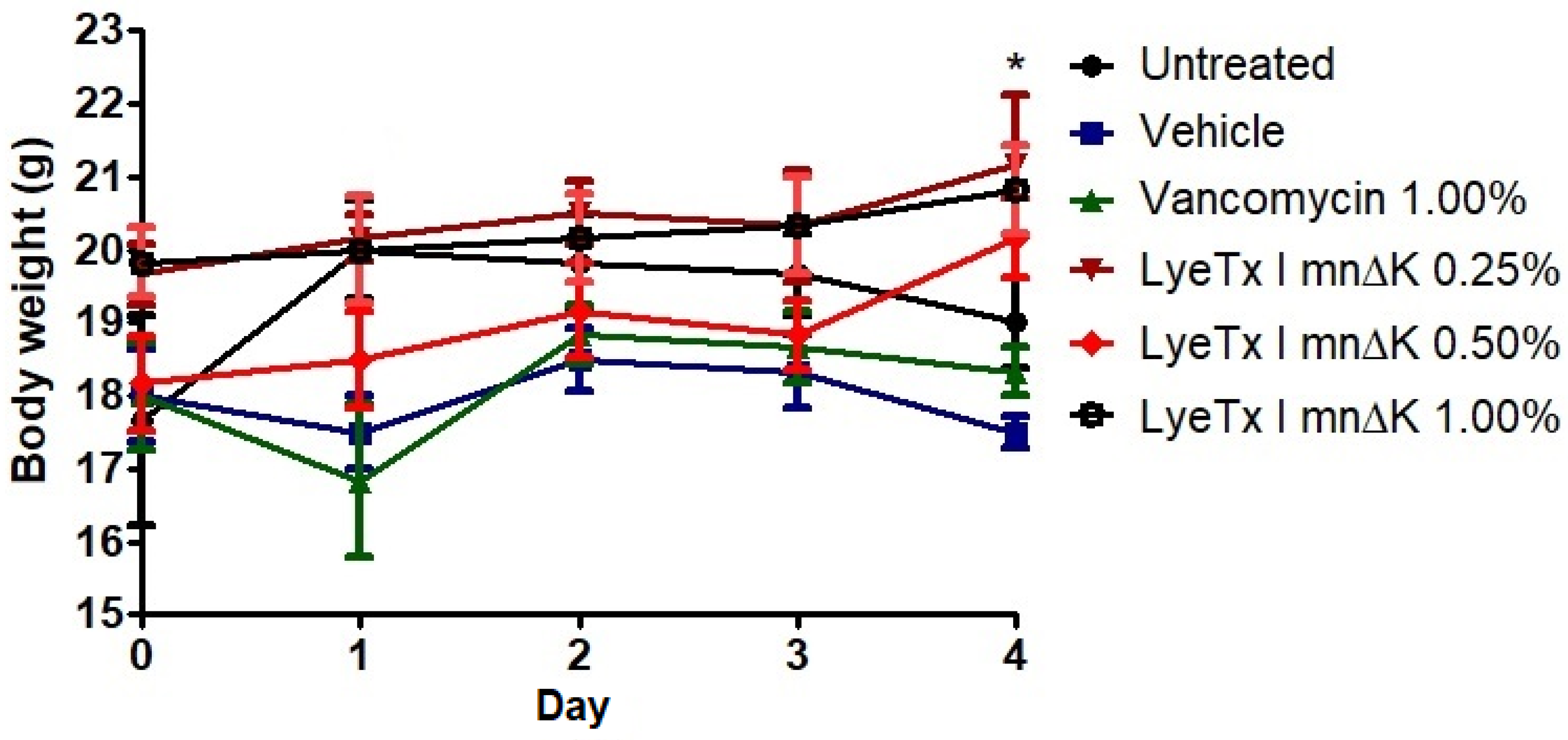
Figure 11.
Effect of the peptide on changes in body weight in mice infected with methicillin-resistant Staphylococcus aureus (MRSA). An asterisk (*) indicates a statistically significant difference between LyeTx I mnΔK 0.50% and untreated group with p-value <0.05. All results were analyzed by one-way ANOVA with Dunnett’s post-test.
Figure 11.
Effect of the peptide on changes in body weight in mice infected with methicillin-resistant Staphylococcus aureus (MRSA). An asterisk (*) indicates a statistically significant difference between LyeTx I mnΔK 0.50% and untreated group with p-value <0.05. All results were analyzed by one-way ANOVA with Dunnett’s post-test.
Table 1.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of LyeTx I mnΔK and vancomycin against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA).
Table 1.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of LyeTx I mnΔK and vancomycin against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA).
Microorganism |
Clinical origin |
Antibacterial activity (µM) |
| LyeTx I mnΔK |
Vancomycin |
| MIC |
MBC |
MIC |
MBC |
|
S. aureus 11 |
Infected wound |
8 |
32 |
1 |
8 |
|
S. aureus 29 |
Infected wound |
8 |
16 |
1 |
2 |
|
S. aureus 130 |
Infected wound |
8 |
32 |
1 |
2 |
|
S. aureus 366 |
Infected wound |
16 |
32 |
1 |
8 |
|
S. aureus 524 |
Infected wound |
16 |
32 |
1 |
2 |
|
S. aureus 526 |
Infected wound |
16 |
32 |
1 |
4 |
| MRSA USA300 |
Infected wound |
8 |
16 |
1 |
2 |
| MIC50 |
8 |
1 |
| MBC50 |
32 |
2 |
Table 2.
Range of fractional inhibitory concentration (FIC) and fractional inhibitory concentration index (FICI) of LyeTx I mn∆K in combination with vancomycin or oxacillin against methicillin-resistant Staphylococcus aureus (MRSA).
Table 2.
Range of fractional inhibitory concentration (FIC) and fractional inhibitory concentration index (FICI) of LyeTx I mn∆K in combination with vancomycin or oxacillin against methicillin-resistant Staphylococcus aureus (MRSA).
Class |
Antimicrobials |
FIC |
FICI
(ΣFIC) |
Effect |
| LyeTx I mnΔK |
Antimicrobial |
| Glycopeptide |
Vancomycin |
1.00 |
0.50 |
1.50 |
Indifferent |
| β-lactam |
Oxacillin |
1.00 |
0.02 |
1.02 |
Indifferent |
Table 3.
Resensitization of methicillin-resistant Staphylococcus aureus (MRSA) to vancomycin and oxacillin using a sub-inhibitory concentration (1/4x MIC) of LyeTx I mnΔK for one hour.
Table 3.
Resensitization of methicillin-resistant Staphylococcus aureus (MRSA) to vancomycin and oxacillin using a sub-inhibitory concentration (1/4x MIC) of LyeTx I mnΔK for one hour.
| Antibacterial |
MICs (µg/mL) |
Fold resensitization |
| Not exposed to LyeTx I mnΔK |
Exposed to LyeTx I mnΔK |
| Vancomycin |
1.0 |
0.5 |
2 |
| Oxacillin |
128 |
64 |
2 |
Table 4.
Effect of LyeTx I mn∆K on kobs for the leakage of calcein from POPG:CL and POPG:CL:LysylPOPG LUVs.
Table 4.
Effect of LyeTx I mn∆K on kobs for the leakage of calcein from POPG:CL and POPG:CL:LysylPOPG LUVs.
| LyeTx I mn∆K (mM) |
POPG:CL |
POPG:CL:Lysyl:POPG |
%calcein
released |
SD* |
kobs (s-1) x10-2
|
SD*
x10-4
|
%calcein
released |
SD* |
kobs (s-1) x10-2
|
SD*
x10-4
|
| 4 |
28.51 |
2.50 |
0.78 |
5.01 |
15.98 |
1.06 |
0.629 |
2.23 |
| 8 |
55.11 |
1.83 |
1.01 |
2.82 |
33.74 |
1.01 |
0.615 |
1.56 |
| 18 |
72.23 |
1.49 |
1.19 |
3.81 |
67.73 |
1.30 |
0.755 |
1.13 |
| 32 |
79.45 |
3.82 |
1.46 |
4.36 |
75.17 |
2.20 |
0.853 |
7.10 |
| 64 |
82.17 |
2.04 |
2.16 |
12.5 |
77.23 |
1.99 |
1.225 |
4.89 |
Table 5.
Sample composition used for hydrodynamic diameter (Dh) and zeta potential ( P) measurements.
Table 5.
Sample composition used for hydrodynamic diameter (Dh) and zeta potential ( P) measurements.
| Sample |
Vpep ( L) |
VLUVs ( L) |
Vbuffer ( L) |
[LyeTx I mn K] ( M) |
| 1 |
0 |
400 |
400 |
0 |
| 2 |
4 |
400 |
396 |
5 |
| 3 |
8 |
400 |
392 |
10 |
| 4 |
12 |
400 |
388 |
15 |
| 5 |
16 |
400 |
384 |
20 |
| 6 |
24 |
400 |
376 |
30 |
| 7 |
32 |
400 |
368 |
40 |
| 8 |
40 |
400 |
360 |
50 |
| 9 |
50 |
400 |
350 |
62.5 |
| 10 |
60 |
400 |
340 |
75 |
Table 6.
Samples composition used for calcein release measurements.
Table 6.
Samples composition used for calcein release measurements.
| Sample |
Vpep (μL) |
VLUVs (μL) |
Vbuffer (μL) |
[LyeTx I mnΔK] (μM) |
| 1 |
0 |
150 |
150 |
0 |
| 2 |
5 |
150 |
145 |
4,2 |
| 3 |
10 |
150 |
140 |
8,3 |
| 4 |
20 |
150 |
130 |
16,7 |
| 5 |
40 |
150 |
110 |
33,3 |
| 6 |
80 |
150 |
70 |
66,7 |
Table 7.
Components used for the formulation of LyeTx I mnΔK.
Table 7.
Components used for the formulation of LyeTx I mnΔK.
| Component |
Concentration |
| Hydroxyethylcellulose (Natrosol®) |
2.2% |
| Sodium metabisulfite |
0.6% |
| Methylparaben (Nipagin®) |
0.2% |
| Distilled water |
q.s. |