Submitted:
26 January 2024
Posted:
26 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
References
- Almetwally, A.; Bin-Jumah, A. Ambient air pollution and its influence on human health and welfare: an overview. Environ Sci Pollut Res Int 2020, 27, 24815–24830. [Google Scholar] [CrossRef]
- Valverde, M.; Granados, A.; Milić, M.; Ceppi, M.; Sollano, L.; Bonassi, S.; Rojas, E. Effect of Air Pollution on the Basal DNA Damage of Mother–Newborn Couples of México City. Toxics 2023, 11, 766. [Google Scholar] [CrossRef]
- Glinianaia SV, R.J., Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology 2004, 15, 36–45. [CrossRef]
- Maisonet M, C.A., Misra D, Jaakkola JJK. A review of the literature on the effects of ambient air pollution on fetal growth. Environmental Research 2004, 95, 106–115. [CrossRef]
- O'Neill, M.; Osornio-Vargas, A.R.; Buxton, M.; Sanchez, B.; Rojas-Bracho, L.; Castillo-Castrejon, M.; Mordhukovich, I.; Brown, D.; Vadillo-Ortega, F. Air pollution, inflammation and preterm birth in Mexico City: study design and methods. Science of Total Environment 2013, 448, 79–83. [Google Scholar] [CrossRef]
- Ritz B, W.M., Hoggatt KJ, Ghosh JKC. Ambient air pollution and preterm birth in the Environment and Pregnancy Outcomes Study at the University of California, Los Angeles. American Journal of Epidemiology DOI: 10.1093/aje/kwm181(American Journal of Epidemiology Advance Access published August 4, 2007). American Journal of Epidemiology 2007, 166, 1045–1052. [CrossRef]
- Naclerio, R.; Ansotegui, I.; Bousquet, J.; Canonica, W.; D'Amato, G.; Rosario, N.; Pawankar, R.; Peden, D.; Bergmann, K.; Bielory, L.; Caraballo, L.; Cecchi, L.; A, C.; Rouadi, P. International expert consensus on the management of allergic rhinitis (AR) aggravated by air pollutants: impact of air pollution on patients with AR: current knowledge and future strategies. World Allergy Organization Journal 2020, 13, 100106. [Google Scholar] [CrossRef]
- Nel AE, D.-S.D.; Li, N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Current Opinion in Pulmonary Medicine 2001, 7, 20–26. [Google Scholar] [CrossRef]
- Saldiva PH, C.R. Saldiva PH, C.R., Coull BA, Stearns RC, Lawrence J, Murthy GG, et al. Lung inflammation induced by concentrated ambient air particles is related to particle composition. American Journal of Respiratory and Critical Care Medicine 2002, 165, 1610–1617. [Google Scholar] [CrossRef]
- Contiero, P.; Boffi, R.; Tagliabue, G.; Scaburri, A.; Tittarelli, A.; Bertoldi, M.; Borgini, A.; Favia, I.; Ruprecht, A.; Maiorino, A.; Voza, A.; Ripoll Pons, M.; Cau, A.; Demarco, C.; Allegri, F.; Tresoldi, F.; Ciccarelli, M. A case-crossover study to investigate the effects of atmospheric particulate matter concentrations, season, and air temperature on accident and emergency presentations for cardiovascular events in Northern Italy. Int J Environ Res Public Health 2019, 16, 4627. [Google Scholar] [CrossRef]
- Delfino, R.; Sioutas, C.; Malik, S. Potential role of ultrafine particles in association between airborne particle mass and cardiovascular health. Environmental Health Perspectives 2005, 113, 934–946. [Google Scholar] [CrossRef]
- Mann, J.; Tager, I.; Lurmann, F.; Segal, M.; Quesenberry, C.; MM, L. Air pollution and hospital admissions for ischemic heart disease: a prospective study and metanalysis. Environmental Health Perspectives 2002, 110, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.; Burnet, R.; Thurston, G.; Thun, M.; Calle, E.; Krewski, D. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef]
- Alfaro-Moreno, E.; Ponce de Leon, S.; Osornio-Vargas, A.; Garcia-Cuellar, C.; Martinez, L.; Rosas, I. Potential toxic effects associated to metals and endotoxin present in PM10: an ancillary study using multivariate analysis. Inhalation Toxicology 2007, 19, 49–53. [Google Scholar] [CrossRef]
- Peters A, F.M., Doring A, Immervoll T, Wichmann HE, Hutchinson WL, et al. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. European Heart Journal 2001, 22, 1198–1204. [CrossRef]
- Risom L, M.P., Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutation Research 2005, 592, 119–137. [CrossRef]
- Bello-Medina, P.; Rodríguez-Martínez, E.; Prado-Alcalá, R.; Rivas-Arancibia, S. Ozone pollution, oxidative stress, synaptic plasticity, and neurodegeneration. Neurologia (English Edition) 2021;in press.
- Ovrevik, J.; Refsnes, M.; Lag, M.; Holme, J.; Schwarze, P. Activation of pro-inflammatory responses in the airway mucosa cells by particulate matter. Oxidant- and non-oxidant-mediated triggering mechanisms. Biomolecules 2015, 5, 1399–1440. [Google Scholar]
- Rouadi, P.; Idriss, S.; Naclerio, R.; Peden, D.; Ansotegui, I.; Canonica, G.; Gonzalez-Diaz, S.; Rosario Filho, N.; Ivancevich, J.; Hellings, P.; Murrieta-Aguttes, M.; Zaitoun, F.; Irani, C.; Karam, M.; Bousquet, J. Immunopathological features of air pollution and its impact on inflammatory airways diseases (IAD). World Allergy Organ J 2020, 13, 100467. [Google Scholar] [CrossRef]
- Li, N.; Georas, S.; Alexis, N.; Fritz, P.; Xia, T. A work report on ultrafine particles (American Academy of Allergy, Asthma and Immunology): why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J Allergy Clin Immunol 2016, 138, 386–396. [Google Scholar] [CrossRef]
- Lowther, S.; Jones, K.; Wang, X.; Whyatt, J.; Wild, O.; Booker, D. Particulate matter measurement indoors: a review of metrics, sensors, needs, and applications. Environ Sci Technol 2019, 53, 11644–11656. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Perez-Guevara, F.; Roy, P.; Elizalde-Martinez, I.; Shruti, V. Impacts of the COVID-19 lockdown on air quality and its association with human mortality trends in megalopolis Mexico City. Air Qual Atmosph Health 2021, 14, 553–562. [Google Scholar] [CrossRef]
- Lopez-Feldman, A.; Heres, D.; Marquez-Padilla, F. Air pollution exposure and COVID-19: a look at mortality in Mexico City using individual-level data. Science of Total Environment 2021, 756, 143929. [Google Scholar] [CrossRef]
- Lin, Y.; Lai, C.; Chu, C. Air pollution diffusion simulation and seasonal spatial risk analysis for industrial areas. Environmental Research 2020, 194, 110693. [Google Scholar] [CrossRef]
- Sarkar, S.; Rivas-Santiago, C.; Ibironke, O.; Carranza, C.; Meng, Q.; Osornio-Vargas, A.; Zhang, J.; Torres, M.; Chow, J.; Watson, J.; Ohman-Strickland, P.; Schwander, S. Season and size of urban particulate matter differentially affect cytotoxicity and human immune responses to Mycobacterium tuberculosis. PlosOne 2019, 14, e0219122.
- Lyu, Y.; Su, S.; Wang, B.; Zhu, X.; Wang, X.; Zeng, E.; Xing, B.; Tao, S. Seasonal and spatial variations in the chemical components and the cellular effects of particulate matter collected in Northern China. The Science of the total environment 2018, 627, 1627–1637. [Google Scholar] [CrossRef]
- Manzano-León, N.; Quintana, R.; Sánchez, B.; Serrano, J.; Vega, E.; Osornio, A. Variation in the composition and in vitro pro-inflammatory effect of urban particulate matter from different sites. J Biochem Mol Toxicol 2013, 27, 87–97. [Google Scholar] [CrossRef]
- Mirowsky, J.; Hickey, C.; Horton, L.; Blaustein, M.; K, G. The effect of particle size, location, and season on urban and rural particulate matter toxicity. Inhal Toxicol 2013, 25, 747–757. [Google Scholar] [CrossRef]
- Mamkhezri, J.; Bohara, A.K.; Islas Camargo, A. Air pollution and daily mortality in the Mexico City Metropolitan Area. Atmósfera 2020, 33, 249–267. [Google Scholar] [CrossRef]
- World Health Organization. WHO ambient /outdoor) air quality database. In: WHO, ed. Geneva, Switzerland: WHO; 2013.
- Astudillo-García, C.; Rodriguez-Villamizar, L.; Cortez-Lugo, M.; Cruz -De la Cruz, J.; Fernandez -Niño, J. Air pollution and suicide in Mexico City: a time series analysis, 2000-2016. International Environmental Research and Public Health 2019, 16, 2971. [Google Scholar] [CrossRef]
- SMA-GDF. Inventario de emisiones de la Zona Metropolitana del Valle de Mexico 2010. in: Secretaría del Medio Ambiente G.d.D.F., ed. Ciudad de México: Secretaría del Medio Ambiente,Gobierno del D.F.; 2012.
- Manzano-Leon, N.; Serrano-Lomelín, J.; Sanchez, B.; Quintana-Belmares, R.; Vega, E.; Osornio-Vargas, A. TNF-alpha and IL-6 responses to particulate matter in vitro: variation according to PM size, season, and polycyclic aromatic hydrocarbon and soil content. Environmental Health Perspectives 2016, 124, 406–412. [Google Scholar] [CrossRef]
- Zeb, B.; Alam, K.; Sorooshian, A.; Blaschke, T.; Ahmad, I.; Shahid, I. On the morphology and composition of particulate matter in an urban environment. Aerosol Air Qual Res 2018, 18, 1431–1447. [Google Scholar]
- INE Instituto Nacional de Ecología. Cuarto almanaque de datos y tendencias de la calidad del aire en 20 ciudades mexicanas (2000-2009) (1ra ed.). Ciudad de México: Gobierno Federal, SEMARNAT. 2011.
- Palacio, F.; Apodaca, M.; Crisci, J. Análisis multivariado para datos biológicos, teoría y su aplicación utilizando el lenguaje R ed^eds. Mexico City: Vázquez Mazzini Editores; 2020.
- Bosshart, H.; Heizelmann, M. THP-1 cells as a model for human monocytes. Ann Transl Med 2016, 4, 438. [Google Scholar] [CrossRef]
- Chanput, W.; Mess, J.; Wichers, H. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Kimura, A.; Naka, T.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fuji-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. Journal of Experimental Medicine 2009, 206, 2027–2035. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Salado, A.; Vaquera-Huerta, H.; Aguirre-Salado, C.; Reyes-Mora, S.; Olvera-Cervantes, A. Developing a hierarchical model for the spatial analysis of PM10 pollution extremes in the Mexico City metropolitan area. Int J Environ Res Public Health 2017, 14, 734. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Dailey, L.; Soukup, J.; Grambow, S.; Devlin, R.; Huang, Y. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ Health Perspect 2005, 113, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Corona-Vazquez, T.; Flores, R.; Rodríguez, V.; Cervantes-Arriaga, A. Air pollution,multiple sclerosis and its relevance to Mexico City. Archives of medical research 2019, 50, 111–112. [Google Scholar] [CrossRef]
- Gutierrez, I.; Calderon Nepomuceno, D.; Gutierrez Cruz, D.; Aquina Verga, E. Correlación entre diferentes contaminantes atmosféricos de la Ciudad de México y el área metropolitana. Ciencia Ergo Sum Revista Científica Multidisciplinaria de Prospectiva 2020, 27, 1. [Google Scholar]
- Secretaría del Medio Ambiente, G.d.D.F. Informe climatológico ambiental del Valle de México. in: Secretaría del Medio Ambiente G.d.D.F., ed. Ciudad de México: Secretaría del Medio Ambiente, Gobierno del D.F. 2005. [Google Scholar]
- Januszek, R.; Staszczak, B.; Siudak, Z.; Bartus, J.; Plens, K.; Bartus, S.; Dudek, D. The relationship between increased air pollution expressed as PM10 concentration and the frequency of percutaneous coronary. Environ Sci Pollut Res 2020, 27, 21320–21330. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.; Rice, A.; Lindroos, P.; O'Brien, P.; Dreher, K.; Rosas, I.; Alfaro-Moreno, E.; Osornio-Vargas, A. Induction of the lung myofibroblast PDGF receptor system by urban ambient particles from Mexico City. American Journal of Respiratory Cell and Molecular Biology 1998, 19, 672–680. [Google Scholar] [CrossRef]
- Rosas Perez I, S.J. Rosas Perez I, S.J., Alfaro-Moreno E, Baumgardner D, Garcia-Cuellar C, Martin Del Campo JM, Raga GB, Castillejos M, Colin RD, Osornio Vargas AR. Relations between PM10 composition and cell toxicity: a multivariate and graphical approach. Chemosphere 2007, 67, 1218–1228. [Google Scholar]
- Chirino, Y.; Sánchez-Perez, Y.; Osornio-Vargas, A.; Rosas, I.; García-Cuellar, C. Sampling and composition of airborne particulate matter (PM10) from two locations of Mexico City. Data in Brief 2015, 4, 353–356. [Google Scholar] [CrossRef]
- Alfaro-Moreno, E.; Martínez, L.; García-Cuellar, C.; Bonner, J.; Murray, J.; Rosas, I.; Rosales, S.; Osornio-Vargas, A. Biologic effects induced in vitro by PM10 from three zones of Mexico City. Environmental Health Perspectives 2002, 110, 715–720. [Google Scholar] [CrossRef]
- Tian, Y.; Ke, S.; Denison, MS.; Rabson, AB.; Gallo, MA. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999, 274, 510–5. [Google Scholar] [CrossRef]
- Fardel, O. Cytokines as molecular targets for aryl hydrocarbon receptor ligands: implications for toxicity and xenobiotic detoxification. Expert Opin Drug Metab Toxicol. 2013, 9, 141–52. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, T.; Abruzzo, P.M.; Bolotta, A. More than a cell biosensor: aryl hydrocarbon receptor at the intersection of physiology and inflammation. Am J Physiol Cell Physiol. 2020, 318, C1078–C1082. [Google Scholar] [CrossRef] [PubMed]
- Huai, W.; Zhao, R.; Song, H.; Zhao, J.; Zhang, L.; Gao, C.; Han, L.; Zhao, W. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nat Commun. 2014, 5, 4738. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Rabson, AB.; Germino, JF.; Gallo, MA.; Tian, Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 2001, 276, 39638–44. [Google Scholar] [CrossRef] [PubMed]
- Guarneros, M.; López-Rivera, C.; Gonsebatt, M.E.; Alcaraz-Zubeldia, M.; Hummel, H.; Schriever, V.A.; Valdez, B.; Hudson, R. Metal-containing particulate matter and associated reduced olfatory identification ability in children from an area of high atmospheric exposure in Mexico City. Chemical Senses 2020, 45, 59–67. [Google Scholar] [CrossRef]
- Maciel-Ruiz, J.A.; López-Rivera, C.; Robles-Morales, R.; Veloz-Martínez, M.G.; López-Arellano, R.; Rodríguez-Patiño, G.; Petrosyan, P.; Govezensky, T.; Salazar, A.M.; Ostrosky-Wegman, P.; Montero-Montoya, R.; Gonsebatt, M.E. Prenatal exposure to particulate matter and ozone: bulky DNA adducts, plasma isoprostanes, allele risk variants, and neonate susceptibility in the Mexico City Metropolitan Area. Environmental and Molecular Mutagenesis 2019, 60, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Jeaniean, M.; Bind, M.; Roux, J. Ozone, NO2 and PM10 are associated with the occurrence of multiple sclerosis relapses. Evidence from seasonal multi-pollutant analyses. Environment Research 2018, 163, 43–52. [Google Scholar]
- Roux, J.; Bard, D.; LePabic, E. Air pollution by particulate matter PM10 may trigger multiple sclerosis relapses. Environ Res 2017, 156, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Buxton, M.A.; Perng, W.; Tellez-Rojo, M.M.; Rodríguez-Carmona, Y.; Cantoral, A.; Sánchez, B.N.; Rivera-González, L.O.; Gronlund, C.J.; Shivappa, N.; Hébert, J.R.; O'Neill, M.S.; Peterson, K.E. Particulate matter exposure, dietary inflammatory index and preterm birth in Mexico city, Mexico. Environ Res. 2020, 189, 109852. [Google Scholar] [CrossRef]
- Oliva-Sánchez, P.; Landeros-López, S.; Velazquez-Trejo, D.; Martínez-Kobeh, J.P.; Vadillo-Ortega, F. COVID-19 epidemiological indicators and exposition to airborne particle matter in Mexico City. Environm Res 2021;(submitted).
- Antonio-Villa, N.E.; Bello-Chavolla, O.Y.; Fermín-Martínez, C.A.; Aburto, J.M.; Fernández-Chirino, L.; Ramírez-García, D.; Pisanty-Alatorre, J.; González-Díaz, A.; Vargas-Vázquez, A.; Barquera, S.; Gutiérrez-Robledo, L.M.; Seiglie, J.A. Socio-demographic inequalities and excess non-COVID-19 mortality during the COVID-19 pandemic: a data-driven analysis of 1 069 174 death certificates in Mexico. Int J Epidemiol 2022, 51, 1711–1721. [Google Scholar] [CrossRef]
- Carlsten, C.; Salvi, S.; Wong, G.W.K.; Chung, K.F. Personal strategies to minimize effects of air pollution on respiratory health: advice for providers, patients and the public. Eur Respir J 2020, 55, 902056. [Google Scholar] [CrossRef]
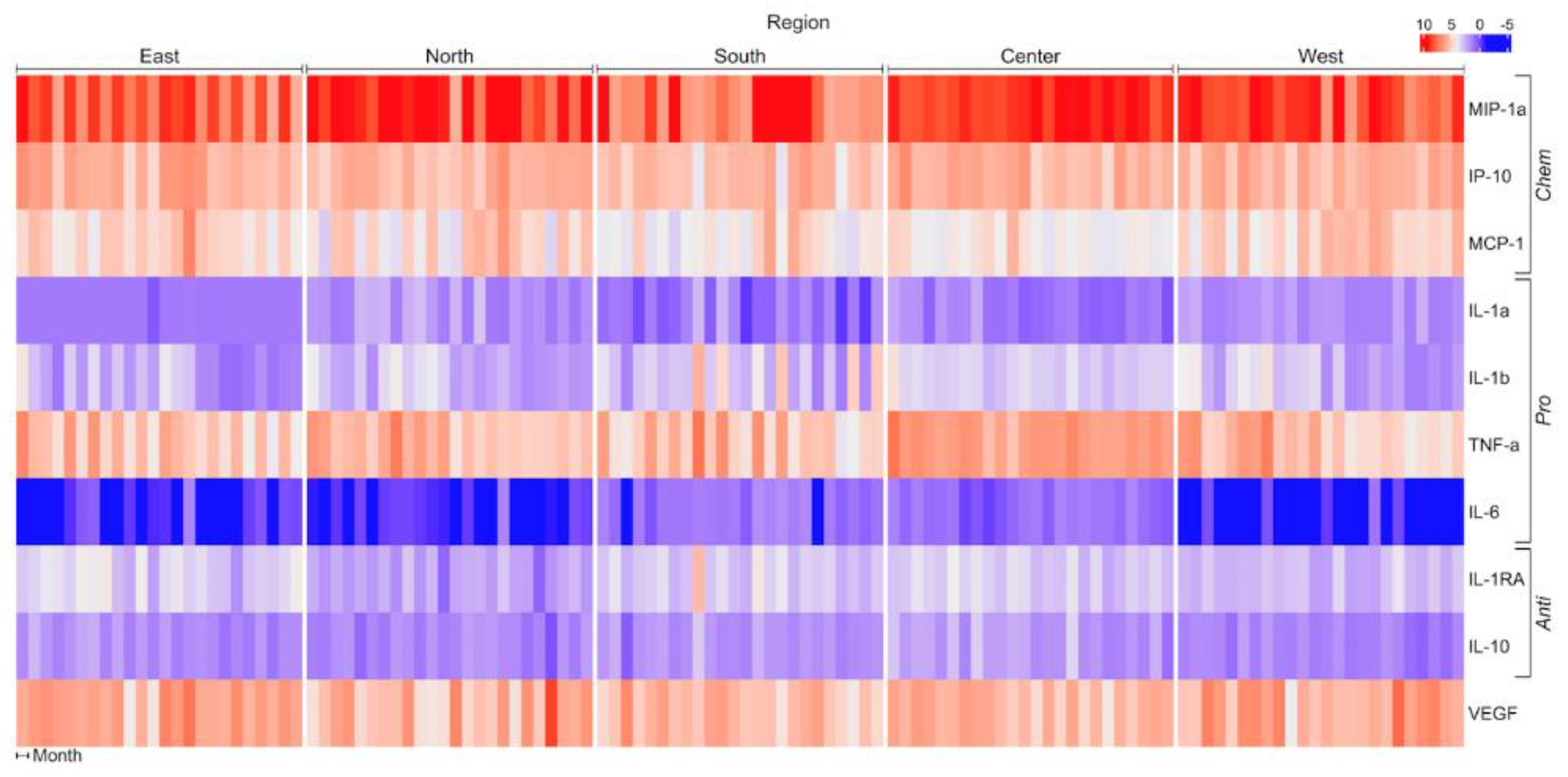
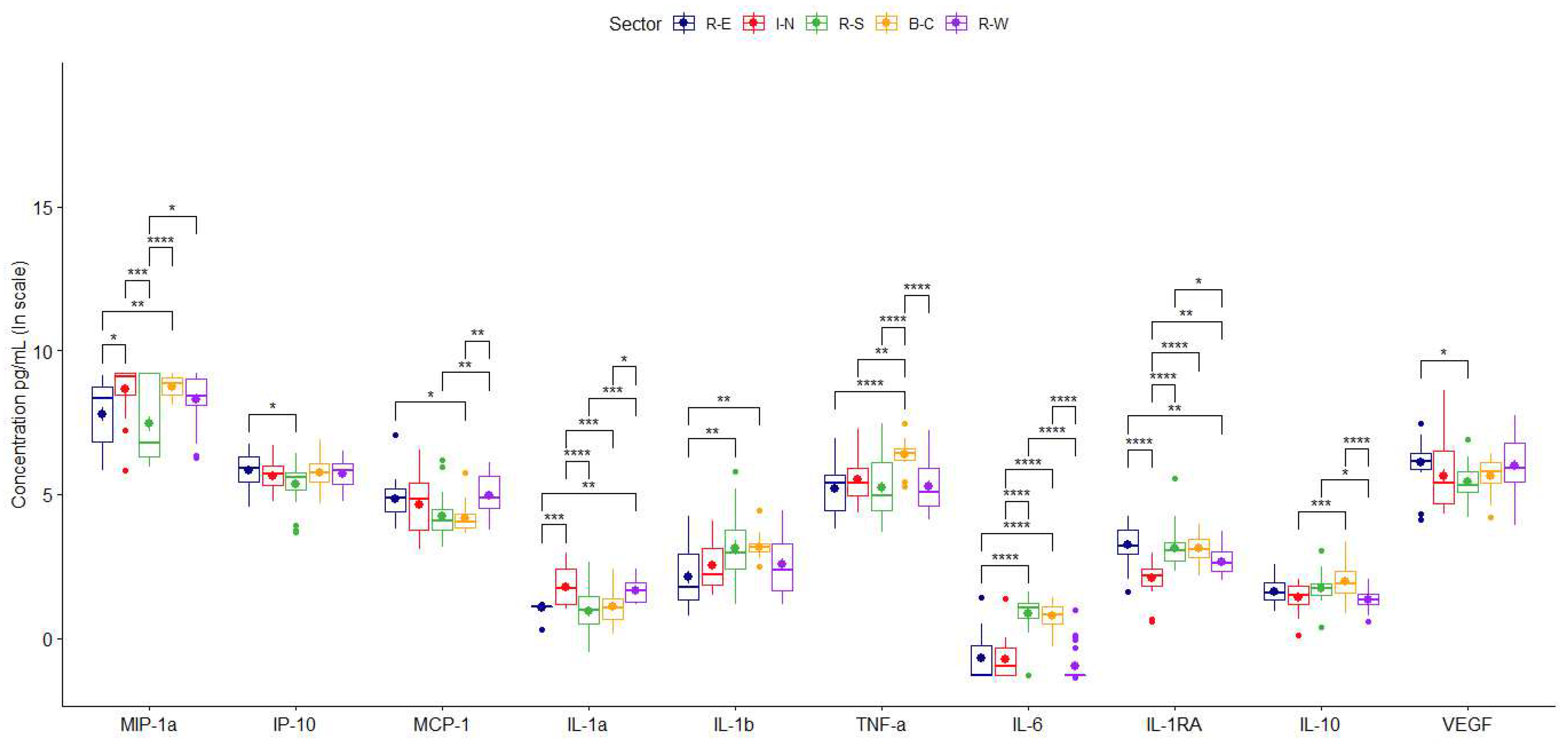
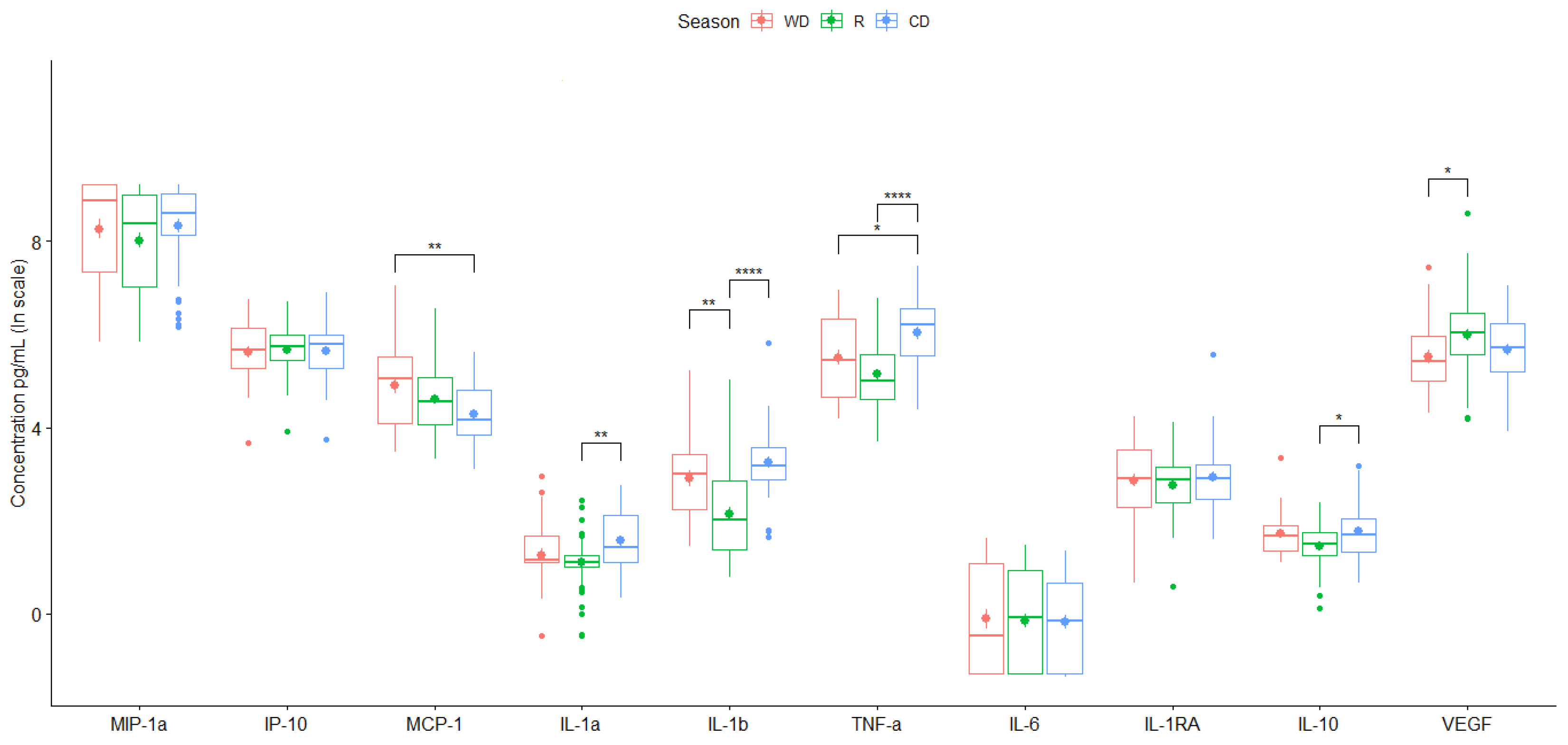

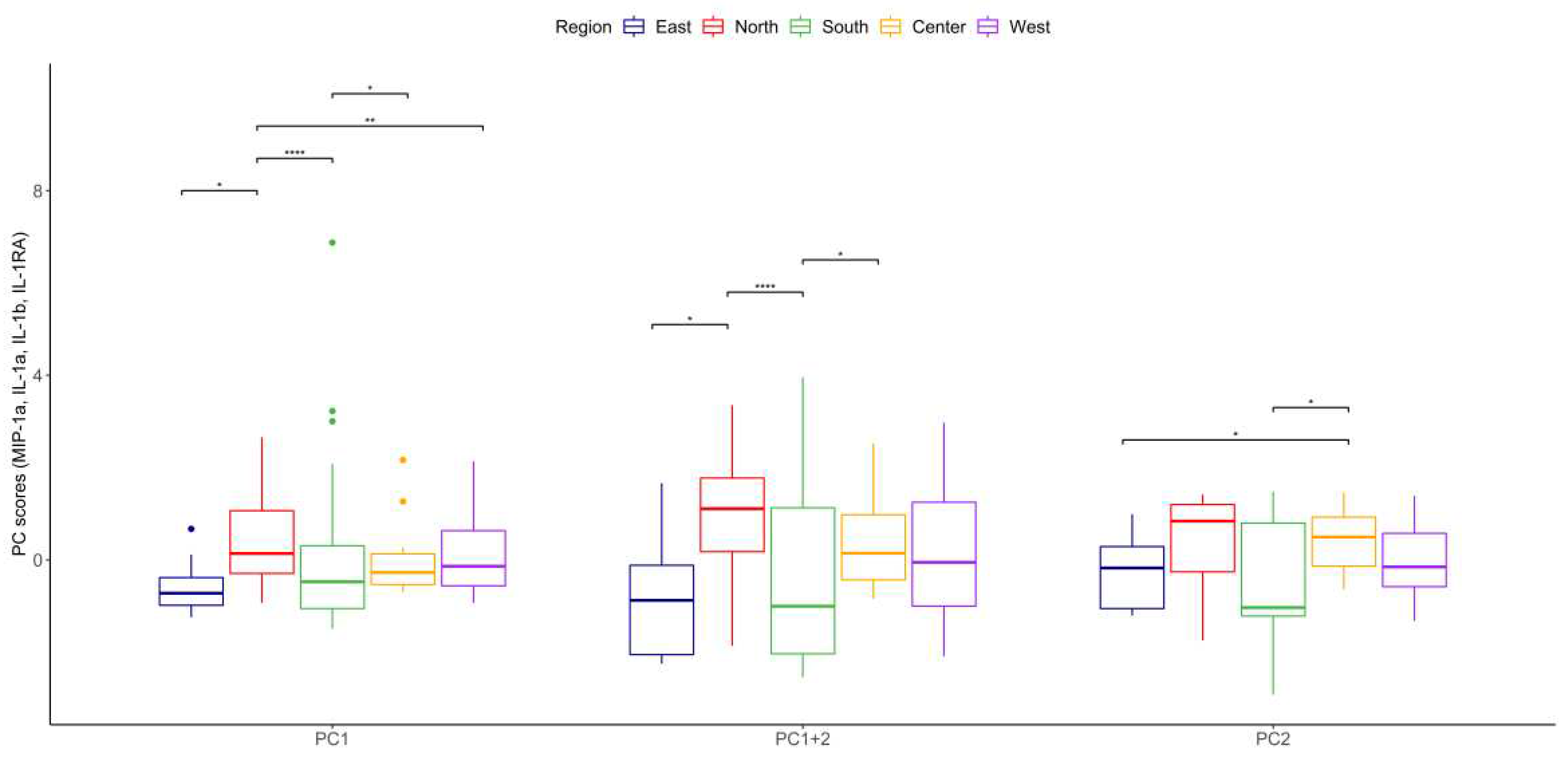
| Sector | |||||
|---|---|---|---|---|---|
| Residential-East (n = 24) |
Industrial-North (n = 24) |
Residential-South (n = 24) |
Business-Center (n = 24) |
Residential-West (n = 24) |
|
| Cytokine | Mean (95% CIa) |
Mean (95% CIa) |
Mean (95% CIa) |
Mean (95% CIa) |
Mean (95% CIa) |
| Chemotactic | |||||
| MIP-1α | 3753.16 (2558.64, 4947.68) |
7208.64 (5737.58, 8679.70) |
3731.48 (1933.75, 5529.22) |
6687.41 (5671.14, 7703.68) |
5371.56 (3996.32, 6746.81) |
| IP-10 | 401.28 (305.08, 497.48) |
314.71 (249.20, 380.22) |
251.75 (193.87, 309.63) |
360.32 (278.25, 442.39) |
341.20 (273.55, 408.85) |
| MCP-1 | 170.07 (78.26, 261.89) |
155.29 (92.50, 218.08) |
98.79 (52.01, 145.57) |
74.78 (51.14, 98.43) |
175.82 (128.74, 222.89) |
| Pro-inflammatory | |||||
| IL-1α | 2.93 (2.78, 3.07) |
7.11 (5.10, 9.12) |
3.57 (2.10, 5.05) |
3.57 (2.53, 4.60) |
5.62 (4.55, 6.69) |
| IL-1β | 13.96 (7.11, 20.81) |
17.65 (10.94, 24.37) |
52.41 (19.31, 85.52) |
26.38 (20.29, 32.48) |
21.14 (11.94, 30.34) |
| TNF-α | 266.62 (160.49, 372.76) |
323.55 (197.03, 450.06) |
318.79 (152.66, 484.91) |
652.74 (525, 780.47) |
301.23 (164.04, 438.43) |
| IL-6 | 0.71 (0.35, 1.06) |
0.64 (0.33, 0.96) |
2.79 (2.26, 3.31) |
2.37 (1.97, 2.77) |
0.49 (0.26, 0.72) |
| Anti-inflammatory | |||||
| IL-1RA | 30.59 (23.32, 37.86) |
9.13 (7.26, 11.01) |
33.46 (12.26, 54.67) |
24.50 (20.01, 28.98) |
15.68 (12.36, 18.99) |
| IL-10 | 5.58 (4.48, 6.68) |
4.55 (3.81, 5.29) |
6.46 (4.82, 8.11) |
8.95 (6.17, 11.73) |
4.01 (3.40, 4.62) |
| Growth factor | |||||
| VEGF | 551.93 (405.15, 698.70) |
566.49 (110.62, 1022.35) |
283.30 (193.44, 373.16) |
324.72 (259.72, 389.73) |
569.17 (353.70, 784.64) |
| Sector | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R-E, I-N | R-E, R-S | R-E, B-C | R-E, R-W | I-N, R-S | I-N, B-C | I-N, R-W | R-S, B-C | R-S, R-W | B-C, R-W | ||
| Chemotactic | |||||||||||
| MIP-1α |
DBM (95% CI) |
0.87 (0.09, 1.64) |
-0.32 (-1.10, 0.45) |
0.96 (0.19, 1.74) |
0.53 (-0.25, 1.30) |
-1.19 (-1.96, -0.42) |
0.10 (-0.68, 0.87) |
-0.34 (-1.11, 0.43) |
1.29 (0.51, 2.06) |
0.85 (0.08, 1.62) |
-0.44 (-1.21, 0.34) |
| p-value | 1.97e-02* | 7.75e-01 | 6.76e-03** | 3.28e-01 | 3.92e-04*** | 9.97e-01 | 7.42e-01 | 9.99e-05**** | 2.35e-02* | 5.22e-01 | |
| IP-10 |
DBM (95% CI) |
-0.18 (-0.64, 0.29) |
-0.48 (-0.95, -0.02) |
-0.06 (-0.53, 0.41) |
-0.11 (0.57, 0.36) |
-0.31 (-0.77, 0.16) |
0.12 (-0.35, 0.58) |
0.07 (-0.39, 0.54) |
0.42 (-0.04, 0.89) |
0.38 (-0.09, 0.85) |
-0.05 (-0.51, 0.42) |
| p-value | 8.29e-01 | 3.83e-02* | 9.97e-01 | 9.71e-01 | 3.68e-01 | 9.56e-01 | 9.93e-01 | 9.37e-02 | 1.70e-01 | 9.99e-01 | |
| MCP-1 |
DBM (95% CI) |
-0.18 (-0.75, 0.40) |
-0.57 (-1.15, 0.01) |
-0.66 (-1.23, -0.08) |
0.13 (-0.45, 0.71) |
-0.39 (-0.97, 0.19) |
-0.48 (-1.06, 0.10) |
0.31 (-0.27, 0.88) |
-0.09 (-0.66, 0.49) |
0.70 (0.12, 1.28) |
0.79 (0.21, 1.36) |
| p-value | 9.15e-01 | 5.58e-02 | 1.75e-02* | 9.71e-01 | 3.33e-01 | 1.52e-01 | 5.83e-01 | 9.93e-01 | 9.41e-03** | 2.36e-03** | |
| Pro-inflammatory | |||||||||||
| IL-1α |
DBM (95% CI) |
0.70 (0.24, 1.16) |
-0.14 (-0.60, 0.32) |
0.03 (-0.43, 0.49) |
0.57 (0.12, 1.03) |
-0.84 (-1.30, -0.38) |
-0.67 (-1.13, -0.21) |
-0.13 (-0.59, 0.33) |
0.17 (-0.29, 0.63) |
0.71 (0.25, 1.17) |
0.54 (0.08, 1.00) |
| p-value | 4.30e-04 | 9.18e-01 | 1.00e+00 | 6.44e-03** | 1.44e-05**** | 9.02e-04*** | 9.39e-01 | 8.37e-01 | 3.28e-04*** | 1.20e-02* | |
| IL-1β |
DBM (95% CI) |
0.42 (-0.34, 1.19) |
1.02 (0.26, 1.78) |
1.07 (0.30, 1.83) |
0.44 (-0.32, 1.20) |
0.60 (-0.16, 1.36) |
0.64 (-0.12, 1.41) |
0.02 (-0.74, 0.78) |
0.04 (-0.72, 0.81) |
-0.58 (-1.34, 0.18) |
-0.62 (-1.39, 0.14) |
| p-value | 5.45e-01 | 2.94e-03** | 1.69e-03** | 4.99e-01 | 1.96e-01 | 1.41e-01 | 1.00e+00 | 1.00e+00 | 2.25e-01 | 1.64e-01 | |
| TNF-α |
DBM (95% CI) |
0.29 (-0.35, 0.94) |
0.02 (-0.62, 0.67) |
1.18 (0.53, 1.83) |
0.07 (-0.58, 0.71) |
-0.27 (-0.92, 0.38) |
0.88 (0.24, 1.53) |
-0.23 (-0.87, 0.42) |
1.16 (0.51, 1.80) |
0.04 (-0.60, 0.69) |
-1.11 (-1.76, -0.46) |
| p-value | 7.18e-01 | 1.00e+00 | 1.70e-05**** | 9.99e-01 | 7.75e-01 | 2.25e-03** | 8.68e-01 | 2.57e-05**** | 1.00e+00 | 5.62e-05**** | |
| IL-6 |
DBM (95% CI) |
-0.02 (-0.55, 0.51) |
1.55 (1.02, 2.09) |
1.48 (0.95, 2.01) |
-0.28 (-0.81, 0.25) |
1.58 (1.04, 2.11) |
1.50 (0.97, 2.03) |
-0.26 (-0.79, 0.27) |
-0.08 (-0.61, 0.45) |
-1.84 (-2.37, -1.31) |
-1.76 (-2.29, -1.23) |
| p-value | 1.00e+00 | 5.88e-12**** | 4.67e-11**** | 5.80e-01 | 3.34e-12**** | 2.66e-11**** | 6.49e-01 | 9.95e-01 | 4.79e-14**** | 6.59e-14**** | |
| Anti-inflammatory | |||||||||||
| IL-1RA |
DBM (95% CI) |
-1.17 (-1.63 -0.71) |
-0.11 (-0.57, 0.35) |
-0.13 (-0.59, 0.32) |
-0.59 (-1.05, -0.13) |
1.06 (0.60, 1.52) |
1.03 (0.57, 1.49) |
0.58 (0.12, 1.04) |
-0.03 (-0.49, 0.43) |
-0.48 (-0.94, -0.02) |
-0.46 (-0.92, 0.00) |
| p-value | 1.46e-09**** | 9.66e-01 | 9.26e-01 | 4.73e-03** | 3.57e-08**** | 7.85e-08**** | 6.30e-03** | 1.00e+00 | 3.39e-02* | 5.26e-02 | |
| IL-10 |
DBM (95% CI) |
-0.20 (-0.58, 0.18) |
0.11 (-0.27, 0.50) |
0.36 (-0.02, 0.75) |
-0.30 (-0.68, 0.08) |
0.31 (-0.07, 0.70) |
0.56 (0.18, 0.95) |
-0.10 (-0.48, 0.28) |
0.25 (-0.14, 0.63) |
-0.41 (-0.80, -0.03) |
-0.66 (-1.05 -0.28) |
| p-value | 5.97e-01 | 9.25e-01 | 7.52e-02 | 2.00e-01 | 1.64e-01 | 8.45e-04*** | 9.52e-01 | 3.83e-01 | 2.82e-02* | 5.17e-05**** | |
| Growth factor | |||||||||||
| VEGF |
DBM (95% CI) |
-0.48 (-1.12, 0.17) |
-0.69 (-1.34, -0.05) |
-0.47 (-1.12, 0.17) |
-0.11 (-0.76, 0.53) |
-0.21 (-0.86, 0.43) |
0.00 (-0.64, 0.65) |
0.37 (-0.28, 1.01) |
0.22 (-0.43, 0.86) |
0.58 (-0.06, 1.23) |
0.36 (-0.28, 1.01) |
| p-value | 2.47e-01 | 2.89e-02* | 2.56e-01 | 9.89e-01 | 8.88e-01 | 1.00e+00 | 5.18e-01 | 8.81e-01 | 9.93e-02 | 5.30e-01 | |
| Season | |||
|---|---|---|---|
| Warm-Dry (n = 30) |
Rainy (n = 50) |
Cold-Dry (n = 40) |
|
| Cytokine | Mean (95% CIa) |
Mean (95% CIa) |
Mean (95% CIa) |
| Chemotactic | |||
| MIP-1α | 5918.32 (4339.06, 7497.59) |
4817.26 (3320.05, 6314.48) |
5591.04 (4231.61, 6950.47) |
| IP-10 | 337.36 (250.81, 423.91) |
329.24 (265.28, 393.19) |
336.99 (255.69, 418.30) |
| MCP-1 | 197.98 (109.46, 286.51) |
135.27 (83.42, 187.13) |
87.27 (63.10, 111.44) |
| Pro-inflammatory | |||
| IL-1α | 4.55 (2.89, 6.21) |
3.51 (2.68, 4.33) |
5.88 (4.26, 7.51) |
| IL-1β | 28.93 (14.02, 43.83) |
15.65 (4.03, 27.26) |
37.67 (16.24, 59.10) |
| TNF-α | 348.83 (230.86, 466.81) |
236.71 (148.80, 324.63) |
560.24 (383.38, 737.10) |
| IL-6 | 1.60 (0.93, 2.27) |
1.40 (0.87, 1.93) |
1.25 (0.79, 1.72) |
| Anti-inflammatory | |||
| IL-1RA | 22.80 (16.19, 29.41) |
18.79 (14.09, 23.49) |
27.43 (10.66, 44.20) |
| IL-10 | 6.35 (4.39, 8.31) |
4.70 (3.86, 5.54) |
7.09 (4.99, 9.19) |
| Growth factor | |||
| VEGF | 358.02 (203.39, 512.65) |
590.60 (261.16, 920.03) |
370.60 (263.45, 477.76) |
| Season | ||||
|---|---|---|---|---|
| WD, R | WD, CD | R, CD | ||
| Chemotactic | ||||
| MIP-1α |
DBM (95% CI) |
-0.25 (-0.84, 0.34) |
0.06 (-0.56, 0.67) |
0.31 (-0.23, 0.85) |
| p-value | 5.78e-01 | 9.72e-01 | 3.71e-01 | |
| IP-10 |
DBM (95% CI) |
0.06 (-0.27, 0.39) |
0.03 (-0.32, 0.38) |
-0.03 (-0.33, 0.27) |
| p-value | 9.03e-01 | 9.76e-01 | 9.71e-01 | |
| MCP-1 |
DBM (95% CI) |
-0.29 (-0.69, 0.12) |
-0.61 (-1.04, -0.19) |
-0.33 (-0.70, 0.05) |
| p-value | 2.21e-01 | 2.46e-03** | 9.91e-02 | |
| Pro-inflammatory | ||||
| IL-1α |
DBM (95% CI) |
-0.15 (-0.50, 0.20) |
0.30 (-0.06, 0.67) |
0.45 (0.13, 0.77) |
| p-value | 5.68e-01 | 1.18e-01 | 2.83e-03** | |
| IL-1β |
DBM (95% CI) |
-0.76 (-1.26, -0.27) |
0.34 (-0.17, 0.86) |
1.11 (0.65, 1.56) |
| p-value | 1.12e-03** | 2.61e-01 | 1.98e-07**** | |
| TNF-α |
DBM (95% CI) |
-0.36 (-0.82, 0.09) |
0.52 (0.05, 1.00) |
0.89 (0.47, 1.31) |
| p-value | 1.46e-01 | 2.82e-02* | 5.62e-06**** | |
| IL-6 |
DBM (95% CI) |
-0.04 (-0.62, 0.53) |
-0.07 (-0.67, 0.53) |
-0.03 (-0.55, 0.50) |
| p-value | 9.82e-01 | 9.59e-01 | 9.93e-01 | |
| Anti-inflammatory | ||||
| IL-1RA |
DBM (95% CI) |
-0.11 (-0.50, 0.28) |
0.07 (-0.34, 0.48) |
0.19 (-0.17, 0.55) |
| p-value | 7.79e-01 | 9.04e-01 | 4.42e-01 | |
| IL-10 |
DBM (95% CI) |
-0.26 (-0.54, 0.02) |
0.06 (-0.23, 0.35) |
0.32 (0.06, 0.58) |
| p-value | 7.67e-02 | 8.79e-01 | 1.11e-02* | |
| Growth factor | ||||
| VEGF |
DBM (95% CI) |
0.46 (0.02, 0.91) |
0.14 (-0.33, 0.61) |
-0.32 (-0.73, 0.09) |
| p-value | 4.08e-02* | 7.52e-01 | 1.57e-01 | |
| Eigenvalue | Percentage of Variance | Cumulative Percentage of Variance | |
|---|---|---|---|
| MIP-1α, | 1.7923532 | 44.808831 | 44.80883 |
| IL-1α | 1.0874345 | 27.185863 | 71.99469 |
| IL-1β | 0.8309943 | 20.774857 | 92.76955 |
| IL-1RA | 0.2892180 | 7.230449 | 100.00000 |
| Dim.1 | Dim.2 | Dim.3 | Dim.4 | |
|---|---|---|---|---|
| IL-1a | 0.50658333 | 0.4456352 | -0.7324773 | 0.09088234 |
| IL-1b | 0.89789048 | 0.1045237 | 0.2021667 | -0.37682372 |
| MIP-1a | -0.08845469 | 0.8931446 | 0.4266453 | 0.11154469 |
| IL-1RA | 0.84952635 | -0.2832159 | 0.2675326 | 0.35569656 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





