1. Introduction
Cryptococcosis is a systemic disease that affects animals and people worldwide caused by an encapsulated yeast species of the genus
Cryptococcus with predilection for the nervous and respiratory systems [
1]. The infection in dogs is frequently caused by
Cryptococcus neoformans and
Cryptococcus gattii. Clinical signs depend on the sites of infection, but the involvement of critical organs, such as the CNS, eyes, gastrointestinal tract, myocardium, adrenal glands, and pancreas in dogs with cryptococcosis is frequently observed [
2]. Even though the reported incidence of cryptococcosis is lower in dogs than in cats, CNS involvement may be more common in dogs, with widespread dissemination to other organs [
3].
Macrophages are crucial for cryptococcosis control. It has been demonstrated that macrophage and dendritic cell depletion significantly reduced mice survival after infection with
C. neoformans. Besides, the Trojan horse hypothesis suggest that fungus entry into the blood brain barrier through dissemination within the macrophage [
4,
5]. In this context for
C. neoformans, a Trojan horse mechanism for dissemination is supported by the results obtained after depletion of alveolar macrophages, preventing brain dissemination [
6]. In addition, increased brain fungal burden was observed in experiments with mice injected with
ex vivo infected macrophages [
7].
Although numerous proteins are important in interaction with macrophages [
8], capsular polysaccharides have shown an important ability to deactivate the immune system [
9]. The capsular polysaccharides of
C. neoformans are the main virulence factor. The capsule is composed of glucuronoxylomannan (GXM),
glucuronoxylomanogalactan (GXMGal) and small amounts of mannoproteins. GXM represents 90% of the capsule, GXMGal around 8% and mannoproteins have been identified (2%) was not studied in detail yet [
10].
Previous studies have demonstrated that capsular polysaccharides as GXM and GXMGal were able to induce impairment of macrophage activity, demonstrated by modulation of proinflammatory cytokines, apoptosis induction and modulated production of neutrophil extracellular traps (NETs) by human neutrophils [
11,
12]. GXM can be captured by different receptors particularly TLRs, CD14, CD18 and FcγRIIB [
13]. Inside the macrophage, GXM produces multiple effects, for example, reduction of the APC function [
14], increase FasL and deregulation of pro-inflammatory and anti-inflammatory secretion of cytokines. Indeed, low doses of GXMGal can be able to induce FasL expression and inhibit proliferation in macrophage cell line, indicating that GXMGal is more potent to produce immunomodulatory effects [
11].
Most data in the literature refer to analyzes carried out in mouse macrophages, clearly showing a lack of information on the effect of capsular polysaccharides from
C. neoformans. In general, the polysaccharides seem to exert an immunomodulatory effect on several effective functions of murine macrophages [
8,
11,
15,
16]. However, there are no reports on the possible effects of these polysaccharides on canine macrophages. Understanding these mechanisms may bring light on the understanding of the initial moments of the interaction with
C. neoformans. Therefore, in this study the objective was to evaluate the ability of the purified capsular polysaccharides from
C. neoformans to modulate the activity of canine macrophage cell line DH82.
2. Materials and Methods
2.1. DH82 Cell Line Culture
Canine macrophages DH82 (ATCC) were grown in 75 cm2 tissue culture flasks (T75) (Nunc, Roskilde, Denmark) with Dulbecco’s Modified Eagle Medium (DMEM; Sigma-Aldrich) supplemented with 10% fetal calf serum (FCS; Gibco), 1% MEM non-essential amino acids (Sigma-Aldrich), 100 μg/mL of streptomycin and 100 Units/mL of penicillin and 2 mM of l-glutamine were cultured at 37 0C with 5% CO2. Subcultures were carried out once a week when they reached a confluence of 95–100%. Cells were released with 0.25% of trypsin (Difco) and 1 mM of EDTA (Sigma-Aldrich), harvested, and washed twice in HBSS by centrifugation at room temperature for 10 min at 250 g. The supernatant was discarded, cell pellet was resuspended in DMEM containing 10% FCS, cultured into new T75 flasks, and culture medium was changed after 3 days in culture. Before to use in all assays, cells’ viability was verified by trypan blue exclusion.
2.2. Isolation and Purification of C. neoformans Capsular Polysaccharides (GXMGal and GXM)
Polysaccharides were isolated and purified as previously described [
15]. Briefly, cells grown in defined liquid medium at 37
0C with continuous shaking (100 rpm) for 5 days were removed through centrifugation (12,000
g for 1 h at 4
0C), and the capsular polysaccharides in the supernatant were precipitated after adding three volumes of cold ethanol. To separate GXMGal from mannoproteins, the capsular polysaccharides were fractionated using lectin-affinity chromatography on a XK-26 column (Pharmacia) packed with 70 mL Concanavalin A-Sepharose 4B (Pharmacia) at 4
0C. The unbound polysaccharide fractions, containing GXMGal, were localized through a phenol-sulfuric reaction, pooled, dialyzed against distilled water and lyophilized. Pure GXMGal was obtained through anion-exchange chromatography on a MonoQ (HR16/10) column using a 50 mL super loop.
GXM was purified by differential precipitation with CTAB. Briefly, capsular polysaccharides isolated from the culture supernatant by precipitation with ethanol were dissolved in 0.2 M NaCl (10 mg ml-1) and CTAB (3 mg mg-1 of polysaccharides) was added slowly. Then, a solution of 0.05% CTAB was added and the GXM was selectively precipitated. The precipitate was collected by centrifugation and washed with 2% acetic acid in ethanol then in 90% ethanol. The precipitate was dissolved in 1 M NaCl, and three volumes of ethanol were added to precipitate the GXM. The precipitate was centrifuged, washed with 2% acetic acid in ethanol and then with ethanol, dissolved in water and lyophilized.
To eliminate potential LPS contamination, 10 mg of GXMGal or GXM preparations were dissolved in LPS-free water and further purified through chromatography on a column of Polimixin B-Agarose (Sigma) equilibrated with LPS free-water. Purified GXMGal or GXM were eluted with 12 mL of LPS free water, recovered and lyophilized.
2.3. Cytotoxicity Assay
The verification of the possible toxic effects of capsular polysaccharides (GXMGal, GXM and total capsule) on canine macrophages DH82 was performed using the XTT colorimetric assay (Sigma), which quantifies mitochondrial metabolism and respiratory chain activity in the presence of the electron acceptor PMS. DH82 cells (1x105/well) were cultured in 96-well plates and incubated with GXMGal, GXM and total capsule at concentrations of 10, 50 and 100 µg/mL for 24 h and 48 h. After incubation, an XTT solution (1.8 mg XTT, 75 µL PMS and 1425 µL PBS) was prepared and 50 µL was added per well. Cultures were incubated for 3 h and quantification was performed using a 490 nm filter in a Spectramax M3 reader.
2.4. Assessment of Binding and Phagocytosis
DH82 cells were plated on glass coverslips (1x10
4 cells/well) and incubated overnight with different concentrations (10, 50 and 100 µg/mL) of GXM, GXMGal and total capsule. The used concentrations of polysaccharides were based on our previous work [
11,
12]. After that,
Saccharomyces cerevisiae yeast was added to the cells in a 10:1 ratio for 40 minutes (binding assay) or 4 h (phagocytosis assay) and incubated at 37
0C and 5% CO
2. After incubation, the cells were washed twice with PBS to remove non- binding and non-phagocytosed yeasts. The coverslips were washed with HBSS, fixed with methanol, and stained with Diff-Quick (Thermo Fisher, Waltham, MA, USA). Yeasts were counted at 100x oil immersion on a (Olympus) microscope. The number of yeasts was estimated in 100 cells per coverslip, and the frequency was compared among six coverslips per time point.
2.5. Viability of Yeasts after Phagocytosis
Canine DH82 macrophages were grown in 24-well culture plates (1x10
4 cells/well) and incubated with the capsular polysaccharides and total capsule for 24 h at the concentrations described above. After this period,
S. cerevisiae yeasts were added at a ratio of 10:1 (yeasts/macrophage) and incubated for 4 h. Subsequently, the cultures were washed twice with PBS and lysed with ice water (500 µL). The lysate was diluted 1:10 in 1x PBS. The 30 µL volume of the diluted lysate was seeded in Petri dishes containing Sabouraud agar. The plates were incubated at 37
0C for 48 h and then the colony forming units were counted [
16].
2.6. Reactive Oxygen Species (ROS) Detection
The quantification of ROS intracellular levels was made by oxidation of non-fluorescent 2′,7′-dichlorofluorescein probe, delivered as diacetate form (DCFH-DA), to the fluorescent product 2′,7′-dichlorofluorescein [
17]. Macrophages DH82 were seeded in 96-well plate (5x10
4 cells/well), incubated with polysaccharides (GXMGal, GXM and capsule) 100 µg/mL, stimulated or not with LPS (400 ng/mL, Sigma-Aldrich) and recombinant IFN-γ (1.5 ng/mL, Serotech) and after 24 h later cells were washed and loaded with 10 μM DCFH-DA (Invitrogen) for 20 minutes at 37
0C. Untreated and unstimulated cells were used as a negative control. After incubation, the cells were washed, and fluorescence was measured (excitation = 485 nm excitation; λ emission = 535 nm) in an FLx800 Fluorescence Microplate Reader (BioTek).
2.7. Real-Time RT-PCR Quantification
DH82 macrophage (1x106 cells) was plated and incubated with GXMGal, GXM and capsule (100 µg/mL), stimulated or not with LPS (400 ng/mL) and IFN-γ (1.5 ng/mL) for 24 h. After this time, total RNA was extracted with RNeasy Plus Mini Kit (Qiagen 74134, Germany), 1 μg aliquot was reverse transcribed to first-strand cDNA with ImProm-II (Promega) and oligo(dT) primer according to the manufacturer's instructions. The DNA sequences of the primers used were IL-6-F: 5′-GCGTCTTCCCTCATGACC-3′, IL-12-R: 5′-GGGTGCCAGTCCAACTCTAC-3′, IL-6-F:5′-GGGAAAGCAGTAGCCATCAC-3′,IL-6-R:5′CAGGACCCCAGCTATGAACT-3′,TGF-β-F:5′-CGAAGCCCTCGACTCC-3′,TGF-β-R:5′TGGCTGYCCTTTGATGTCAC-3′, GAPDH-F: 5′-TGCACCACCAACTGCTTAGC-3′ and GAPDH-R: 5′-GGCATGGACTGTGGTCATGAG-3′. qRT-PCR data from the experiments were normalized using Gapdh primers as an endogenous control. Amplicon specificity was carefully verified by the presence of a single melting temperature peak in dissociation curves run after real-time RT-PCR. Real-time quantitative RT-PCR (qRT-PCR) was performed via the Applied Biosystems StepOne™ detection system (Applied Biosystems) using GoTaq® qPCR Master Mix (Promega Corp., Madison, WI, USA). All expression ratios were computed via the analysis of relative gene expression ΔΔCt method through the StepOne software version 2.0 (Applied Biosystems).
All real-time PCR reactions were previously standardized. Including both the concentration of primers in relation to the melting curve and a standard curve between the endogen used and the targets, to achieve efficiency across all targets. It was only after these tests that we carried out the analysis of the relative expression in relation to the endogenous one, which in this case is GAPDH. Among all samples tested, the Ct (“Cycle Threshold”) was similar and close to less than 1 log, thus being considered a good endogenous target to normalize during reactions.
2.8. MHC Class II Expression
DH82 macrophages were grown in 6-well culture plates (1x106 cells/well) and incubated for 24 h with the capsular polysaccharides (100 µg/mL), LPS (400 ng/mL) and IFN-γ (1.5 ng /mL). After 24 h, the cells were detached, washed, adjusted to a concentration of 5x105 cells/tube, and incubated with blocking buffer (CD16/CD32 Fc Block-eBioscience) for 15 min on ice to prevent non-specific antibody binding to Fc receptors. Cells were stained with anti-dog MHC class II-FITC (Serotech). All washing steps were performed with PBS containing 3% FCS and 0.02% sodium azide. Data was acquired (10,000 events), evaluated on FACSCalibur™ cytometer and analyzed using CellQuest® software (BD Biosciences, Heidelberg, Germany).
2.9. Assay of Protein Phosphorylation
DH82 macrophages were grown in 6-well culture plates (1x10
6cells/well) and incubated for 24 h with the polysaccharides GXMGal, GXM and total capsule (100 µg/mL), stimulated with canine IFN-γ (1.5 ng/mL) and LPS (400 ng/ml). The western blotting protocol has been described [
18]. The following antibodies were used: anti-ERK (4696S - Cell Signaling), anti-pERK (4370S-CellSignaling), anti-PPAR-γ (2435 - Cell Signaling) and anti-β actin (Sigma-Aldrich) as primary antibody and anti-IgG (whole molecule)-peroxidase (Sigma-Aldrich) as secondary antibody. Reactions were developed using a chemiluminescence kit (ECL Western Blotting Substrate-Promega-w1015) according to the manufacturer's instructions. The band densitometry of western blotting was analyzed by the Scion Image software 4.0.3.2.
4. Discussion
Different models been extensively studied to reveal the mechanisms of interaction between host cells and parasitic agents [
23,
24,
25]. For many infectious agents, the type of interaction with host cells can determine the resolution or success of the infection. Among the host cells, macrophages stand out for their importance in innate and adaptive immunity [
8,
25].
Cryptococcus neoformans is an infectious agent capable of replicating within macrophages and this has been reported by several authors [
26,
27,
28]. Furthermore, it was demonstrated that macrophages could be used by
C. neoformans as a Trojan horse to transport yeast to strategic points in the body, such as the CNS [
7,
29].
Although several studies point to the relevance of the interaction of macrophages with
C. neoformans, there is no study carried out with the aim of understanding the effect of the interaction of the capsular components of
C. neoformans with canine macrophages. In this work we used the canine macrophage DH82 cell line and our group has demonstrated the importance of this cell line in the interaction with parasites as a replacement method [
30,
31]. Using DH82 canine macrophages treated with purified
C. neoformans polysaccharides, we demonstrated for the first time their immunomodulatory effects. Previous work has shown that purified polysaccharides from
C. neoformans can lead to death by apoptosis of murine macrophages [
11]. Thus, we first treated canine macrophages DH82 with different concentrations of GXMGal, GXM and total capsule with incubation of 24 and 48h to see if the polysaccharides would have any toxic effect on canine macrophages. Surprisingly, it was not possible to observe a change in cell viability at any of the concentrations used. This data suggests that macrophages from different species may show variations in resistance to toxic effects resulting from contact with capsular polysaccharides.
The ability to binding and phagocytose infectious agents is a fundamental microbiological function for macrophages. However, it is known that components of the
C. neoformans capsule inhibit phagocytic activity and may compromise efficient elimination by this mechanism [
16,
32]. To assess whether purified polysaccharides would reproduce this effect, we incubated canine macrophages DH82 with GXMGal, GXM and whole capsule and offered Saccharomyces cerevisiae yeasts. We observed that the polysaccharides were able to inhibit the ability of macrophages to binding to yeasts, as well as phagocytose. The polysaccharides indistinctly led to a decrease in phagocytic activity. We observed a decrease in the number of yeasts attached to macrophages, number of cells that phagocytosed and number of phagocytosed yeasts. This data corroborates findings from other authors who previously showed that the
C. neoformans capsule can inhibit phagocytosis by macrophages through various mechanisms [
33,
34,
35].
Based on the observation that GXMGal, GXM and the total capsule of
C. neoformans influenced phagocytosis, we investigated whether phagocytosed
S. cerevisiae yeasts would undergo fungicidal action by DH82 macrophages by counting CFU of recovered yeasts after phagocytosis. Experiments performed on murine macrophages [
16], suggest vesicles secreted by
C. neoformans influence the amount of CFU produced by recovered yeasts after being phagocytosed. In our experiment, the high number of yeast colonies recovered from cells treated (100 µg/mL) with polysaccharides suggests a relationship with a modulating action of the microbicidal activities of the macrophage by the polysaccharides, consistent with previous work that showed inhibition of the production of pro-inflammatory cytokines [
11] and can be extrapolated to the physiological model, where the impairment of macrophages is related to inhibition of the immune system leading to a greater fungal burden [
4,
5,
11,
14,
36]. The presence of numerous CFU in macrophages with polysaccharides suggests that they decrease the macrophage's fungicidal activity.
Despite the reduced rate of phagocytosis in the presence of the capsular polysaccharides of
C. neoformans, it was shown that the yeasts phagocytosed by these macrophages remained viable and able to grow. For this reason, we analyzed a microbicidal mechanism of oxidant production by phagocytes. ROS play a crucial role in protection against fungal infections, as observed in patients with chronic granulomatous disease (CGD) which are very susceptible to fungal infections [
37,
38]. Furthermore, the decrease in ROS production may compromise the differentiation of T lymphocytes to the Th1 profile, which is the protective profile [
39]. ROS is a factor that participates in the inflammatory process observed in canine cryptococcosis associated with excessive granuloma formation [
3,
40,
41]. Our results show that polysaccharides were able to inhibit ROS production in DH82 macrophages, suggesting that the inhibitory effect on ROS production may have favored yeast survival.
After phagocytosis, within the phagosome, yeasts are under the action of low pH, ROS, reactive nitrogen species, and nutrients deprivation [
42]. These challenges are neutralized by yeast through powerful mechanisms. Phagocytosed yeasts can upregulate gene expression of oxidative stress enzymes, starvation responses, and the autophagic machinery [
43,
44]. In a model of NADPH oxidase-null mice, cryptococcal infection is contained and the fungal load in both brain and lung is decreased [
39], suggesting that inflammatory ROS are prejudicial also to the host.
Although ROS act in the death of fungi, this usually happens in a pro-inflammatory environment [
45], in our model, where canine macrophages treated with polysaccharides showed ROS inhibition, this suppressor environment, points to the important role of ROS in the microbicidal activity of DH82 canine macrophages.
Macrophages act in the processing and presentation of antigens, providing the adaptive immune response. Thus, the expression of MHC molecules is crucial, because through the expression of MHC-II, macrophages are upregulated, acting as APC and functioning as fundamental elements in the activation of lymphocytes, which, when stimulated, will lead to the adaptative immune response [
46]. DH82 macrophages treated with capsular polysaccharides did not increase the basal expression of MHC-II, contributing to the non-responsiveness of the immune system imposed by
C. neoformans on the host [
47]. The basal expression of MHC-II presented in our results is similar to that found previously [
48], but slightly lower [
49,
50]. DH82 canine macrophages stimulated with LPS and IFN-γ increase the expression of MHC-II, due to the already known capacity that these factors exert on the increase of the expression of this protein [
49,
50]. However, we observed statistically significant decrease in MHC class II expression in the presence of GXMGal, indicating a possible suppression of the DH82 canine macrophage response mediated by this purified
C. neoformans polysaccharide, data shown for the first time in this cell type, since most of the works refer to GXM [
14]. However, it is known that the
C. neoformans cell induces the expression of MHC-II in glial cells when in contact with the fungus [
51].
Macrophages produce cytokines that mediate and direct the immune response to an infection. In cryptococcosis, the crucial role of this function has been demonstrated, the polarization of macrophages, through the cytokine profile, influencing the course of infection by
C. neoformans [
52]. We evaluated the cytokines IL-12 and IL-6 that help protect the host and TGF-β that can contribute to the permanence of the pathogen, since cryptococcosis is a balance infection between Th1 and Th2 profiles [
53].
Our results show that GXMGal, GXM and the total capsule inhibit the expression of IL-12, IL-6 and TGF-β. Although non-stimulated DH82 macrophages do not alter the expression of these cytokines, we clearly saw that when stimulated these cells have a significant reduction in the expression of IL-12 and IL-6. On the other hand, TGF-β had its expression increased in non-stimulated cells and reduced in response to stimuli. This modulation in cytokine production suggests that the increase in TGF-β in initial moments of contact with capsular polysaccharides may compromise the production of pro-inflammatory cytokines in canine DH82 macrophages. The inhibition of pro-inflammatory cytokines such as IL-12, which is fundamental in autocrine signaling in the activation of macrophages [
54], as well as the inhibition of IL-6 can be determinant not only for macrophage activation, but also in the induction of the adaptive response [
55]. Thus, the action of polysaccharides may compromise the activation of macrophages. Previous works have demonstrated the anti-inflammatory role being predominantly exerted by GXM [
11,
47], however, our results suggest that, for canine macrophages DH82, GXMGal, GXM and total capsule exert the same inhibitory effect on the expression of the evaluated cytokines.
The results presented so far lead to the profile of canine DH82 macrophage suppression, with this, we investigated the intracellular signaling in the face of stimulation with the capsular polysaccharides of
C. neoformans. The results obtained show that GXMGal, GXM or the total capsule do not induce ERK phosphorylation, which occurs in the presence of LPS and IFN-γ. We also observed a decrease in cell phosphorylation in the presence of GXM, showing once again the suppressive characteristic exerted by polysaccharides, since ERK is part of the large family of MAPK that are involved in several cellular mechanisms, including proliferation and cell activation upon stimulation [
56,
57]. Another intracellular marker investigated was PPAR-γ, a nuclear factor that is activated in the presence of suppressive stimuli [
21,
58]. Based on the already known activation that LPS and IFN-γ cause in macrophages, we observed through the increase in PPAR-γ expression that capsular polysaccharides, especially GXM of
C. neoformans induce the suppression of canine macrophages DH82.
All aspects evaluated in the present study, such as decreased phagocytosis, inhibition of ROS, IL-12 and IL-6 production, increased TGF-β production, combined with decreased MHC class II expression, suggest that polysaccharides lead to the impairment of fundamental microbicidal activities of macrophages. In addition, the evaluation of intracellular signaling revealed that in the presence of polysaccharides there is a reduction in ERK phosphorylation and an increase in PPAR-γ expression, reinforcing the suppressor aspect exerted by the polysaccharides on canine macrophages DH82. These data may contribute to the understanding that the impairment of the microbicidal activities of macrophages could contribute to the permanence and spread of the fungus in the canine host.
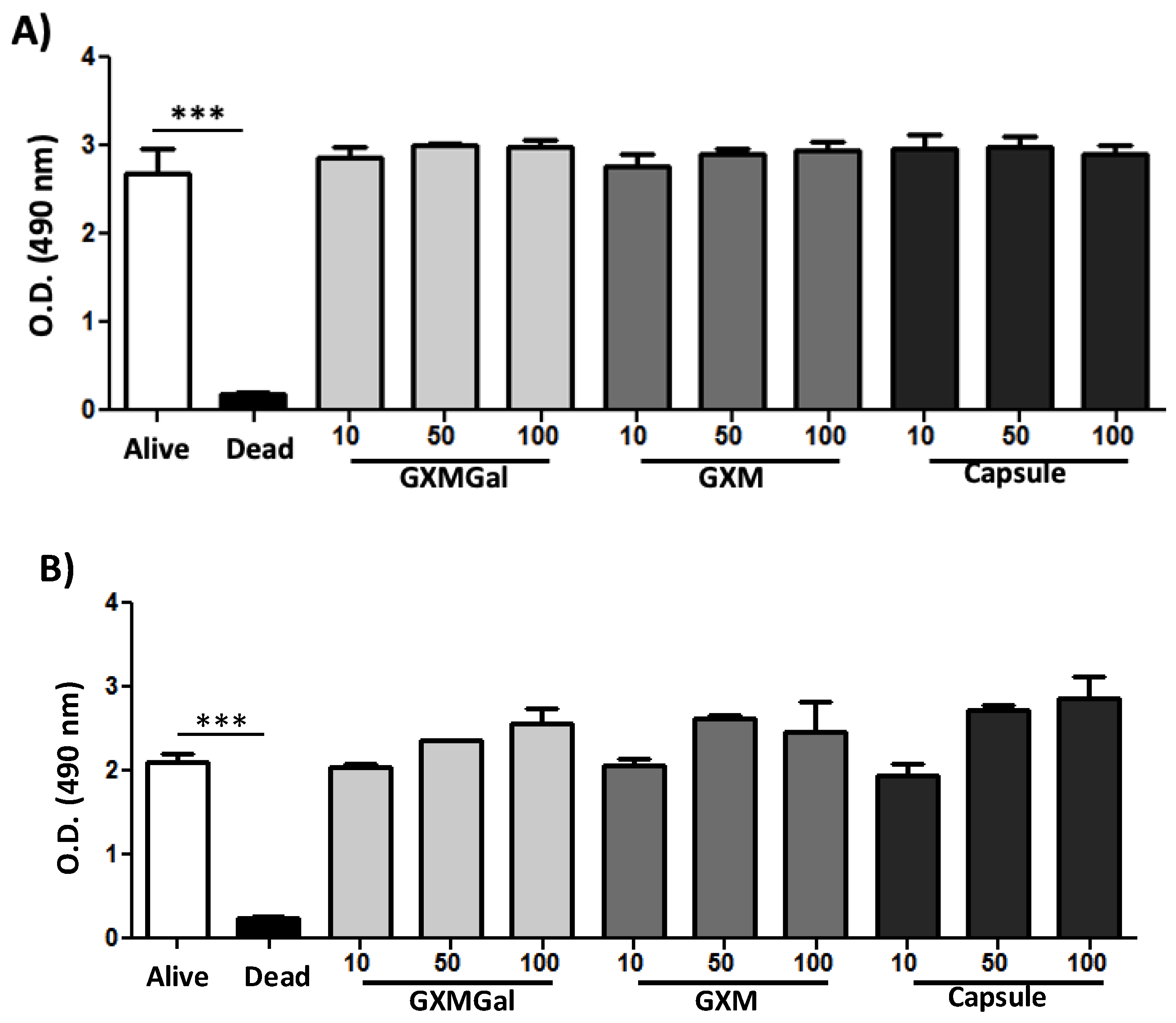
Figure 1.
Effect of purified polysaccharides and the total capsule on the viability of DH82 cells. Canine macrophage DH82 cultures were grown at a concentration of 105 cells/well and incubated for 24 h (A) and 48 h (B). Cells were treated with GXMGal, GXM and total capsule at the indicated concentrations (μg/mL). The dead control received the addition of 0,1% of Triton before the addition of XTT. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
Figure 1.
Effect of purified polysaccharides and the total capsule on the viability of DH82 cells. Canine macrophage DH82 cultures were grown at a concentration of 105 cells/well and incubated for 24 h (A) and 48 h (B). Cells were treated with GXMGal, GXM and total capsule at the indicated concentrations (μg/mL). The dead control received the addition of 0,1% of Triton before the addition of XTT. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
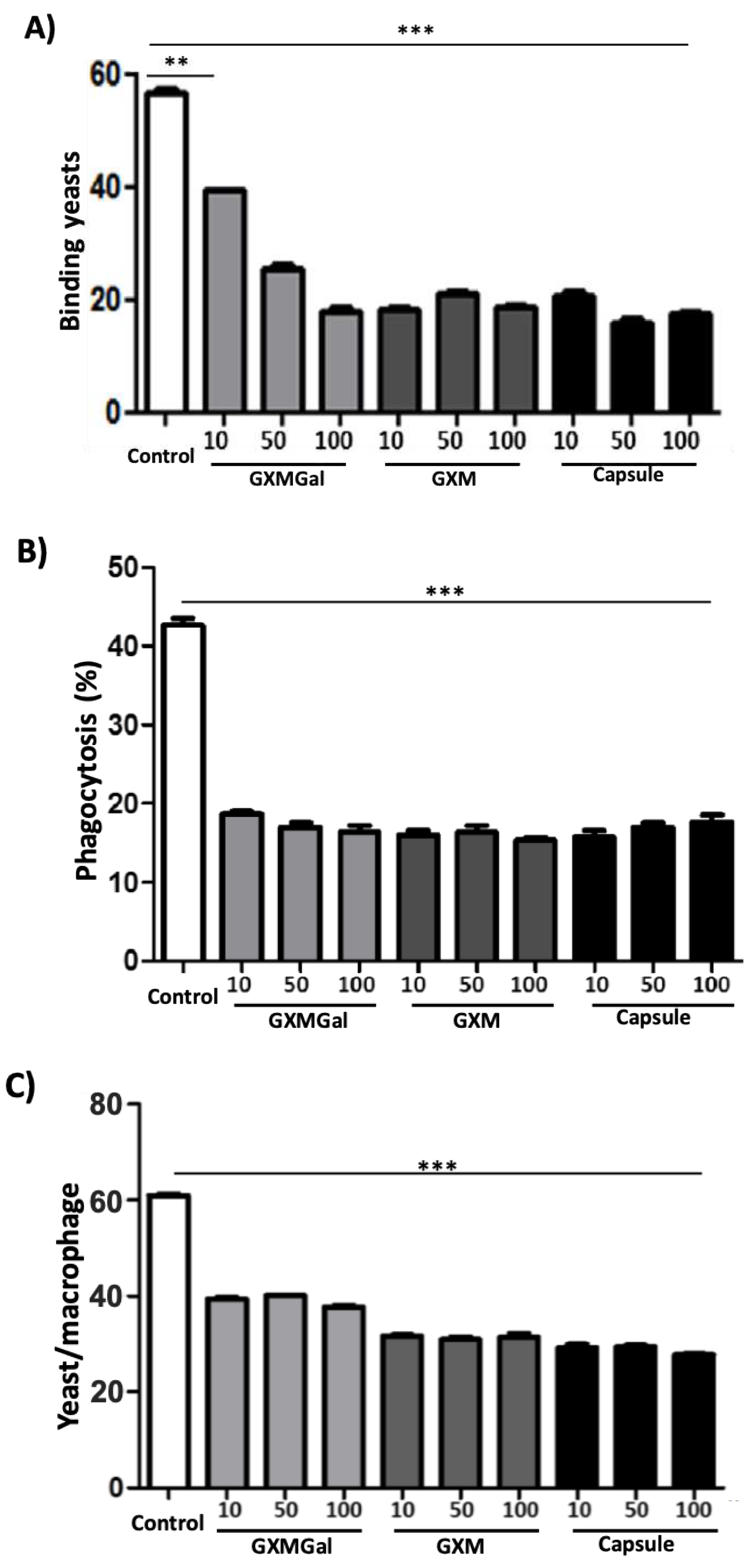
Figure 2.
Adherence and phagocytosis by DH82 in the presence of capsular polysaccharides of C. neoformans. DH82 cells were plated on a glass coverslip (1x104 cells/well) and incubated with GXMGal, GXM and capsule at the indicated concentrations (μg/mL). After 24 h, S. cerevisiae (10x104 cells/well) was added to the culture. After 40 minutes for the evaluation of binding (A), 4h to evaluate the percentage of cells that phagocytized (B) and number of phagocytosed yeasts (C). The cells were then stained, and the count was performed at 100x magnification. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
Figure 2.
Adherence and phagocytosis by DH82 in the presence of capsular polysaccharides of C. neoformans. DH82 cells were plated on a glass coverslip (1x104 cells/well) and incubated with GXMGal, GXM and capsule at the indicated concentrations (μg/mL). After 24 h, S. cerevisiae (10x104 cells/well) was added to the culture. After 40 minutes for the evaluation of binding (A), 4h to evaluate the percentage of cells that phagocytized (B) and number of phagocytosed yeasts (C). The cells were then stained, and the count was performed at 100x magnification. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
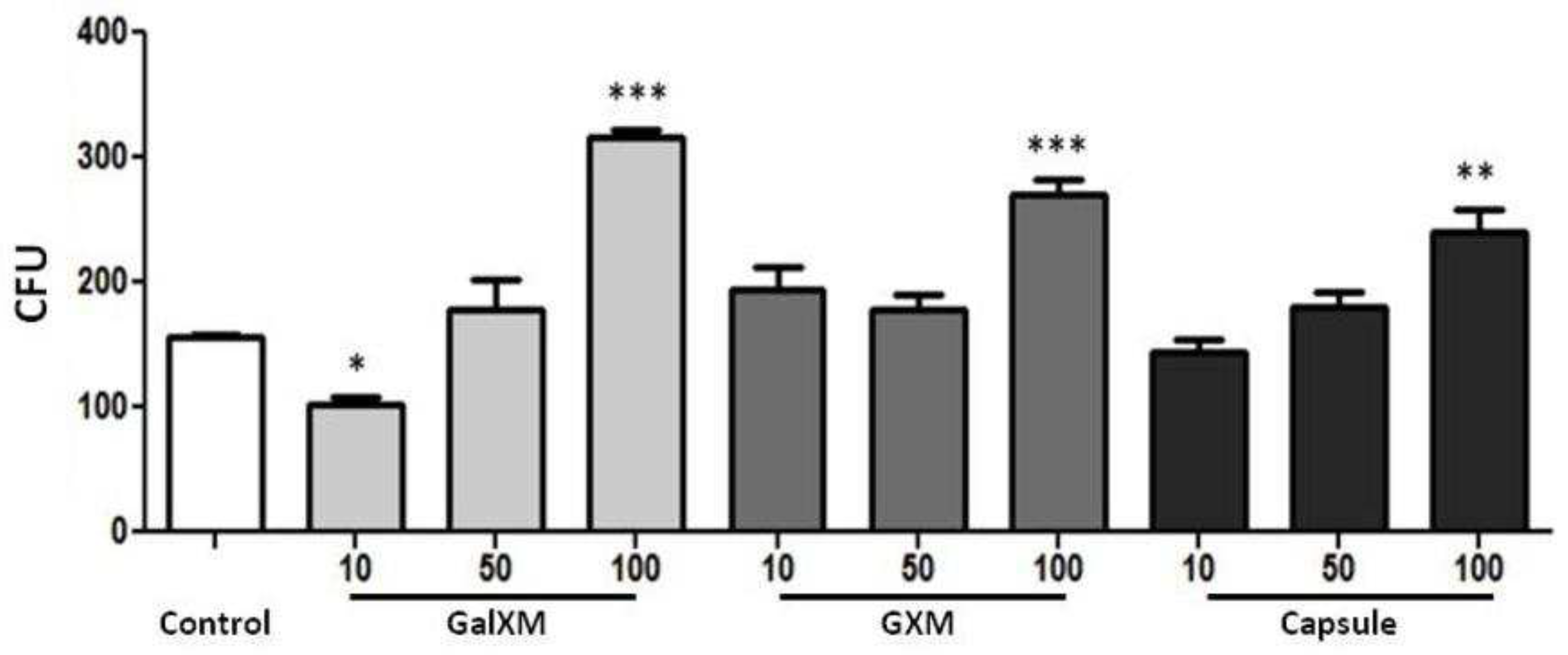
Figure 3.
Fungicidal activity of canine macrophages DH82 treated with purified polysaccharides from C. neoformans. DH82 cell cultures were plated (1x104 cells/well) and treated with GXMGal, GXM or capsule at the indicated concentrations (μg/mL). Yeasts of S. cerevisiae (10x104cells/well) were added to the culture and incubated for 4 h. After incubation, the macrophages were lysed, and the recovered yeasts were seeded on Sabouraud agar and incubated for 3 days. After incubation, colony forming units were counted. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
Figure 3.
Fungicidal activity of canine macrophages DH82 treated with purified polysaccharides from C. neoformans. DH82 cell cultures were plated (1x104 cells/well) and treated with GXMGal, GXM or capsule at the indicated concentrations (μg/mL). Yeasts of S. cerevisiae (10x104cells/well) were added to the culture and incubated for 4 h. After incubation, the macrophages were lysed, and the recovered yeasts were seeded on Sabouraud agar and incubated for 3 days. After incubation, colony forming units were counted. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
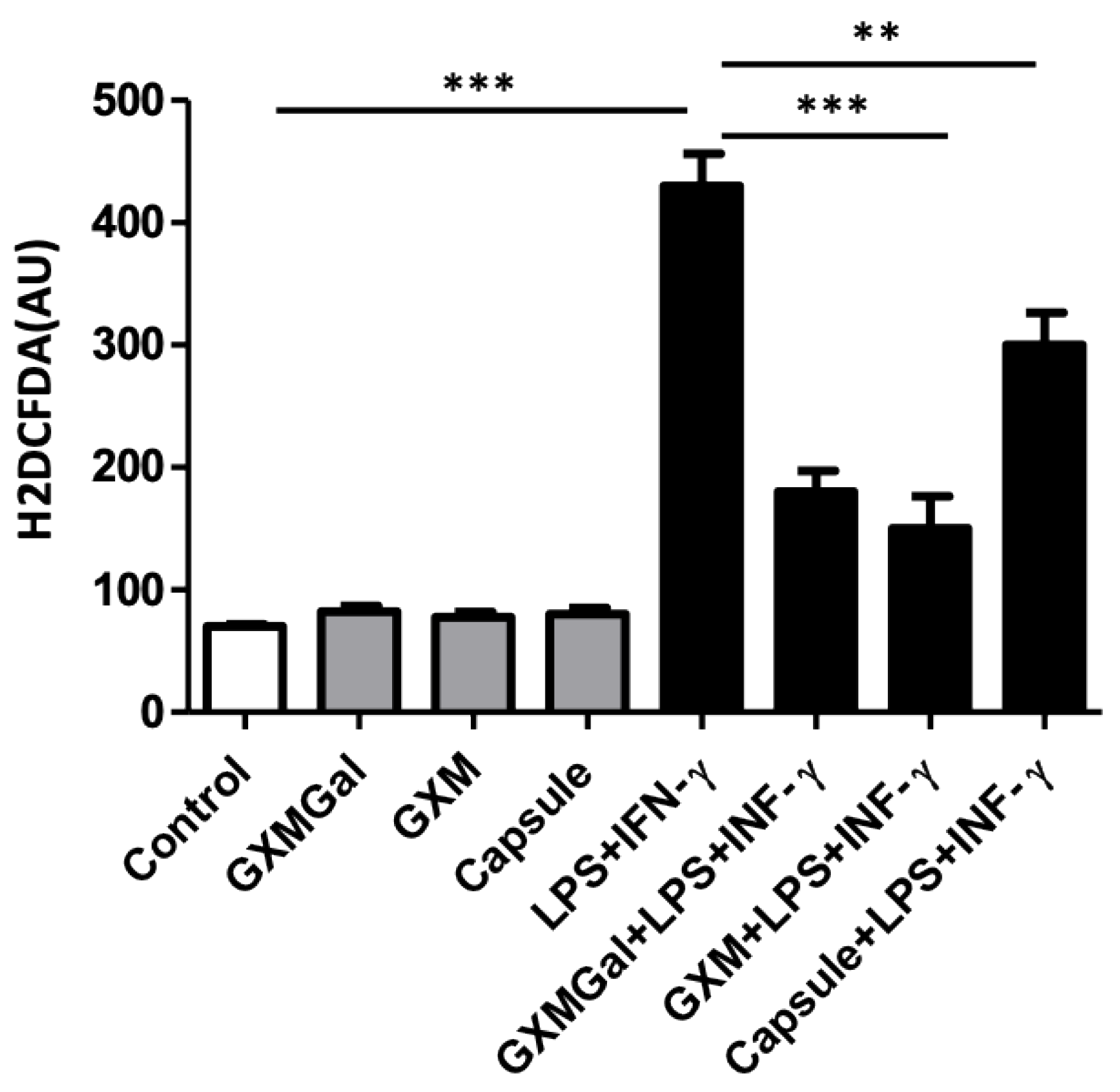
Figure 4.
Reactive species of oxygen (ROS) production by DH82 cells treated with purified capsular polysaccharides from C. neoformans. DH82 canine macrophages were cultured (5x104/mL) and incubated with H2DCFDA, followed by washing the cells were treated with GXMGal, GXM or capsule (100 μg/mL) and some cultures were stimulated with LPS (400 ng/mL) and IFN-γ (1.5 ng/mL) for 24 h. All cultures were performed in triplicate are shown as the mean arbitrary fluorescence units (AU) + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
Figure 4.
Reactive species of oxygen (ROS) production by DH82 cells treated with purified capsular polysaccharides from C. neoformans. DH82 canine macrophages were cultured (5x104/mL) and incubated with H2DCFDA, followed by washing the cells were treated with GXMGal, GXM or capsule (100 μg/mL) and some cultures were stimulated with LPS (400 ng/mL) and IFN-γ (1.5 ng/mL) for 24 h. All cultures were performed in triplicate are shown as the mean arbitrary fluorescence units (AU) + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
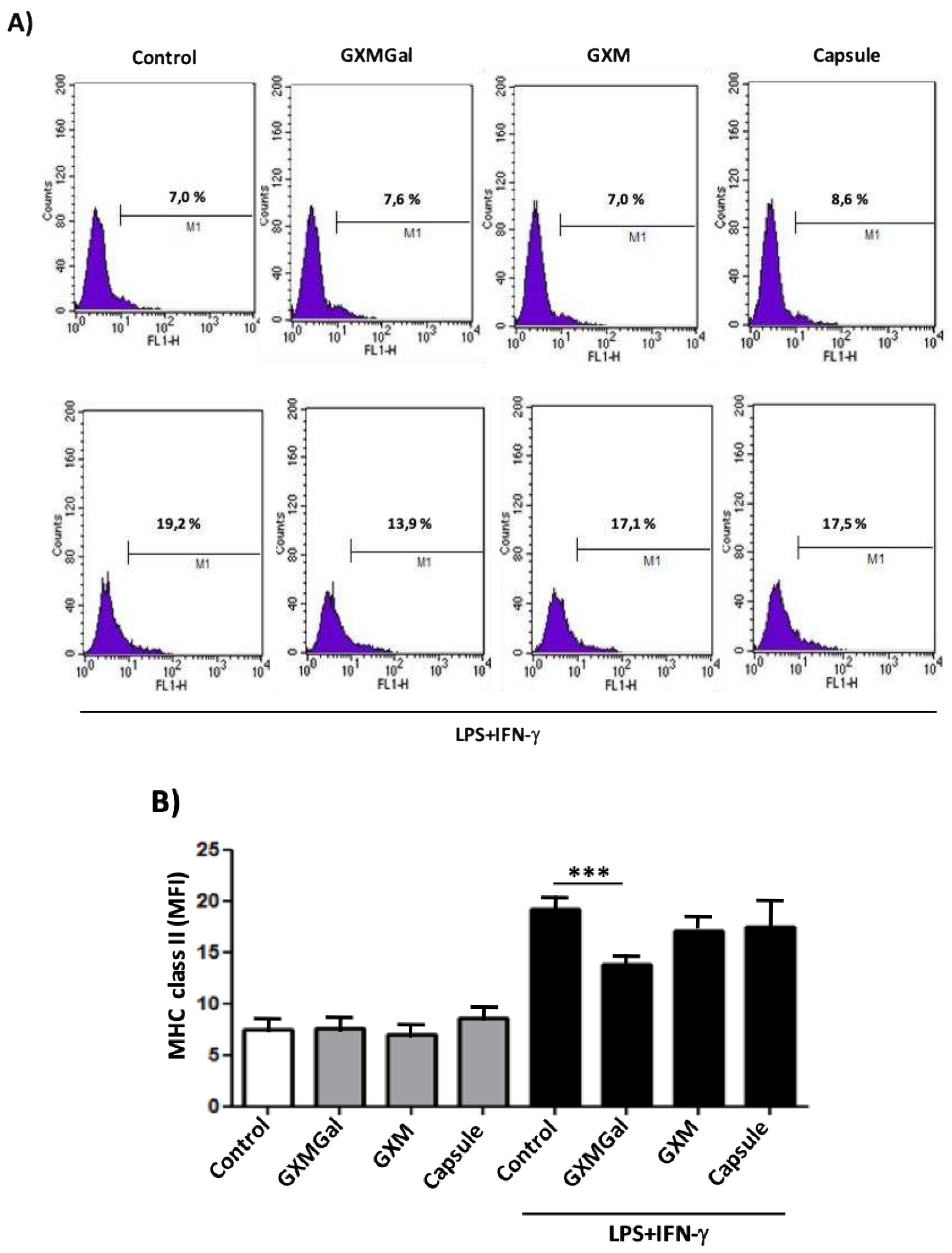
Figure 5.
Measurement of MHC class II expression in canine macrophage DH82 treated with powdered polysaccharides from C. neoformans. DH82 macrophages (5x105/mL) were treated with GXMGal, GXM (100 μg/mL). Some cultures were stimulated with LPS (400 ng/mL) and IFN-γ (1.5 ng/mL). After 24 h, the cells were prepared for analysis by flow cytometry. The M1 marker shows the percentage of cells identified by MHC class II (A, and B). All cultures were performed in triplicate are shown as the mean arbitrary fluorescence units (AU) + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
Figure 5.
Measurement of MHC class II expression in canine macrophage DH82 treated with powdered polysaccharides from C. neoformans. DH82 macrophages (5x105/mL) were treated with GXMGal, GXM (100 μg/mL). Some cultures were stimulated with LPS (400 ng/mL) and IFN-γ (1.5 ng/mL). After 24 h, the cells were prepared for analysis by flow cytometry. The M1 marker shows the percentage of cells identified by MHC class II (A, and B). All cultures were performed in triplicate are shown as the mean arbitrary fluorescence units (AU) + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
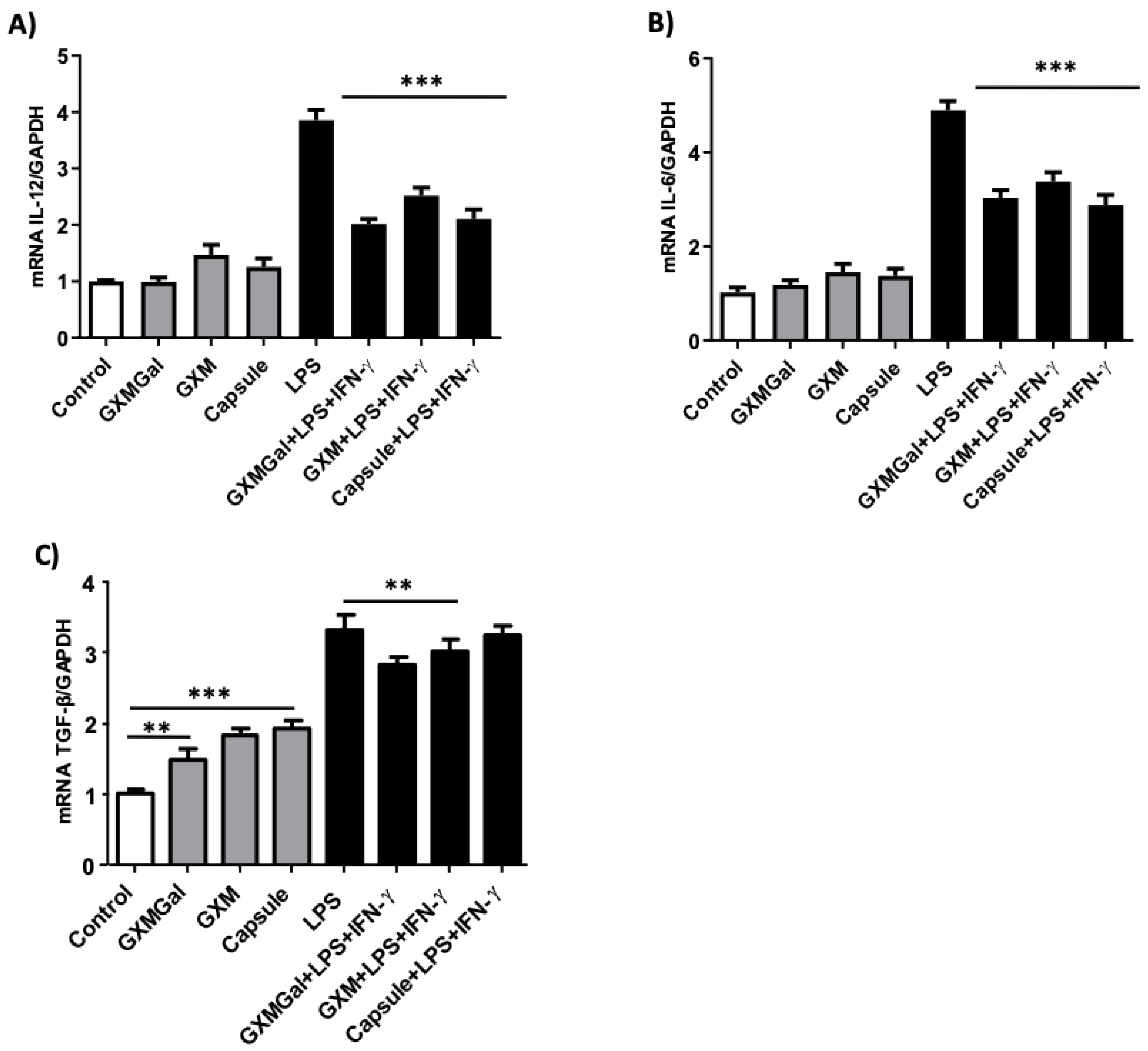
Figure 6.
Cytokine expression of the canine macrophage DH82. Cytokine profile in canine macrophage DH82 (1x106/mL) were plated and treated with GXMGal, GXM and capsule (100 μg/mL). Some cultures were stimulated with LPS (400 ng/mL) and IFN-γ (1.5 ng/mL). After 24 h of incubation, the cells were processed and mRNA for the cytokines quantified by qRT-PCR. Data from the experiments were normalized using GADPH primers as an endogenous control. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
Figure 6.
Cytokine expression of the canine macrophage DH82. Cytokine profile in canine macrophage DH82 (1x106/mL) were plated and treated with GXMGal, GXM and capsule (100 μg/mL). Some cultures were stimulated with LPS (400 ng/mL) and IFN-γ (1.5 ng/mL). After 24 h of incubation, the cells were processed and mRNA for the cytokines quantified by qRT-PCR. Data from the experiments were normalized using GADPH primers as an endogenous control. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
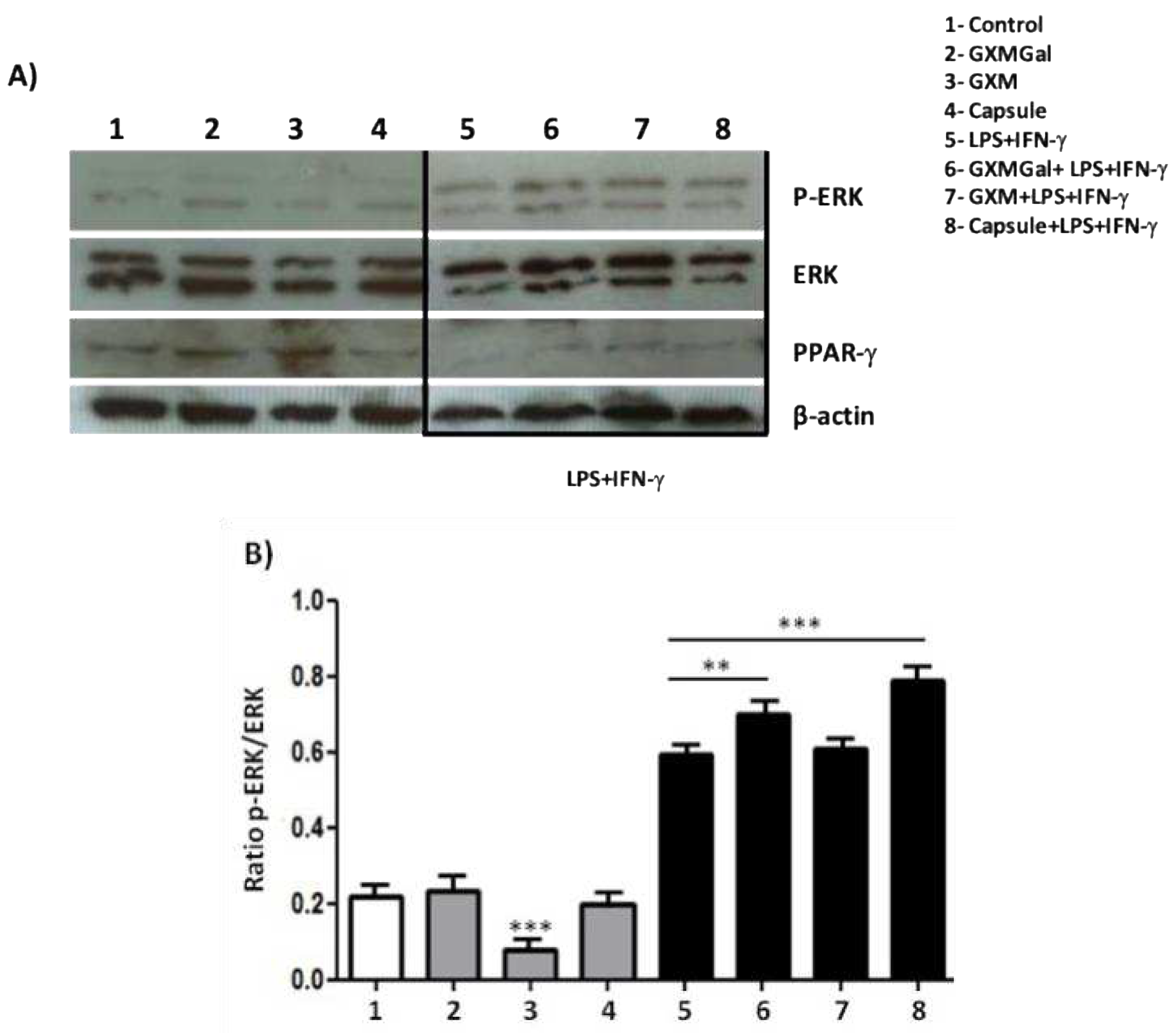
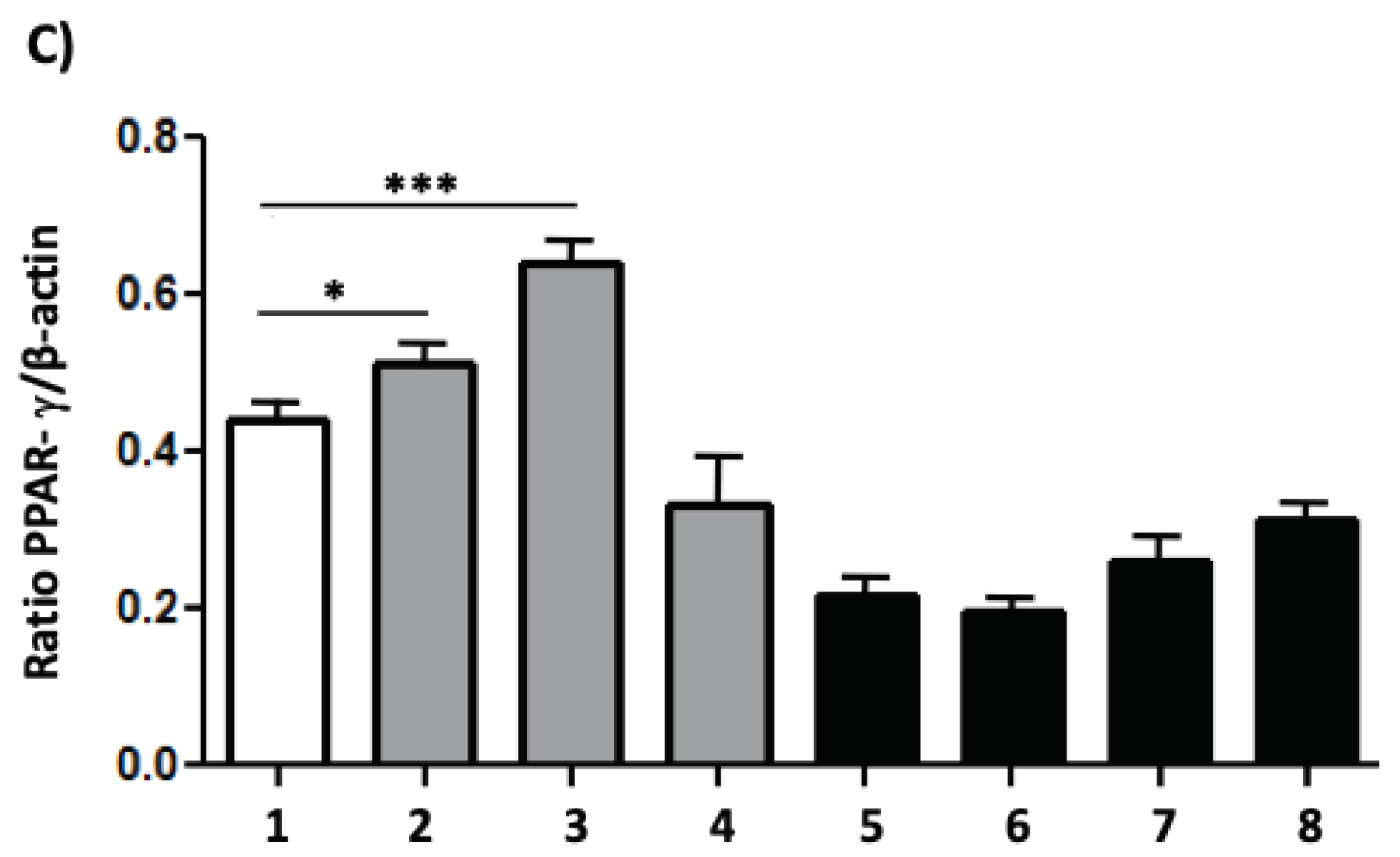
Figure 7.
MAPK phosphorylation and PPAR- γ activation. Canine macrophage DH82 (5x105) were plated and treated GXMGal, GXM and capsule (100 μg/mL). Some cultures were stimulated with LPS and IFN-γ. After 24 h of incubation whole-cell lysates were loaded onto SDS-PAGE gels. The blot was run and probed with the following antibodies: (A) anti-phospho ERK antibody and (B) anti-PPAR-γ antibody. The same blots were then stripped and reprobed with antibodies to non-phosphorylated proteins to determine absolute protein levels. Bar graphics shows the ratio of phosphorylated and total proteins. The band densitometry of western blotting was analyzed by the Scion Image software 4.0.3.2. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).
Figure 7.
MAPK phosphorylation and PPAR- γ activation. Canine macrophage DH82 (5x105) were plated and treated GXMGal, GXM and capsule (100 μg/mL). Some cultures were stimulated with LPS and IFN-γ. After 24 h of incubation whole-cell lysates were loaded onto SDS-PAGE gels. The blot was run and probed with the following antibodies: (A) anti-phospho ERK antibody and (B) anti-PPAR-γ antibody. The same blots were then stripped and reprobed with antibodies to non-phosphorylated proteins to determine absolute protein levels. Bar graphics shows the ratio of phosphorylated and total proteins. The band densitometry of western blotting was analyzed by the Scion Image software 4.0.3.2. All cultures were performed in triplicate and bars show the mean + SD. Statistical analysis was performed by t-test from representative results of three similar experiment (*p<0.05, **p<0.01, ***p<0.001).