Submitted:
23 January 2024
Posted:
23 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mechanism and Application of PET-CT Scan
2.1. Imaging Principles and Clinical Application
2.2. False positive and false negative PET/CT: Causes and Probabilities
2.2.1. Caveats in interpreting PET-CT in individuals with esophageal cancer in Table 2
| Causes of False-Positive Findings | Casuses of False-Negative Findings |
|---|---|
| Infections/Inflammatroy lesions | Lesion dependent |
| Radiation-induced liver disease (RILD) | Small tumors (<8-10 mm) |
| Radiation pneumonitis | Low metabolic activity of the tumor |
| (Postobstructive) pneumonia/abscess | The presence of a treatment-induced decrease in tumor metabolism |
| Mycobacterial or fungal infection | Technique limitation |
| Granulomatous disorders (sarcoidosis, Wegener) | Hyperglycemia |
| Chronic nonspecific lymphadenitis | Paravenous FDG injection |
| (Rheumatoid) arthritis | Excessive time between injection and scanning |
| Occupational exposure (anthracosilicosis) | Low resolution or motion artifacts |
| Bronchiectasis | |
| Organizing pneumonia | |
| Reflux esophagitis | |
| Iatrogenic causes | |
| Invasive procedure (puncture, biopsy) | |
| Talc pleurodesis | |
| Radiation esophagitis and pneumonitis | |
| Bone marrow expansion postchemotherapy | |
| Colony-stimulating factors | |
| Thymic hyperplasia postchemotherapy | |
| Benign mass lesions | |
| Salivary gland adenoma (Whartin) | |
| Thyroid adenoma | |
| Adrenal adenoma | |
| Colorectal dysplastic polyps | |
| Focal physiological FDG uptake | |
| Gastrointestinal tract | |
| Muscle activity | |
| Brown fat | |
| Unilateral vocal cord activity | |
| Arherosclerotic plaques |
2.2.3. Common non-malignant pathological conditions showing increased uptake of FDG before therapy
3. Current Treatment Protocol of Esophageal Cancer Involving True Liver Metastasis and False Liver Metastasis
3.1. Current Standard Procedure of Treatment for Esophageal Cancer in Figure 1

3.2. Reports of The Reference of True and False Metastasis for Restaging after nCRT
3.2.1. Case Reports Series and Cohort Study
| Table 4. Overview of current case reports. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (Year) | Age | TNM-pathology | Chemotherapy | Radiotherapy dose-Modality | Delay CRT to PET | PET | CT | MR | Biopsy | Liver Tests | Follow-up |
| Iyer et al.[2] (2007) | 63 | NA-adeno | NA | 50.4 Gy - 3D conformal | 6w | Nodular | Well-defined, low attenuation | - | Perop | AP↑ | NA |
| Iyer et al.[2] (2007) | NA | NA-NA | NA | 50.4 Gy - 3D conformal | 6w | Nodular | Well-defined, low attenuation | - | NA | AP↑ | NA |
| Nakahara et al.[3] (2008) | 50 | uT3N M2 1(bone) - NA | Docetaxel weekly (20mg/m2) | 46 Gy + boost 14 Gy - AP-RT | 4w | Wedge-shaped | Well-defined, low attenuation + band-likelesion (≈zoneof < 40Gy) | - | NA | AP↑ | 4months |
| DeLappe et al.[5] (2009) | 61 | uT3N M1 0 - NA | 4 cycli (apirubicine þ oxaliplatin + capcetabine) + 3 cycli (docetaxel þ irinotecan) + concurrent 5-FU | 50.4 Gy - IMRT | 5w | Ill-defined nodular | Patchy defined, mixed attenuation, heterogeneous enhancement of left liver | - | CT-guided + perop | NA | NA |
| Wong et al.[6] (2012) | 58 | NA - NA | NA | 50.4 Gy - AP-RT | 6w | Nodular with linear distribution | Patchy-defined, low attenuation in segment 2 and 3 | - | NA | Normal | NA |
| Rabe et al.[8] (2015) | 53 | uT3N M1 0 - squamous | 5 cycli (carboplatin + paclitaxel) | 50.4 Gy - 3D conformal | 2w | Nodular | Well-defined, low attenation | Hyperintens T2-weighted | Perop | AP↑ | 12months |
| Current case (2015) | 42 | uT2N M1 0 - adeno | Concurrent Oxaliplatin + 5-FU | 45 Gy - 3D conformal | 4w | Nodular | Patchy-defined, low attenuationin segment 2 | Hyperintens T2-weighted | Perop | Normal | 18months |
4. RILD Inducing False PET-CT
4.1. Implications of Increased FDG Uptake
4.2. The Formation and Classification of RILD
4.3. Occurrence and Duration of RILD
4.4. Incidence of RILD
4.4.1. If Tumor Cell Type Pose Different Risk Factors for RILD: Squamous-Cell Carcinoma (SCC) and Adeno Carcinoma in Table 4
| Risk Factor | Squamous-Cell Carcinoma | Adenocarcinoma |
|---|---|---|
| Tobacco use | +++ | ++ |
| Alcohol use | +++ | - |
| Barrett’s esophagus | - | ++++ |
| Weekly reflux symptoms | - | +++ |
| Obesity | - | ++ |
| Poverty | ++ | - |
| Achalasia | +++ | - |
| Caustic injury to the esophagus | ++++ | - |
| Nonepidermolytic palmoplantar keratoderma (tylosis) | ++++ | - |
| Plummer–Vinson syndrome | ++++ | - |
| History of head and neck cancer | ++++ | - |
| Frequent consumption of extremely hot beverages | + | - |
4.4.2. Gender, age, race
4.5. Molecule Biology of RILD
4.6. Influence of RILD
4.6.1. Dose of Radiotherapy
4.6.2. Synergic Effect with Chemotherapy
4.6.3. Underlying Liver Disease Is Vulnerable to RILD
4.7. Confirmation of RILD- Challenges in Characterizing RILD with Imaging
4.7.1. Sono of Liver and Its Sensitivity
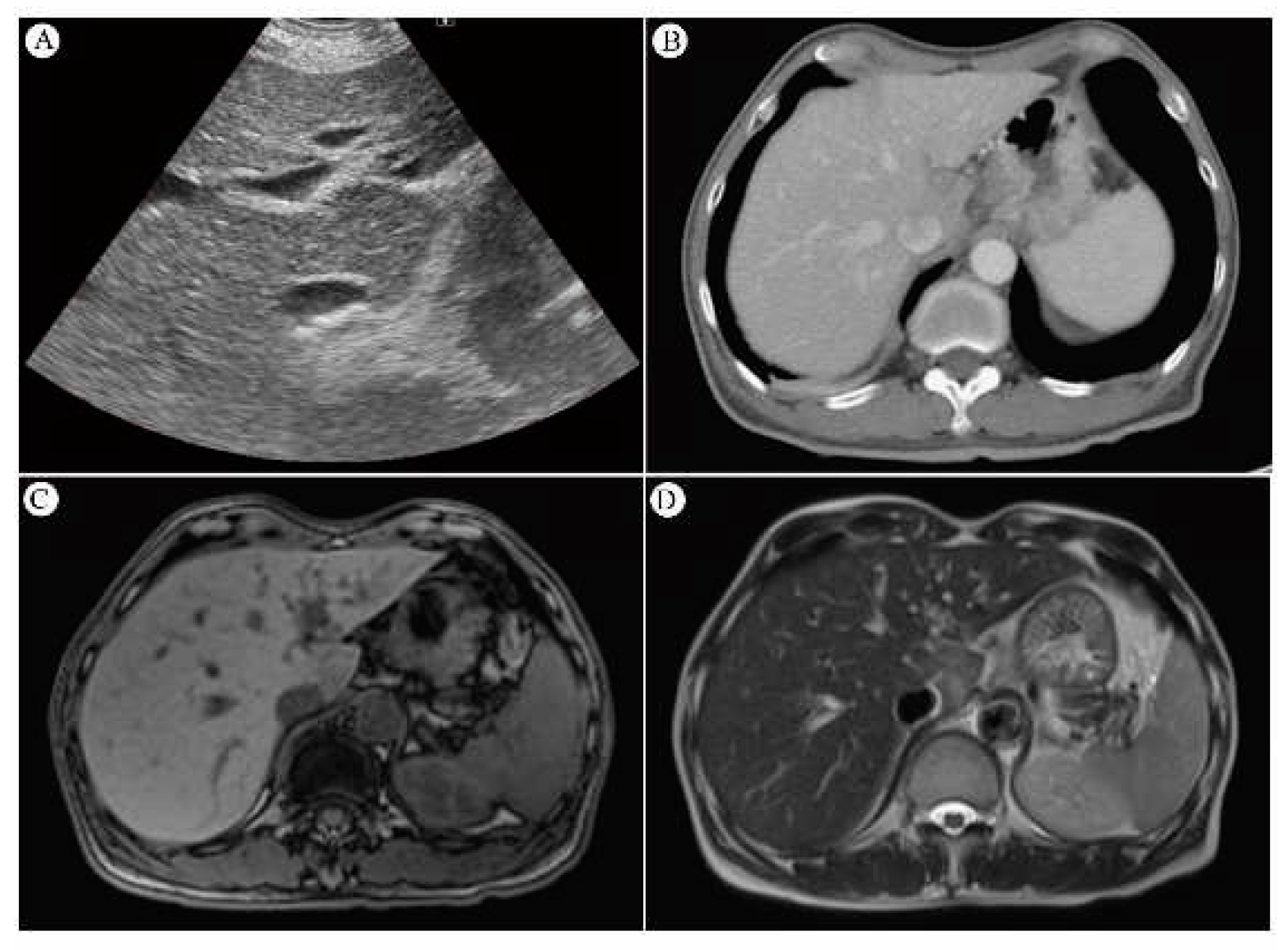
4.7.2. CT Scan and Its Sensitivity
4.7.3. MRI and Its Sensitivity
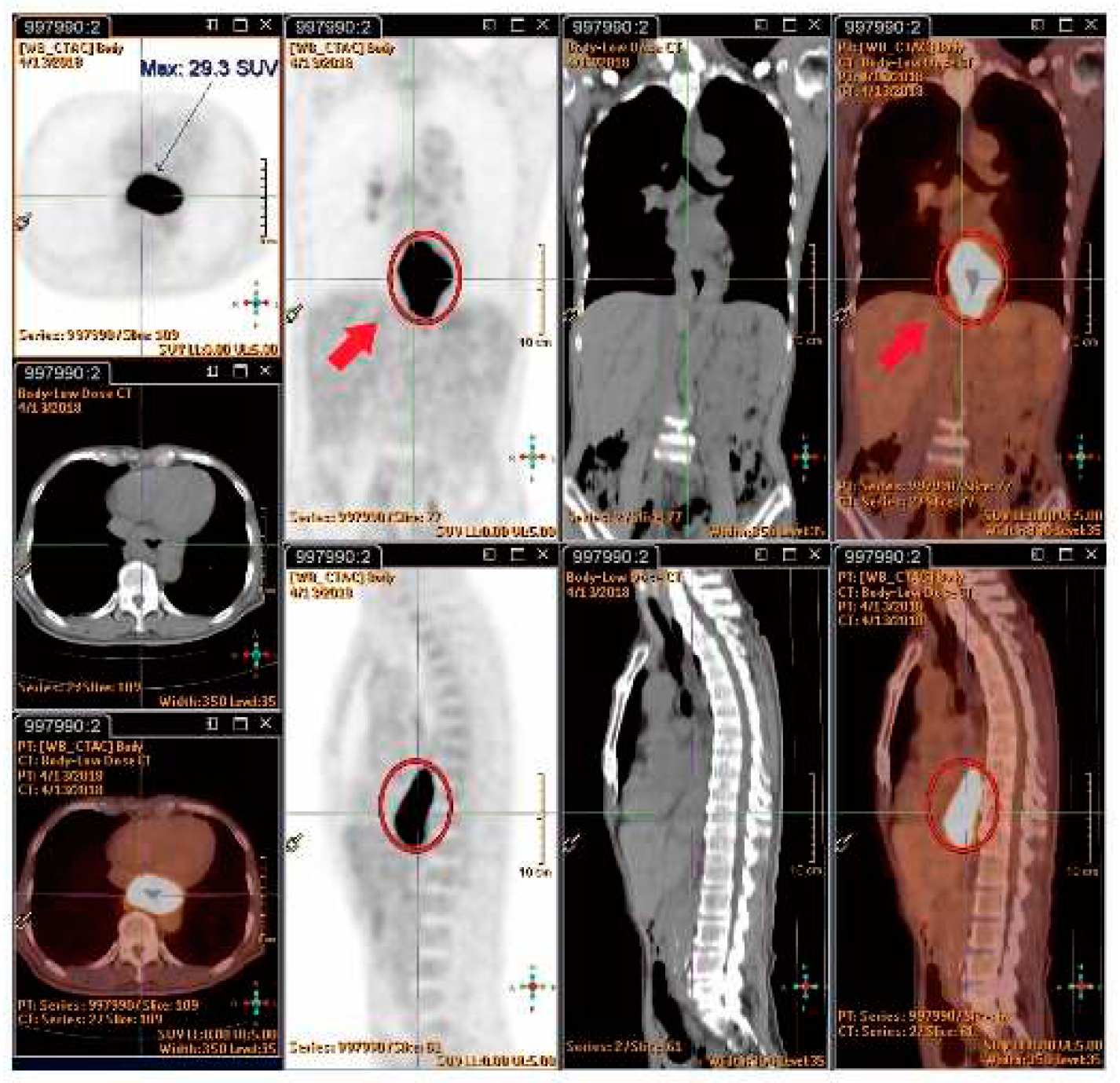
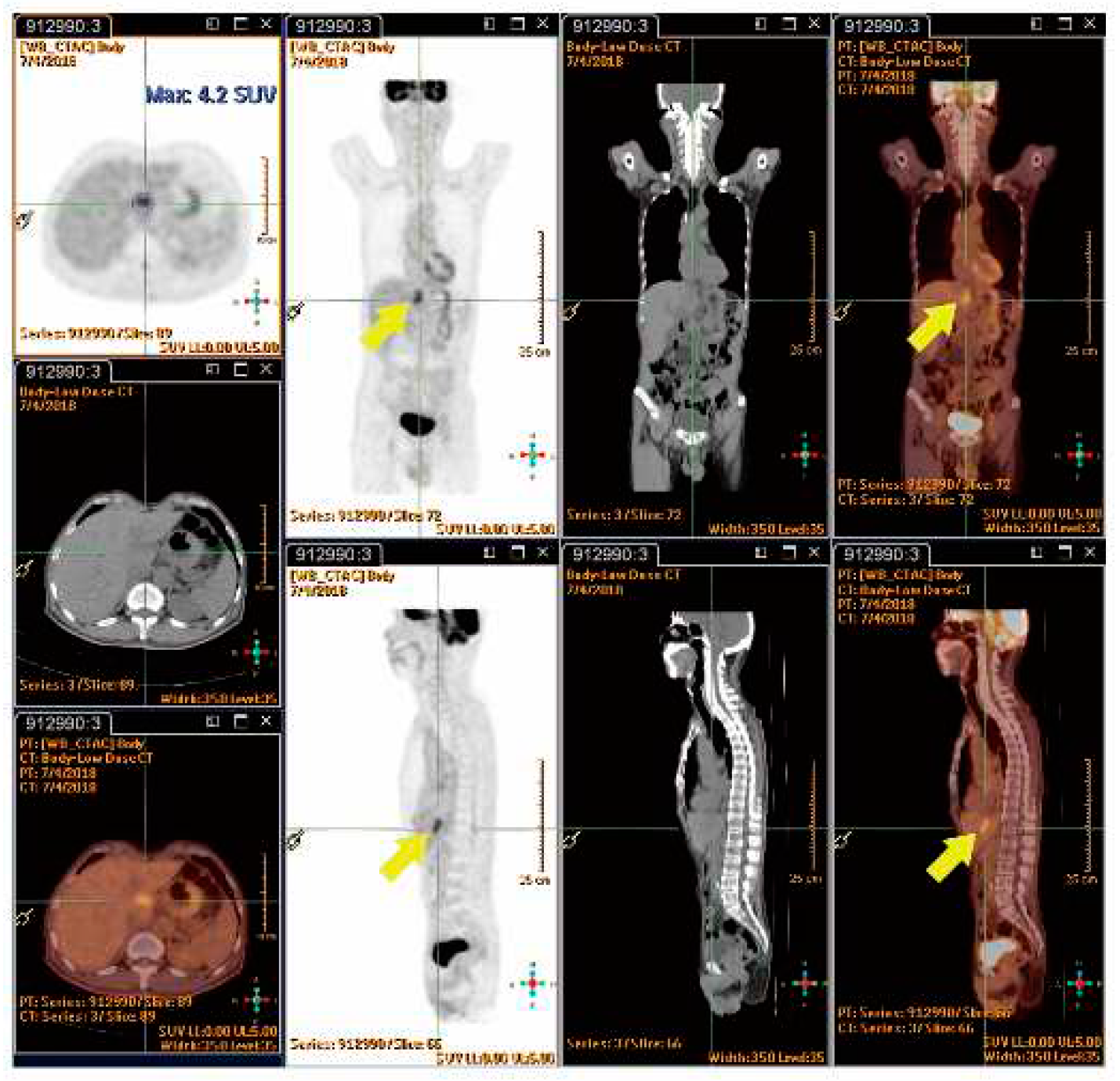
4.7.4. SUVmax value of PET/CT Serve as Indicator of RILD?
4.7.5. Biopsy for Diagnosis of Liver- Options by Sono-guiding, CT-guiding, Open biopsy or Clinical Observation
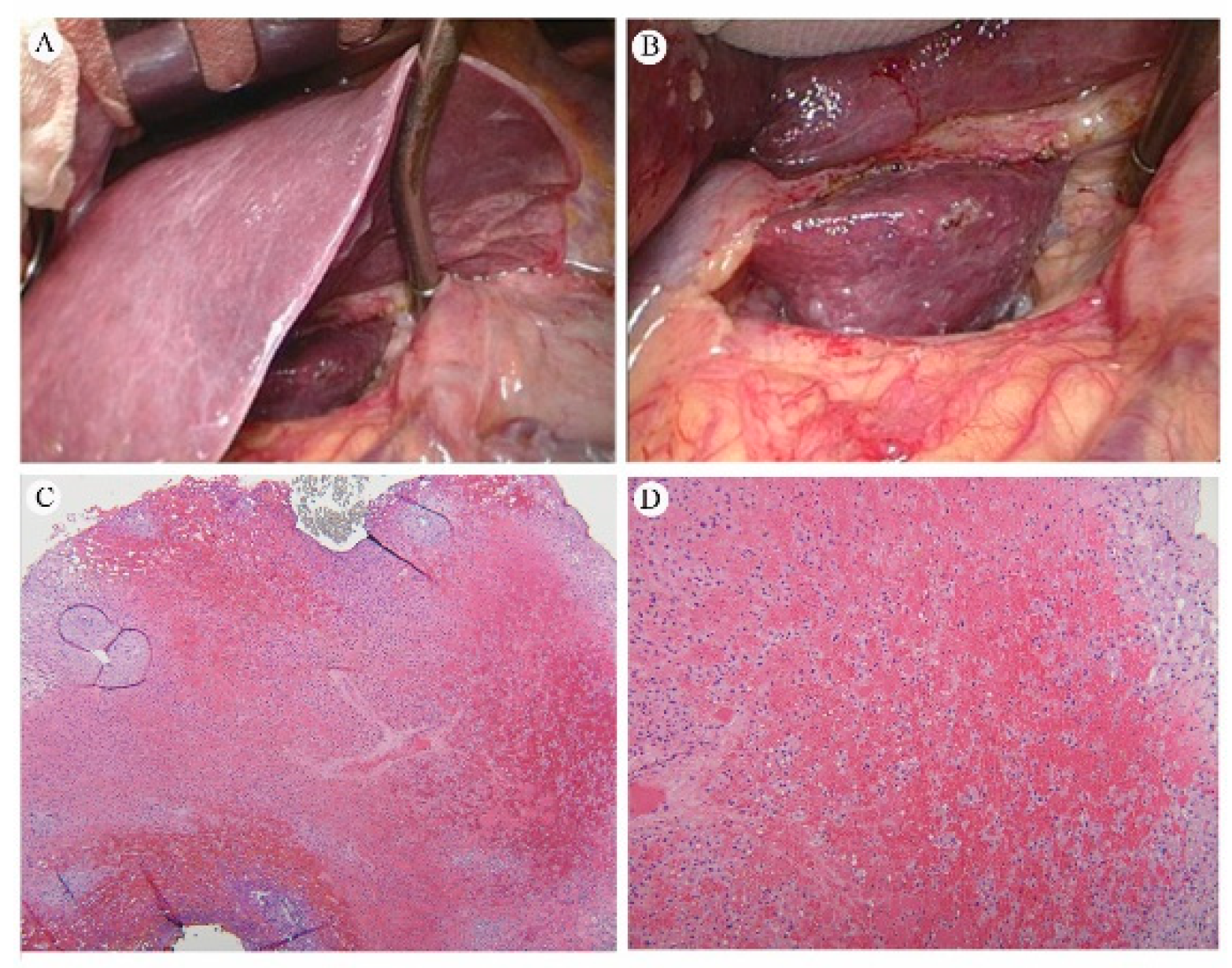
4.7.5.1. Pathology of RILD
4.7.5.2. Gross Appearance and Microscopy of RILD
5. Overview of Literature Review
| Author (Year) | Gender | Age (Range) | Race | Chemoradiotherapy | Liver function* | Stage | Esophageal cancer | |||||
| Neoadjuvant | Dose | Medicine | SCC | Adeno | Other | |||||||
| 1 | Rabe et al. (2016) [12] | F | 53 | NA | Yes | 50.4Gy | Carboplatin and paclitaxel | Yes | T3N1M0-->T2-weighted | 1 | 0 | 0 |
| 2 | Iyer et al. (2007) [10,13] | 24M /2F | 54 (41-78) | NA | Yes | 50.4 Gy/ patient | NA | Yes | NA | 2 | 24 | 0 |
| 3 | Daly et al. (2007) [11] | 74.2%M /25.8%F, n=5044 | 67.3 | 76.8% Non-Hispanic Caucasian, 19.2% African American, 4.0% Hispanic | NA | NA | NA | NA | Clinical stage - 0 (2.2%), I (14.1%), II (23.0%), III (22.1%), IV (38.7%). | 51.60% | 41.90% | 0 |
| 4 | Nakahara et al. (2008) [12] | M | 50 | NA | Yes | Up to a total of 46 Gy with an additional boost irradiation of 14 Gy. | Docetaxel | Yes | diagnosed with esophageal cancer with lymph node and bone metastases | NA | NA | 0 |
| 5 | Voncken et al. (2018) [13] | M | 50 | NA | Yes | 50.4 Gy in 23 fractions with weekly carboplatin and paclitaxel | Carboplatin and paclitaxel | NA | T3N1M0 | 1 | 0 | 0 |
| M | 62 | Not specified | Yes | 41.4 Gy in 23 fractions with weekly carboplatin and paclitaxel | carboplatin and paclitaxel | N0 | T3N0M0 | 0 | 62 | 0 | ||
| M | 41 | NA | Yes | 41.4 Gy in 23 fractions with weekly carboplatin and paclitaxel | Carboplatin and paclitaxel | No | T3N1M0 | 0 | 41 | 0 | ||
| M | 59 | NA | Yes | 50 Gy | Cisplatin and 5-fluorouracil | No | T3N1M0 | 0 | 1 | 0 | ||
| M | 49 | NA | Yes | 41.4 Gy | Carboplatin and paclitaxel | No | T3N1M0 | 0 | 1 | 0 | ||
| 6 | Stiekema et al. (2014) [6] | 60M /16F | 63 (46-80) | NA | Yes | 50 Gy or 50/ 50.4 Gy | 5-FU/cisplatin or carboplatin and paclitaxel | NA | NA | 14 | 60 | 2 (Undifferentiated) |
| 24M /2F | 63 (46-80) | NA | Yes | 50 Gy (n= 21) or 41.4 Gy (n=50) or 50.4 Gy (n=5) | 5-FU/cisplatin (n=21) or carboplatin and paclitaxel (n= 55) | NA | NA | 9 | 39 | |||
| 7 | Grant et al. (2014) [14] | 93M /19F | 57 (28-81) | NA | Yes | 41.4-50.4 Gy | NA | NA | NA | 21 | 97 | 4 |
| 8 | Wieder et al. (2004) [15] | 27M /11F | 60 (46-73) | NA | Yes | 40 Gy | Fluorouracil | NA | NA | 38 | 0 | 0 |
| 17 | DeLappe et al. (2009) [41] | M | 61 | NA | Yes | 50.4Gy | NA | NA | T3N1M0 | 0 | 1 | 0 |
| 18 | Shai et al. (2020) [] | M | 66 | Asian | Yes | 5000cGy | NA | No | T3N1M0 | 1 | 0 | 0 |
| 19 | Demey et al. (2016) | M | 42 | NA | Yes | 45Gy | Oxaliplatin, levofolinic acid and 5-FU | No | uT2N1M0 | 0 | 1 | 0 |
| 20 | Anderegg et al. (2015) | 76.3%M, n=156 | 65 (34-83) | NA | Yes | 41.4 Gy | Carboplatin and paclitaxel (n=139) or Cbp, Ptx and panitumumab (n=17) | NA | NA | 29 | 126 | 1 |
| 21 | Voncken et al. (2018) | M | 50 | NA | Yes | 50.4 Gy | Cbp and Ptx | NA | T3N1M0 | 1 | 0 | 0 |
| M | 62 | NA | Yes | 41.4 Gy | Cbp and Ptx | No | T3N0M0 | 0 | 1 | 0 | ||
| M | 41 | NA | Yes | 41.4 Gy | Carboplatin and Ptx | No | T3N1M0 | 0 | 1 | 0 | ||
| M | 59 | NA | Yes | 50 Gy | Cis and 5-FU | No | T3N1M0 | 0 | 1 | 0 | ||
| M | 49 | NA | Yes | 41.4 Gy | Cbp and Ptx | No | T3N1M0 | 0 | 1 | 0 | ||
| M | 75 | NA | Yes | 50 Gy | Carboplatin/ etoposide | No | T2N1M0 | 0 | 0 | 1 | ||
| 22 | Goense et al. (2018) | 675M /108F | <65 (n=425), ≧65 (n=358) | NA | Yes | 45 Gy or 50.4 Gy | Oxaliplatin / 5-FU or Docetaxel / 5-FU or Capecitabine / 5-FU or other | NA | NA | 111 | 672 | 0 |
| 23 | Gabriel et al. (2017) | 234M /24F | 61.5 | NA | Yes | 50.4 Gy | Cis and Iri/ Cbp and Ptx or Oxaliplatin/capecitabine or 5-FU and Cis | NA | NA | 39 | 219 | 0 |
| 24 | Yuekai et al. (2020) | 76M /48F | 56 (25-82) | NA | NA | NA | NA | NA | NA | 20 | 69 | 35 |
| 25 | Blom et al. (2011) | 40 male/ 10 female, n=50 | 61 (56-67) | NA | Yes | 50.4 Gy | Cis and 5-FU | NA | Stages II to IVa | 9 | 40 | 1 |
| 26 | Cerfolio et al. (2005) | 41 male/ 7 female, n=48 | 68 (48-76) | NA | Yes | <50Gy (n=22), >50Gy (n=26) | NA | NA | Stages I to Ivb | 5 | 43 | 0 |
6. Conclusions and Future Prospective
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Sjoquist, K.M.; Burmeister, B.H.; Smithers, B.M.; Zalcberg, J.R.; Simes, R.J.; Barbour, A.; Gebski, V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011, 12, 681–692. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015, 16, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.M.; Fry, W.A.; Little, A.G.; Winchester, D.P.; McKee, R.F.; Stewart, A.K.; Fremgen, A.M. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000, 190, 562–572. [Google Scholar] [CrossRef]
- Nakahara, T.; Takagi, Y.; Takemasa, K.; Mitsui, Y.; Tsuyuki, A.; Shigematsu, N.; Kubo, A. Dose-related fluorodeoxyglucose uptake in acute radiation-induced hepatitis. Eur J Gastroenterol Hepatol 2008, 20, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Monjazeb, A.M.; Riedlinger, G.; Aklilu, M.; Geisinger, K.R.; Mishra, G.; Isom, S.; Clark, P.; Levine, E.A.; Blackstock, A.W. Outcomes of patients with esophageal cancer staged with [¹⁸F]fluorodeoxyglucose positron emission tomography (FDG-PET): can postchemoradiotherapy FDG-PET predict the utility of resection? J Clin Oncol 2010, 28, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- Smithers, B.M.; Couper, G.C.; Thomas, J.M.; Wong, D.; Gotley, D.C.; Martin, I.; Harvey, J.A.; Thomson, D.B.; Walpole, E.T.; Watts, N.; et al. Positron emission tomography and pathological evidence of response to neoadjuvant therapy in adenocarcinoma of the esophagus. Dis Esophagus 2008, 21, 151–158. [Google Scholar] [CrossRef]
- Blom, R.L.; Schreurs, W.M.; Belgers, H.J.; Oostenbrug, L.E.; Vliegen, R.F.; Sosef, M.N. The value of post-neoadjuvant therapy PET-CT in the detection of interval metastases in esophageal carcinoma. Eur J Surg Oncol 2011, 37, 774–778. [Google Scholar] [CrossRef]
- Bruzzi, J.F.; Munden, R.F.; Truong, M.T.; Marom, E.M.; Sabloff, B.S.; Gladish, G.W.; Iyer, R.B.; Pan, T.S.; Macapinlac, H.A.; Erasmus, J.J. PET/CT of esophageal cancer: its role in clinical management. Radiographics 2007, 27, 1635–1652. [Google Scholar] [CrossRef]
- Stiekema, J.; Vermeulen, D.; Vegt, E.; Voncken, F.E.; Aleman, B.M.; Sanders, J.; Boot, H.; van Sandick, J.W. Detecting interval metastases and response assessment using 18F-FDG PET/CT after neoadjuvant chemoradiotherapy for esophageal cancer. Clin Nucl Med 2014, 39, 862–867. [Google Scholar] [CrossRef]
- Goense, L.; Ruurda, J.P.; Carter, B.W.; Fang, P.; Ho, L.; Meijer, G.J.; van Hillegersberg, R.; Hofstetter, W.L.; Lin, S.H. Prediction and diagnosis of interval metastasis after neoadjuvant chemoradiotherapy for oesophageal cancer using (18)F-FDG PET/CT. Eur J Nucl Med Mol Imaging 2018, 45, 1742–1751. [Google Scholar] [CrossRef]
- Rabe, T.M.; Yokoo, T.; Meyer, J.; Kernstine, K.H., Sr.; Wang, D.; Khatri, G. Radiation-Induced Liver Injury Mimicking Metastatic Disease in a Patient With Esophageal Cancer: Correlation of Positron Emission Tomography/Computed Tomography With Magnetic Resonance Imaging and Literature Review. J Comput Assist Tomogr 2016, 40, 560–563. [Google Scholar] [CrossRef]
- Iyer, R.B.; Balachandran, A.; Bruzzi, J.F.; Johnson, V.; Macapinlac, H.A.; Munden, R.F. PET/CT and hepatic radiation injury in esophageal cancer patients. Cancer Imaging 2007, 7, 189–194. [Google Scholar] [CrossRef]
- Voncken, F.E.M.; Aleman, B.M.P.; van Dieren, J.M.; Grootscholten, C.; Lalezari, F.; van Sandick, J.W.; Steinberg, J.D.; Vegt, E. Radiation-induced liver injury mimicking liver metastases on FDG-PET-CT after chemoradiotherapy for esophageal cancer: A retrospective study and literature review. Strahlenther Onkol 2018, 194, 156–163. [Google Scholar] [CrossRef]
- Reed, G.B., Jr.; Cox, A.J., Jr. The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol 1966, 48, 597–611. [Google Scholar]
- Benson, R.; Madan, R.; Kilambi, R.; Chander, S. Radiation induced liver disease: A clinical update. J Egypt Natl Canc Inst 2016, 28, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y. Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med 2017, 49, e359. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.S.; Robertson, J.M.; Anscher, M.S.; Jirtle, R.L.; Ensminger, W.D.; Fajardo, L.F. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995, 31, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Hizawa, K.; Yoshida, M.; Kitamuro, T.; Akagi, G.; Kagawa, K.; Fukuda, F. HEPATIC INJURY FOLLOWING IRRADIATION--A MORPHOLOGIC STUDY. Tokushima J Exp Med 1963, 10, 240–251. [Google Scholar] [PubMed]
- Chou, C.H.; Chen, P.J.; Lee, P.H.; Cheng, A.L.; Hsu, H.C.; Cheng, J.C. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin Cancer Res 2007, 13, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.; Alnaji, R.; Du, W.; Attwood, K.; Kukar, M.; Hochwald, S. Effectiveness of Repeat 18F-Fluorodeoxyglucose Positron Emission Tomography Computerized Tomography (PET-CT) Scan in Identifying Interval Metastases for Patients with Esophageal Cancer. Ann Surg Oncol 2017, 24, 1739–1746. [Google Scholar] [CrossRef]
- Grant, M.J.; Didier, R.A.; Stevens, J.S.; Beyder, D.D.; Hunter, J.G.; Thomas, C.R.; Coakley, F.V. Radiation-induced liver disease as a mimic of liver metastases at serial PET/CT during neoadjuvant chemoradiation of distal esophageal cancer. Abdom Imaging 2014, 39, 963–968. [Google Scholar] [CrossRef]
- Som, P.; Atkins, H.L.; Bandoypadhyay, D.; Fowler, J.S.; MacGregor, R.R.; Matsui, K.; Oster, Z.H.; Sacker, D.F.; Shiue, C.Y.; Turner, H.; et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med 1980, 21, 670–675. [Google Scholar] [CrossRef]
- Liu, Y.; Ghesani, N.V.; Zuckier, L.S. Physiology and pathophysiology of incidental findings detected on FDG-PET scintigraphy. Semin Nucl Med 2010, 40, 294–315. [Google Scholar] [CrossRef]
- von Schulthess, G.K.; Steinert, H.C.; Hany, T.F. Integrated PET/CT: current applications and future directions. Radiology 2006, 238, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, T.M.; Meltzer, C.C.; Townsend, D.W. PET/CT: form and function. Radiology 2007, 242, 360–385. [Google Scholar] [CrossRef] [PubMed]
- Hillner, B.E.; Siegel, B.A.; Liu, D.; Shields, A.F.; Gareen, I.F.; Hanna, L.; Stine, S.H.; Coleman, R.E. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol 2008, 26, 2155–2161. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Krause, B.J.; Heusner, T.A.; Boy, C.; Bockisch, A.; Antoch, G. PET/CT for the staging and follow-up of patients with malignancies. Eur J Radiol 2009, 70, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Metser, U.; Miller, E.; Lerman, H.; Even-Sapir, E. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: characterization and incidence. AJR Am J Roentgenol 2007, 189, 1203–1210. [Google Scholar] [CrossRef]
- Beatty, J.S.; Williams, H.T.; Aldridge, B.A.; Hughes, M.P.; Vasudeva, V.S.; Gucwa, A.L.; David, G.S.; Lind, D.S.; Kruse, E.J.; McLoughlin, J.M. Incidental PET/CT findings in the cancer patient: how should they be managed? Surgery 2009, 146, 274–281. [Google Scholar] [CrossRef]
- Culverwell, A.D.; Scarsbrook, A.F.; Chowdhury, F.U. False-positive uptake on 2-[¹⁸F]-fluoro-2-deoxy-D-glucose (FDG) positron-emission tomography/computed tomography (PET/CT) in oncological imaging. Clin Radiol 2011, 66, 366–382. [Google Scholar] [CrossRef]
- Chowdhury, F.U.; Sheerin, F.; Bradley, K.M.; Gleeson, F.V. Sarcoid-like reaction to malignancy on whole-body integrated (18)F-FDG PET/CT: prevalence and disease pattern. Clin Radiol 2009, 64, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Jacene, H.A.; Stearns, V.; Wahl, R.L. Lymphadenopathy resulting from acute hepatitis C infection mimicking metastatic breast carcinoma on FDG PET/CT. Clin Nucl Med 2006, 31, 379–381. [Google Scholar] [CrossRef]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Heeren, P.A.; Jager, P.L.; Bongaerts, F.; van Dullemen, H.; Sluiter, W.; Plukker, J.T. Detection of distant metastases in esophageal cancer with (18)F-FDG PET. J Nucl Med 2004, 45, 980–987. [Google Scholar]
- Nijkamp, J.; Rossi, M.; Lebesque, J.; Belderbos, J.; van den Heuvel, M.; Kwint, M.; Uyterlinde, W.; Vogel, W.; Sonke, J.J. Relating acute esophagitis to radiotherapy dose using FDG-PET in concurrent chemo-radiotherapy for locally advanced non-small cell lung cancer. Radiother Oncol 2013, 106, 118–123. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Lyall, A. Identifying and distinguishing treatment effects and complications from malignancy at FDG PET/CT. Radiographics 2013, 33, 1817–1834. [Google Scholar] [CrossRef]
- Bruzzi, J.F.; Swisher, S.G.; Truong, M.T.; Munden, R.F.; Hofstetter, W.L.; Macapinlac, H.A.; Correa, A.M.; Mawlawi, O.; Ajani, J.A.; Komaki, R.R.; et al. Detection of interval distant metastases: clinical utility of integrated CT-PET imaging in patients with esophageal carcinoma after neoadjuvant therapy. Cancer 2007, 109, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Flamen, P.; Van Cutsem, E.; Lerut, A.; Cambier, J.P.; Haustermans, K.; Bormans, G.; De Leyn, P.; Van Raemdonck, D.; De Wever, W.; Ectors, N.; et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol 2002, 13, 361–368. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; Bryant, A.S.; Ohja, B.; Bartolucci, A.A.; Eloubeidi, M.A. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg 2005, 129, 1232–1241. [Google Scholar] [CrossRef]
- Weber, W.A.; Ott, K.; Becker, K.; Dittler, H.J.; Helmberger, H.; Avril, N.E.; Meisetschläger, G.; Busch, R.; Siewert, J.R.; Schwaiger, M.; et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 2001, 19, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.M.; Gillies, R.S.; Franklin, J.M.; Teoh, E.J.; Jones, G.E.; di Carlo, S.; Gleeson, F.V.; Maynard, N.D.; Bradley, K.M.; Middleton, M.R. Restaging oesophageal cancer after neoadjuvant therapy with (18)F-FDG PET-CT: identifying interval metastases and predicting incurable disease at surgery. Eur Radiol 2016, 26, 3519–3533. [Google Scholar] [CrossRef]
- Levine, E.A.; Farmer, M.R.; Clark, P.; Mishra, G.; Ho, C.; Geisinger, K.R.; Melin, S.A.; Lovato, J.; Oaks, T.; Blackstock, A.W. Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg 2006, 243, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Demey, K.; Van Veer, H.; Nafteux, P.; Deroose, C.M.; Haustermans, K.; Coolen, J.; Vandecaveye, V.; Coosemans, W.; Van Cutsem, E. Hepatic radiation injury mimicking metastasis in distal esophageal cancer(). Acta Chir Belg 2017, 117, 250–255. [Google Scholar] [CrossRef] [PubMed]
- DeLappe, E.M.; Truong, M.T.; Bruzzi, J.F.; Swisher, S.G.; Rohren, E.M. Hepatic radiation injury mimicking a metastasis on positron-emission tomography/computed tomography in a patient with esophageal carcinoma. J Thorac Oncol 2009, 4, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Luk, W.H.; Au-Yeung, A.W.; Loke, T.K. Imaging patterns of liver uptakes on PET scan: pearls and pitfalls. Nucl Med Rev Cent East Eur 2013, 16, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Shai, S.E.; Lin, Y.H.; Lai, Y.L.; Tang, H.W.; Hsieh, Y.W.; Hung, S.C. Phantom simulation of liver metastasis on a positron emission tomography with computed tomography scan after neoadjuvant chemoradiotherapy for distal esophageal cancer: a case report. J Med Case Rep 2020, 14, 106. [Google Scholar] [CrossRef]
- Pan, C.C.; Kavanagh, B.D.; Dawson, L.A.; Li, X.A.; Das, S.K.; Miften, M.; Ten Haken, R.K. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010, 76, S94–S100. [Google Scholar] [CrossRef] [PubMed]
- Sempoux, C.; Horsmans, Y.; Geubel, A.; Fraikin, J.; Van Beers, B.E.; Gigot, J.F.; Lerut, J.; Rahier, J. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology 1997, 26, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, L.F.; Colby, T.V. Pathogenesis of veno-occlusive liver disease after radiation. Arch Pathol Lab Med 1980, 104, 584–588. [Google Scholar]
- Dawson, L.A.; Normolle, D.; Balter, J.M.; McGinn, C.J.; Lawrence, T.S.; Ten Haken, R.K. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002, 53, 810–821. [Google Scholar] [CrossRef]
- Dawson, L.A.; Ten Haken, R.K. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol 2005, 15, 279–283. [Google Scholar] [CrossRef]
- Yamasaki, S.A.; Marn, C.S.; Francis, I.R.; Robertson, J.M.; Lawrence, T.S. High-dose localized radiation therapy for treatment of hepatic malignant tumors: CT findings and their relation to radiation hepatitis. AJR Am J Roentgenol 1995, 165, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Munden, R.F.; Erasmus, J.J.; Smythe, W.R.; Madewell, J.E.; Forster, K.M.; Stevens, C.W. Radiation injury to the liver after intensity-modulated radiation therapy in patients with mesothelioma: an unusual CT appearance. AJR Am J Roentgenol 2005, 184, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Enzinger, P.C.; Mayer, R.J. Esophageal cancer. N Engl J Med 2003, 349, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.E.; Friedman, S.L. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol 2011, 25, 195–206. [Google Scholar] [CrossRef]
- Wieder, H.A.; Brücher, B.L.; Zimmermann, F.; Becker, K.; Lordick, F.; Beer, A.; Schwaiger, M.; Fink, U.; Siewert, J.R.; Stein, H.J.; et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol 2004, 22, 900–908. [Google Scholar] [CrossRef] [PubMed]
- King, P.D.; Perry, M.C. Hepatotoxicity of chemotherapy. Oncologist 2001, 6, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Guha, C.; Kavanagh, B.D. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol 2011, 21, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, R.B., Jr.; Moss, A.A.; Quivey, J.M.; Federle, M.P.; Wara, W.M. CT of radiation-induced hepatic injury. AJR Am J Roentgenol 1980, 135, 445–448. [Google Scholar] [CrossRef]
- Unger, E.C.; Lee, J.K.; Weyman, P.J. CT and MR imaging of radiation hepatitis. J Comput Assist Tomogr 1987, 11, 264–268. [Google Scholar] [CrossRef]
- Kwek, J.W.; Iyer, R.B.; Dunnington, J.; Faria, S.; Silverman, P.M. Spectrum of imaging findings in the abdomen after radiotherapy. AJR Am J Roentgenol 2006, 187, 1204–1211. [Google Scholar] [CrossRef]
- Itai, Y.; Murata, S.; Kurosaki, Y. Straight border sign of the liver: spectrum of CT appearances and causes. Radiographics 1995, 15, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Vernuccio, F.; Cannella, R.; Bartolotta, T.V.; Galia, M.; Tang, A.; Brancatelli, G. Advances in liver US, CT, and MRI: moving toward the future. Eur Radiol Exp 2021, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.; Anthony, M.P.; Lan Khong, P. Hepatic radiation injury in distal esophageal carcinoma: a case report. Clin Nucl Med 2012, 37, 709–711. [Google Scholar] [CrossRef]
- Viswanathan, C.; Truong, M.T.; Sagebiel, T.L.; Bronstein, Y.; Vikram, R.; Patnana, M.; Silverman, P.M.; Bhosale, P.R. Abdominal and pelvic complications of nonoperative oncologic therapy. Radiographics 2014, 34, 941–961. [Google Scholar] [CrossRef]
- Jelvehgaran, P.; Steinberg, J.D.; Khmelinskii, A.; Borst, G.; Song, J.Y.; de Wit, N.; de Bruin, D.M.; van Herk, M. Evaluation of acute esophageal radiation-induced damage using magnetic resonance imaging: a feasibility study in mice. Radiat Oncol 2019, 14, 188. [Google Scholar] [CrossRef]
- Seidensticker, M.; Burak, M.; Kalinski, T.; Garlipp, B.; Koelble, K.; Wust, P.; Antweiler, K.; Seidensticker, R.; Mohnike, K.; Pech, M.; et al. Radiation-induced liver damage: correlation of histopathology with hepatobiliary magnetic resonance imaging, a feasibility study. Cardiovasc Intervent Radiol 2015, 38, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, R.; Ota, H.; Takanami, K.; Ichinose, A.; Matsushita, H.; Saito, H.; Takase, K.; Jingu, K. MRI findings of radiation-induced myocardial damage in patients with oesophageal cancer. Clin Radiol 2014, 69, 1273–1279. [Google Scholar] [CrossRef]
- Machann, W.; Beer, M.; Breunig, M.; Störk, S.; Angermann, C.; Seufert, I.; Schwab, F.; Kölbl, O.; Flentje, M.; Vordermark, D. Cardiac magnetic resonance imaging findings in 20-year survivors of mediastinal radiotherapy for Hodgkin′s disease. Int J Radiat Oncol Biol Phys 2011, 79, 1117–1123. [Google Scholar] [CrossRef]
- Yankelevitz, D.F.; Henschke, C.I.; Knapp, P.H.; Nisce, L.; Yi, Y.; Cahill, P. Effect of radiation therapy on thoracic and lumbar bone marrow: evaluation with MR imaging. AJR Am J Roentgenol 1991, 157, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Henning, T.; Link, T.M. MR imaging of therapy-induced changes of bone marrow. Eur Radiol 2007, 17, 743–761. [Google Scholar] [CrossRef] [PubMed]

| Parameter | 18F-FDG PET-CT |
|---|---|
| Sensitivity (%) [95%CI] | 65/87 (74.7%) [64.3–83.4] |
| Specificity (%) [95%CI] | 652/696 (93.7%) [91.6–95.4] |
| Positive predictive value (%) [95%CI] | 65/109 (59.6%) [52.0–66.9] |
| Negative predictive value (%) [95%CI] | 652/674 (96.7%) [95.4–97.7] |
| Diagnostic accuracy | 91.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





