1. Introduction
Bipolar disorder (BD) is a frequent disorder with a high suicide rate and an impact on patients' functioning [
1,
2]. It is defined by the recurrence of depressive and manic or mixed episodes. One of the most impacted domains in BD is emotional processing whose disruptions underlie many symptoms. Even during remitted phases, BD patients experience more frequent and intense emotions in response to environmental conditions relative to healthy subjects, which leads to mood instability [
3]. Emotional hyper-reactivity and mood instability have a detrimental impact on functioning, relapses, and suicide attempts [
4,
5]. Patients with BD use maladaptive forms of emotion regulation strategies such as rumination and dampening, compared with controls [
6,
7]. The current physiological consensual model of BD assumes that a dysfunction in prefrontal cortex regulatory processes of the limbic structures is the main cause of the disease [
8,
9].
Emotional regulation (ER) may be automatic or controlled. Two well-studied controlled or voluntary emotion regulation strategies are attentional distraction and cognitive reappraisal [
10,
11,
12,
13]. In distraction, an external cognitive task is used to put away the attention from a specific emotional stimulus. Reappraisal consists of reinterpreting a situation to reduce its emotional impact. Several studies reported attenuated self-reported negative or positive affects after distraction and reappraisal [
11,
14,
15].
Temporal characteristics of these two emotion regulation strategies in BD patients might be provided by electroencephalography (EEG). Current EEG studies have assessed the time course of emotion regulation processes in healthy participants through cognitive reappraisal [
16,
17,
18] and distraction [
19]. These studies have focused more specifically on a late event-related potential (ERP), called the late positive potential (LPP). The LPP is a positive slow modulation of the ERP with a posterior midline scalp distribution and an onset around 250 msec after stimulus presentation. In emotional studies, the LPP is usually thought to mark both emotional reactivity and regulation. Importantly, the LPP is highly sensitive to the emotional intensity of stimuli and is larger for both pleasant and unpleasant than for neutral stimuli [
20,
21]. Both ER strategies were successful in reducing LPP magnitudes to emotional stimuli. Specifically, the LPP is reliably smaller when an unpleasant stimulus is cognitively evaluated in a neutral compared to a negative manner [
16,
17]. Distraction began modulating the LPP from its very beginning whereas reappraisal began modulating the LPP later [
12,
19]. To our knowledge, no study was interested in time course of ER strategies in BD patients.
The aim of this EEG study was to investigate the neurochronometry of two emotion regulation strategies, distraction and reappraisal, in euthymic patients with bipolar disorder. For that purpose, we used an established experimental paradigm [
12,
14]. We hypothesized exaggerated emotional responses in BD patients in self-reports and a larger amplitude of the LPP in passive viewing compared with healthy controls. Similarly, emotion regulation deficits are expected, reflected in the less attenuation of LPP in emotion regulation conditions.
2. Materials and Methods
2.1. Participants
Bipolar patients were recruited from a psychiatric hospital (Créteil, France). The study was described in detail to the patients, and written informed consent was obtained from all participants. Fourteen bipolar patients (seven men and seven women; mean age = 39.5 years, SD = 11.06) and thirteen volunteers (seven men and six women; mean age = 28.5 years, SD = 12.7) took part in this experiment. Euthymic state was defined by a score <12 on both the Montgomery-Åsberg Depression Scale [
22] and the Young Mania Rating Scale [
23]. Healthy controls had no history of Axis I psychiatric disorders. Subjects with a neurological history were excluded. All participants were right-handed according to a handedness questionnaire [
24], had normal or corrected-to-normal vision. The study was approved by the research ethical committee of Paris Descartes University.
2.2. Stimulus material
In this study we used the same material as in Schönfelder et al. study [
12]. Emotion-inducing stimuli were 90 color photographs drawn from the International Affective Picture System (IAPS) [
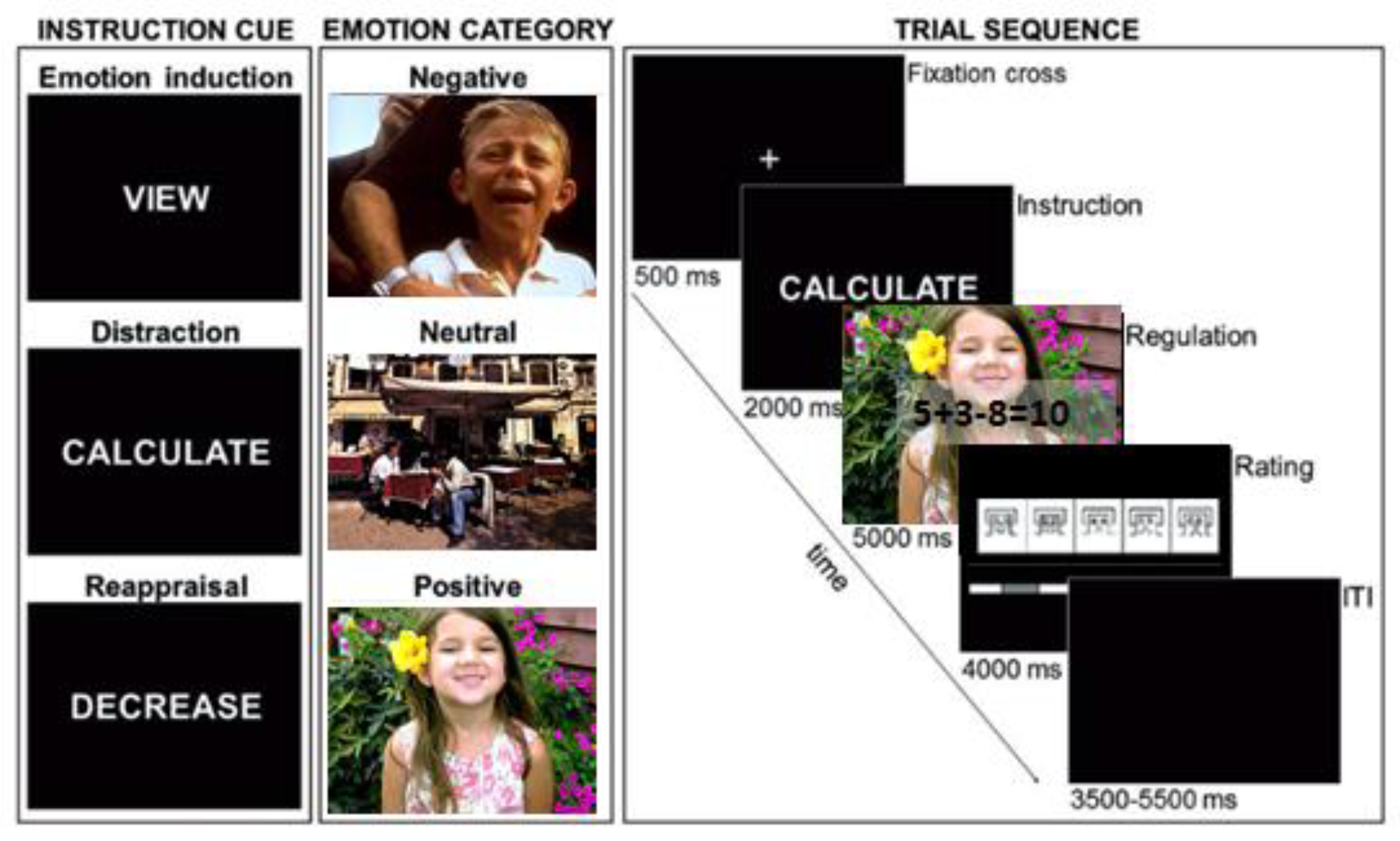
25]. Negative and positive stimuli were highly arousing, and neutral stimuli were rated low in arousal. Thus, 30 positive pictures (for example, exciting sport or happy family scenes), 30 negative pictures (human violence, accidents) and 30 neutral pictures (for example, people doing ordinary activities) were selected. For distraction, participants had to solve 3-operand arithmetic equations, including one subtraction and one addition (e.g., 8 + 3 – 7 = 5) and to indicate via button press as quickly and accurately as possible whether the displayed solution was correct or incorrect. Half of all equations were incorrect and were constructed to differ by 1 from the correct answer (
Figure 1).
2.3. Experimental task
The experiment consisted of three experimental conditions, a passive viewing condition and two regulation conditions, one with reappraisal strategy and the other with distraction strategy (
Figure 1). The purpose of the passive viewing block was to establish the basic effect of emotional reactivity on the LPP. In the reappraisal condition, participants were given reappraisal instructions. The experimenter explained the concept of reappraisal as reinterpreting an unpleasant or a pleasant picture to decrease emotional impact. In the distraction condition, participants were asked to answer to an arithmetic task. Before each picture presentation, a single-word instruction (VIEW, CALCULATE or REGULATE) was presented, signaling the strategy to be used during the following trial.
During passive viewing (VIEW), participants were asked to view the picture attentively and without trying to change upcoming emotional responses.
During distraction (CALCULATE), participants were asked to solve the concurrently presented mathematical equation and to indicate via button press whether the displayed solution was correct or incorrect whilst ignoring the background picture.
During reappraisal (REGULATE), participants were asked to cognitively diminish their emotional reactions by distancing themselves from the picture, by becoming a detached, uninvolved observer, or by thinking that the depicted situation is not real. Each picture was presented in the VIEW, CALCULATE and REGULATE condition, except for the neutral pictures that were not presented for reappraisal to not confuse participants by asking them to lower an emotional response to a non-affective stimulus. Each participant received a different pseudo-randomized trial order with no more than three trials of the same valence category or regulation instruction appearing consecutively. To practice the ER strategies, a train block was proposed before the experiment. Participant’s responses were reviewed by the experimenters until they felt sure that the ER instructions were correctly understood.
Time course of a trial was the following one: a fixation cross (500 ms) was presented on the center of the screen, following by instruction cue (2000 ms) signaled the participants to regulate their emotions according to the practiced strategies or to simply watch the picture and was then replaced with the picture (5000 ms) (see
Figure 1). For distraction trials, arithmetic problems were additionally presented as transparent overlay on the picture to allow for a solution of the problem. After picture offset, participants rated their current emotional experience on a 9-point scale using the Self-Assessment Manikin scale for valence [
26] ranging from pleasant (1) via neutral (5) to unpleasant (9). A variable inter-trial interval (3500–5500 ms) was presented prior to presentation of the next trial to permit recovery on all physiological measures. A total of five experimental blocks, separated by a brief rest, were administered. The complete experiment consisted of 240 trials and lasted about 65 min.
2.4. Electrophysiological recordings
EEG was recorded from 64 electrodes, placed according to the international 10–20 system mounted in an elastic cap (ActiCap, Brain Products) and recorded with the Brain Vision Recorder, Brain Products. All channels were referenced online against FCz. For data analysis, channels were re-referenced to an average reference. Electrode impedances were kept below 25 kΩ. Data were recorded at a sampling rate of 1000 Hz. An online band-pass filter of 0.01– 100 Hz was used. Then, the data were filtered offline (IIR-Butterworth filter) with a high-pass filter at 1 Hz (slope 24 dB/octave) and a low-pass filter for 40 Hz (slope 24 dB/octave). On the continuous data, automatic artifact detection for non-ocular artifacts was conducted (Maximum amplitude difference in interval of 200 ms: 300 μV, maximum gradient voltage step: 70 μV/ms, lowest allowed activity in interval of 100 ms: 0.5 μV). The continuous data were epoched at -250 to 1200 msec relative to stimulus onset and were baseline-corrected relative to the average of the prestimulus interval (-250 to 0 msec). Grand-average ERPs were finally generated by computing the mean ERPs across participants in each condition. Data processing was performed offline using Brain Vision Analyser 2 software (Brain Products).
Based on previous studies [
12,
16], LPP was quantified as mean level of activity at an electrode cluster consisting of CP1, CPz, CP2, Pz for the entire picture duration with the following time windows: 600-800 ms, 1000-2000ms, 2000-3000ms, 3000-4000ms et 4000-5000ms.
2.5. Data Analysis
Subjective emotional state ratings and ERP were analyzed with Stastitica. Behavioral data and ERP responses were analyzed to evaluate emotional reactivity during free-viewing trials. Repeated-Measures ANOVAs were performed with within-subject factor Emotion (positive, negative, neutral) and between-subject factor Group (patients with bipolar disorder, healthy controls). To evaluate regulation effects, we performed repeated-measures ANOVAs with Condition (view, distraction, reappraisal), Emotion (positive, negative) as within-subject factors and Group (healthy controls, patients with bipolar disorder) as between-subject factor.
All ANOVA results were Greenhouse-Geisser corrected if assumption of sphericity was violated. Effects with a significance level of <0.05 were treated as statistically significant. Post-hoc multiple comparisons were carried out using Bonferroni-adjusted corrections.
Figure 1.
Illustration of the different experimental conditions (view, distraction and reappraisal) and trial sequence.
Figure 1.
Illustration of the different experimental conditions (view, distraction and reappraisal) and trial sequence.
3. Results
3.1. Effect of emotional induction
3.1.1. Subjective assessment
Passive view
The ANOVA showed a significant effect of Emotion (F(2,50) = 109.72; p <.001; η2 =0.81). Negative and positive trials differed from each other and from neutral trials indicating successful emotion induction. Emotional pictures (positive and negative) were experienced as more pleasant or more aversive than neutral pictures (all p < .001). (
Figure 2). There were no group effects regarding BD patients and controls (p > .05).
Subjective assessment of emotional regulation
ANOVA revealed a significant Condition × Emotion interaction (
F(2,50) = 23.32;
p < .001;
η2 = 0.48). Post-hoc analyses revealed that, emotional pictures were rated less negative or positive during distraction and reappraisal as compared with the free viewing condition, this effect being larger for negative pictures (all
p < .001). No difference was found between distraction and reappraisal conditions (
p > .05). There were no group effects regarding BD patients and controls (
p > .05) (
Figure 3).
3.1.2. ERP responses on passive view
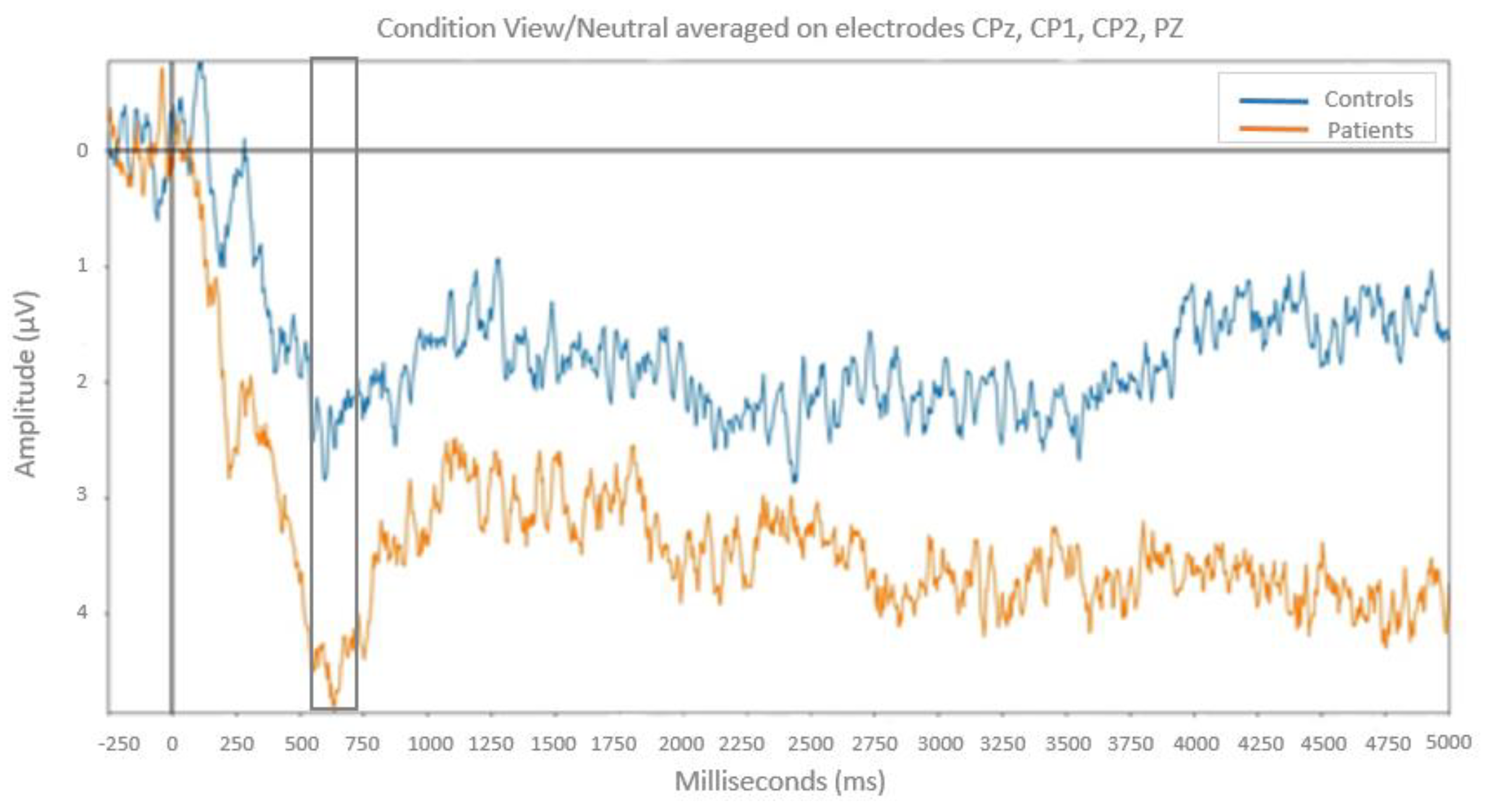
On the 600-800 ms time window: the ANOVA revealed a significant Group × Emotion interaction (
F(2,50) = 10.25;
p < .001,
MSe = 2.66;
η2 = 0.29). Post-hoc analyses indicated that for neutral pictures LPP amplitude is higher in patients with bipolar disorder than in healthy controls (
F(1,25) = 5.93;
p < .05;
MSe = 2.57). No group difference was found for positive and negative pictures (
p > 0.05). (
Figure 4)
3.1.3. ERP responses in emotional regulation
On the 600-800 ms time window: The ANOVA revealed a significant Condition × Emotion (F(2,50) = 3.62; p < .05; MSe = 0.74, η2 = 0.13). Planned comparisons showed greater LPP amplitude for negative pictures in free-viewing condition than in distraction condition (F(1,25) = 5.15; p < .05; MSe = 1.75) meaning that distraction condition is able to reduce the response intensity. However, there were no effects of group regarding BD patients and controls.
Moreover, in reappraisal conditions, for the control participants, LPP amplitude is reduced compared to the free-viewing condition, in an early time window for negative emotions and in a later time window for positive emotions. This effect is not observed in patients with bipolar disorder neither for positive nor for negative emotions.
4. Discussion
The aim of this study was to investigate behavioral and electrophysiological markers of two emotion regulation strategies, distraction and reappraisal, in euthymic patients with bipolar disorder. Patients were asked to report subjective assessments while EEG was recorded. The subjective evaluations showed no differences between healthy controls and patients with bipolar disorder as shown in previous studies [
11,
12,
27] but we found some electrophysiological differences that are interesting to discuss. Behavioral results showed that both patients and controls experience less intense emotions in response to positive and negative emotion-inducing IAPS pictures in both the distraction and reappraisal conditions compared with passive viewing.
At the neurophysiological level, during passive view, the LPP, i.e., an ERP component thought to reflect regulation processes, was larger for neutral pictures but not for pictures with an emotional valence (positive and negative) in patients with bipolar disorder compared with healthy controls. This is in favor of less regulation of emotional processes when the emotional signal is not clearly determined. Bipolar patients during euthymic period fail to mobilize emotional regulatory processes when facing neutral stimuli, whereas there is not difference in comparison to controls subjects to regulate emotional processes in front of stimuli with a clear emotional valence. These results are consistent with previous studies showing emotional hyper arousal in euthymic bipolar patients compared to control subjects when facing neutral stimuli. Conversely with the M’Bailara study [
28], we did not find this result using qualitative behavioral measures (Likert scales). This can be explained by the fact that our sample size is much smaller than the one of M'Bailara's study where the number of subjects was 90 for the control group and 55 for the group of bipolar patients, which seems to indicate that subjective tests are less sensitive in detecting this phenomenon than electrophysiological marker [
28,
29,
30]. Our results are in line with previous studies showing discrepancies between behavioral and neural activation measures in euthymic patients [
11,
31,
32,
33]. These results confirmed that failure of emotional regulation is a predominant feature of bipolar disorder [
34,
35]. It would be interesting to see if the dysregulation of emotional processes to neutral stimuli in euthymic bipolar patients extends to other types of stimuli (positive and negative) depending on the thymic episodes.
Regarding ERP measures LPP amplitude were reduced in distraction condition compared with free-viewing especially for negative emotions in patients with BD as well as in controls. It is concordant with previous findings by [
11] who did not find any differences between patients and controls in distraction condition meaning that patients can regulate emotional responses as well as controls.
Concerning the method of regulation by reappraisal, our study highlights a difference of temporality in the activation of emotional processes according to the valence of the images, between the control subjects and the bipolar patients. We found that LPP were reduced compared with free-viewing in controls but not in patients in an early time window for negative emotions and in a later time window for positive emotions. The controls thus seem to have a faster regulation process for negative stimuli than for positive stimuli, whereas the patients do not have a differentiated process depending on the valence.
These results are to be related to the results of imaging studies showing overall abnormalities in the emotion regulation system in bipolar patients. In [
11], BD patients showed a pattern of limbic hyperactivation and altered connectivity with frontal regions, resulting in an emotion regulation deficit with respect to amygdala downregulation during the reappraisal condition, mediated by reduced altered connectivity between OFC and amygdala. This regulation deficit was only present in the reappraisal condition and not during distraction. Moreover, reappraisal deficits are also found in population at risk for bipolar disorder [
11], and in patients with schizophrenia [
36]. In another study comparing both strategies [
37], found that patients with BD showed aberrantly increased intra-network and inter-network connectivity in the default mode network during distraction compared to the reappraisal condition, which indicates that these strategies can be distinguished by specific activity patterns in largescale brain networks.
5. Limitations
Certain limitations should be considered. First, main limitation of the present study is the sample size, resulting in reduced power. It may be necessary to further replicated with a larger sample size. Finally, reappraisal is more difficult to operationalize than distraction. Participants may not always succeed in implementing this strategy during the experiment. A recent study highlighted the fact that individuals prefer using distraction in the presence of high-intensity stimuli while they prefer using reappraisal in the case of low-intensity emotional stimuli [
38].
However, our results seem confirm that distraction may be a resource that patients with BD during euthymic phases can exploit, while they may not benefit from applying reappraisal [
39].
Funding
This work was supported by the Labex Empirical Foundations of Linguistics (EFL) of the Université Paris Cité which financed the purchase of a mobile EEG system used to collect the data.
Acknowledgments
We are grateful to Henri Mondor Hospital for giving us access to patients and for providing us with an experimental room in which to make EEG recordings. We would also like to thank the Labex Empirical Foundations of Linguistics (EFL) at the Université Paris Cité for providing us with the mobile EEG system.
References
- Merikangas, K.R., Jin, R., He, J., et al., 2011. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psych 68, 241–251. [CrossRef]
- Vieta, E., Berk, M., Schulze, T.G., Carvalho, A.F., Suppes, T., Calabrese, J.R., Grande, I., 2018. Bipolar disorders. Nat Rev Dis Primers 4(1), 1-16.
- Henry, C., Van den Bulke, D., Bellivier, F., Roy, I., Swendsen, J., M'Bailara, K., 2008. Affective lability and affect intensity as core dimensions of bipolar disorders during euthymic period. Psychiatry Res 159, 1–6. [CrossRef]
- Dargél, A.A., Godin, O., Etain, B., Hirakata, V., Azorin, J.M., M’Bailara, K., ... FACE-BD collaborators, 2017. Emotional reactivity, functioning, and C-reactive protein alterations in remitted bipolar patients: clinical relevance of a dimensional approach. Aust N Z J Psychiatry 51(8), 788-798. [CrossRef]
- Strejilevich, S.A., Martino, D.J., Murru, A., Teitelbaum, J., Fassi, G., Marengo, E., ... Colom, F., 2013. Mood instability and functional recovery in bipolar disorders. Acta Psych Scand 128(3), 194-202. [CrossRef]
- Dodd, A., Lockwood, E., Mansell, W., Palmier-Claus, J., 2019. Emotion regulation strategies in bipolar disorder: A systematic and critical review. J Affect Disord 246, 262-284. [CrossRef]
- Lima, I.M., Peckham, A.D., Johnson, S.L., 2018. Cognitive deficits in bipolar disorders: Implications for emotion. Clin Psychol Rev 59, 126-136. [CrossRef]
- Phillips, M.L., Swartz, H.A., 2014. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 171(8), 829-843. [CrossRef]
- Strakowski, S.M., Adler, C.M., Almeida, J., Altshuler, L.L., Blumberg, H.P., Chang, K.D., ... Townsend, J.D., 2012. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord 14(4), 313-325. [CrossRef]
- Heissler, J., Kanske, P., Schonfelder, S., Wessa, M., 2013. Inefficiency of emotion regulation as vulnerability marker for bipolar disorder: Evidence from healthy individuals with hypomanic personality. J Affect Disord 152-154, 83–90. [CrossRef]
- Kanske, P., Schonfelder, S., Forneck, F., Wessa, M., 2015. Impaired regulation of emotion: neural correlates of reappraisal and distraction in bipolar disorder and unaffected relatives. Transl Psychiatry 5, 497. [CrossRef]
- Schönfelder, S., Kanske, P., Heissler, J., Wessa, M., 2014. Time course of emotion-related responding during distraction and reappraisal. Soc Cog Affect Neurosci 9(9), 1310-1319. [CrossRef]
- Zilverstand, A., Parvaz, M.A., Goldstein, R.Z., 2017. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage 151, 105-116. [CrossRef]
- Kanske, P., Heissler, J., Schonfelder, S., Bongers, A., Wessa, M., 2011. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex 21(6), 1379–88. [CrossRef]
- Van Dillen, L.F., Koole, S.L., 2007. Clearing the Mind: A Working Memory Model of Distraction from Negative Mood. Emotion 7(4), 715-23.
- Foti, D., Hajcak, G., 2008. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. J Cog Neurosci 20(6), 977–88. [CrossRef]
- Hajcak, G., Nieuwenhuis, S., 2006. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci 6(4), 291–7. [CrossRef]
- Moser, J.S., Hajcak, G., Bukay, E., Simons, R.F., 2006. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology 43(3), 292–6. [CrossRef]
- Thiruchselvam, R., Blechert, J., Sheppes, G., Rydstrom, A., Gross, J.J., 2011. The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biol Psychol 87(1), 84–92. [CrossRef]
- Cuthbert, B.N., Schupp, H.T., Bradley, M.M., Birbaumer, N., Lang, P.J., 2000. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol Psychol 52, 95-111. [CrossRef]
- Schupp, H.T., Cuthbert, B.N., Bradley, M., Cacioppo, J.T., Ito, T., Lang, P.J., 2000. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology 37, 257-261.
- Montgomery, S.A., Åsberg, M., 1979. A new depression scale designed to be sensitive to change. BJPsych 134(4), 382-389. [CrossRef]
- Young, R.C., Biggs, J.T., Ziegler, V.E., Meyer, D.A., 1978. A rating scale for mania: reliability, validity and sensitivity. BJPsych 133, 429-435. [CrossRef]
- Dorthe, N.J., Blumental, T.D., Jason, D.R., Lantz, P.E. 1995. The use of the next-of-kin in assessing handedness. Percept Mot Skills 81(1), 203-208. [CrossRef]
- Lang, P.J., Bradley, M.M., Cuthbert, B.N., 2005. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical report A-6 Gainesville, FL: Center of Research in Psychophysiology, University of Florida.
- Bradley, M.M., Lang, P.J. 1994. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry 25(1), 49-59. [CrossRef]
- Trotti, R.L., Parker, D.A., Sabatinelli, D., Tamminga, C.A., Gershon, E.S., Keedy, S.K., ... Clementz, B.A., 2020. Electrophysiological correlates of emotional scene processing in bipolar disorder. J Psychiat Res 120, 83-90. [CrossRef]
- M’Bailara, K., Demotes-Mainard, J., Swendsen, J., Mathieu, F., Leboyer, M., Henry, C., 2009. Emotional hyper-reactivity in normothymic bipolar patients. Bipol Disord 11(1), 63-69. [CrossRef]
- Giakoumaki, S.G., Bitsios, P., Frangou, S., Roussos, P., Aasen, I., Galea, A., Kumari, V., 2010. Low baseline startle and deficient affective startle modulation in remitted bipolar disorder patients and their unaffected siblings. Psychophysiology 47(4), 659-668. [CrossRef]
- Rich, B.A., Vinton, D.T., Roberson-Nay, R., Hommer, R.E., Berghorst, L.H., McClure, E.B., ... Leibenluft, E., 2006. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Nati Acad Sciences 103(23), 8900-8905. [CrossRef]
- Corbalán, F., Beaulieu, S., Armony, J.L., 2015. Emotion regulation in bipolar disorder type I: an fMRI study. Psychological medicine 45(12), 2521-2531. [CrossRef]
- Townsend, J.D., Torrisi, S.J., Lieberman, M.D., Sugar, C.A., Bookheimer, S.Y., Altshuler, L.L., 2013. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry 73, 127-135.
- Zhang, L., Opmeer, E.M., Van der Meer, L., Aleman, A., Ćurčić-Blake, B., Ruhé, H.G., 2018. Altered frontal-amygdala effective connectivity during effortful emotion regulation in bipolar disorder. Bipolar Disord 20(4), 349-358. [CrossRef]
- Townsend, J., Altshuler, L.L., 2012. Emotion processing and regulation in bipolar disorder: a review. Bipol Disord 14(4), 326-339. [CrossRef]
- Wessa, M., Linke, J., 2009. Emotional processing in bipolar disorder: behavioural and neuroimaging findings. Int Rev Psychiatry, 21(4), 357–67. [CrossRef]
- Bartolomeo, L.A., Culbreth, A.J., Ossenfort, K.L., Strauss, G.P., 2020. Neurophysiological evidence for emotion regulation impairment in schizophrenia: The role of visual attention and cognitive effort. J Abnorm Psychol 129(6), 670. [CrossRef]
- Lois, G., Gerchen, M.F., Kirsch, P., Kanske, P., Schönfelder, S., Wessa, M., 2017. Large-scale network functional interactions during distraction and reappraisal in remitted bipolar and unipolar patients. Bipol Disord 19(6), 487-495. [CrossRef]
- Shafir, R., Thiruchselvam, R., Suri, G., Gross, J.J., Sheppes, G., 2016. Neural processing of emotional intensity predicts emotion regulation choice. Soc Cog Affect Neurosci 11(12), 1863-1871. [CrossRef]
- Kurtz, M., Mohring, P., Förster, K., Bauer, M., Kanske, P., 2021. Deficits in explicit emotion regulation in bipolar disorder: a systematic review. Int J Bipol Disord 9(1), 1-23. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).