Submitted:
26 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. X-ray Crystallography
2.3. Experimental Procedures
3. Results and discussion
3.1. Synthesis and Structural Elucidation of Compounds 1a, 1b – 4a, 4b
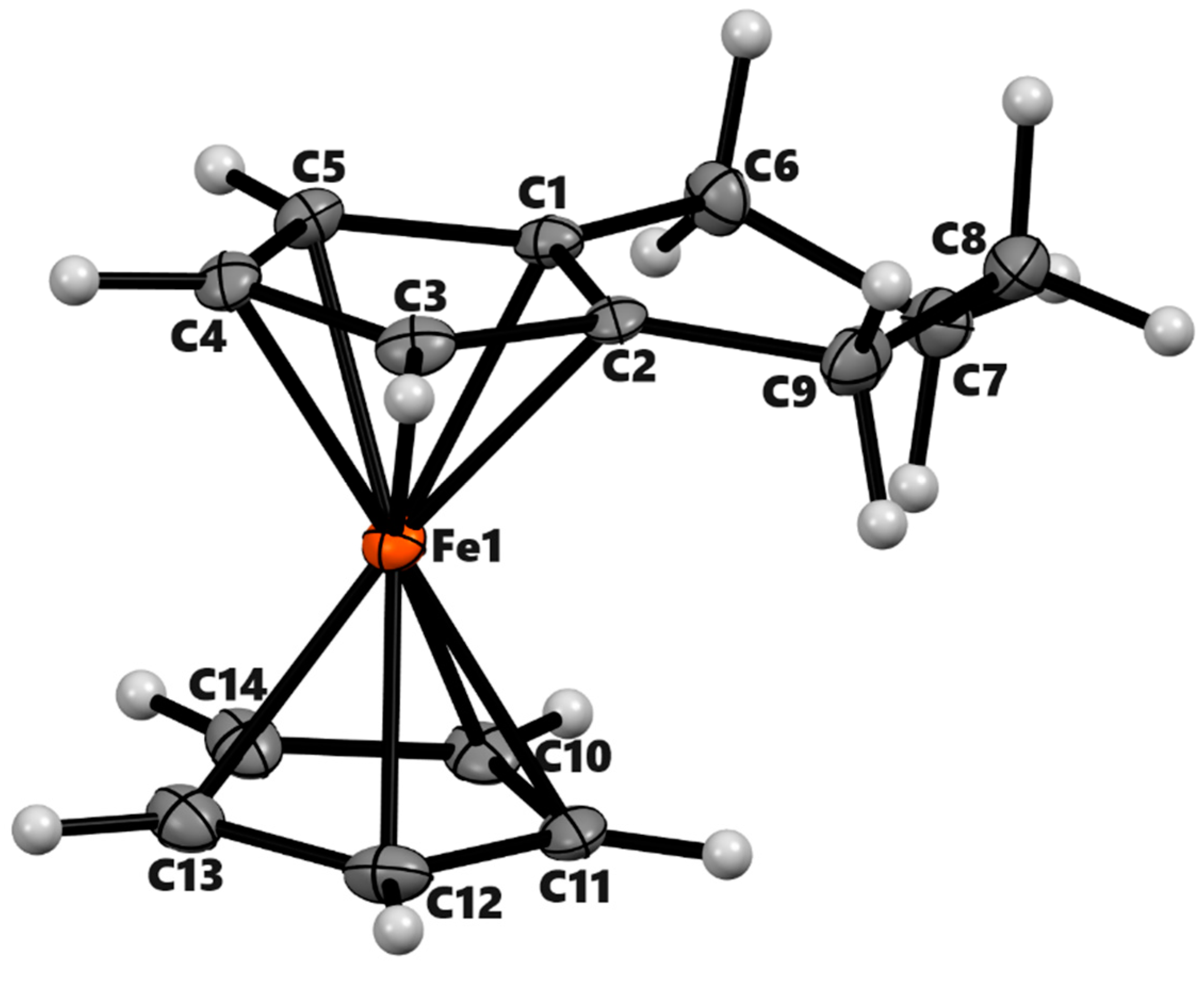
3.2. X-ray Crystal Structures
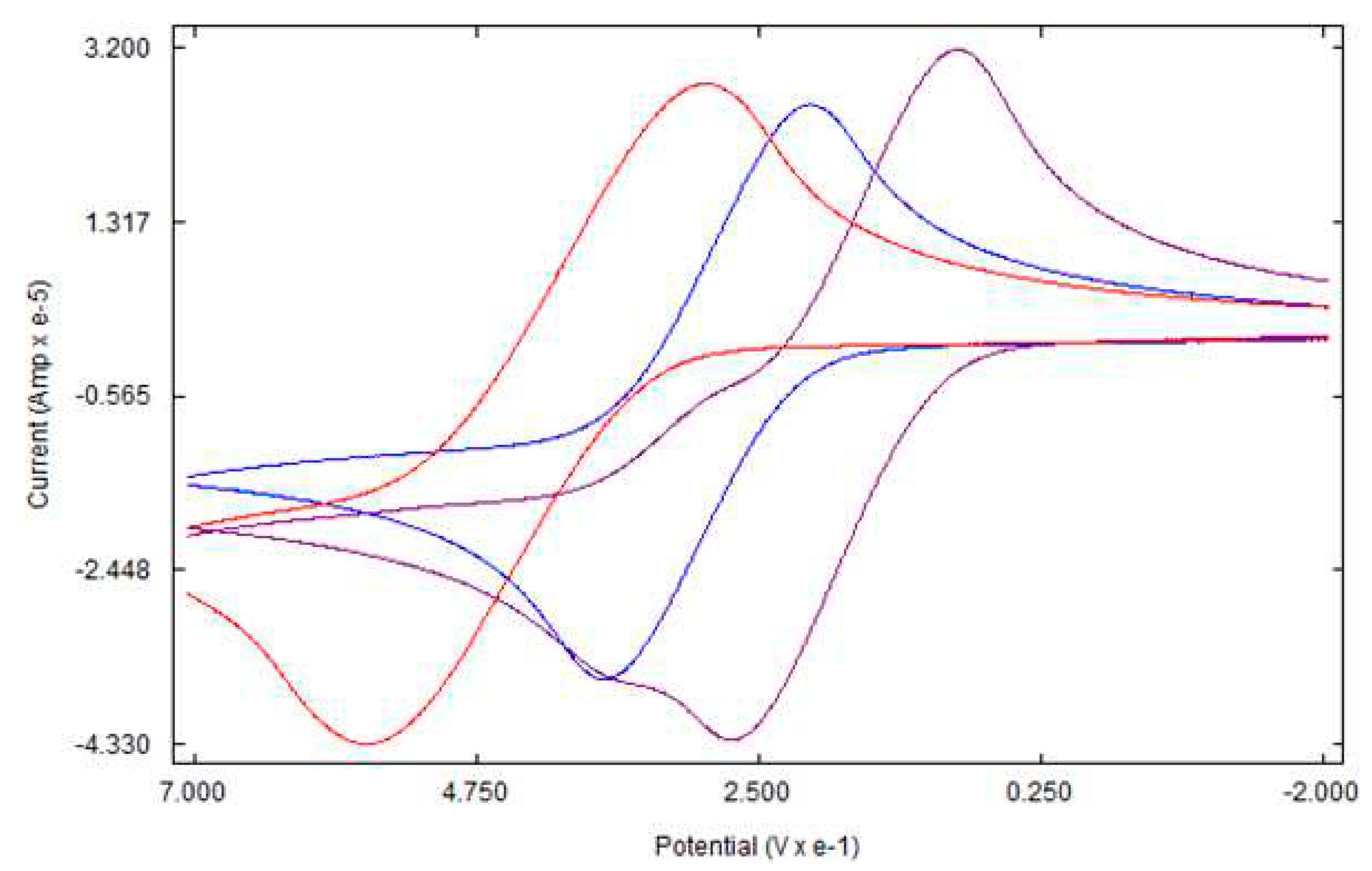
3.3. Electrochemical studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Astruc, D. Why is ferrocene so exceptional? European Journal of Inorganic Chemistry 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Kealy, T.J.; Pauson, P.L. A New Type of Organo-Iron Compound. Nature 1951, 168, 1039–1040. [Google Scholar] [CrossRef]
- Yang, X.; Rominger, F.; Mastalerz, M. Benzo-Fused Perylene Oligomers with up to 13 Linearly Annulated Rings. Angewandte Chemie International Edition 2021, 60, 7941–7946. [Google Scholar] [CrossRef]
- Anthony, J.E. The larger acenes: versatile organic semiconductors. Angewandte Chemie International Edition 2008, 47, 452–483. [Google Scholar] [CrossRef] [PubMed]
- Hewison, L.; Crook, S.H.; Johnson, T.R.; Mann, B.E.; Adams, H.; Plant, S.E.; Sawle, P.; Motterlini, R. Iron indenyl carbonyl compounds: CO-releasing molecules. Dalton Transactions 2010, 39, 8967–8975. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, H.K.; Lee, S.S.; Chung, Y.K. Convenient Synthesis of Mixed Ferrocenes. Organometallics 1997, 16, 304–306. [Google Scholar] [CrossRef]
- Perekalin, D.S.; Babak, M.V.; Karslyan, E.E.; Nelyubina, Y.V.; Kudinov, A.R. Synthesis of iron complexes [(η5-indenyl)FeL3]+ from the readily available [(η5-indenyl)Fe(η6-indene)]+. Inorganica Chemica Acta 2012, 392, 73–76. [Google Scholar] [CrossRef]
- Shirokii, V.L.; Knizhnikov, V.A.; Konovalov, T.P.; Zubreichuk, Z.P.; Erdman, A.A.; Nefedov, S.E.; Eremenko, I.L.; Yanovskii, A.I.; Struchkov, Y.T.; Maier, N.A. Electrochemical synthesis of π-indenyl-π-(3)-1,2-dicarbollyliron(III). Izvestiya Akademi Nauk, Seriya Khimicheskaya 1993, 764–765. [Google Scholar] [CrossRef]
- Belmont, J.A.; Wrighton, M.S. Photochemical conversion of (η5-C5H5)Fe(CO)2(η1-C5H5) and related complexes to ferrocene and related derivatives: reactivity of the monocarbonyl intermediate. Organometallics 1986, 5, 1421. [Google Scholar] [CrossRef]
- Curnow, O.J.; Fern, G.M. Synthesis, structures and spectroelectrochemistry of methyl-substituted bis(η5-indenyl)iron(II) complexes. Journal of Organometallic Chemistry 2005, 690, 3018–3026. [Google Scholar] [CrossRef]
- Pokharel, U.R. Organometallic heterocycles and acene-quinone complexes of ruthenium, iron and manganese. PhD dissertation, University of Kentucky, 2012.
- Pokharel, U.R.; Selegue, J.P.; Parkin, S. Ruthenocene 1,2-dicarboxylic acid, carboxylic anhydride, and acid chloride: A facile route to metallocene-fused acenequinones. Organometallics 2011, 30, 3254–3256. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Curby, R.J., Jr. Ferrocene bridging and homoannular cyclizations. Journal of the American Chemical Society 1957, 79, 3290–3291. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallographica Section A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallographica Section C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Patwa, A.N.; Gupta, S.; Gonnade, R.G.; Kumar, V.A.; Bhadbhade, M.M.; Ganesh, K.N. Ferrocene-linked thymine/uracil conjugates: base pairing directed self-assembly and supramolecular packing. Journal of Organic Chemistry 2008, 73, 1508–1515. [Google Scholar] [CrossRef]
- Apreutesei, D.; Lisa, G.; Hurduc, N.; Scutaru, D. Synthesis and un-isotherm kinetic study of some ferrocene acids. Central European Journal of Chemistry 2004, 2, 553–562. [Google Scholar] [CrossRef]
- Overbaugh, S.C.; Allen, C.F.H.; Martin, E.L.; Fieser, L.F. γ-Phenylbutyric acid. Org. Synth. 1935, XV, 64. [Google Scholar] [CrossRef]
- Weißenbacher, M.; Sturm, T.; Kalchhauser, H.; Kratky, C.; Weissensteiner, W. Synthesis and characterization of novel aminophosphine ligands based on ferrocenodecaline backbones. Monatshefte für Chemie 2002, 133, 991–1009. [Google Scholar] [CrossRef]
- Huffman, J.; Rabb, D. Notes- The Preparation of 1,2-(α-Ketotetramethylene)ferrocene. The Journal of Organic Chemistry 1961, 26, 3588–3589. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Curby, R.J., Jr.; Gustafson, D.H.; Harrison, K.G.; Bozak, R.E.; Bublitz, D.E. Organic chemistry of ferrocene. V. Cyclization of ω-ferrocenylaliphatic acids. Journal of the American Chemical Society 1962, 84, 3263. [Google Scholar] [CrossRef]
- King, R.B.; Bisnette, M.B. π-Cyclopentadienyl-π-indenyliron. Angewandte Chemie International 1963, 75, 642. [Google Scholar] [CrossRef]
- Bernhard, Y.; Gilbert, J.; Bousquet, T.; Favrelle-Huret, A.; Zinck, P.; Pellegrini, S.; Pelinski, L. One-Pot Synthesis of 2,5-Disubstituted Furans through In Situ Formation of Allenes and Enolization Cascade. European Journal of Organic Chemistry 2019, 2019, 7870–7873. [Google Scholar] [CrossRef]
- Liu, G.; He, H.; Wang, J. Ferrocene redox controlled reversible immobilization of ruthenium carbene in ionic liquid: a versatile catalyst for ring-closing metathesis. Advanced Synthesis & Catalysis 2009, 351, 1610–1620. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Vol'kenau, N.A.; Vil'chevskaya, V.D. Intramolecular acylation in the ferrocene series. Cyclization of γ-ferrocenyl substituted acids and oxo acids. Doklady Akademii Nauk SSSR 1958, 118, 512. [Google Scholar]
- Osiecki, J.H.; Hoffman, C.J.; Hollis, D.P. Organometallic compounds. Ruthenium and iron derivatives of indene. Journal of Organometallic Chemistry 1965, 3, 107. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Vol'kenau, N.A.; Vil'chevskaya, V.D. Intramolecular acylation in the ferrocene series. Doklady Akademii Nauk SSSR 1956, 111, 362–364. [Google Scholar]
- Hallam, B.F.; Pauson, P.L. Ferrocene derivatives. IV. Indenyl- and tetrahydroindenyliron carbonyls. Journal of the Chemical Society 1958, 646. [Google Scholar] [CrossRef]
- Brandon, R.L.; Osiecki, J.H.; Ottenberg, A. Reactions of metallocenes with electron acceptors. Journal of Organic Chemistry 1966, 31, 1214. [Google Scholar] [CrossRef]
- Fleischer, E.B.; Hawkinson, S.W. The structure of α-keto-1,5-tetramethyleneferrocene. Acta Crystallographica 1967, 22, 376–381. [Google Scholar] [CrossRef]
- Schlögl, K. Stereochemistry of Metallocenes. In Topics in Stereochemistry; 1967; pp. 39–91. [Google Scholar]
- Paramasivam, S.; Purushothaman, S.; Seshadri, P.R.; Raghunathan, R. (E)-1-Ferrocenyl-3-[2-(2-hydroxyethoxy)phenyl]prop-2-en-1-one. Acta Crystallographica Section E 2013, 69, m144. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; He, Z.; Xu, L.; Huang, S. 2-Amino-4-(4-chlorophenyl)-6-ferrocenylpyridine-3-carbonitrile. Acta Crystallographica Section E 2008, 64, m730. [Google Scholar] [CrossRef]
- Bratych, N.; Hassall, K.; White, J. Redetermination of the structure of diferrocenyl ketone at low temperature. Acta Crystallographica Section E 2003, 59, m33–m35. [Google Scholar] [CrossRef]
- Erben, M.; Ruzicka, A.; Vinklarek, J.; Stava, V.; Handlir, K. 1'-Acetylferrocene-1-carbonitrile. Acta Crystallographica Section E 2007, 63, m2145–m2146. [Google Scholar] [CrossRef]
- Erben, M.; Vinklarek, J.; Ruzicka, A. Acetylferrocene-2-chloro-1-ferrocenylethanone (1/1). Acta Crystallographica Section E 2011, 67, m1447–m1448. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.T.; Pople, J. General definition of ring puckering coordinates. Journal of the American Chemical Society 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Okuda, J.; Albach, R.W.; Herdtweck, E.; Wagner, F.E. Substituent effects in multiply trimethylsilyl-substituted ferrocenes. Molecular structure of 1,1′,2,2′,4,4′-hexakis(trimethylsilyl)ferrocenium tetrafluoroborate. Polyhedron 1991, 10, 1741–1748. [Google Scholar] [CrossRef]
- Tateaki, O.; Kazuo, O.; Tadashi, F.; Shunsuke, M.; Taeko, I.; Akira, K.; Nobuyuki, T. Electrochemical properties of ferrocenophanes. I. Voltammetric studies on the oxidation of mono-, di-, and tri-bridged ferrocenophanes in acetonitrile. Bulletin of the Chemical Society of Japan 1981, 54, 3723–3726. [Google Scholar] [CrossRef]
- Kuwana, T.; Bublitz, D.E.; Hoh, G. Chronopotentiometric Studies on the Oxidation of Ferrocene, Ruthenocene, Osmocene and Some of their Derivatives. Journal of the American Chemical Society 1960, 82, 5811–5817. [Google Scholar] [CrossRef]
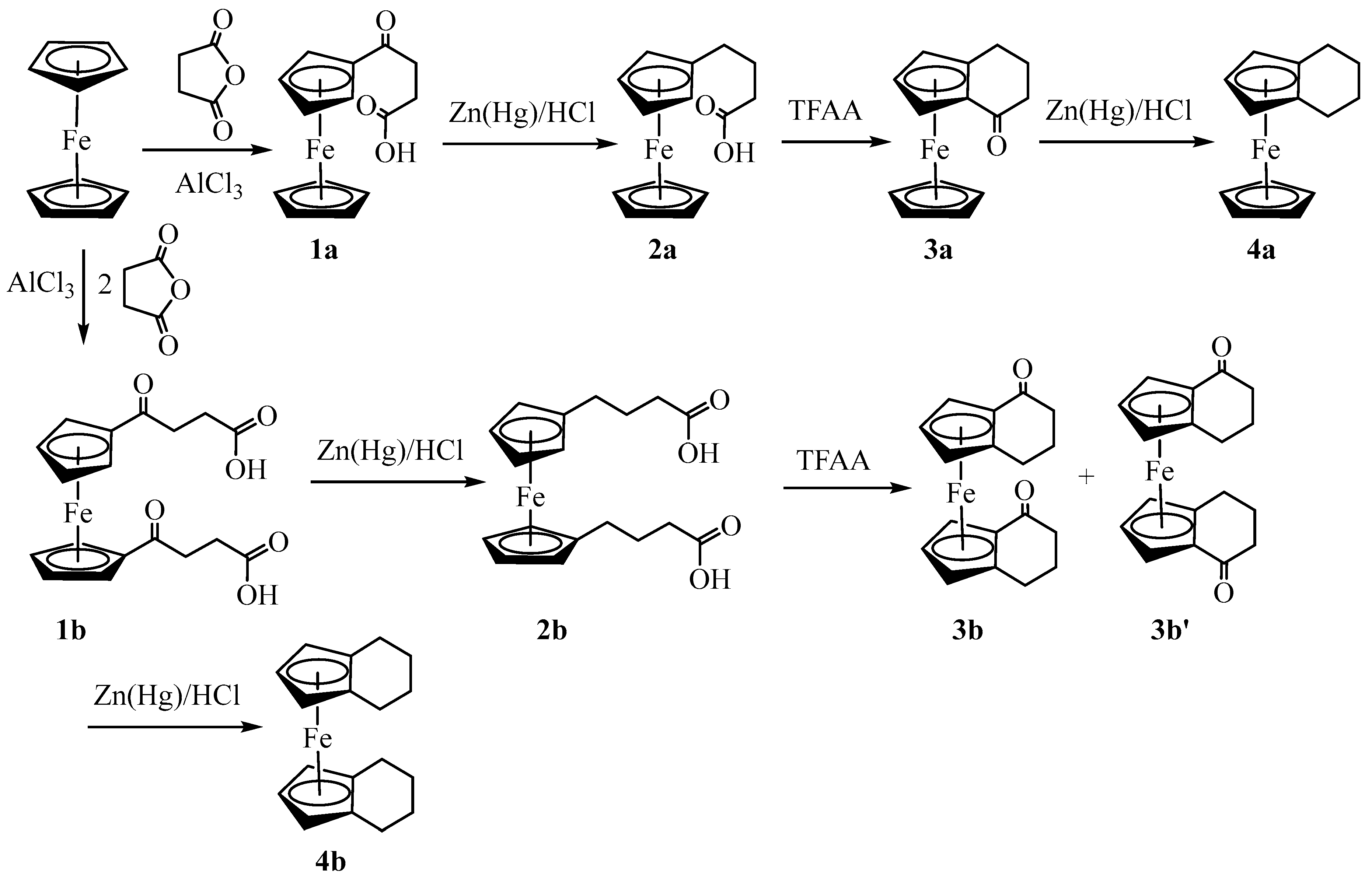
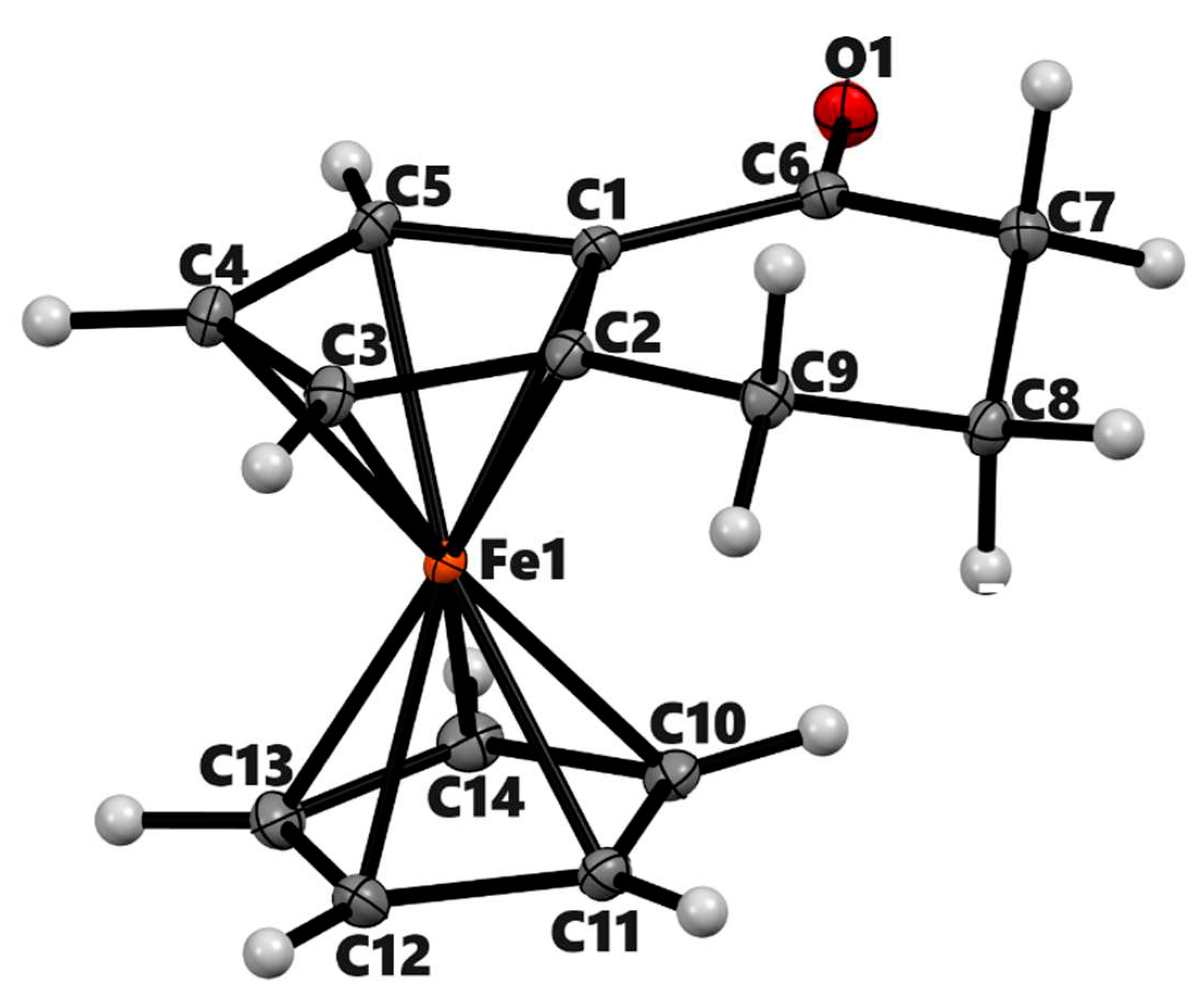
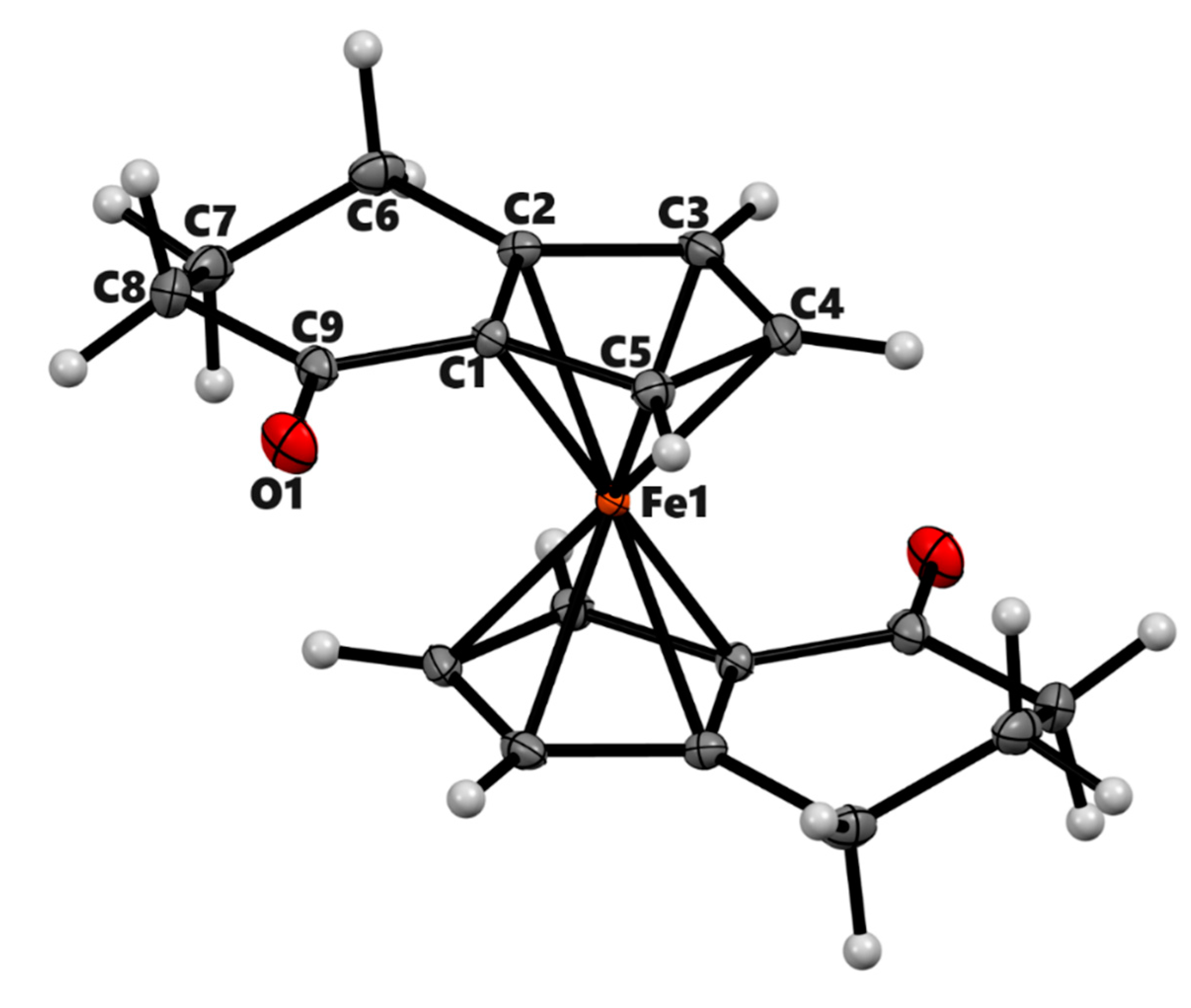
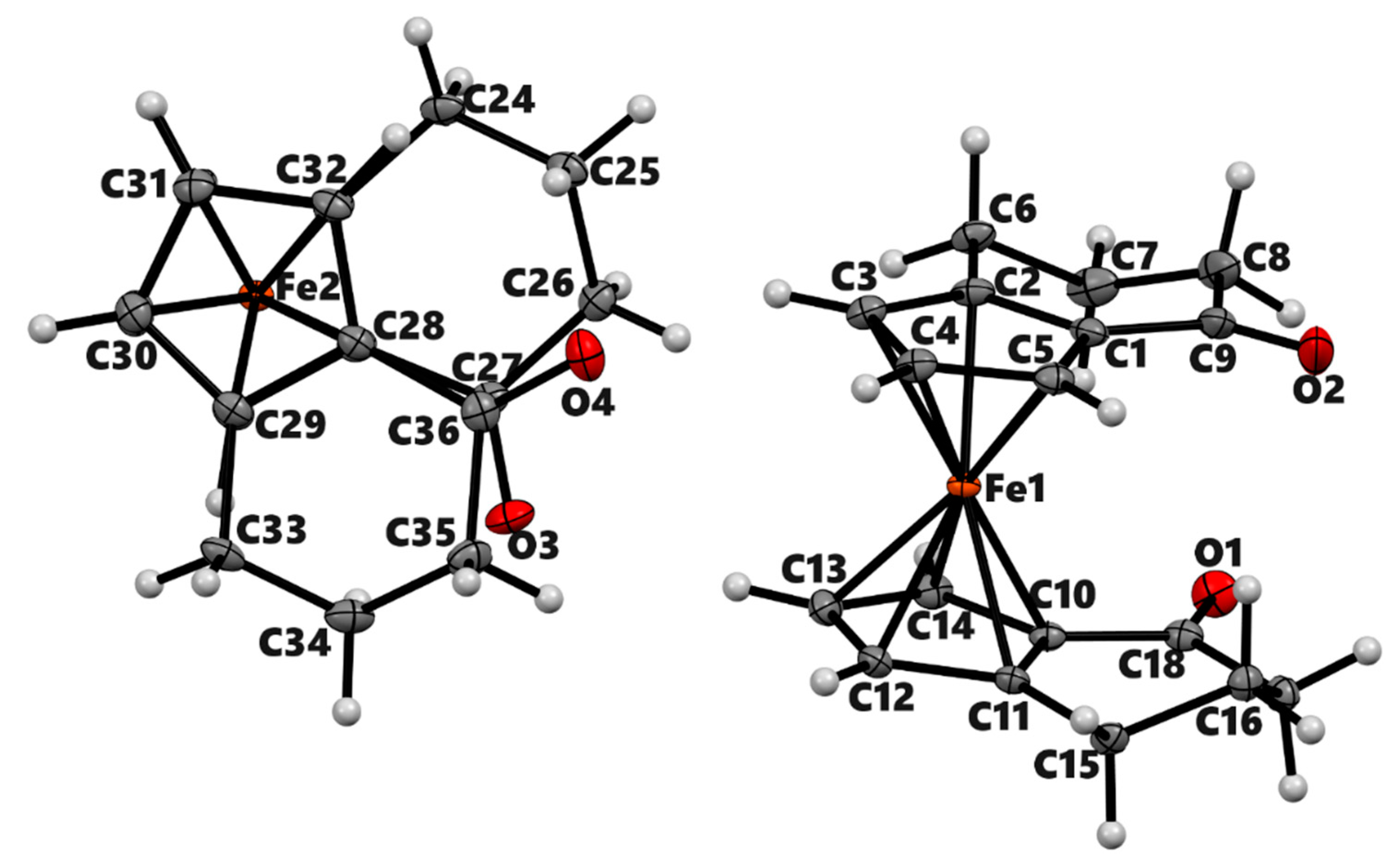
| 3a | 3b (meso) | 3b' (racemic) | 4a | |
| Chemical formula | C14H14FeO | C18H18FeO2 | C18H18FeO2 | C14H16Fe |
| Mr | 254.1 | 322.17 | 322.17 | 240.12 |
| Deposition No. | CCDC 2322201 | CCDC 2322202 | CCDC 2322203 | CCDC 2322204 |
| Crystal system, space group | Triclinic, P-1 | Monoclinic, P21/n | Monoclinic, P21/n | Monoclinic, P21/c |
| Temperature (K) | 90 | 90 | 90 | 90 |
| a, b, c (Å) | 6.5983 (4), 7.7105 (4), 11.8843 (7) | 7.422 (2), 7.551 (2), 12.366 (4) | 13.6414 (6), 14.7516 (6), 13.8763 (6) | 7.661 (3), 9.506 (4), 14.642 (6) |
| α, β, γ (°) | 108.140 (3), 90.461 (3), 108.897 (3) | 90, 100.397 (14), 90 | 90, 99.432 (2), 90 | 90, 95.574 (6), 90 |
| V (Å3) | 539.61 (6) | 681.6 (3) | 2754.6 (2) | 1061.4 (7) |
| Z | 2 | 2 | 8 | 4 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα | Mo Kα |
| µ (mm−1) | 1.37 | 1.11 | 1.10 | 1.38 |
| Crystal size (mm) | 0.16 × 0.15 × 0.06 | 0.36 × 0.20 × 0.11 | 0.44 × 0.39 × 0.36 | 0.15 × 0.11 × 0.06 |
| Diffractometer | Bruker Kappa APEX-II DUO | Bruker Kappa APEX-II | Bruker Kappa APEX-II DUO | Bruker Kappa APEX-II DUO |
| Absorption correction | Multi-scan | Multi-scan | Multi-scan | Multi-scan |
| Tmin, Tmax | 0.863, 0.922 | 0.750, 0.888 | 0.657, 0.749 | 0.753, 0.922 |
| No. of measured, independent and | 22162, 9304, 7739 | 22653, 5593, 4567 | 74998, 21250, 16339 | 16912, 3242, 1949 |
| observed [I > 2σ(I)] reflections | ||||
| Rint | 0.025 | 0.026 | 0.028 | 0.139 |
| (sin θ/λ)max (Å−1) | 1.042 | 1.018 | 0.974 | 0.716 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.034, 0.081, 1.04 | 0.030, 0.084, 1.07 | 0.033, 0.090, 1.05 | 0.052, 0.100, 1.00 |
| No. of reflections | 9304 | 5593 | 21250 | 3242 |
| No. of parameters | 187 | 97 | 400 | 136 |
| No. of restraints | 0 | 0 | 9 | 0 |
| H-atom treatment | Only H-atom coordinates refined | H-atom parameters constrained | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.38, −0.75 | 1.05, −0.77 | 1.47, −0.53 | 0.66, −0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




