Submitted:
11 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
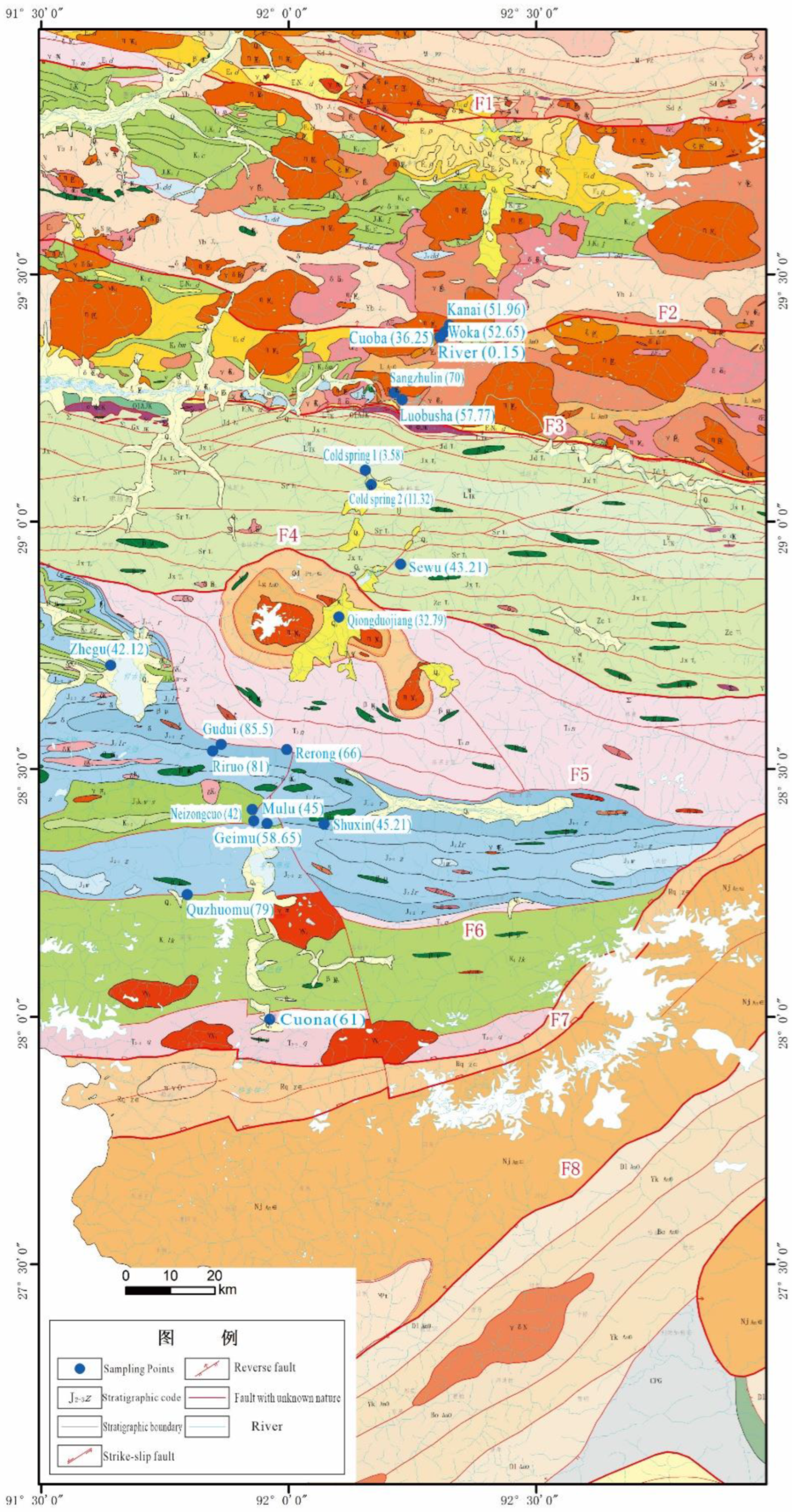
2. Overview of the Study Area
3. Materials and Methods
4. Results
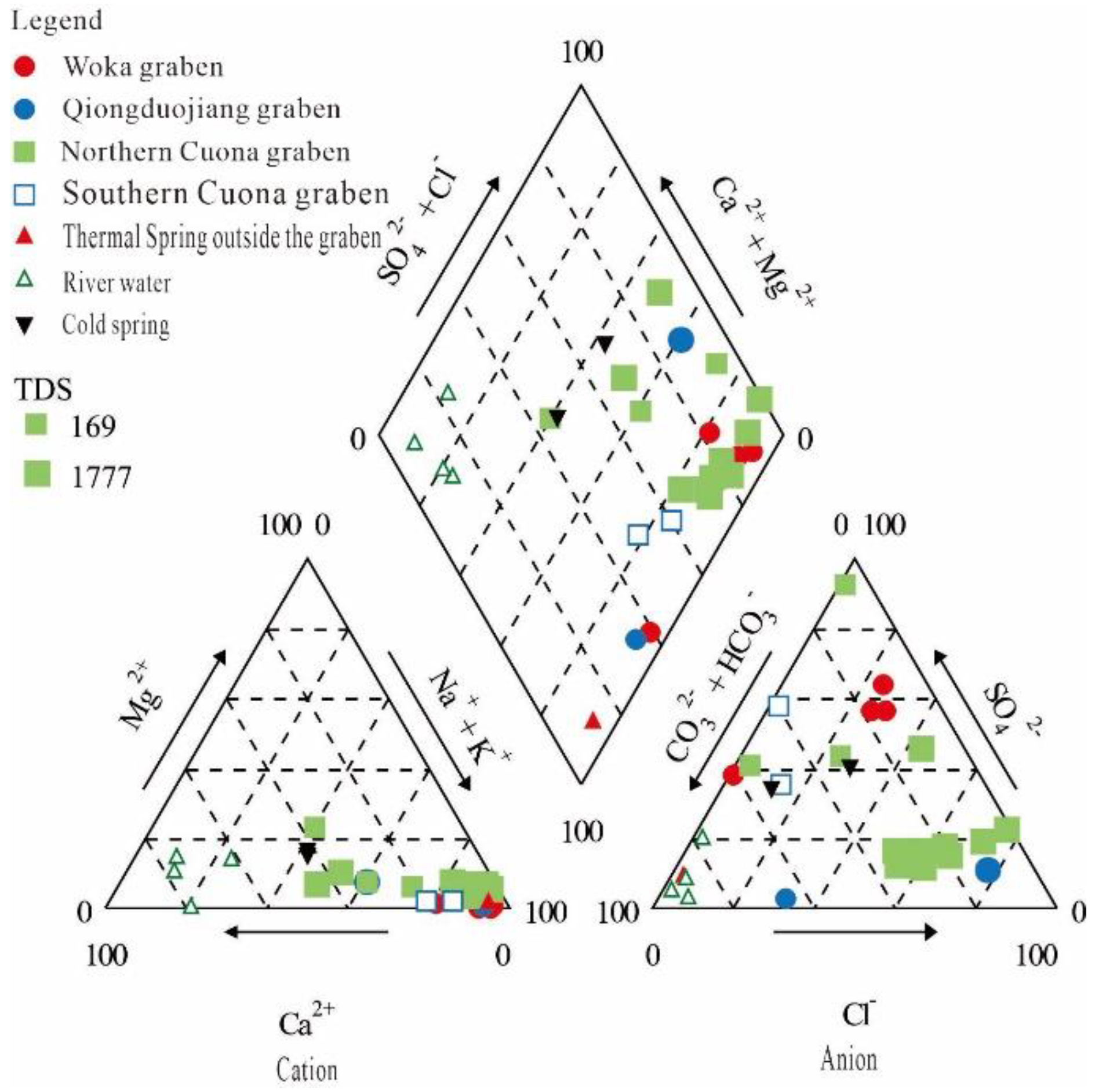
4.1. Major Elements
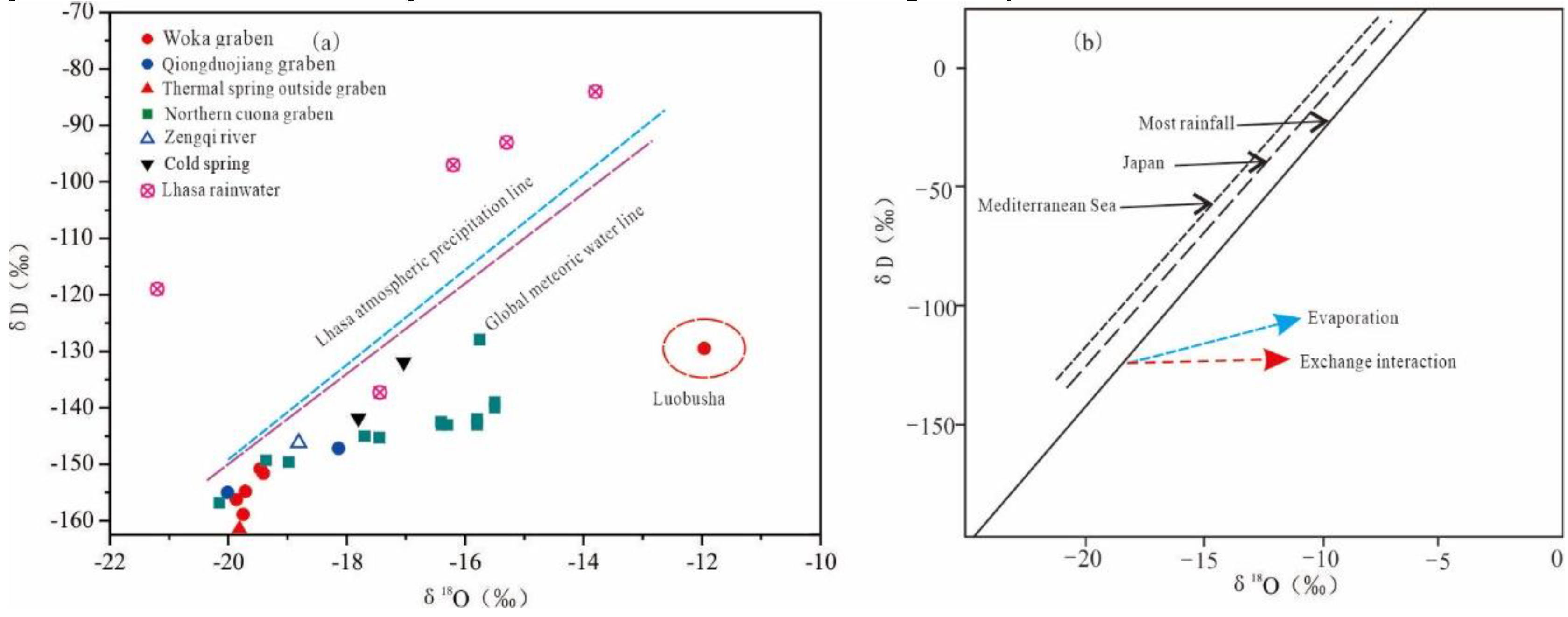
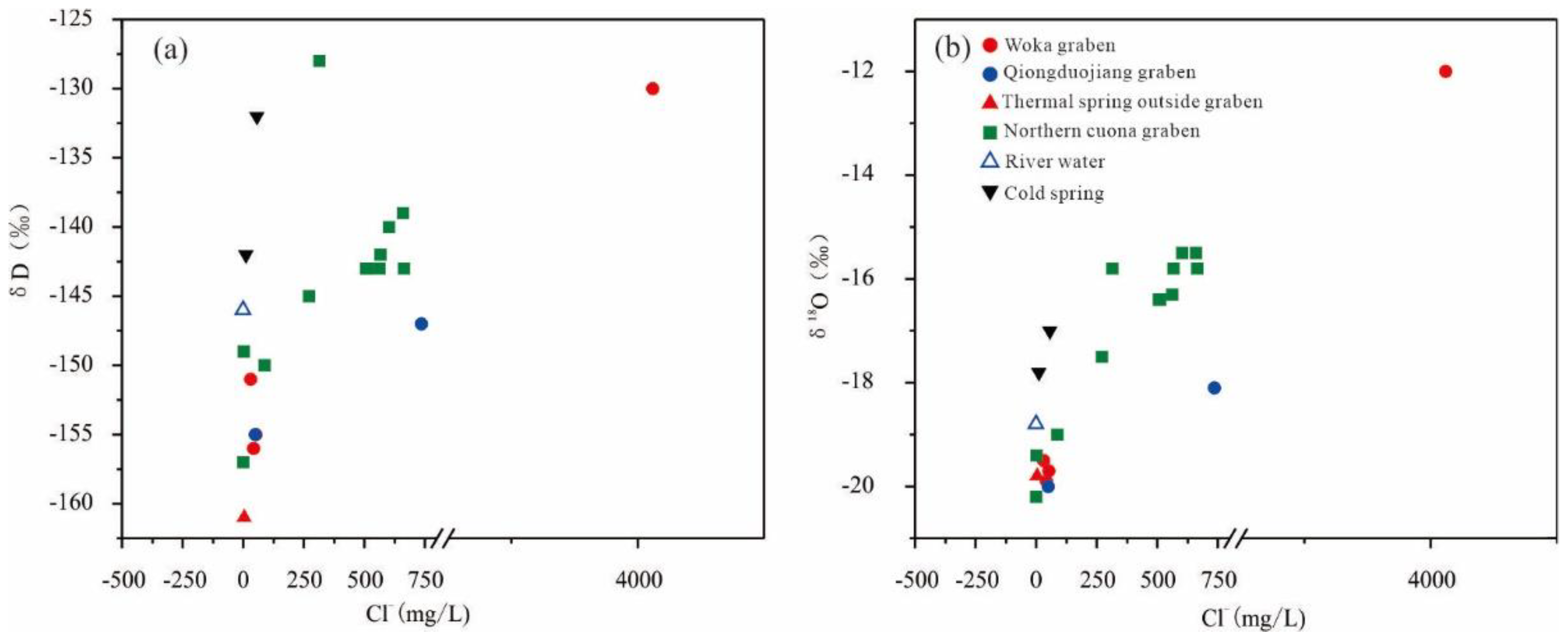
4.2. Isotopic Characteristics
5. Discussion
5.1. Recharge Source and Elevation of Geothermal Water
5.2. Subsurface Retention Time and Occurrence Environment of Geothermal Water
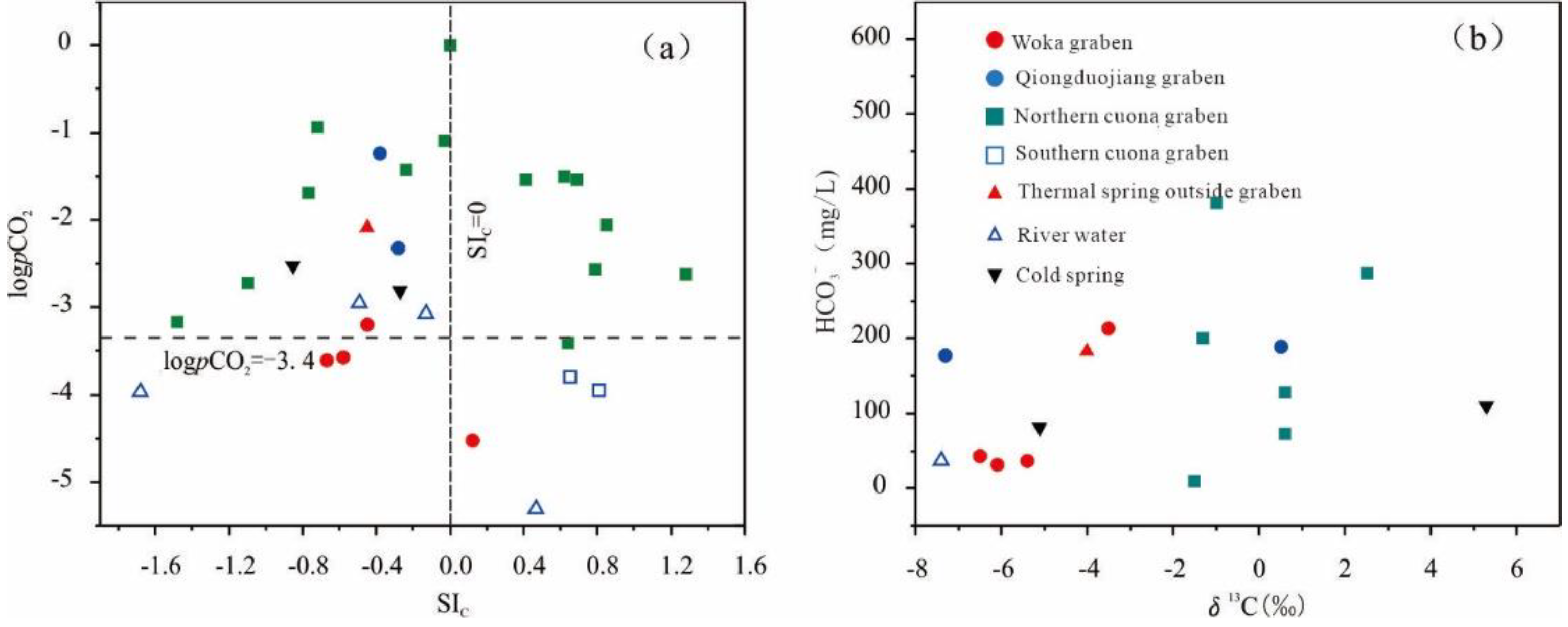
5.3. Carbon Sources of Groundwater
5.4. Migration Pathways Indicated by Carbon Isotopes in Geothermal Water
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.X.; Wang, J.Y.; Deng, X. Geothermal system type map and brief explanation in China. Geol. Sci. 1996, 2, 114–121. [Google Scholar]
- Wang, G.L.; Zhang, W.; Liang, J.Y.; et al. Evaluation of geothermal resource potential in China. Acta Geol. Sin. 2017, 38, 449–459. [Google Scholar]
- Yang, B.; Li, W.; Xiong, J.; et al. Health Risk Assessment of Heavy Metals in Soil of Lalu Wetland Based on Monte Carlo Simulation and ACPS-MLR. Water 2023, 15, 4223. [Google Scholar] [CrossRef]
- White, D. Hydrology, activity, and heat flow of the Steamboat Springs thermal system, Washoe County, Nevada; Center for Integrated Data Analytics: Wisconsin, 1968; pp. 1–116. [Google Scholar]
- Gu, W.Z.; Pang, Z.H.; Wang, Q.J.; et al. Isotope Hydrology; Science Press: Beijing, 2011. [Google Scholar]
- Li, H.; Liu, M.; Jiao, T.; et al. Using Clustering, Geochemical Modeling, and a Decision Tree for the Hydrogeochemical Characterization of Groundwater in an In Situ Leaching Uranium Deposit in Bayan-Uul, Northern China. Water 2023, 15, 4234. [Google Scholar] [CrossRef]
- Sultan, K.; Faraj, T.; Zaman, Q. Ferric Oxyhydroxylsulfate Precipitation Improves Water Quality in an Acid Mining Lake: A Hydrogeochemical Investigation. Water 2023, 15, 4273. [Google Scholar] [CrossRef]
- Craig, H. Standards for reporting concentrations of deuterium and oxygen-18 in natural waters. Science 1961, 133, 1833–1834. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.H.; Hou, F.G.; Ni, B.L. Hydrogen and oxygen stable isotopes in precipitation in China. Chin. Sci. Bull. 1983, 13, 801–806. [Google Scholar]
- Ning, A.F.; Yin, G.; Liu, T.C. Distribution characteristics of atmospheric precipitation isotopes in the Lhasa River region. Acta Mineral. Sin. 2000, 20, 95–99. [Google Scholar]
- Zheng, S.H.; Zhang, Z.F.; Ni, B.L.; et al. Hydrogen and oxygen stable isotope study of geothermal waters in Tibet. Acta Sci. Nat. Univ. Pekin. 1982, 1, 99–106. [Google Scholar]
- Liu, Z.F.; Tian, L.D.; Yao, T.D.; et al. Temporal and spatial variations of δ18O in precipitation in the Yarlung Zangbo River basin. Acta Geogr. Sin. 2007, 62, 510–517. [Google Scholar] [CrossRef]
- Torres-Martínez, J.A.; Mora, A.; Knappett, P.S.K.; et al. Tracking nitrate and sulfate sources in groundwater of an urbanized valley using a multi-tracer approach combined with a Bayesian isotope mixing model. Water Res. 2020, 182, 115962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Genty, D.; Sirieix, C.; et al. Quantitative assessments of moisture sources and temperature governing rainfall δ18O from 20 years' monitoring records in SW-France: Importance for isotopic-based climate reconstructions. J. Hydrol. 2021, 591, 125327. [Google Scholar] [CrossRef]
- Yin, G.; Ni, S.J.; Zhang, Q.C. Deuterium excess parameter and its hydrogeological significance - A case study of Jiuzhaigou and Yele in Sichuan. J. Chengdu Univ. Technol. 2001, 28, 251–254. [Google Scholar]
- Wang, S.Q. Hydrogeochemical processes and formation mechanisms of high-temperature geothermal systems in Tibet; China University of Geosciences: Beijing, 2017. [Google Scholar]
- Xiao, K.; Shen, L.C.; Wang, P. Hydrogen and oxygen isotopes of lakes and geothermal waters in the arid region of southern Tibet. Environ. Sci. 2014, 35, 2952–2958. [Google Scholar]
- Ren, K.; Pan, X.D.; Zeng, J.; et al. Carbon isotope geochemical characteristics of groundwater under different land uses in karst areas and their ecological significance. Environ. Sci. 2019, 40, 4523–4531. [Google Scholar]
- Shangguan, Z.G.; Gao, S.J. Carbon isotope seismogeochemical characteristics of hot springs in the Dianxi experimental field. Earthquake 1987, 6, 27–37. [Google Scholar]
- Liu, Z.H.; Yuan, D.X.; He, S.Y.; et al. Geochemical characteristics of the geothermal CO2-water-carbonate rock system and its CO2 sources - A case study of Huanglonggou Kangding in Sichuan and Xiagei in Yunnan. Sci. China (Ser. D) 2000, 30, 209–214. [Google Scholar]
- Zhang, Y.H. Genesis and exploitation of the Kangding-Moxi segment of the Xianyu River fault geothermal system; Chengdu University of Technology: Chengdu, China, 2018. [Google Scholar]
- Frondini, F.; Caliro, S.; Cardellini, C.; et al. Carbon dioxide degassing and thermal energy release in the Monte Amiata volcanic-geothermal area (Italy). Appl. Geochem. 2009, 24, 860–875. [Google Scholar] [CrossRef]
- Shen, L.C.; Wu, K.Y.; Xiao, Q.; et al. CO2 degassing in the geothermal anomaly area of Tibet: A case study of Langju and Dagejia geothermal areas. Chin. Sci. Bull. 2011, 56, 2198–2208. [Google Scholar]
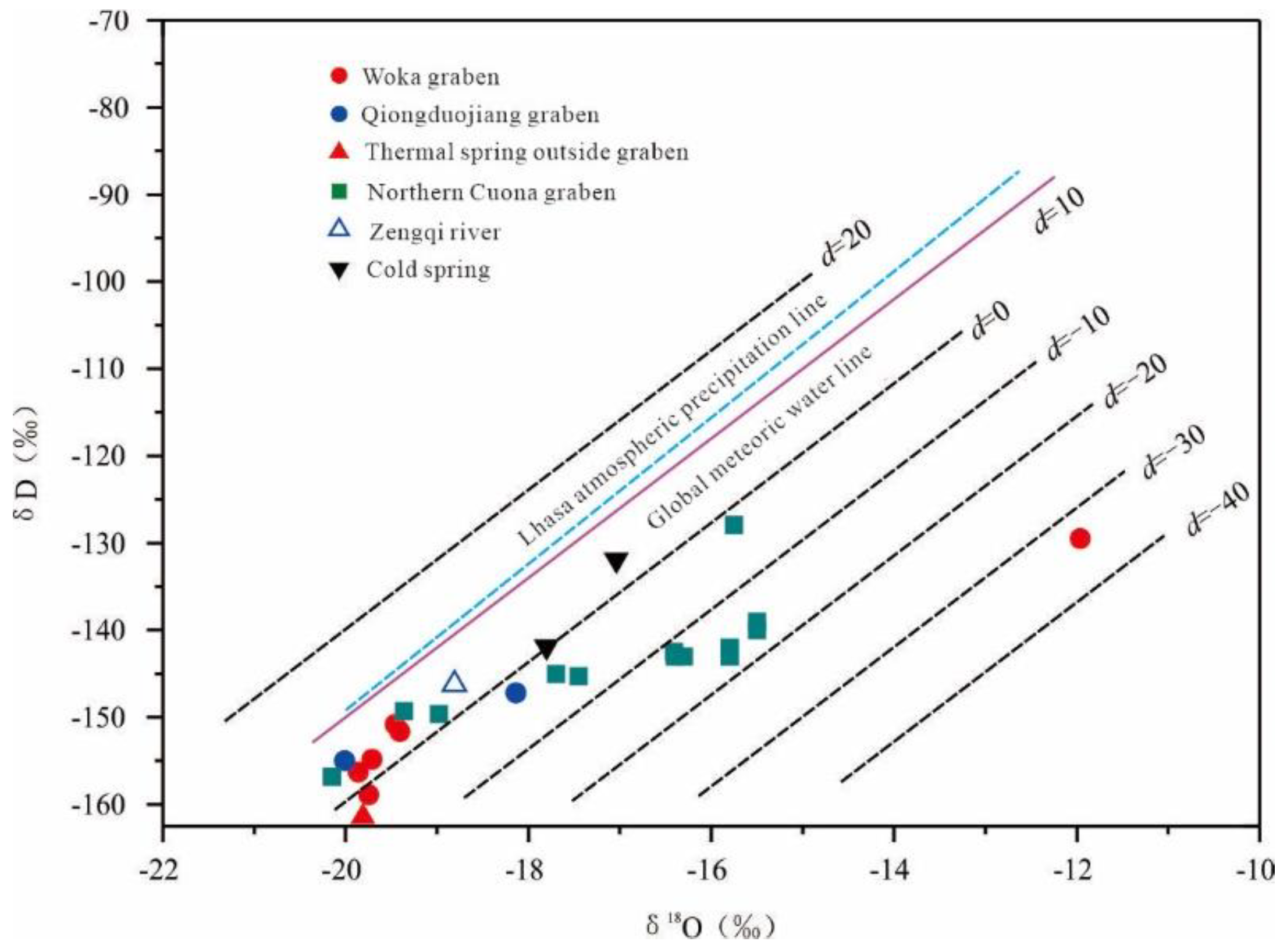
| Location | Thermal Spring | δD/‰ | δ18O/‰ | Elevation /m | Reference Value /‰ | d Value /‰ |
|---|---|---|---|---|---|---|
| Woka graben | Kanai | -156 | -19.9 | 3933 | -126 | 2.6 |
| Cuo Ba | -151 | -19.5 | 3988 | -126 | 4.9 | |
| Woka | -155 | -19.7 | 3920 | -126 | 2.9 | |
| Qiongduojiang graben | Sewu | -155 | -20.0 | 4400 | -126 | 5.1 |
| Qiongduojiang | -147 | -18.1 | 4440 | -126 | -2.1 | |
| Geothermal water outside the grabens | Zhegu | -161 | -19.8 | 4600 | -126 | -2.9 |
| Northern Cuona graben | Riruo | -143 | -16.4 | 4440 | -126 | -11.2 |
| Re Rong | -145 | -17.7 | 4248 | -126 | -3.4 | |
| GuDui Q003 | -143 | -16.4 | 4388 | -126 | -11.8 | |
| GuDui Q006 | -140 | -15.5 | 4388 | -126 | -16.0 | |
| GuDui Q007 | -143 | -16.3 | 4388 | -126 | -12.6 | |
| GuDui Q012 | -143 | -15.8 | 4388 | -126 | -16.6 | |
| GuDui Q010 | -142 | -15.8 | 4388 | -126 | -15.6 | |
| GuDui ZK251 | -139 | -15.5 | / | -126 | -15.0 | |
| Neizong Cuo | -157 | -20.2 | 5020 | -126 | 4.4 | |
| Geimu | -149 | -19.4 | 4950 | -126 | 5.6 | |
| Mulu | -145 | -17.5 | 4700 | -126 | -5.7 | |
| Shuxin | -150 | -19.0 | 4320 | -126 | 2.2 | |
| Qu Zhuo Mu | -128 | -15.8 | 4360 | -126 | -1.9 | |
| Maximum Value | -128 | -12.0 | 5020 | -126 | 5.6 | |
| Minimum Value | -161 | -20.2 | 3568 | -126 | -33.8 | |
| Average Value | -147 | -17.7 | 4317 | -126 | -5.4 | |
| Zengqiqu | River Water | -146 | -18.8 | 4028 | -126 | 4.3 |
| Cold-spring water | Cold spring 1 | -132 | -17.0 | 3954 | -126 | 4.4 |
| Cold spring 2 | -142 | -17.8 | 3875 | -126 | 0.5 |
| Number | Region Name | Recharge Elevation(m) | Surrounding Peaks |
|---|---|---|---|
| 1 | Woka graben | 5100-5300 m, averaging 5200 m | 5200- 5500m |
| 2 | Qiongduojiang graben | 5300-5500 m | 5200- 5700m, with the highest peak at 6635 m (La Xiangbo Qingri Peak) |
| 3 | Northern Cuona graben | 4500-6200 m, averaging 5450 m | 4300~6400m, with the highest peak at 6537 m (Kongbu Gangri Peak) |
| 4 | Southern Cuona graben | Not calculated | \ |
| 5 | Extrathermal-Zhegu | 6000m | 6635 m (La Xiangbo Qingri Peak) |
| Location | Thermal Spring | SIC | SID | SIG | log(pCO2) | δ13C | Metamorphic carbon proportion | Mantle-derived carbon proportion |
|---|---|---|---|---|---|---|---|---|
| Woka graben | Kanai | -0.67 | -3.27 | -2.41 | -3.61 | -6.1 | 13% | 87% |
| Woka | -0.58 | -2.73 | -2.32 | -3.57 | -6.5 | 10% | 90% | |
| Cuoba | -0.45 | -2.04 | -2.17 | -3.20 | -5.4 | 20% | 80% | |
| Sangzhuling | 0.12 | -0.40 | -3.35 | -4.53 | / | / | ||
| Qiongduojiang graben | Sewu | -0.28 | -1.20 | -3.59 | -2.32 | -7.3 | 2% | 98% |
| Qiongduojiang | -0.24 | -0.97 | -1.28 | -1.43 | 0.5 | 76% | 24% | |
| Northern Cuona graben | Riruo | -1.10 | -2.82 | -1.53 | -2.72 | 71% | 29% | |
| Gudui Q004 | 0.41 | 0.84 | -1.79 | -1.54 | -1.0 | 62% | 38% | |
| Gudui Q005 | 0.64 | 1.49 | -2.09 | -3.41 | / | / | / | |
| Gudui Q006 | 0.79 | 1.86 | -2.23 | -2.57 | / | / | / | |
| Gudui Q007 | 0.62 | 1.25 | -1.80 | -1.50 | / | / | / | |
| Gudui H008 | 1.28 | 2.30 | -1.63 | -2.62 | / | / | / | |
| Gudui Q010 | 0.69 | 1.34 | -1.65 | -1.54 | / | / | / | |
| Gudui ZK251 | 0.00 | 0.00 | 0.00 | 0.00 | / | / | / | |
| Neizongcuo | -1.48 | -3.36 | -1.75 | -3.16 | -1.5 | 58% | 42% | |
| Geimu | -0.77 | -1.71 | -2.08 | -1.69 | 0.6 | 77% | 23% | |
| Mulu | -0.03 | -0.48 | -1.35 | -1.09 | 2.5 | 95% | 5% | |
| Shuxin | -0.72 | -1.93 | -1.27 | -0.94 | -1.3 | 60% | 40% | |
| Quzhuomu | 0.85 | 0.69 | -0.59 | -2.06 | 0.6 | 77% | 23% | |
| Southern Cuona graben | Cuona spring | 0.65 | 0.55 | -2.24 | -3.79 | / | / | / |
| Cuona-Drilling | 0.81 | 0.79 | -2.46 | -3.95 | / | / | / | |
| Geothermal water outside the grabens | Zhegu | -0.45 | -1.42 | -3.13 | -2.09 | -4.0 | 33% | 67% |
| River | Zengqi river | 0.47 | -0.05 | -3.60 | -5.31 | -7.4 | / | / |
| Jiaboxiong river | -0.13 | -0.99 | -3.21 | -3.08 | / | / | / | |
| Qiena river | -1.68 | -99.99 | -4.97 | -3.97 | / | / | / | |
| Cuona river | -0.49 | -1.89 | -2.63 | -2.95 | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




