1. Introduction
The quality of veterinary medicinal products (VMP) is essential for efficient disease management. Therefore, poor-quality VMP may lead to increased morbidity, mortality, and the emergence of antimicrobial resistance and pose a risk to animals and humans. Poor quality VMPs include substandard VMP, caused by poor manufacturing or distribution practices, and falsifications, prompted by a deliberate intent to fraud [
1]. Different studies [
2,
3,
4] have defined substandard drugs as "genuine medicines which have not passed the standard and quality testing protocol set for them". Falsified products are therefore a type of substandard drug [
5]. The World Health Organization (WHO) defines falsified medicines as "drugs that are deliberately and fraudulently produced and/or mislabeled with respect to identity and/or source to make it appear to be a genuine product″ [
6]. According to new research from WHO, 1 in 10 medical products circulating in low- and middle-income countries is either substandard or falsified [
4]. This is probably due to inadequate regulation and governance that are compounded by unethical practices by manufacturers, importers, wholesalers, retailers, and practitioners.
These poor-quality products may contain unknown medication concentrations (for example with the wrong active ingredients, without active ingredients, with insufficient or too many active ingredients, and/or with fake/falsified packaging) as well as potentially hazardous impurities (for example heavy metals and unlabeled drug substances). When these products are used, serious side effects such as lack of disease control, worsening of disease, severe reactions, or even death may occur. Administration of falsified antibiotics (or for example parasiticides/anthelmintics) can enhance antimicrobial resistance, as well as the risks of treatment failure and disease spread. Also, substandard and falsified drugs represent an expanding issue throughout developing countries [
4]. However, empirical evidence on VMP falsification is lacking in distribution systems. Thus, the use of falsified VMP should be examined and investigated. Different techniques such as high-performance liquid chromatography or gas chromatography coupled to detection systems such as ultraviolet spectroscopy, mass spectrometry, fluorescence, or chemiluminescence, have been used to test the quality of VMP in order to maintain an appropriate assurance of VMP quality [
5]. Nevertheless, these techniques usually provide high sensitivity and selectivity but require high grade instruments, solvents, and expertise, and finally, they become more and more expensive and only a few laboratories in some countries are currently available to perform them [
5]. Consequently, the development and use of simple, easier, and faster tests should facilitate a balance between the need to increase the extent of VMP testing on the one hand, and the need to contain costs on the other. The Global Pharma Health Fund (GPHF)-Minilab offers inexpensive analytical techniques primarily based on (i) thin layer chromatography (TLC) for rapid drug quality verification and falsified medicines detection and (ii) physical testing for a quick check on visual appearance, powder/capsule/tablets/bolus weight, and deficiencies in VMP release [
6]. Thus, the GPHF-Minilab could close the capacity gap on drug quality testing in countries where the means for an effective drug quality control system are not fully in place or where full testing is expensive, hardly accessible, or time-consuming. The GPHF-Minilab will enable importers, wholesalers, retailers, practitioners, and regulatory bodies to protect themselves against the menace of dangerous trade in spurious and dodgy VMP. This may serve as an important source of information about the quality of VMP available to animals. It is also vital that planning effective interventions improve the quality of VMP. Hence, this study is pioneering in Rwanda to fill knowledge gaps on substandard and falsified VMP available on the Rwandan market using GPHF-Minilab®.
2. Results
2.1. Overview of Collected VMP Samples
As shown in
Table 1, 130 VMP samples from 13 APIs were collected in the course of this study. A total of 60 VMP samples from 6 APIs (Clavulanic acid/Amoxicillin, Tetracycline hydrochloride, cloxacillin, Cefalexin, Cefazolin sodium salt, Amoxicillin, and Ampicillin Trihydrate) were excluded from the TLC testing, most frequently because their single TLC test protocols for the respective dosage form are currently not available in the manual accompanying the GPHF-Minilab.
Of the 130 VMP samples included in the physical testing, 50.0% (n=65) were collected from licensed veterinary retail pharmacies whereas the other 50.0% (n=65) were collected from non-licensed veterinary retail pharmacies in five districts of Rwanda.
Table 4.
Overview of collected VMP samples.
Table 4.
Overview of collected VMP samples.
| Reference standards API |
Dosage form |
Number of samples per API
|
Source of samples |
Total number of samples collected |
| Licensed veterinary retail pharmacy |
Non-licensed veterinary retail pharmacy |
| Clavulanic acid/Amoxicillin* |
Injectable |
2 |
1 |
1 |
10 |
Sulfamethoxazole/
Trimethoprim |
Powder |
2 |
1 |
1 |
10 |
| Doxycycline (as hyclate) |
Powder |
2 |
1 |
1 |
10 |
| Tetracycline hydrochloride* |
Eye Ointment |
2 |
1 |
1 |
10 |
| Gentamycin sulfate, |
Injectable |
2 |
1 |
1 |
10 |
| Cloxacillin* |
Intramammary Suspension |
2 |
1 |
1 |
10 |
| Cefalexin* |
Injectable |
2 |
1 |
1 |
10 |
| Cefazolin sodium salt |
Powder |
2 |
1 |
1 |
10 |
| Albendazole |
Bolus |
2 |
1 |
1 |
10 |
| Mebendazole |
Bolus |
2 |
1 |
1 |
10 |
| Praziquantel |
Bolus |
2 |
1 |
1 |
10 |
| Amoxicillin* |
Injectable |
2 |
1 |
1 |
10 |
| Ampicillin* |
Smooth Sterile Cream |
2 |
1 |
1 |
10 |
All sampled veterinary retail shop owners were registered with both the Rwanda Council of Veterinary Doctors (RCVD) and the Rwanda FDA. None of the sampled veterinary retail shop owners had training in veterinary pharmacy management in the last one year. The last time visited by Rwanda FDA and RCVD was 1 to 6 months and more than 12 months, respectively.
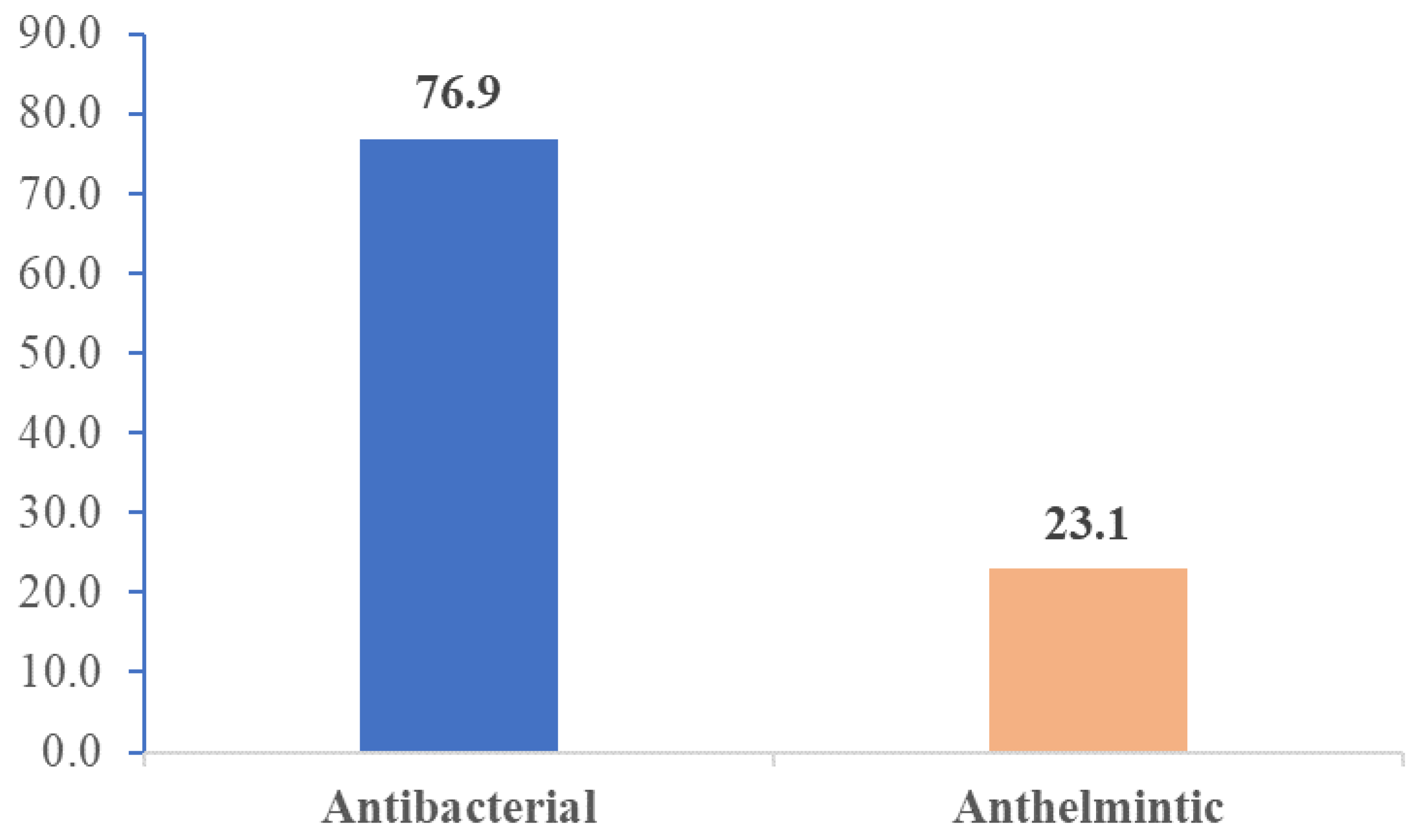
Figure 1 presents the therapeutic categories of the 130 VMP samples. 76.9% (n=100) of VMP samples were medicines for the treatment of infectious diseases whereas 23.1% (n=30) were anthelminthic veterinary drugs.
2.2. Results of VMP Sample Analysis
2.2.1. Physical Testing
The physical testing of VMP samples (n=130) encompassing antibacterial (n=100) and anthelmintics (n=30) was performed. The descriptions of the physical characteristics of samples observed during visual inspection are presented in
Table 2. Overall, the findings of visual inspection revealed that none of the assessed VMP samples showed defects in physical characteristics, packaging, and/or labeling information.
The results of the weight verification and disintegration test categorized based on the source of samples, APIs, and veterinary therapeutic category are depicted in
Table 3. The results of the weight verification test indicated that all VMP samples match the VMP’s label claim. Furthermore, all the boluses placed into a wide neck bottle vessel containing 100 mL of warm tap water at approximately 37˚C were fully disintegrated within 30 minutes
2.3. TLC Testing
In total, 7 different APIs were tested according to the single TLC test protocols of the GPHF Minilab manual. A detailed overview of the different APIs and dosage forms of the VMP samples included in the TLC analysis is given in
Table 4.
Among 70 VMPs from 7 APIs analyzed using TLC protocols, 10 VMPs (14.3%) from one API (Sulfamethoxazole/Trimethoprim) from the same brand failed to comply with TLC result specifications as indicated in
Table 5.
Table 6 presents the detailed results from the TLC analysis conducted on 7 different APIs. Among the tested APIs, one (1) was qualified in this study as probably a falsified API.
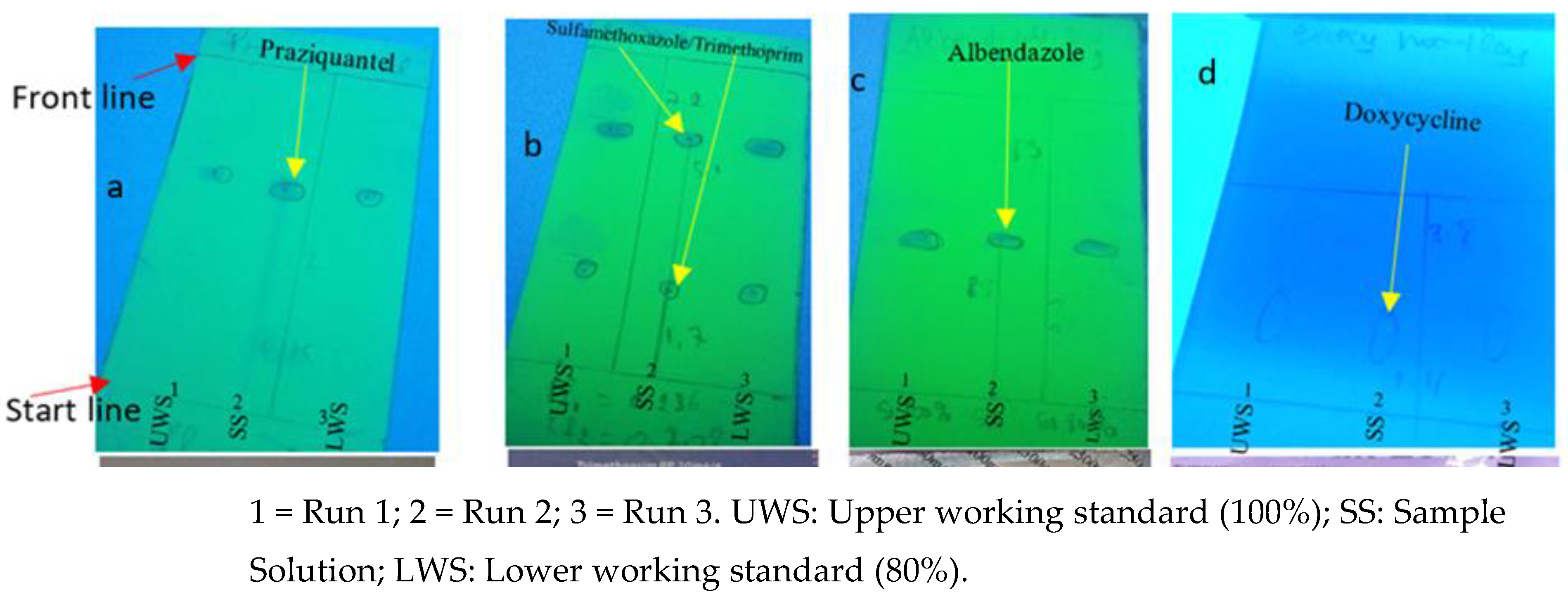
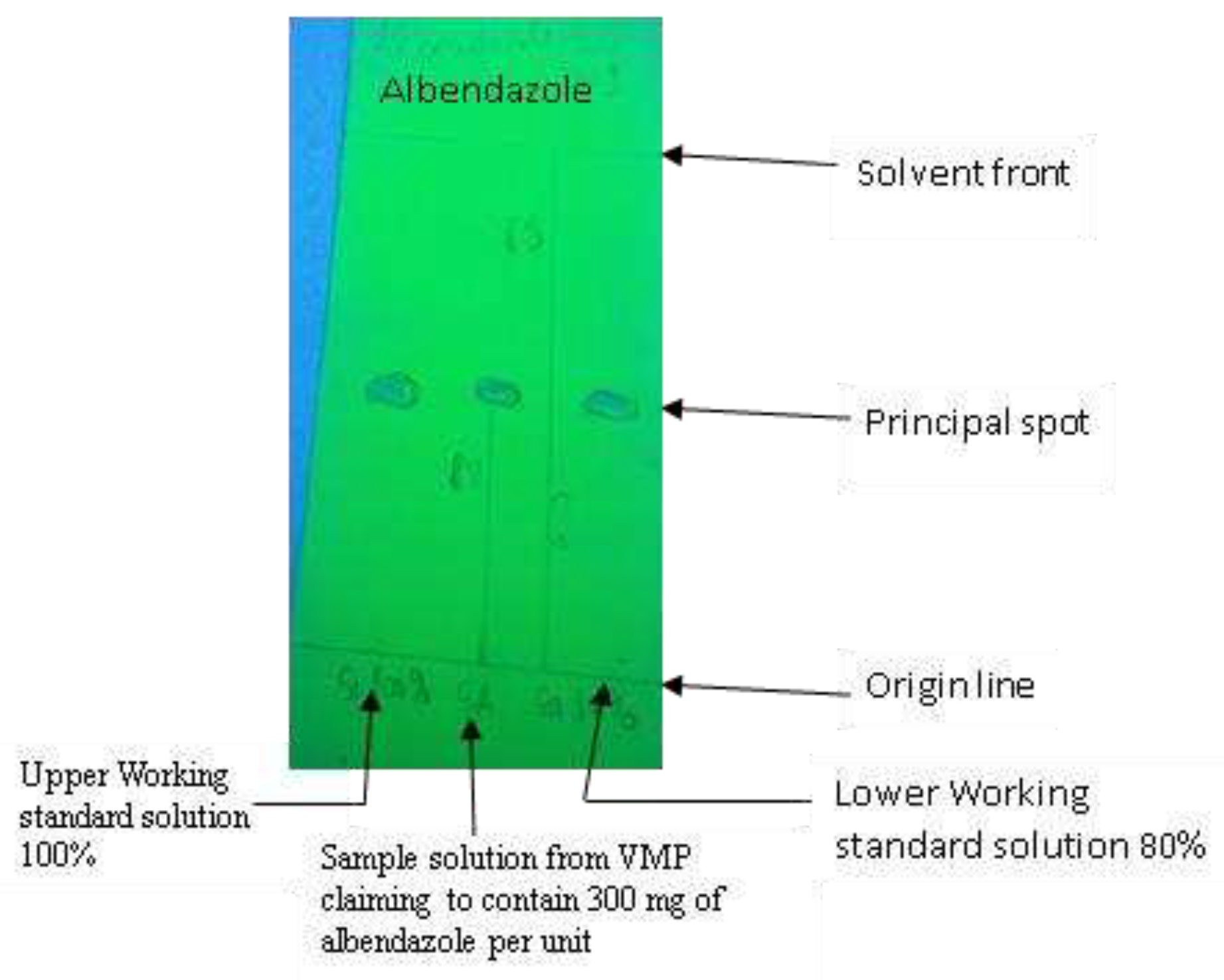
As presented in
Figure 2b, one (1) API (Sulfamethoxazole/Trimethoprim) had differences in spot size and intensity compared to test solutions, respectively, upper working standard and low working standard. This result indicates differences in drug concentrations. Here (
Figure 2b), the sample solution spot on run number 2 fails to meet the size and intensity of the reference spots on run number 1 and 3 representing the higher (100%) and lower (80%) standards, respectively. Failing to meet this range of drug concentrations means that the product fails to meet the label claim on potency.
3. Discussion
Authors Quality-assured VMP are critical in preventing and mitigating diseases and preventing the emergency of resistance, as well as reducing risks attributed to the use of poor-quality VMP. In recent years, there has been growing awareness of the threats to individual and public health represented by poor-quality medicines for human use, but the field of VMP remains relatively neglected. In Rwanda, owing to the prevalence of infectious animal diseases [
8,
9,
10], VMPs such as antibiotics, anthelmintics, antiprotozoals, and acaricides are widely used [
11]. However, there is scarce information regarding the quality of VMP circulating in the market.
This study is a pioneer application of the physical testing and thin-layer chromatography (TLC) test through the Global Pharma Health Fund (GPHF)-Minilab in identifying falsified VMP circulating on the Rwandan market. The approach has not been used previously in evaluating the quality of VMP.
In the current study, a total of 60 VMP samples from 6 active pharmaceutical ingredients (APIs) (Clavulanic acid/Amoxicillin, Tetracycline hydrochloride, cloxacillin, Cefalexin, Cefazolin sodium salt, Amoxicillin, and Ampicillin Trihydrate) were excluded from the TLC testing, most frequently because their single TLC test protocols for the respective dosage form are currently not available in the manual accompanying the GPHF-Minilab. This could be attributed to the fact that the current GPHF-Minilab manual contains a collection of 100 TLC test protocols for 100 essential APIs including a multitude of solid and liquid formulations, salt forms, and fixed-dose combination products most used in human medicine [
6]. Only 22 (22.0%) APIs are related to VMP in different dosage forms. Furthermore, the current single TLC test protocols were mostly developed based on tablets/bolus and capsules [
6]. This finding corroborates with the observations of [
12] in Ethiopia that a total of 136 out of 2055 samples (6.6%) were excluded from the TLC analysis, most frequently because they represented oral liquid dosage forms. This was due to the non-existence of GPHF-Minilab protocols for oral liquid dosage forms.
The results of this study revealed that none of the assessed VMP samples failed physical tests (visual inspection, weight verification, and disintegration test). This finding could be related to the regular visit of veterinary drug shops by regulatory bodies namely Rwanda FDA and Rwanda Council of Veterinary Doctors. On the other hand, the regulation of the VMP supply chain in Rwanda could explain this finding. The VMP supply chain in Rwanda comprises different actors (manufacturers, wholesalers, distributors, retailers, and end-users). VMP importers are registered with the Rwanda FDA and run large-scale businesses that deal directly with international manufacturers/companies. Imported VMP are physically checked by the Rwanda FDA and then importers sell these VMP to primary, secondary, and tertiary distributors/wholesalers and retailers. In the sample districts, all non/licensed veterinary retailer shops bought the VMPs from distributors and/or wholesalers especially located in Nyarugenge district, Kigali city. Conversely, the defects in the physical characteristics of the VMP samples were reported from Ethiopia [
13] as the result of visual inspection. This difference should be supported by the policy framework regulating the VMP supply chain in these countries.
In the current study, 10 samples (Sulfamethoxazole/Trimethoprim) out of 130 (7.7%) passed the visual inspections but later failed to comply with TLC result specifications. This is supported by the findings of Tefera et al. [
13], they reported that 60 samples out of 953 (6.3%) passed the visual inspections, but later failed to comply with assay result specifications.
The current finding indicates the probability of the presence of falsified VMP circulating in Rwanda. This could be linked to irregular chemical analysis of VMPs by the Rwanda FDA for regulatory inspection or post-market surveillance of VMPs despite their regular visit to the veterinary retailer shops sampled. On the other hand, the observed poor quality of VMP (Sulfamethoxazole/Trimethoprim) may reflect the failure of manufacturers to comply with good manufacturing practices, or failure to implement adequate storage and distribution practices along the veterinary supply chain. In fact, it is difficult to distinguish quality problems caused by poor practices at manufacturing sites versus those caused by poor practices along the distribution chains.
Given the fact that Sulfamethoxazole/Trimethoprim is an essential medicine widely used in the poultry industry in Rwanda, the quality failure observed for this VMP could jeopardize the existing efforts of the veterinary services. Therefore, this suggests the need for stronger control and monitoring of the quality of VMP during production, procurement, distribution/ supply, storage, and post-market- surveillance.
4. Materials and Methods
4.1. Study Location
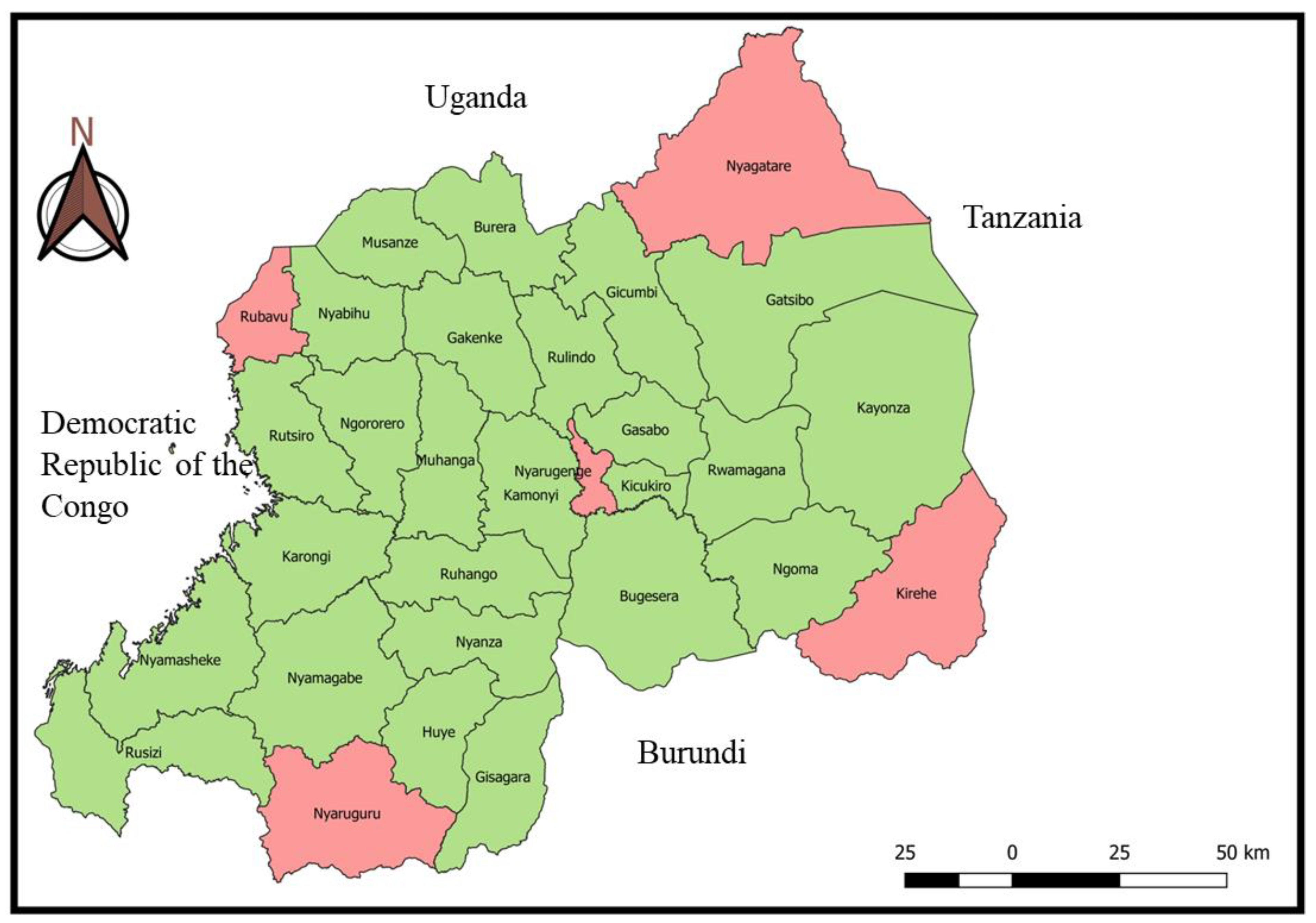
VMP were collected in a cross-sectional study from veterinary retail pharmacies located in five districts in four provinces of Rwanda, during May and June 2023. These five (5) districts are bordering four (4) neighbouring countries (Rubavu bordering the Democratic Republic of the Congo, Nyaruguru bordering Burundi, Kirehe bordering Tanzania, Nyagatare bordering Uganda), and one (1) district (Nyarugenge) in the city of Kigali (
Figure 3).
The VMP retailers were chosen because they mainly supply drugs to animal health service providers and livestock farmers. In addition, they are the most country widely accessed outlets for VMP. The choice of sampling locations was first based on the existing unofficial circuits of acquisition of veterinary drugs in Rwanda (mainly in districts at the borders). Acquisition of veterinary drugs through illegal circuits was reported in districts at the borders of Rwanda specifically in the Eastern province [Rwanda Food and Drugs Authority (Rwanda FDA), 2022]. Also, the illegal drug sellers in boutiques, village markets, and local shops get the veterinary medicine products from retailers and black markets inside and outside the country, especially in the area close to borders where smuggles illegally introduce veterinary medicinal products from neighboring countries and distribute them to farmer’s community living near the borders (Ndayisenga, 2009). Second, the Nyarugenge district in Kigali City was included in the investigation because of the high number of veterinary retail pharmacies.
4.2. Sampling Framework
From each district, two veterinary retail pharmacies (licensed and non-licensed) were randomly selected from a list of all retail pharmacies using a random number selection. In each selected veterinary retail pharmacy, the VMP that were sampled had to contain the active pharmaceutical ingredients (APIs) that are commonly used in cattle, sheep, goats, swine, and/or poultry. These APIs are listed in
Table 1. The choice of VMP purchased and collected was based on the following criteria: (i) on previous studies that have established low efficacy or resistance to these products in animals [8-10], (ii) on the capacity of GPHF-Minilab™ to be used™ [
6], and (iii) on the frequency of their importation into the country according to Rwanda FDA (Manishimwe et al., 2022) or other studies that have established VMP on the Rwandan market. There were 30 APIs standards within the GPHF-Minilab, of which 43.3% (n=13) were found in the Rwandan market during the period of sample collection (
Table 7). Thus, a total of 130 VMP containing 13 APIs across five districts (
Figure 1) were purchased, collected, and transported to the Veterinary Laboratory of the School of Animal Sciences and Veterinary Medicine, Nyagatare Campus, University of Rwanda for analysis (
Table 7).
4.3. Storage and Transportation of VMP Samples
Storage and transportation of the collected samples to the testing laboratory were done as quickly and straight as possible so as not to jeopardize the quality of the collected VMP samples. Therefore, they were kept in their original packaging whenever possible and under storage conditions as specified on the label to avoid breakage and contamination during transport and detailed information was filled in the sample information collection form/questionnaire (Annex 1). Each collected VMP sample was coded for traceability. Sample code included API name, sampling site, and sampling date. Coded samples with their respective sample information collection form were kept in the labeled sampling envelope and sealed.
4.4. Metadata Collected on the VMP Samples and Veterinary Retail Shops
Detailed information on the VMP samples and veterinary retail pharmacies was collected using a predesigned sample information collection form or questionnaire (Annex 1). Briefly, administrative information related to veterinary retail shops included the names and addresses of the owners, sample collection dates, shop type (chain or single owner), size, registration status, licenses, qualification and competency of pharmacy staff (their last training), and last time visited by the Rwanda FDA. In contrast, information about the VMP samples included: container and closure, label (outer and inner packaging), product information leaflet: international nonproprietary name, brand name, dosage form, dosage statement, strength, number of units per container, manufacturer’s full address, date of manufacture and expiry, batch number, APIs, transportation and storage conditions, indication of use, physical characteristics, indicated/targeted species, and packaging conditions. Additionally, spelling mistakes or grammatical errors and the VMP samples' visual appearance were recorded.
Before testing, collected VMP samples were stored in the laboratory storage facilities under appropriate conditions.
4.5. Analysis of Veterinary Medicinal Products Samples
The GPHF-Minilab manual contains protocols for the analysis of 100 APIs mainly in the forms of tablets/bolus, capsules, and/or injectables, as well as for frequently fixed combinations of these APIs [
6]. Therefore, after purchasing, collecting, and transporting VMP, we observed that six (46.2%) APIs had no Minilab’s Thin-Layer Chromatography (TLC) test protocols for the respective dosage forms that were available on the market during the period of VMP sampling (
Table 8).
Therefore, the quality of VMP samples using GPHF-Minilab involves a four-stage test plan that employs very simple physical and chemical analytical techniques [
6,
7]: (i) a visual inspection scheme of solid dosage forms, including associated packaging material, for early rejection of the more crudely presented VMP counterfeits, (ii) a disintegration test for a preliminary assessment of deficiencies related to VMP solubility and availability, (iii) A quick check of the fill and total weight serves as an early indicator for the detection of false information related to the drug content, and (iv) easy-to-use thin-layer chromatography as a chemical test for rapid verification of label claims regarding drug identity [
6].
4.5.1. Physical Testing of VMP Samples
The physical testing was a visual observation of the parameters of each VMP sample, weight verification, and disintegration test (if applicable). The latter test is not applicable for injectables, dry syrups, creams, ointments, suspensions, and chewable tablets.
A visual inspection test was conducted by examining dosage forms and packaging material to detect obvious and gross product faults as specified in the GPHF-Minilab Manual [
6]. Therefore, parameters carefully checked and recorded during the visual inspection included but not limited to deficiencies in labeling, packaging and pack size, dosage forms, strength, manufacturing and expiration dates, warning instructions, batch number, spelling mistakes or grammatical errors, availability and information on the primary and secondary packaging, indications (
Figure 4). In particular, tablets/bolus were checked for unaltered surfaces and color uniformity and undamaged.
The eligibility and correctness of the above information were checked against GPHF-Minilab Manual guidelines [
11]. Thus, the VMP sample was considered as falsified/substandard in case of poor, altered, or absent printing on the packaging material, simple spelling faults, wrong/absent of batch number, false formats for/wrong manufacturing and expiration dates, non-existing addresses for manufacturers, wrong tablets/bolus shapes and color.
A weigh verification consisted of quickly checking on tablet/bolus and powders for injection or suspension mass to see variations and deficiencies in weight against specifications supplied by the genuine manufacturer indicating poor and non-uniform dosing [
6]. Five randomly selected bolus and powders for injection or suspension were weighed on a calibrated electronic pocket balance (KERN CM 60-2N, KERN & SOGH GmbH, Germany) (
Figure 4).
To pass this criterion, the fill weight of bolus or powders for injection or suspension should not fall below the dosage strength claimed on the label.
Next to a visual inspection scheme and verifying the fill and total weight of solid dosage forms, the search on falsified and substandard VMP included a simplified disintegration test to foresee deficiencies in VMP release probably due to poor bolus formulation or storage. Disintegration was defined as that state in which no residue of the tablets/bolus remains in the test solution. Therefore, disintegration testing was carried out as specified in the GPHF Minilab manual [
6]. For that, six boluses per product, chosen at random, were tested according to the basic GPHF-Minilab kit consisting of a wide-neck bottle, alcohol thermometer, and a timer [
7]. Criteria of this test were marked as passed when bolus samples placed into a wide neck bottle vessel containing 100 mL of warm tap water at approximately 37˚C fully disintegrated within 30 minutes (
Figure 5).
4.5.2. Thin-Layer Chromatographic Analysis
Thin-layer chromatography (TLC) was used for the semi-qualitative determination of API present in the dosage forms. TLCs were performed according to the single TLC test protocols of VMP samples as described in the GPHF-Minilab manual [
6]. Briefly, TLC starts by using aluminium chromatoplates pre-coated with silica gel 60 F254 (size of 5 x 10 cm; by Merck, Darmstadt, Germany) to fit into the TLC developing chamber. Tablets/bolus were crushed with a pestle before extraction by wrapping up it into aluminium foil and crushing it down to a fine powder. Next, all solids were dissolved in a known volume of extraction solvent specific to each VMP sampled using a set of various straight pipettes capable of delivering an accurate volume of 0.01 to 25.0 ml of solvent and therefore, the working standard or working sample solution was prepared accordingly. The working standard solution 100% (upper working limit) represented a VMP of good quality containing 100% of API of the VMP sampled whereas a working standard solution 80% (lower working limit) represented a VMP of poor quality containing just 80% of the amount of API of sampled VMP as stated on the VMP’s label. Consequently, API content was determined semi-quantitatively against a standard solution concentrated to an 80% lower specification limit (LSL) and 100% upper specification limit (USL) of the declared amount, respectively [
13].
Using a pencil of soft grade and a ruler, the origin line was marked on a chromatoplate on about 1.5 cm from the bottom edge of the chromatoplate. Two (2) µl of each test and standard solution were applied on chromatoplate using microcapillary pipette. The loaded TLC plate was placed into the development tank and wait till the solvent front has moved about three-quarters of the length of the TLC plate. After that, the plate was removed from the TLC tank and allowed any excess solvent to evaporate and the solvent front was marked on TLC plate. After drying off all solvent, the chromatoplate was first observed at daylight followed by an inspection with UV-light of 254 nm in the dark (
Figure 6).
The center of the spots and both travel distances were expressed in millimeters, the one from the solvent front and the one from the spots were marked using a graduated ruler (
Figure 7). The chromatoplate reading employed the principle of comparing spots of the test sample and reference solutions (
Table 9).
The principal spot obtained with the test sample solution was required to correspond with the chromatographic runs of the standard solution in terms of color, shape, size, intensity, and relative retention factor (Rf) value (
Figure 7). As presented in
Figure 7, Run number 1 represents the sample solution. In contrast, run number 1 and 3 represent the upper/higher (100%) and lower (80%) standard solutions, respectively. Failing to meet this range of drug concentrations means that the product fails to meet the label claim on potency. Also, the differences in travel distances indicate that the test solution contains a different drug.
4.6. Data Analysis
Six APIs (
Table 8) were excluded from TLC testing and statistical analysis because they had no Minilab’s Thin-layer Chromatography test protocols for the respective dosage form [
6]. Thus, single TLC test protocols were available for 7 different APIs. All data were entered and analyzed using Excel by Microsoft Office Professional Plus 2016.
The relative retention factor (Rf) value was computed using the following formula [
6]:
The test sample was considered failed if the Rf value of the test sample was different by more than 10% from that of the standard sample and/or if the intensity of the spot was less than that of a reference containing 80% of the stated amount of the API. In contrast, there was strong evidence that the API in the test and standard solutions were identical if the principal spots of both samples showed the same travel distance. In other words, the VMP sample passed the criteria if the intensity of the principal spots was detected between 80% lower specification limit and 100% upper specification limit. TLC plates were photographed with a smartphone (Samsung Galaxy A72) camera.
5. Conclusions
The results of the present study indicated that 10 samples (Sulfamethoxazole/Trimethoprim) out of 130 (7.7%) passed the visual inspections but later failed to comply with TLC result specifications. Therefore, continued strengthening of the inspection and chemical quality control of VMPs along the supply chain is highly recommended. Furthermore, the VMP sample that GPHF-Minilab™ identified to be substandard and falsified should be sent to a quality control laboratory of Rwanda FDA for confirmation and verification of the API content.
Author Contributions
“Conceptualization, P.F.J., H.R. and N.P.; methodology, L.J., P.F.J., H.R. and N.P.; software, H.R, N.P. and H.J.P; validation, L.J., P.F.J., I.E. and N.M.; formal analysis,H.R, N.P., H.J.P.; investigation, H.R., N.P.; resources, L.J., P.F.J.; data curation, H.R, M.R., I.E. and N.M.; writing—original draft preparation, H.R., N.P. and H.J.P.; writing—review and editing, M.R., I.E., N.M., L.J., P.F.J.; visualization, HR and N.P.; supervision, L.J. and P.F.J.; project administration, H.R.; funding acquisition, L.J and P.F.J. All authors have read and agreed to the published version of the manuscript”.
Funding
This material is based upon work supported by the Food and Agriculture Organization of the United Nations (FAO-Rome-Italy) via a Mars grant.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the School of Animal Sciences and Veterinary Medicine, University of Rwanda, for its laboratory facilities. The same goes for the owners of veterinary retailer shops for their greater cooperation during the VMP purchasing and collection period.
Conflicts of Interest
“The authors declare no conflicts of interest.”
Appendix A
Questionnaire for the study on identifying substandard and falsified veterinary medicinal products on the Rwandan market using GPHF Minilab
- A.
Administrative information related to veterinary retail shops - 1.
Respondent’s name [ ____________________________________] - 2.
Tel. number [__________________________] - 3.
Gender of the respondent [ ____] 1 = Male 2 = Female - 4.
Age of the respondent [ ____ ____] years - 5.
Respondent’s position in Vet Retail shop [ ____] 1 = Owner, 2 =employee, Other (specify) [ _______________] - 6.
Professional [______] 1 = Veterinary Medicine, 2 = Animal health, 3 = Animal Production, 4 - 7.
Education level in the field indicated above [ ____] 1 = Secondary school (A2), 2 = University (BVM), 3 = TVET school (A1) - 8.
VMP retail shops location [ ____] 1 = Urban 2 = Rural - 9.
-
Farmer’s address (i) Village [_________________] (ii) Cell [ ________________]
(iii) Sector [ __________________]
(iv) GPS coordinates (use SW Maps): S_________________
E________________
Picture_________________
- 10.
VMP Retail shop type [_____] 1= Chain, 2 = single - 11.
Size (indicates length and width of the shop) = [ --------- m × …………m) - 12.
Registration into RCVD [______] 1 = yes, 2 = no - 13.
Registration into Rwanda FDA [________] 1 = yes, 2 = no - 14.
Licensed by RCVD [_____] 1 = yes, 2 = no - 15.
Licensed by Rwanda FDA [_____] 1 = yes, 2 = no - 16.
Have u had a training on pharmacy management in last one year [ ____] 1 = yes, No = 2 - 17.
What was the last time visited by the Rwanda FDA? - a)
1-6 month 0 - b)
6-12 month 0 - c)
More than 12 month 0 - 18.
What was the last time visited by the RCVD? - a)
1-6 month 0 - b)
6-12 month 0 - c)
More than 12 month 0 -
- B.
Information about the VMP samples - 1.
Type of VMP sampled [______] 1 = antibiotics, 2 = anthelmintic, 3 = steroid preparations, 4 = antiprotozoal, 5 = Other (please specify) [__________________] - 2.
Registered VMP by Rwanda FDA [______] 1 = Registered, 2 = unregistered - 3.
Sampling site [________________________________] - 4.
Sampling date [____ _____ ____] - 5.
Presence of Outer label [______] 1 = yes, 2 = no - 6.
Presence of Inner packaging [_____] 1 = yes, 2 = no - 7.
Product information leaflet: - a)
International non-proprietary name [____________________________] - b)
Brand name [ _______________________________________] - c)
Dosage form [_________] 1 = Injectable/ Solution, 2 powder, 3 = bolus/ Tablet, 4= Water Soluble, 5 = Drops, 6= Eye ointment, 7 = Cream, - d)
Dosage statement [_______________________________] - e)
Strength [____________________________________] eg. 20 mg/ml solution for injection for dogs or 100mg - f)
Number of units per container [_______________________________________] - g)
Manufacturer’s full address - o
Country [________________________] - o
Box [____ _____ _____] - o
Email _______________________________ - o
Website___________________________________ - h)
Date of manufacture [____ ____ ____] - i)
Date of expiry [ ____ ____ ____] - j)
Batch/lot number [ ____ ___- ____] - k)
Active pharmaceutical ingredients [__________________________________________] - l)
Transportation conditions [ ____________________________________________] - m)
Storage conditions [ ___________________________________________________] - n)
Indication of use [____________________________________________] - o)
Physical characteristics [________________________________________________] - p)
Indicated/targeted species [_______] 1 = cattle, 2 = goats, 3 = sheep, 4 = dog, 5 = pig, 6 =poultry, 7 = other (please specify) _______________________________________ - q)
Packaging conditions - r)
Pack size - s)
Spelling mistakes or grammatical error |
| We thank you for your time and appreciate your thoughtful answers to our questions. |
| END OF THE QUESTIONNAIRE |
References
- World Health Organization. (22–31 May 2017). Seventieth World Health Assembly. Annex A70/23. Appendix 3. Geneva, Switzerland. Available: https://www.who.int/medicines/regulation/ssffc/A70_23-en1.pdf?ua=1.
- Videau JV, Fundafunda B. 2000. Generic drugs: the hidden issues of quality and cost: general policy issues. WHO drug information 2000; 14(2): 77-81. https://apps.who.int/iris/handle/10665/58129.
- World Health Organization. 1999. Quality assurance of pharmaceuticals, vol 2. World Health Organization, Geneva, Switzerland.
-
https://apps.who.int/iris/bitstream/handle/10665/43532/9789241547086_eng.pdf?sequence=1&isAllowed=y.
- World Health Organization. 2018. Substandard and falsified medical products https://www.who.int/news-room/fact-sheets/detail/substandard-and-falsified-medical-products.
- Ingrid M.E. Bakker-’t Hart, Dana Ohana, Bastiaan J. Venhuis, 2021. Current challenges in the detection and analysis of falsified medicines. [CrossRef]
- Richard W.O. Jahnke and Kornelia Dwornik, 2020. A concise Quality Control Guide on Essential Drugs and other Medicines. Review and Extension 2020. Physical Testing and Thin-Layer Chromatography.
- Richard W.O. Jahnke and Gabriele Kusters, 2001. Low-cost quality assurance of Medicines using the GPHF-minilab. [CrossRef]
- Nyabinwa, P., Kashongwe, O.B., Habimana, J.P. et al. Estimating prevalence of endometritis in smallholder zero-grazed dairy cows in Rwanda. Trop Anim Health Prod 52, 3135–3145 (2020). [CrossRef]
- Jean Baptiste Twagirayezu & Vestine Musanayire & Lydia Murerwa & Mohamed Moctar Mouliom Mouiche & Jean Nepomuscene Hakizimana & Pascal Nyabinwa, 2021. "Prevalence of Subclinical Mastitis and its Effects on Reproductive Performance in Dairy Cows during the Postpartum Period in Gasabo District, Rwanda," International Journal of Veterinary Sciences Research, Conscientia Beam, vol. 6(1), pages 1-13.
- JB Twagirayezu, V Musanayire, L Murerwa, JN Hakizimana, P Nyabinwa. Postpartum clinical diseases of dairy cows managed on smallholder production system in Gasabo district, Rwanda. Int J Vet Sci Anim Husbandry 2021;6(2):15-22. [CrossRef]
- Manishimwe R, Moncada PM, Musanayire V, Shyaka A, Morgan Scott H, Loneragan GH. Antibiotic-resistant Escherichia coli and Salmonella from the faeces of food animals in the Eastern province of Rwanda. Animals 2021;11:1–17. [CrossRef]
- Gnegel, G., Häfele-Abah, C., Neci, R. et al. Surveillance for substandard and falsified medicines by local faith-based organizations in 13 low- and middle-income countries using the GPHF Minilab. Sci Rep 12, 13095 (2022). [CrossRef]
- Tefera, B., Bacha, B., Belew, S. et al. Study on identification, assay and organoleptic quality of veterinary medicines in Ethiopia. J of Pharm Policy and Pract 15, 17 (2022). [CrossRef]
- Ndayisenga, F (2009). Analyse de la distribution et dela qualite des medicaments veterinaires au Rwanda. https://www.beep.ird.fr/collect/eismv/index/assoc/TD09-37.dir/TD09-37.pdf.
- Manishimwe, R., Ndayisenga, B., Habimana, R., Mwikarago, I. E., Habiyaremye, T., Shyaka, A., ... & Bienvenu, E. (2022). Consumption Trends in Antibiotics for Veterinary Use in Rwanda: A Retrospective Study between 2019 and 2021. [CrossRef]
- Rwanda Food Drugs and Authority (Rwanda FDA), 2022. https://rwandafda.gov.rw/wp_content/uploads/2022/11/Medicine%20safety%20bulletin%20version%202.pdfAuthor 1, A.B.; Author 2, C.D. Title of the article. Abbreviated Journal Name Year, Volume, page range.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



 Minilab protocol exists
Minilab protocol exists  Minilab protocol doesn’t exist.
Minilab protocol doesn’t exist.



