Introduction
Osteoarthritis (OA) is a joint disorder characterized primarily by degenerative changes in joint cartilage. The key features of OA include an imbalance in the extracellular matrix (ECM) metabolism of chondrocytes (Wieland et al. 2005). Within the ECM of cartilage, proteoglycans (PG) are the major constituents, and type II collagen (COL2A1) forms the scaffold structure of chondrocytes. A disintegrin and metalloprotease with thrombospondin motifs (ADAMTS) and matrix metalloproteinases (MMPs) are the main matrix-degrading enzymes in joint cartilage (Hui et al. 2012). They can directly degrade PG and COL2A1. Among them, ADAMTS-4 and ADAMTS-5 are the most potent and are considered the primary PG hydrolases (Henrotin et al. 2016,Zeng et al. 2006,Song et al. 2007). Additionally, MMP-1 and MMP-13 primarily participate in the degradation of COL2A1 in chondrocyte ECM, while MMP-3 can activate the precursors of MMP-1 and MMP-13, promoting ECM degradation collectively (Hu and Ecker 2021).
Usnic acid (UA) was primarily found in lichen species such as genera Usnea, Evernia, and Alectoria (K 2002). UA has exhibited biological activities including anti-inflammatory, antioxidant, and wound healing-promoting effects (Araújo et al. 2015). Vijayakumar et al. conducted acute and chronic experiments using UA in rodent models, revealing anti-inflammatory effects (Reddy 2000). UA has been shown to enhance the nuclear factor erythroid-2-related factor 2 (Nrf2) gene and protein nuclear translocation (Fernandez-Moriano et al. 2017), along with upregulation of antioxidant enzymes like Heme Oxygenase 1 (HO-1), NADPH: Quinone Oxidoreductase 1 (NQO1), and Superoxide Dismutase (SOD) (Krajka-Kuźniak et al. 2021,Li and Zhang 2020).
Nrf2 signaling pathway serves to counteract reactive oxygen species (ROS) and reactive nitrogen species (RNS) by regulating the balance between oxidative and antioxidative systems, thereby preventing oxidative damage (Sivandzade et al. 2019,Ma 2013, Ding et al. 2015). Upon activation, the Nrf2 pathway upregulates the expression of HO-1 and NQO1, enhancing cellular antioxidant capacity (Qiao, Jiang and Gao 2018). Simultaneously, it suppresses the activation of the NF-κB signaling pathway, alleviating cellular inflammation and providing further cellular protection to impede disease progression (Jia et al. 2017, Tang et al. 2018, Jung and Kwak 2010). HO-1, categorized as an antioxidant enzyme, produces degradation products that collectively inhibit inflammatory reactions, thereby exhibiting antioxidative effects (Alcaraz and Ferrándiz 2020). Chen et al. demonstrated that the Nrf2 pathway can modulate HO-1 expression in synovial cells, mitigating inflammatory responses and decelerating cartilage degradation (Chen et al. 2019). During cellular oxidative stress, NQO1 acts by preventing ROS generation, thereby reducing cellular ROS levels, exerting antioxidative effects (Dinkova-Kostova and Talalay 2010).
The NF-κB signaling pathway is considered to be highly associated with OA (Rigoglou and Papavassiliou 2013, Oeckinghaus and Ghosh 2009). The NF-κB pathway can function independently or interact with other signaling pathways, inducing the expression of inflammatory mediators such as IL-6, Tumor Necrosis Factor-α (TNF-α), Cyclooxygenase-2 (COX-2), Prostaglandin E2(PGE2), and inducible nitric oxide synthase (iNOS) (Midwood et al. 2009, Ge et al. 2011, Goldring MB 2011), which in turn affect chondrocyte metabolism and stimulate the secretion of matrix-degrading enzymes. Previous studies have demonstrated that UA downregulates the levels of IL-1β, IL-6, TNF-α, COX-2, and iNOS, thus suppressing inflammation, by inhibiting the NF-κB signaling pathway (Jin, Li and He 2008, Pazdziora et al. 2023). Odabasoglu et al. found that UA can enhance the expression of antioxidants like SOD, reduce iNOS activity, inhibit oxidative damage (Odabasoglu et al. 2006).
However, the use of UA in the treatment of osteoarthritis has rarely been reported.This study based on in vitro cultured rat chondrocytes, aims to explore the protective effect and molecular mechanism of UA on OA. By comprehensively analyzing the regulatory effects of UA on rat chondrocytes and identifying the optimal dosages, this research holds crucial significance for future drug development, clinical trials, and the study of relevant target drugs.
Material and Method
Antibodies and Reagents
INOS, COX-2, COL2A1, ADAMTS-4, Nrf2, HO-1, NQO1, p65, p-p65, IκBα, p-IκBα, GAPDH, and Lamin B were purchased from ABconal (Massachusetts, USA); MMP1, MMP3, and MMP13 were purchased from Affinity (USA); UA (HY-N0656, purity: 98.69%) and the phosphatase inhibitor were procured from Med Chem Express (New Jersey, USA).
Experimental Animal
Sprague-Dawley (SD) pregnant rats were obtained from the Liaoning Changsheng Biomedical Experimental Animal Center. Prior to the experiment, rats were housed in a well-ventilated and appropriately humidified environment with free access to water and food, and bedding was changed regularly. The experimental subjects consisted of 14-21-day-old lactating SD rats. All SD rats used in this study were conducted under the guidance of the Animal Ethics Review Committee, in accordance with relevant regulations for ethical review of animal experiments.
Experimental Animal Grouping and Surgery
In this experimental study, thirty male rats were randomly assigned to three groups: Control Group (n=10), Model Group (n=10), and Usnic acid Group (n=10). The Control Group underwent a skin incision without excision of any ligaments or menisci. The Model Group and Usnic acid Group employed the Anterior Cruciate Ligament Transection (ACLT) method to induce OA in rats. Both the Control and Model Groups were maintained under standard conditions, while the Usnic acid Group received abdominal injections of Usnic acid (50 mg/kg) following the induction of OA.
Histology and Immunohistochemical Analysis
12 weeks after the mold was made, samples (blood and joints) were taken from the right hind limbs of each group of rats. The tissues were fixed and embedded in paraffin for slicing. Safranin O-fast green staining was used to observe the pathological changes in the tibial cartilage, femoral cartilage, and bones. Immunohistochemical analysis was performed using previously described methods. Rabbit primary antibodies and diaminobenzidine (DAB) substrate kit were used for semi-quantitative analysis of each index. Under the pathological microscope, MMP-3, MMP-13, ADAMTS-4, Aggrecan, and COL2A1 expression in chondrocyte extracellular matrix appeared yellow-brown. Then, we used Image-Pro Plus software version 6.0 (Media Cybernetics, Rockville, MD, USA) to analyze the data from all groups.
Isolation and Cultivation of Primary Chondrocytes
Detailed experimental steps are shown in Annex 1. The cell culture medium was changed on days 2-3, and passage was performed when cell confluence reached 80%. The experiments were conducted using the second passage chondrocytes. Control Group: Normal cultured chondrocytes for 24 hours; IL-1β Group: Chondrocytes induced with 10 ng/mL IL-1β for 24 hours; UA Groups (2.5, 5, 10): Chondrocytes co-treated with 10 ng/mL IL-1β and 2.5, 5, and 10 μM UA for 24 hours; Negative Control Group (NC): Chondrocytes co-treated with IL-1β and 10μM ML385 for 24 hours(Ma et al. 2022); Inhibitor Group (ML385): Chondrocytes co-treated with IL-1β, ML385, and 10 μM UA for 24 hours.
Enzyme Linked Immunosorbent Assay (ELISA)
After collecting the culture supernatant, the levels of IL-6, TNF-α, and PGE2 in the chondrocyte secretions were determined following the protocols provided by the rat IL-6 ELISA kit, rat TNF-α ELISA kit (Shanghai BlueGene Biotech Co., Ltd.), and rat PGE2 ELISA kit (Nanjing Jiancheng Bioengineering Institute). Standard curves and linear regression equations were generated using ELISA Calc based on the optical density (OD) values, and the concentrations of IL-6, TNF-α, and PGE2 in each group were calculated. According to the manufacturer's instructions, the levels of INOS ((Nanjing Jiancheng Bioengineering Institute)), COX-2, PGE2, and TNF-α in rat serum were detected using an ELISA kit.
The Assessment of UA's Impact on Chondrocyte Viability
The effect of different concentrations of UA on the viability of the second-generation chondrocytes was determined using the CCK-8 assay kit (Shanghai BlueGene Biotech Co., Ltd.). The experimental groups were as follows: Blank control group: chondrocytes without any treatment; Vehicle control group: cells + 0.1% DMSO (Sigma, Germany); UA group: cells + UA (concentrations of 2.5 μM, 5 μM, 10 μM), with 3 parallel wells set up for each concentration. UA was dissolved in DMSO, with the DMSO concentration not exceeding 0.1%.
Extraction of Total RNA from Chondrocytes and Real-Time Fluorescent Quantitative PCR (qPCR)
Total RNA was extracted using a total RNA extraction kit (Tiangen, Beijing, China) following the manufacturer's instructions. The RNA concentration and purity were determined using a micro-UV spectrophotometer (Thermo, Massachusetts, USA). The reverse transcription system was established according to the instructions of the reverse transcription kit (Kogen, Tokyo, Japan), and reverse transcription was performed using a PCR instrument. The obtained cDNA was stored at -80°C. Reaction system: SuperReal PreMix, 10 μL; cDNA, 2 μL; forward primer, 0.6 μL; reverse primer, 0.6 μL; DEPC water, 6.8 μL. After centrifugation at 2000 rpm for 30 s in a PCR plate, the reaction was carried out on a LightCycler®480 fluorescence quantitative PCR instrument (Roche, Germany), with primer sequences as shown in Table 2-1. The 2-ΔΔCT method was used to calculate the relative gene expression levels.
Table 2-1.
Primer sequence.
Table 2-1.
Primer sequence.
| Gene Name |
Sequence |
|
IL-6
|
Forward:5’-AGGAGTGGCTAAGGACCAAGACC-3’
Reverse:5’-TGCCGAGTAGACCTCATAGTGACC-3’ |
|
TNF-α
|
Forward:5’-GCATGATCCGAGATGTGGAACTGG-3’
Reverse:5’-CGCCACGAGCAGGAATGAGAAG-3’ |
PGE2
INOS
|
Forward:5’-AGAAGCTGATAATCGGGT-3’
Reverse:5’-CCTAGTGAAGGATCC-3’
Forward:5’-AAGAGACGCACAGGCAGAGG-3’
Reverse:5’-AGCAGGCACACGCAATGATG-3’ |
|
COX-2
|
Forward:5’-AGAAGCGAGGACCTGGGTTCAG-3’
Reverse:5’-ACACCTCTCCACCGATGACCTG-3’ |
|
COL II
|
Forward:5’-GAACGGCGGCTTCCACTTCAG-3’
Reverse:5’-GCTTCGTCCAGGTAGGCAATGC-3’ |
|
ADAMTS-4
|
Forward:5’-CACCGAACCGACCTCTTCAA-3’
Reverse:5’-GAGTTCCATCTGCCACCCGT-3’ |
|
MMP-1
|
Forward:5’-GCTTAGCCTTCCTTTGCTGTTGC-3’
Reverse:5’-GACGTCTTCACCCAAGTTGTAGTAG-3’ |
|
MMP-3
|
Forward:5’-TTTGGCCGTCTCTTCCATCC-3’
Reverse:5’-GCATCGATCTTCTGGACGGT-3’ |
|
MMP-13
|
Forward:5’-TTCTGGTCTTCTGGCACACG-3’
Reverse:5’-TGGAGCTGCTTGTCCAGGT-3’ |
Nrf2
HO-1
NQO1
|
Forward:5’-AATATCCAGGGCAAGCGACTC-3’
Reverse:5’-CAGCACATCCAGACAGACACC-3’
Forward:5’- CTTCCCGAGCATCGACAAC -3’
Reverse:5’-CTGTCACCCTGTGCTTGACC-3’
Forward:5’-GCCATCATTTGGGCAAGTCC-3’
Reverse:5’-TCCTTGTGGAACAAAGGCGA-3’ |
|
GAPDH
|
Forward:5’-GATGCCCCCATGTTTGTGAT-3’
Reverse:5’-GGCATGGACTGTGGTCATGAG-3’ |
Chondrocyte Protein Extraction and Western Blotting (WB)
The proteins from chondrocytes were extracted according to the instructions of the nuclear protein extraction kit (Beyotime Biotechnology, Shanghai, China) and stored at -80°C. Protein immunoblot experiments were performed using previous methods, and Image J software was used for grayscale analysis to determine the relative protein expression levels.
According to the previous WB method, detect specific proteins. p65, 1:3000; p-p65, 1:2000; IκBα, 1:3000; p-IκBα, 1:2000; Nrf2 1:2000; HO-1, 1:3000; NQO1, 1:2000; iNOS, 1:2000; COX-2, 1:3000; MMP1, 1:2000; MMP3, 1:2000; MMP13, 1:2000; ADAMTS-4, 1:3000; COL2A1, 1:2000; GAPDH, 1:3000; LaminB, 1:3000.
Immunofluorescence (IF)
Sufficiently rinsed with PBS buffer and fixed with 4% paraformaldehyde (30 min). After sufficient rinsing, 0.5% Triton-100 (PBS buffer preparation) was added for 20 min at room temperature, and 3% BSA was blocked at room temperature (20 min), and the blocking solution was discarded. Add primary antibody and incubate at 4℃ overnight; discard primary antibody, add Alexa 488 anti-rabbit IgG fluorescent secondary antibody (37℃, 30 min); rinse with PBS buffer, add DAPI staining solution (37℃, 30 min); discard DAPI staining solution, rinse and add anti-fluorescence quenching sealing solution, and observe by inverted fluorescence microscope.
Statistic Analysis
Statistical analysis in this study was conducted using GraphPad Prism 8.0 software. All values are presented as means ± standard deviation (SD). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. A significance level of P<0.05 was considered statistically significant for differences.
Results
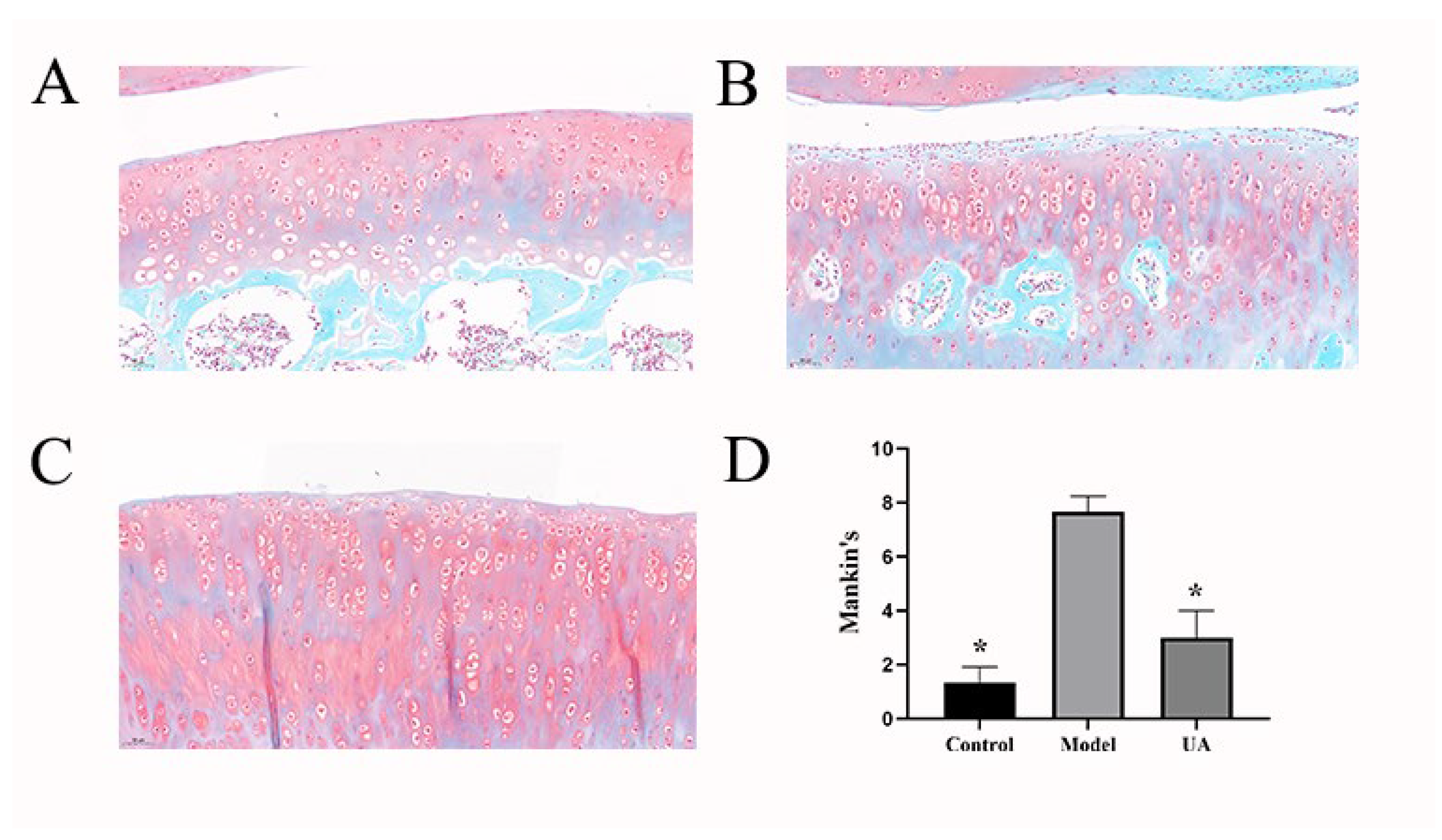
The Effect of UA on Pathological Changes in Articular Cartilage
The results of Safranin O-fast green staining reveal distinctive outcomes following interventions. The control group exhibits a relatively intact cartilaginous structure characterized by a smooth and regular surface, uniform distribution of chondrocytes, and normal red staining of the cartilaginous matrix. In contrast, the model group displays irregularities on the cartilage surface, chondrocyte loss, reduced quantity with clustered distribution, and diminished red staining of the cartilaginous matrix. In the Usnic Acid group, the cartilaginous structure appears relatively intact, with a smooth surface and a minor loss of chondrocytes, which, however, are evenly distributed. In comparison to the model group, the red staining of the cartilaginous matrix is more uniformly distributed. Mankin scores indicate a significantly pronounced difference in cartilage assessments between the control and model groups(*p<0.05). Furthermore, a notable difference is observed between the model group and the group treated with Usnic Acid (*p<0.05). It is evident that the ACLT method effectively induces cartilage degeneration, while administration of Usnic Acid demonstrates a significant ability to impede this progression.
Figure 1.
(A–C) Safranin O-fast green staining was used to visualize pathological changes in articular cartilage in various groups (20 x,50μm). (D) Mankin scoring. *p<0.05, **p<0.01 (compared with the model group).
Figure 1.
(A–C) Safranin O-fast green staining was used to visualize pathological changes in articular cartilage in various groups (20 x,50μm). (D) Mankin scoring. *p<0.05, **p<0.01 (compared with the model group).
The Role of UA in IL-1β-Induced Rat Chondrocytes
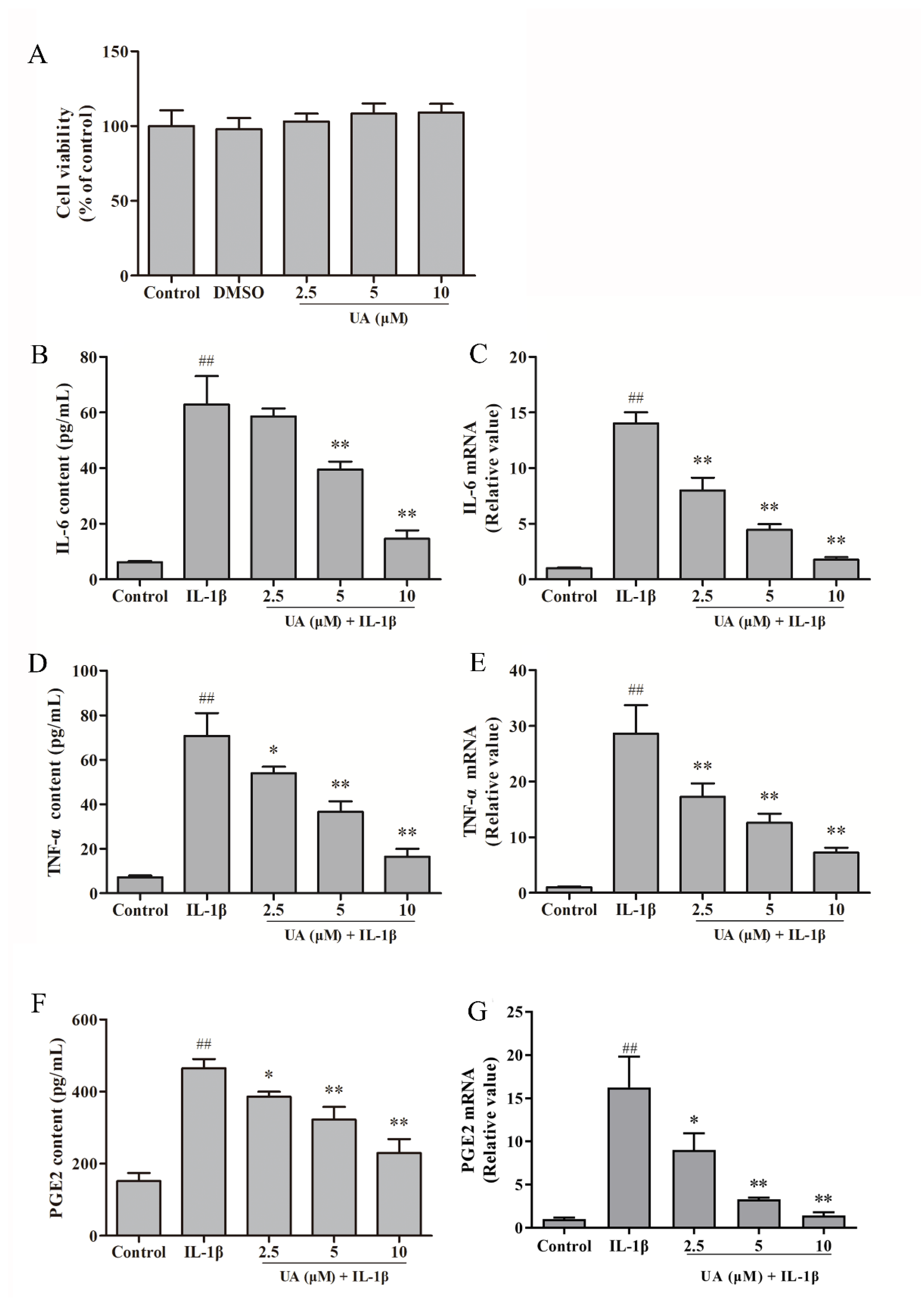
The Impact of Usnic Acid on Chondrocyte Viability and the Levels of IL-6, TNF-α, and PGE2 in Culture Medium
As shown in the
Figure 4A, there was no statistically significant difference in rat chondrocyte viability after 24 hours of incubation with DMSO and different concentrations (2.5 μM, 5 μM, 10 μM) of UA compared to the control group (P>0.05). This indicates that the various concentrations of UA used in this study did not exhibit significant cytotoxicity towards rat chondrocytes.
As shown in the
Figure 4B,D,F, compared to the control group, after 24 hours of IL-1β induction in rat chondrocytes, the expression levels and gene transcription levels of IL-6, TNF-α, and PGE2 in cell secretions were significantly increased (P<0.01). In the
Figure 1C,E,G, in comparison to the model group, the UA group exhibited significantly reduced expression levels and gene transcription levels of IL-6, TNF-α, and PGE2 in cell secretions (P<0.01), in a dose-dependent manner. These results indicate that UA can inhibit the expression of inflammatory mediators in rat chondrocytes induced by 10 ng/mL IL-1β.
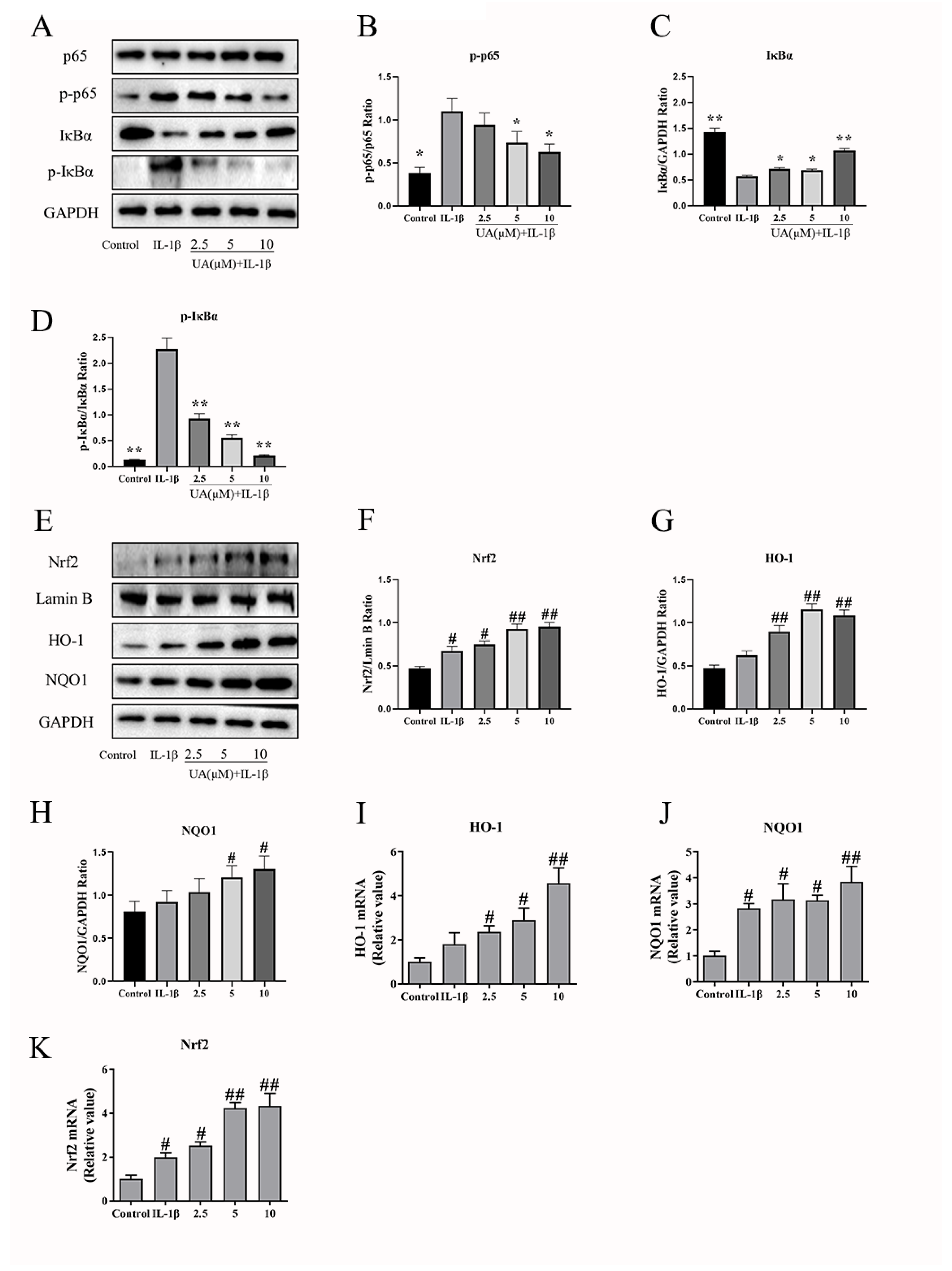
The Effects of UA on the NF-κB and Nrf2 Pathway in IL-1β-Induced Rat Chondrocytes
As shown in
Figure 6A–D, the WB results indicate that, compared to the control group, the IL-1β group exhibited a significant increase in p-p65 levels (P<0.05), a highly significant increase in p-IκBα levels (P<0.01), and a highly significant decrease in IκBα levels (P<0.01). Compared to the model group, all different concentrations of UA groups reduced p-p65 and p-IκBα levels while increasing the levels of IκBα. These results indicate that UA can inhibit the activation of the NF-κB signaling pathway in IL-1β-induced rat chondrocytes. As shown in
Figure 6E–K, WB and qPCR results indicated that compared to the control group, the model group exhibited a significant increase in nuclear Nrf2 levels (P<0.05), as well as elevated expression levels of HO-1 and NQO1. Furthermore, when compared to the control group, the UA group showed further upregulation in the expression of Nrf2, HO-1, and NQO1. These results suggest that UA can enhance the activation of the Nrf2 signaling pathway in IL-1β-induced rat chondrocytes.
The Effects of Nrf2 Inhibition on the Action of UA in IL-1β-Induced Chondrocytes
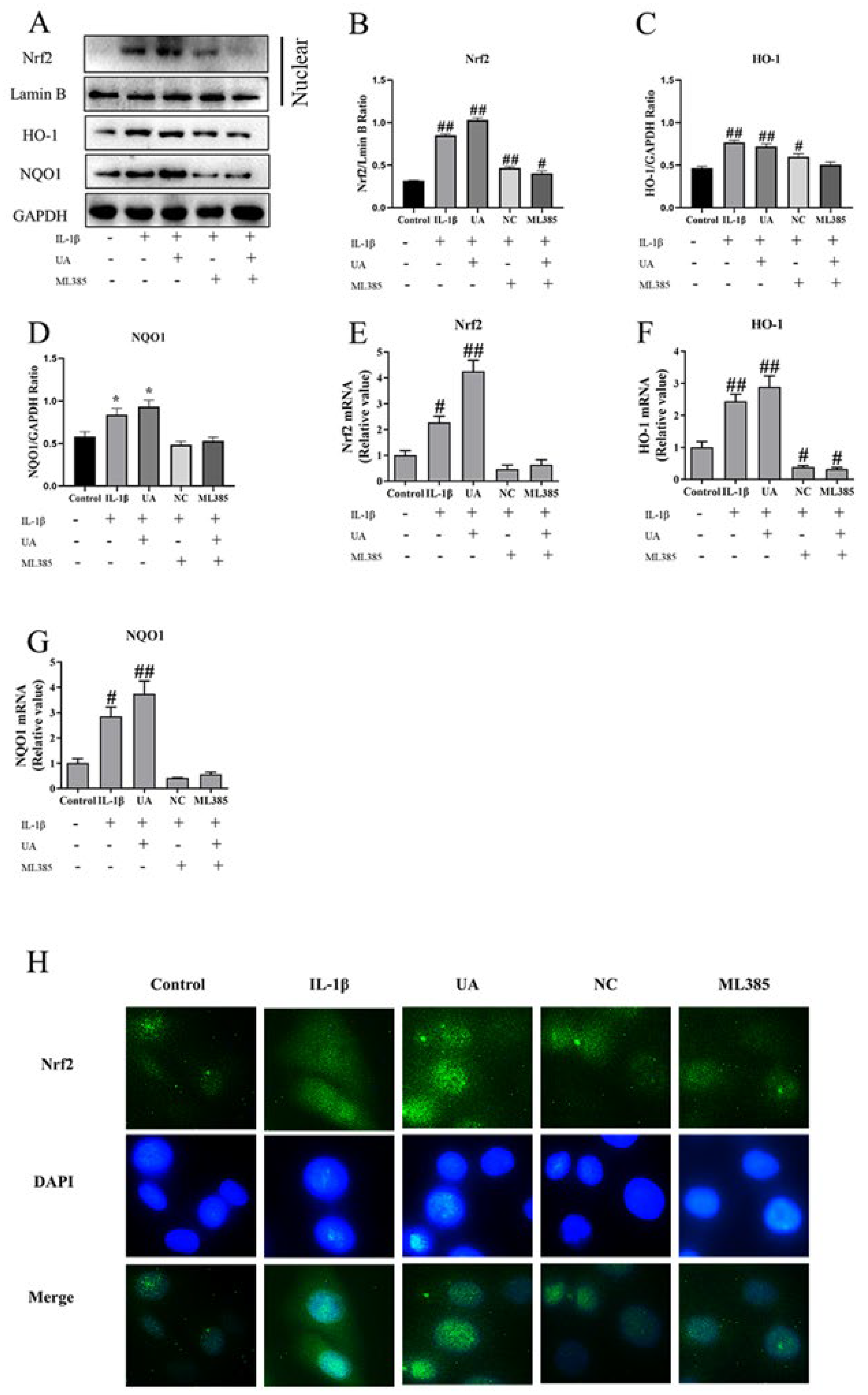
The Impact of Nrf2 Inhibition on the Effects of UA on Nrf2, HO-1, and NQO1 in L-1β-Induced Rat Chondrocytes
As shown in
Figure 7, both WB and qPCR results revealed a significant increase in nuclear Nrf2 levels (P<0.05) in the model group compared to the control group, along with elevated expressions of HO-1 and NQO1. When compared to the control group, the UA group exhibited further increases in the expressions of Nrf2, HO-1, and NQO1. Additionally, when compared to the UA group, the NC group and ML385 group showed significantly reduced protein expression and mRNA transcription levels of Nrf2, HO-1, and NQO1. Furthermore, as shown in Figure H, under fluorescence microscopy, Nrf2 was stained green, and cell nuclei were stained blue with DAPI. The control group had minimal Nrf2 labeling in chondrocyte nuclei, while the IL-1β group and UA group exhibited clear and dense Nrf2 labeling with fluorescence staining. In contrast, the fluorescence staining in the nuclei of cells in the NC group and ML385 group noticeably decreased. The consistency between immunofluorescence, WB, and qPCR results indicates that the addition of the inhibitor ML385 can alter the activating effect of UA on the Nrf2 signaling pathway.
The Impact of Nrf2 Inhibition on the Action of UA in the NFκB Pathway in IL-1β-Induced Rat Chondrocytes
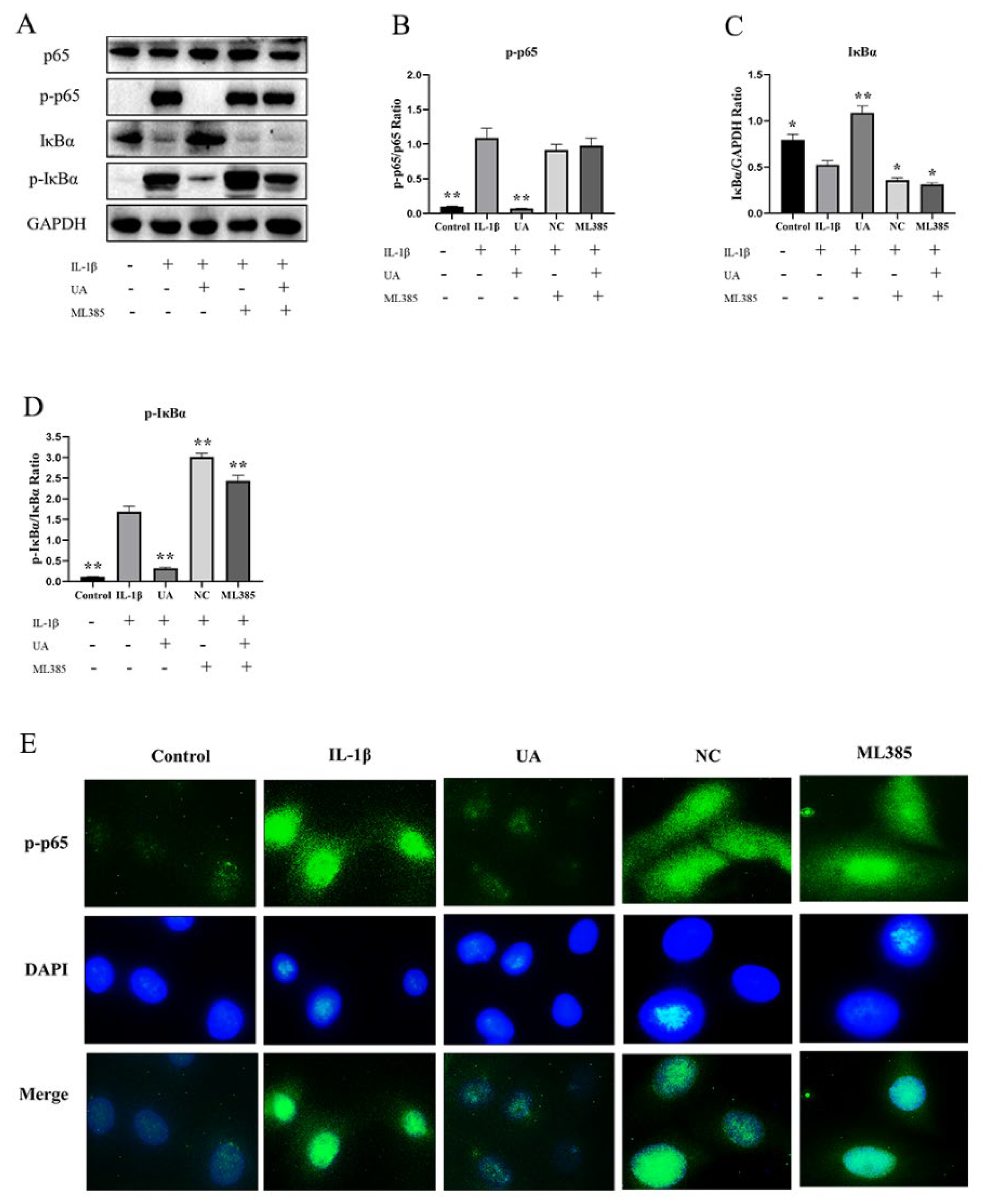
As shown in the
Figure 8, the Western blot results demonstrate that, compared to the control group, the model group had significantly elevated levels of p-p65 and p-IκBα (P<0.01), while the expression level of IκBα was significantly decreased (P<0.05). Compared to the model group, the NC group and the ML385 group showed no significant differences in p-p65 levels, with further increases in p-IκBα levels and significant decreases in IκBα levels. Additionally, as depicted in Figure E, under fluorescent microscopy, p-p65 is stained green, and the cell nuclei are stained blue with DAPI. The control group and the UA group chondrocytes showed no or very few p-p65 markings in their cell nuclei. In contrast, the IL-1β group, along with the NC and ML385 groups, exhibited clear and dense p-p65 markings in their chondrocyte nuclei, showing fluorescent staining. The immunofluorescence results are consistent with the Western blot results, indicating that consistent Nrf2 inhibition can alter the effect of UA on the NFκB signaling pathway.
The Influence of Nrf2 Inhibition on the Action of UA on ADAMTS-4, MMP1, MMP3 and MMP13 in IL-1β-Induced Rat Chondrocytes.
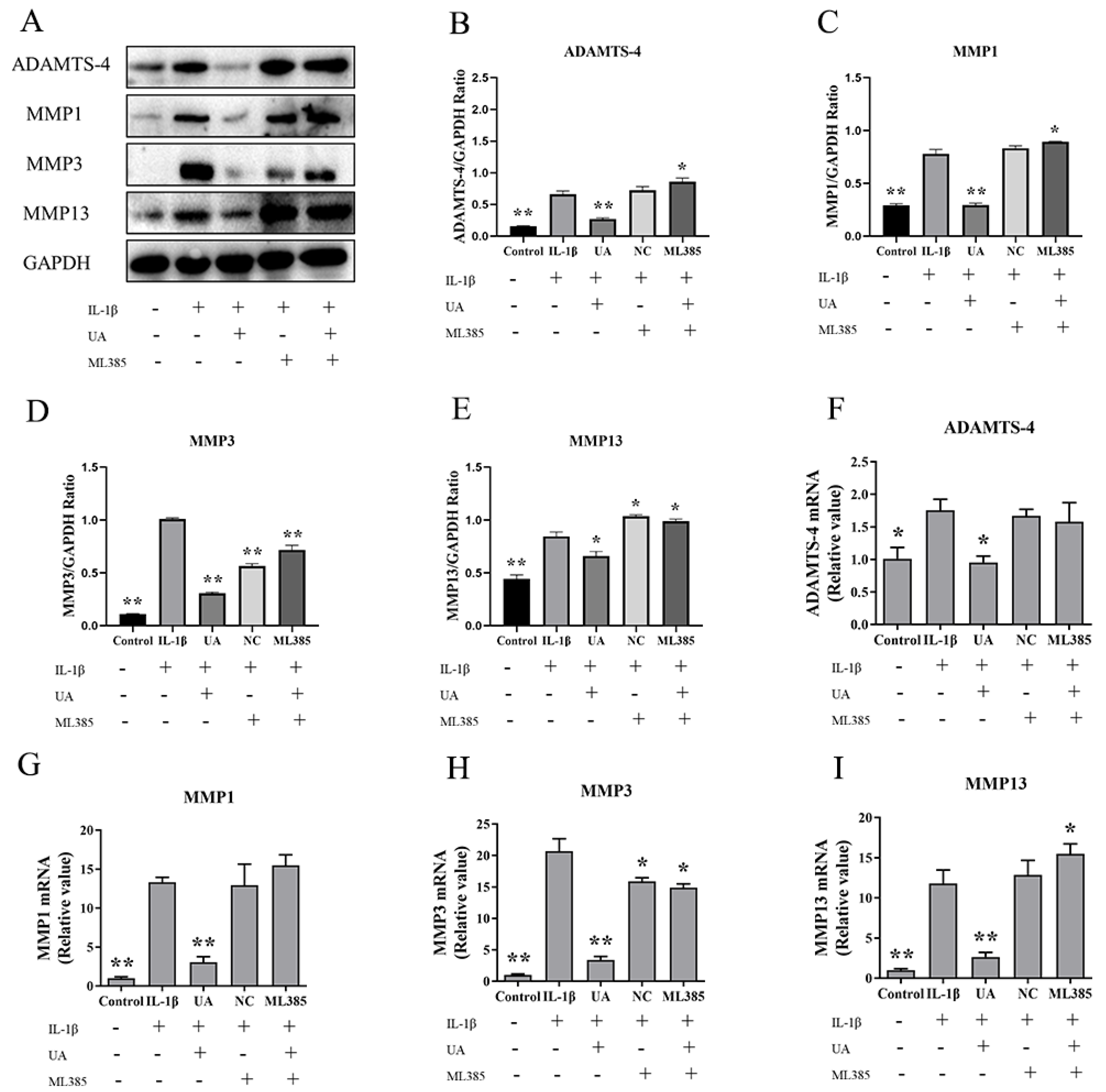
As shown in
Figure 9, both WB and qPCR results demonstrate a significant increase in the levels of ADAMTS-4, MMP1, MMP3, and MMP13 in the model group compared to the control group. Compared to the model group, both the NC group and ML385 group exhibited further increases in ADAMTS-4, MMP1, and MMP13 levels, although the expression of MMP3, while relatively reduced, still remained higher than that in the control and UA groups. This suggests that the inhibition of Nrf2 can reverse the inhibitory effect of UA on matrix degradation in chondrocytes.
The Impact of Nrf2 Inhibition on the Effects of UA on INOS, COX-2, and COL2A1 in IL-1β-Induced Rat Chondrocytes
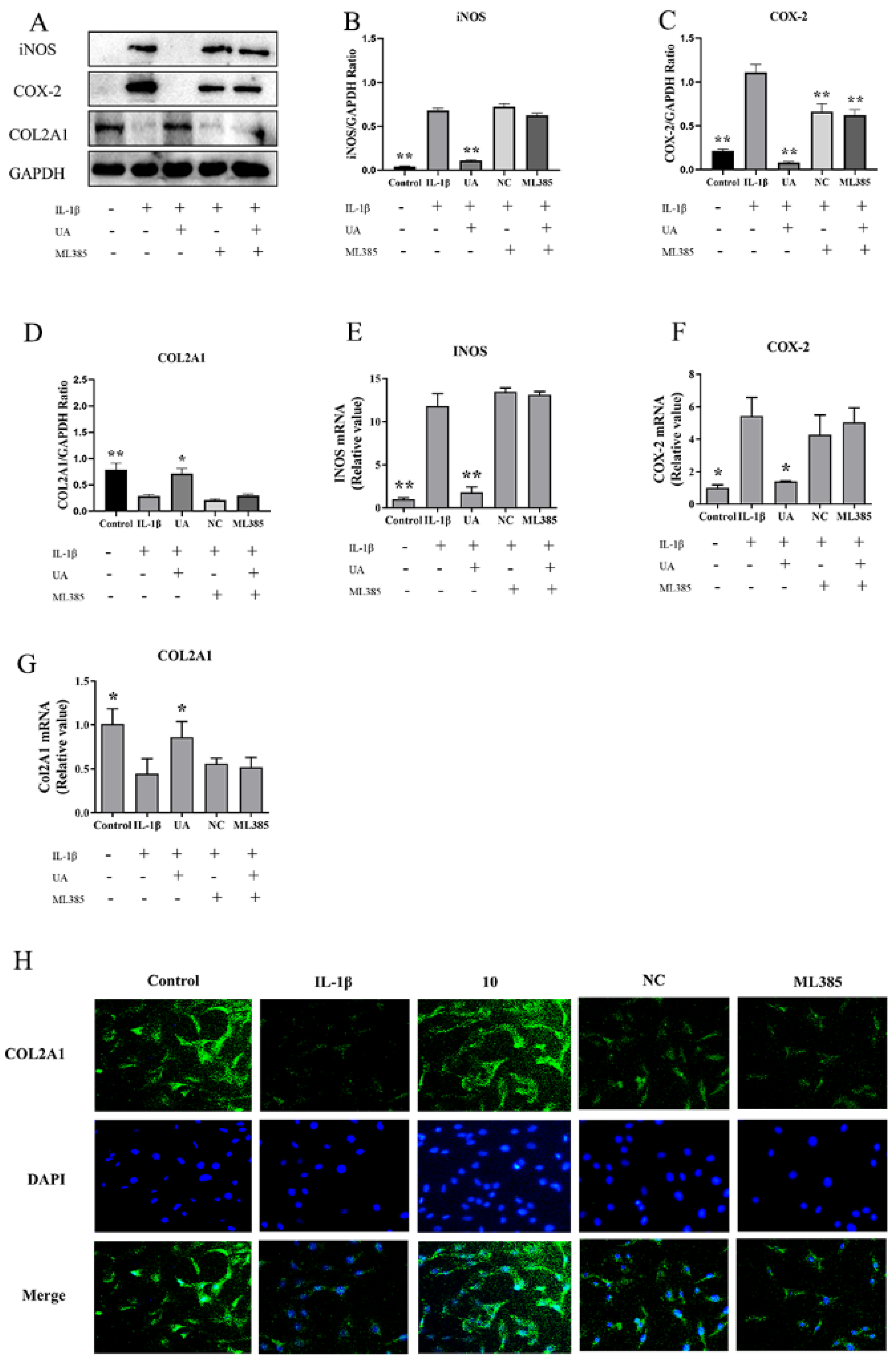
As shown in
Figure 10, both WB and qPCR results indicate that compared to the control group, the model group exhibited significantly elevated levels of INOS and COX-2. In comparison to the model group, the NC group and ML385 group showed no significant differences in INOS and COL2A1 levels, while the COX-2 protein expression decreased but remained at relatively high levels. As shown in Figure H, under fluorescence microscopy, COL2A1 was stained green, and the cell nuclei were stained blue with DAPI. The control group and UA-treated chondrocytes exhibited clear and dense COL2A1 labeling, presenting fluorescent staining. Chondrocytes treated with IL-1β displayed weaker fluorescence staining of COL2A1. The NC group and ML385 group showed similar weaker fluorescence as seen in the IL-1β group. This result is consistent with the WB and qPCR results, indicating that inhibiting Nrf2 can reverse the inhibitory effect of UA on matrix degradation in chondrocytes.
Discussion
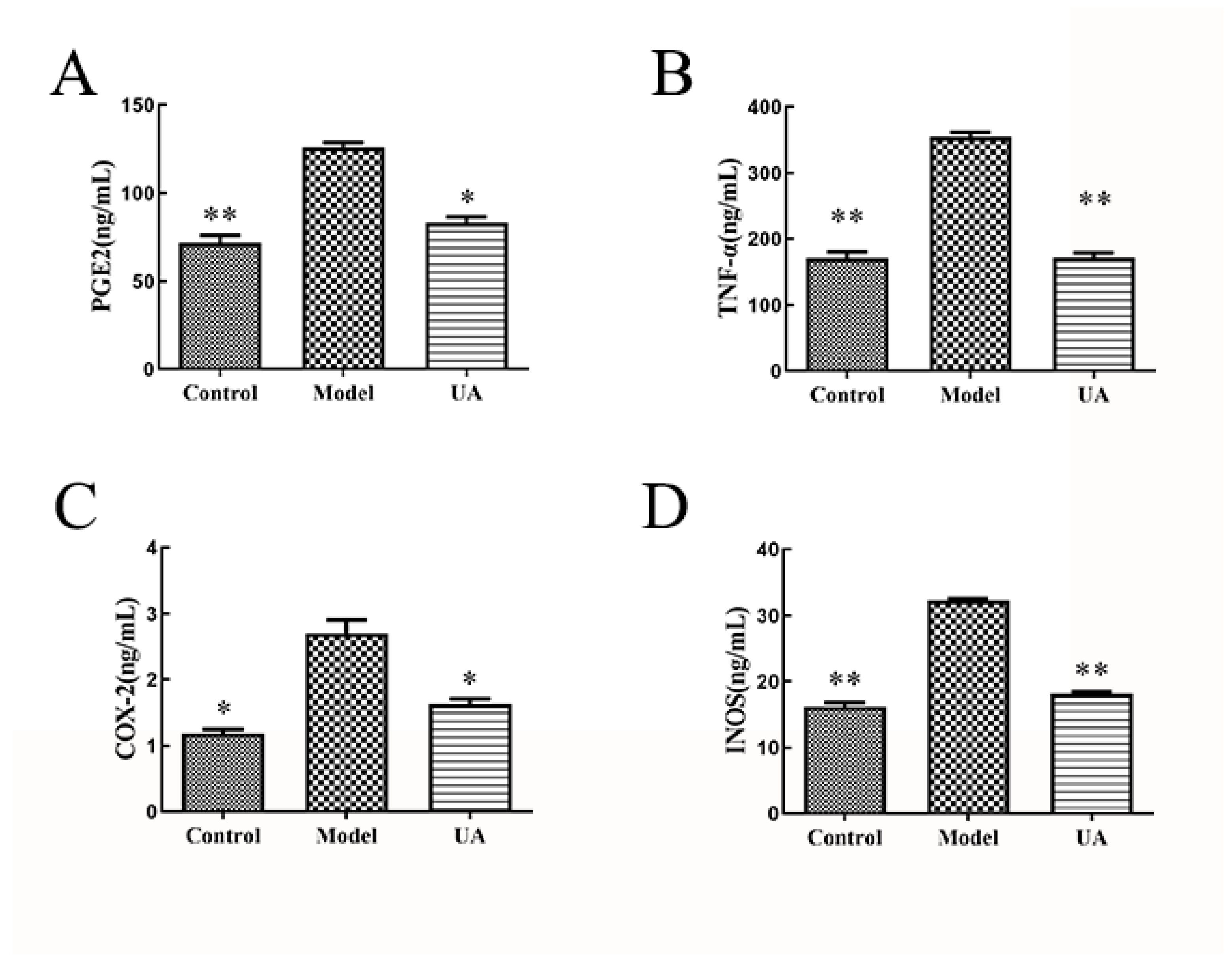
In recent years, the Nrf2/NF-κB signaling pathway is considered a key target in regulating the pathological processes of OA (Bannuru et al. 2018, Taruc-Uy and Lynch 2013). It can mediate oxidative stress and inflammatory responses in chondrocytes, thereby regulating ECM and joint cartilage processes in chondrocytes (Tang et al. 2018, Nandhu et al. 2017). UA exhibits a wide range of pharmacological and biological activities, including anti-inflammatory, antioxidant, and wound healing promotion (Su et al. 2014, Piska et al. 2018). Furthermore, UA is considered a potential treatment for osteoporosis and has a protective effect against bone-destructive diseases (Kim et al. 2018). In vivo experiments demonstrated that Safranin O-Fast Green staining showed less severe articular cartilage damage, minimal loss of chondrocytes, and a more uniform matrix staining in the usnic acid group compared to the model group. Immunohistochemistry results revealed a significant increase in the expression of cartilage-degrading components MMP3, MMP13, and ADAMTS-4, as well as a significant increase in COL2A1 and Aggrecan, protective components in the melatonin group compared to the model group. Additionally, serum ELISA results also demonstrated that usnic acid can reduce the expression of inflammatory mediators such as PGE2, TNF-α, iNOS, and COX-2, as well as oxidative components. Therefore, it can be concluded that intraperitoneal administration of usnic acid at a dose of 50 mg/kg can protect articular cartilage by reducing degradation of the extracellular matrix through its anti-inflammatory and antioxidative effects.
Research has found that UA not only inhibits the NF-κB signaling pathway, downregulating the levels of inflammatory mediators and exerting anti-inflammatory effects, but it can also activate the Nrf2 signaling pathway, increasing the expression of antioxidant enzymes and enhancing cellular antioxidant capacity (Chen et al. 2016, Qi et al. 2020). Therefore, it is hypothesized that UA may exert protective effects in OA through the Nrf2/NF-κB signaling pathway. Thus, this study intends to explore the effects of UA on chondrocytes and, at the same time, uncover the targeted regulatory mechanisms of UA on IL-1β-induced rat chondrocytes based on the Nrf2/NF-κB signaling pathway. IL-1β is a primary factor that disrupts the homeostasis within cartilage and can activate pathological pathways in chondrocytes, leading to joint cartilage damage and ultimately inducing OA (Dinarello 2011, Liu et al. 2019). In this experiment, it was observed that 10 ng/ml IL-1β induced the expression of oxidative markers (INOS, COX-2) and matrix-degrading enzymes (ADAMTS-4, MMP1, MMP3, MMP13) in chondrocytes (Hu et al. 2021). The study also found that IL-1β could activate the Nrf2 and NF-κB signaling pathways in chondrocytes, promoting the expression of inflammatory mediators and matrix-degrading enzymes, inducing an inflammatory response, disrupting the ECM metabolism balance in chondrocytes, and ultimately leading to joint cartilage damage. These findings align with the results of this study. In this research, 10 ng/ml IL-1β significantly promoted IκBα and p65 phosphorylation in chondrocytes, facilitated the translocation of p-p65 into the cell nucleus, and also enhanced the expression of Nrf2, HO-1, and NQO1. These results are consistent with several other studies, indicating that IL-1β induction can successfully establish an in vitro model of OA (Jin et al. 2021, He et al. 2020, Hu et al. 2018).
Anti-inflammatory properties of UA have been widely confirmed (Araújo et al. 2015, Zugic, Tadic and Savic 2020). In in vitro studies, Jin et al. found that UA exerts anti-inflammatory effects by diminishing p65 nuclear translocation and inhibiting IκBα degradation, leading to a significant reduction in TNF-α and iNOS levels (Jin et al. 2008). However, there have been no reported studies on the effects of UA on the expression of inflammatory mediators in chondrocytes. In this study, chondrocytes co-treated with UA and 10 ng/ml IL-1β exhibited a significant decrease in the expression of ADAMTS-4, MMP1, MMP3, MMP13, INOS, and COX-2, along with a significant increase in COL2A1 expression. This suggests that UA can effectively reduce the production of matrix-degrading enzymes, thereby protecting chondrocytes. Nevertheless, the mechanisms through which UA operates in chondrocytes still warrant further investigation. Based on previous literature, we hypothesized that the Nrf2 pathway and the NF-κB pathway might be the mechanisms through which UA exerts its effects. The experimental results demonstrated that UA significantly reduces the levels of p-IκBα and p-p65 induced by IL-1β in chondrocytes. Minimal p-p65 fluorescence labeling was observed in the cell nucleus, and the levels of inflammatory mediators such as IL-6, TNF-α, COX-2, and PGE2 were downregulated. Huang et al. have previously reported that UA can inhibit the NF-κB signaling pathway in vitro, reducing the expression of inflammatory mediators like IL-1β, IL-6, TNF-α, and COX-2, thereby suppressing the inflammatory response (Jin et al. 2008). These findings, in line with our study results, suggest that UA may act by inhibiting the dissociation of p65 from IκBα, thereby restraining p-p65 nuclear translocation and reducing the expression of inflammatory mediators in chondrocytes. This implies that UA might alleviate chondrocyte inflammation, protect chondrocytes, and potentially do so by inhibiting the activation of the NFκB pathway.
The Nrf2 signaling pathway regulates the expression of antioxidant enzyme genes, thus modulating the oxidative-reductive balance within cells and playing a crucial role in protecting against oxidative stress damage in the body (Khan et al. 2017). When activated, it upregulates the expression of HO-1 and NQO1, thereby enhancing the cell's antioxidant capacity(Kim, Lee and Jeong 2020). In this study, treatment with 10 ng/ml IL-1β induced oxidative damage in chondrocytes, resulting in the activation of Nrf2, its translocation into the cell nucleus, and an increase in downstream antioxidant enzymes, including HO-1 and NQO1. Numerous studies have demonstrated that the expression levels of Nrf2 and HO-1 significantly increase following the induction of chondrocytes with 10 ng/ml IL-1β (Khan, Ahmad and Haqqi 2018, Chen et al. 2020). Consistent with findings from studies by Jia and Wang Yongsheng, this study confirms that 10 ng/ml IL-1β can activate the Nrf2 signaling pathway to some extent in chondrocytes. Fernandez et al. discovered that UA promotes the translocation of Nrf2 protein from the cytoplasm to the cell nucleus, upregulates HO-1 levels, and enhances cellular antioxidant capacity (Fernandez-Moriano et al. 2017). Research by Krajka et al. also found that UA can stimulate Nrf2 gene transcription, Nrf2 protein translocation into the nucleus, and enhance NQO1 expression at both the mRNA and protein levels (Krajka-Kuźniak et al. 2021). Similar to the results of Fernandez and Krajka the combined treatment of UA with 10 ng/ml IL-1β further promotes Nrf2 nuclear translocation and enhances the upregulation of HO-1 and NQO1.
Nrf2 serves not only as a critical regulator of the antioxidant system but also as an upstream regulatory entity of the NF-κB signaling pathway. Wardyn's research has shown that Nrf2 gene deficiency enhances NF-κB activity, leading to increased production of inflammatory mediators (Wu et al. 2023). HO-1 can also inhibit the activation of the NF-κB signaling pathway and the production of inflammatory mediators. Based on previous studies (Shen et al. 2023, Wang et al. 2018) .we hypothesized that UA can activate the Nrf2 pathway to subsequently inhibit the activation of the NF-κB signaling pathway, thereby further reducing the generation of inflammatory mediators and decreasing the secretion of matrix-degrading enzymes, alleviating chondrocyte extracellular matrix (ECM) damage to a certain extent. Therefore, we introduced the Nrf2 inhibitor ML385 to further validate the relationship between the Nrf2 pathway, the NF-κB signaling pathway, and chondrocyte matrix degradation. Experimental results showed that with the addition of the inhibitor ML385, the expression levels of Nrf2 significantly decreased, along with reduced expression of HO-1 and NQO1. Conversely, NF-κB was activated, and the expression levels of p-p65 and p-IκBα increased. Furthermore, the components related to chondrocyte matrix degradation (ADAMTS-4, MMP1, MMP3, MMP13, INOS, COX-2) also increased, while COL2A1 expression decreased. These results suggest that UA may activate the Nrf2 signaling pathway, promote the production of the antioxidant enzymes HO-1 and NQO1, and subsequently inhibit the activation of the NF-κB signaling pathway, thereby exerting anti-inflammatory and antioxidant effects and reducing damage to rat chondrocytes.
The results of this study indicate that UA can activate the Nrf2 signaling pathway, upregulate the levels of antioxidant enzymes such as HO-1 and NQO1, and inhibit the activation of the NF-κB signaling pathway. This leads to a reduction in the expression of inflammatory mediators (IL-6, TNF-α, PGE2), matrix-degrading enzymes (ADAMTS-4, MMP-1, MMP-3, MMP-13), and oxidative markers (INOS, COX-2), as well as a decrease in the degradation of COL2A1. These actions exert anti-inflammatory and antioxidant effects, ultimately inhibiting the degradation of ECM in chondrocytes and protecting rat chondrocytes.
While UA exhibits anti-inflammatory and antioxidant effects and has shown protective effects against bone-related disorders, reports on the functional formulations of UA have indicated potential hepatotoxicity with long-term oral administration (Durazo et al. 2004, Sanchez et al. 2006, Yellapu RK 2011). Therefore, further research is needed to investigate its pharmacological and toxicological effects before clinical use, including long-term validation in experimental animal models. This study primarily focused on exploring the mechanisms by which UA modulates ECM degradation in cultured rat chondrocytes via the Nrf2/NF-κB signaling pathways from the perspectives of inflammation and oxidative stress. However, intracellular signaling pathways are intricate, with potential cross-talk and interactions, necessitating further investigation into the connections between these pathways.
Conclusions
(1) In vivo, intraperitoneal injection of usnic acid (50mg/kg) can alleviate pathological changes in articular cartilage by reducing the expression of matrix degradation-related components in chondrocytes, and can also reduce the levels of inflammatory and oxidative-related matrix in the serum.
(2) Usnic acid can downregulate the levels of inflammatory mediators such as IL-6, TNF-α, COX-2, PGE2, as well as matrix-degrading enzymes including ADAMTS-4, MMP-1, MMP-3, and MMP-13 in rat chondrocytes induced by 10 ng/ml IL-1β, thereby protecting chondrocytes through anti-inflammatory and anti-matrix degradation pathways, with the most effective intervention observed at 10 μM usnic acid.
(3) Usnic acid can upregulate the expression levels of Nrf2/HO-1/NQO1 proteins, inhibit p65 phosphorylation and nuclear translocation processes, and exert chondroprotective effects by targeting the Nrf2/NF-κB signaling pathway.
Funding
This research was funded by Central Guided Local Science and Technology Development Special Fund Project - Promotion of Specialized Breeding Technology Services for Sport Horses in Zhaosu County (ZYYD2023C02-3) --------Purchase and feed of laboratory animals. Major Science and Technology Special Project of Xinjiang Uygur Autonomous Region - Integration and Demonstration of Key Technologies for Specialized Strain Breeding of High-Quality Sport Horses (2022A02013-5-1) --------Purchase of experimental materials. Natural Science Foundation of Heilongjiang Province: Mechanisms of Cross-Regulation between Nrf2 and NF-κB Pathways in Inflammatory Injury of Bovine Chondrocytes (LH2023C025). --------Costs for processing articles and software
Contributors
Mingchao Zhao: Writing- Original draft, Writing- Reviewing, Project administration; Xinyu Wang: Conceptualization, Software; Wenyue Yang: Data curation; Qiu Di: Editing; Siyao Li: Validation; Jilang Tang: Visualization; Hong Chen: Supervision; Hongri Ruan: preparation; Xue Miao: Software; Xin Cheng: Software; Sui Xin: Methodology; Junnan Jiang: lnvestigation.; Chengwei Wei: Data curation, Financial support; Hai Li: Financial support, lnvestigation; Jianhua Xiao: Theoretical guidance, financial support
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the animal care and use involved in this study were approved by the protocol of the Ani-mal Welfare Committee of Northeast Agricultural University (Harbin, China).
Conflicts of Interest
The authors declare no conflict of interest.
Annex 1
Isolation and Cultivation of Primary Chondrocytes: After sacrificing 14-21-day-old SD rats, bilateral femoral heads were extracted within a cell laminar flow hood and placed in DMEM/F12 medium without fetal bovine serum (FBS) (containing 100 U/ml penicillin-streptomycin). The femoral heads were then incubated in 0.25% trypsin for 30 minutes. After digestion, the cartilage tissue was minced, followed by additional digestion with 0.2% type II collagenase for 4 hours. Upon complete digestion, an appropriate amount of DMEM/F12 medium containing 10% FBS (with 100 U/ml penicillin-streptomycin) was added to resuspend the cells, which were then seeded into cell culture flasks and placed in a 5% CO2, 37°C cell culture incubator. The cell culture medium was changed on days 2-3, and passage was performed when cell confluence reached 80%. The experiments were conducted using the second passage chondrocytes. Control Group: Normal cultured chondrocytes for 24 hours; IL-1β Group: Chondrocytes induced with 10 ng/mL IL-1β for 24 hours; UA Groups (2.5, 5, 10): Chondrocytes co-treated with 10 ng/mL IL-1β and 2.5, 5, and 10 μM UA for 24 hours; Negative Control Group (NC): Chondrocytes co-treated with IL-1β and ML385 for 24 hours; Inhibitor Group (ML385): Chondrocytes co-treated with IL-1β, ML385, and 10 μM UA for 24 hours.
References
- Alcaraz, M. J. & M. L. Ferrándiz (2020) Relevance of Nrf2 and heme oxygenase-1 in articular diseases. Free Radical Biology and Medicine, 157, 83-93. [CrossRef]
- Araújo, A. A. S., M. G. D. de Melo, T. K. Rabelo, P. S. Nunes, S. L. Santos, M. R. Serafini, M. R. V. Santos, L. J. Quintans-Júnior & D. P. Gelain (2015) Review of the biological properties and toxicity of usnic acid. Natural Product Research, 29, 2167-2180. [CrossRef]
- Bannuru, R. R., M. C. Osani, F. Al-Eid & C. Wang (2018) Efficacy of curcumin and Boswellia for knee osteoarthritis: Systematic review and meta-analysis. Semin Arthritis Rheum, 48, 416-429. [CrossRef]
- Chen, S., Z. Zhang, T. Qing, Z. Ren, D. Yu, L. Couch, B. Ning, N. Mei, L. Shi, W. H. Tolleson & L. Guo (2016) Activation of the Nrf2 signaling pathway in usnic acid-induced toxicity in HepG2 cells. Archives of Toxicology, 91, 1293-1307. [CrossRef]
- Chen, X., M. Gu, J. Jin, C. Ren, Z. Pan, Y. Wu, N. Tian, A. Wu, L. Sun, W. Gao, X. Wang, C. Bei, Y. Zhou & X. Zhang (2020) β-Hydroxyisovalerylshikonin inhibits IL-1β-induced chondrocyte inflammation via Nrf2 and retards osteoarthritis in mice. Food & Function, 11, 10219-10230. [CrossRef]
- Chen, Z., H. Zhong, J. Wei, S. Lin, Z. Zong, F. Gong, X. Huang, J. Sun, P. Li, H. Lin, B. Wei & J. Chu (2019) Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res Ther, 21, 300. [CrossRef]
- Dinarello, C. A. (2011) A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol, 41, 1203-17. [CrossRef]
- Ding, Y., M. Chen, M. Wang, Y. Li & A. Wen (2015) Posttreatment with 11-Keto-beta-Boswellic Acid Ameliorates Cerebral Ischemia-Reperfusion Injury: Nrf2/HO-1 Pathway as a Potential Mechanism. Mol Neurobiol, 52, 1430-1439. [CrossRef]
- Dinkova-Kostova, A. T. & P. Talalay (2010) NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys, 501, 116-23. [CrossRef]
- Durazo, F. A., C. Lassman, S. H. Han, S. Saab, N. P. Lee, M. Kawano, B. Saggi, S. Gordon, D. G. Farmer, H. Yersiz, R. L. Goldstein, M. Ghobrial & R. W. Busuttil (2004) Fulminant liver failure due to usnic acid for weight loss. Am J Gastroenterol, 99, 950-2.
- Fernandez-Moriano, C., P. K. Divakar, A. Crespo & M. P. Gomez-Serranillos (2017) Protective effects of lichen metabolites evernic and usnic acids against redox impairment-mediated cytotoxicity in central nervous system-like cells. Food Chem Toxicol, 105, 262-277. [CrossRef]
- Ge, X. P., Y. H. Gan, C. G. Zhang, C. Y. Zhou, K. T. Ma, J. H. Meng & X. C. Ma (2011) Requirement of the NF-kappaB pathway for induction of Wnt-5A by interleukin-1beta in condylar chondrocytes of the temporomandibular joint: Functional crosstalk between the Wnt-5A and NF-kappaB signaling pathways. Osteoarthritis Cartilage, 19, 111-7. [CrossRef]
- Goldring MB, O. M., Plumb DA, Dragomir C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì RM, Marcu KB. (2011) Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater, 24;21:202-20. [CrossRef]
- He, L., T. He, J. Xing, Q. Zhou, L. Fan, C. Liu, Y. Chen, D. Wu, Z. Tian, B. Liu & L. Rong (2020) Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Research & Therapy, 11. [CrossRef]
- Henrotin, Y., C. Sanchez, A. C. Bay-Jensen & A. Mobasheri (2016) Osteoarthritis biomarkers derived from cartilage extracellular matrix: Current status and future perspectives. Annals of Physical and Rehabilitation Medicine, 59, 145-148. [CrossRef]
- Hu, H., X. Song, Y. Li, T. Ma, H. Bai, M. Zhao, X. Wang, L. Liu & L. Gao (2021) Emodin protects knee joint cartilage in rats through anti-matrix degradation pathway: An in vitro and in vivo study. Life Sciences, 269. [CrossRef]
- Hu, P. F., F. F. Sun, L. F. Jiang, J. P. Bao & L. D. Wu (2018) Paeoniflorin inhibits IL-1β-induced MMP secretion via the NF-κB pathway in chondrocytes. Experimental and Therapeutic Medicine. [CrossRef]
- Hu, Q. & M. Ecker (2021) Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. International Journal of Molecular Sciences, 22. [CrossRef]
- Hui, W., G. J. Litherland, M. S. Elias, G. I. Kitson, T. E. Cawston, A. D. Rowan & D. A. Young (2012) Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Annals of the Rheumatic Diseases, 71, 455-462. [CrossRef]
- Jia, T., J. Qiao, D. Guan & T. Chen (2017) Anti-Inflammatory Effects of Licochalcone A on IL-1beta-Stimulated Human Osteoarthritis Chondrocytes. Inflammation, 40, 1894-1902. [CrossRef]
- Jin, J., X. Lv, B. Wang, C. Ren, J. Jiang, H. Chen, X. Chen, M. Gu, Z. Pan, N. Tian, A. Wu, L. Sun, W. Gao, X. Wang, X. Zhang, Y. Wu, Y. Zhou & P. Evelson (2021) Limonin Inhibits IL-1β-Induced Inflammation and Catabolism in Chondrocytes and Ameliorates Osteoarthritis by Activating Nrf2. Oxidative Medicine and Cellular Longevity, 2021, 1-15. [CrossRef]
- Jin, J. Q., C. Q. Li & L. C. He (2008) Down-regulatory effect of usnic acid on nuclear factor-kappaB-dependent tumor necrosis factor-alpha and inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264.7. Phytother Res, 22, 1605-9. [CrossRef]
- Jung, K. A. & M. K. Kwak (2010) The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules, 15, 7266-91. [CrossRef]
- K, I. (2002) Usnic acid. Phytochemistry, 61(7):729-36.
- Khan, N. M., I. Ahmad & T. M. Haqqi (2018) Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radical Biology and Medicine, 116, 159-171. [CrossRef]
- Khan, N. M., A. Haseeb, M. Y. Ansari, P. Devarapalli, S. Haynie & T. M. Haqqi (2017) Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radical Biology and Medicine, 106, 288-301. [CrossRef]
- Kim, E.-N., H.-S. Lee & G.-S. Jeong (2020) Cudratricusxanthone O Inhibits H2O2-Induced Cell Damage by Activating Nrf2/HO-1 Pathway in Human Chondrocytes. Antioxidants, 9. [CrossRef]
- Kim, K. J., M. H. Jeong, Y. Lee, S. J. Hwang, H. B. Shin, J. S. Hur & Y. J. Son (2018) Effect of Usnic Acid on Osteoclastogenic Activity. J Clin Med, 7. [CrossRef]
- Krajka-Kuźniak, V., J. Paluszczak, R. Kleszcz & W. Baer-Dubowska (2021) (+)-Usnic acid modulates the Nrf2-ARE pathway in FaDu hypopharyngeal carcinoma cells. Molecular and Cellular Biochemistry, 476, 2539-2549. [CrossRef]
- Li, X. Z. & S. N. Zhang (2020) Recent advance in treatment of osteoarthritis by bioactive components from herbal medicine. Chin Med, 15, 80. [CrossRef]
- Liu, F.-C., C.-C. Wang, J.-W. Lu, C.-H. Lee, S.-C. Chen, Y.-J. Ho & Y.-J. Peng (2019) Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients, 11. [CrossRef]
- Ma, Q. (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol, 53, 401-26. [CrossRef]
- Ma, T., L. Jia, J. Zhao, L. Lv, Y. Yu, H. Ruan, X. Song, H. Chen, X. Li, J. Zhang & L. Gao (2022) Ginkgolide C slows the progression of osteoarthritis by activating Nrf2/HO-1 and blocking the NF-κB pathway. Frontiers in Pharmacology, 13. [CrossRef]
- Midwood, K., S. Sacre, A. M. Piccinini, J. Inglis, A. Trebaul, E. Chan, S. Drexler, N. Sofat, M. Kashiwagi, G. Orend, F. Brennan & B. Foxwell (2009) Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med, 15, 774-80. [CrossRef]
- Nandhu, M. S., A. Kwiatkowska, V. Bhaskaran, J. Hayes, B. Hu & M. S. Viapiano (2017) Tumor-derived fibulin-3 activates pro-invasive NF-kappaB signaling in glioblastoma cells and their microenvironment. Oncogene, 36, 4875-4886. [CrossRef]
- Odabasoglu, F., A. Cakir, H. Suleyman, A. Aslan, Y. Bayir, M. Halici & C. Kazaz (2006) Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J Ethnopharmacol, 103, 59-65. [CrossRef]
- Oeckinghaus, A. & S. Ghosh (2009) The NF- B Family of Transcription Factors and Its Regulation. Cold Spring Harbor Perspectives in Biology, 1, a000034-a000034. [CrossRef]
- Pazdziora, W., I. Podolak, M. Grudzinska, P. Pasko, K. Grabowska & A. Galanty (2023) Critical Assessment of the Anti-Inflammatory Potential of Usnic Acid and Its Derivatives-A Review. Life (Basel), 13. [CrossRef]
- Piska, K., A. Galanty, P. Koczurkiewicz, P. Żmudzki, J. Potaczek, I. Podolak & E. Pękala (2018) Usnic acid reactive metabolites formation in human, rat, and mice microsomes. Implication for hepatotoxicity. Food and Chemical Toxicology, 120, 112-118. [CrossRef]
- Qi, W., C. Lu, H. Huang, W. Zhang, S. Song & B. Liu (2020) (+)-Usnic Acid Induces ROS-dependent Apoptosis via Inhibition of Mitochondria Respiratory Chain Complexes and Nrf2 Expression in Lung Squamous Cell Carcinoma. International Journal of Molecular Sciences, 21. [CrossRef]
- Qiao, Y. Q., P. F. Jiang & Y. Z. Gao (2018) Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch Med Sci, 14, 617-624.
- Reddy, C. S. V. S. V. M. K. (2000) Anti-inflammatory activity of (+)-usnic acid. Fitoterapia, 71(5):564-6. [CrossRef]
- Rigoglou, S. & A. G. Papavassiliou (2013) The NF-kappaB signalling pathway in osteoarthritis. Int J Biochem Cell Biol, 45, 2580-4. [CrossRef]
- Sanchez, W., J. T. Maple, L. J. Burgart & P. S. Kamath (2006) Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin Proc, 81, 541-4. [CrossRef]
- Shen, P.-c., S.-H. Huang, Z. M. Liu, C.-C. Lu, S.-H. Chou & Y. C. Tien (2023) Suramin ameliorates osteoarthritis by acting on the Nrf2/HO-1 and NF-κB signaling pathways in chondrocytes and promoting M2 polarization in macrophages. International Immunopharmacology, 120. [CrossRef]
- Sivandzade, F., S. Prasad, A. Bhalerao & L. Cucullo (2019) NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol, 21, 101059. [CrossRef]
- Song, R.-H., M. D. Tortorella, A.-M. Malfait, J. T. Alston, Z. Yang, E. C. Arner & D. W. Griggs (2007) Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis & Rheumatism, 56, 575-585. [CrossRef]
- Su, Z.-Q., Z.-Z. Mo, J.-B. Liao, X.-X. Feng, Y.-Z. Liang, X. Zhang, Y.-H. Liu, X.-Y. Chen, Z.-W. Chen, Z.-R. Su & X.-P. Lai (2014) Usnic acid protects LPS-induced acute lung injury in mice through attenuating inflammatory responses and oxidative stress. International Immunopharmacology, 22, 371-378. [CrossRef]
- Tang, S., Q. Tang, J. Jin, G. Zheng, J. Xu, W. Huang, X. Li, P. Shang & H. Liu (2018) Polydatin inhibits the IL-1beta-induced inflammatory response in human osteoarthritic chondrocytes by activating the Nrf2 signaling pathway and ameliorates murine osteoarthritis. Food Funct, 9, 1701-1712. [CrossRef]
- Taruc-Uy, R. L. & S. A. Lynch (2013) Diagnosis and treatment of osteoarthritis. Prim Care, 40, 821-36, vii.
- Wang, Y., Y. Chen, Y. Chen, B. Zhou, X. Shan & G. Yang (2018) Eriodictyol inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes. Biomedicine & Pharmacotherapy, 107, 1128-1134. [CrossRef]
- Wieland, H. A., M. Michaelis, B. J. Kirschbaum & K. A. Rudolphi (2005) Osteoarthritis - an untreatable disease? Nat Rev Drug Discov, 4, 331-44. [CrossRef]
- Wu, J., H. Li, F. Hu & P. Luo (2023) Stevioside attenuates osteoarthritis via regulating Nrf2/HO-1/NF-κB pathway. Journal of Orthopaedic Translation, 38, 190-202. [CrossRef]
- Yellapu RK, M. V., Grewal P, Fiel M, Schiano T. (2011) Acute liver failure caused by 'fat burners' and dietary supplements: A case report and literature review. Can J Gastroenterol, 25(3):157-60. [CrossRef]
- Zeng, W., C. Corcoran, L. A. Collins-Racie, E. R. LaVallie, E. A. Morris & C. R. Flannery (2006) Glycosaminoglycan-binding properties and aggrecanase activities of truncated ADAMTSs: Comparative analyses with ADAMTS-5, -9, -16 and -18. Biochimica et Biophysica Acta (BBA) - General Subjects, 1760, 517-524. [CrossRef]
- Zugic, A., V. Tadic & S. Savic (2020) Nano- and Microcarriers as Drug Delivery Systems for Usnic Acid: Review of Literature. Pharmaceutics, 12. [CrossRef]
Figure 4.
The effect of UA on the viability of chondrocytes and levels of IL-6, TNF-α, and PGE2 in IL-1β-Induced Rat Chondrocytes. (A) The effect of UA on the viability of chondrocytes. (B,D,F) Levels of IL-6, TNF-α, and PGE2 in cell supernatants; (C,E,G) Gene transcription levels of IL-6, TNF-α, and PGE2 in cells. ## P<0.01 compared to the control group; * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 4.
The effect of UA on the viability of chondrocytes and levels of IL-6, TNF-α, and PGE2 in IL-1β-Induced Rat Chondrocytes. (A) The effect of UA on the viability of chondrocytes. (B,D,F) Levels of IL-6, TNF-α, and PGE2 in cell supernatants; (C,E,G) Gene transcription levels of IL-6, TNF-α, and PGE2 in cells. ## P<0.01 compared to the control group; * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 5.
Figure 2. The impact of UA on matrix degradation-related markers in chondrocytes. (
A–
P) Protein expression levels and mRNA transcription levels of ADAMTS-4, MMP1, MMP3, MMP13 and INOS, COX-2, COL2A1 in chondrocytes. * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 5.
Figure 2. The impact of UA on matrix degradation-related markers in chondrocytes. (
A–
P) Protein expression levels and mRNA transcription levels of ADAMTS-4, MMP1, MMP3, MMP13 and INOS, COX-2, COL2A1 in chondrocytes. * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 6.
The Effects of UA on the NF-κB and Nrf2 Signaling Pathway in Chondrocytes. (A–D) Influence on the protein expression of p65, p-p65, IκBα, and p-IκBα. (E–K) Impact on the protein expression and mRNA transcription of Nrf2, HO-1, and NQO1. # P<0.05, ## P<0.01 compared to the control group. * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 6.
The Effects of UA on the NF-κB and Nrf2 Signaling Pathway in Chondrocytes. (A–D) Influence on the protein expression of p65, p-p65, IκBα, and p-IκBα. (E–K) Impact on the protein expression and mRNA transcription of Nrf2, HO-1, and NQO1. # P<0.05, ## P<0.01 compared to the control group. * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 7.
Effects of inhibiting Nrf2 on the Nrf2 pathway in chondrocytes treated with UA. (A–G) Impact on the protein expression and mRNA transcription of the Nrf2 pathway in chondrocytes. (H) IF assay on the effects of inhibiting Nrf2 on collagen type II in chondrocytes treated with UA (×400) # P<0.05, ## P<0.01 compared to the IL-1β group.
Figure 7.
Effects of inhibiting Nrf2 on the Nrf2 pathway in chondrocytes treated with UA. (A–G) Impact on the protein expression and mRNA transcription of the Nrf2 pathway in chondrocytes. (H) IF assay on the effects of inhibiting Nrf2 on collagen type II in chondrocytes treated with UA (×400) # P<0.05, ## P<0.01 compared to the IL-1β group.
Figure 8.
The Effect of Nrf2 Inhibition on the NF-κB Pathway in Chondrocytes Treated with UA. (A–D) Protein Expression Levels of p65, p-p65, IκBα, and p-IκBα. (E) IF Detection of p-p65 Nuclear Translocation in Chondrocytes (×400). * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 8.
The Effect of Nrf2 Inhibition on the NF-κB Pathway in Chondrocytes Treated with UA. (A–D) Protein Expression Levels of p65, p-p65, IκBα, and p-IκBα. (E) IF Detection of p-p65 Nuclear Translocation in Chondrocytes (×400). * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 9.
Impact of Nrf2 Inhibition on UA-Treated Chondrocytes in Relation to Matrix Degradation Components. (A–I) Effects of UA on the protein expression and mRNA transcription of ADAMTS-4 and MMPs in chondrocytes. * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 9.
Impact of Nrf2 Inhibition on UA-Treated Chondrocytes in Relation to Matrix Degradation Components. (A–I) Effects of UA on the protein expression and mRNA transcription of ADAMTS-4 and MMPs in chondrocytes. * P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 10.
The Impact of Nrf2 Inhibition on UA-Treated Chondrocytes regarding Oxidative Markers and COL2A1. (A–G) Effects on the protein expression and mRNA transcription of INOS, COX-2, and COL2A1. (H) IF detection of the inhibition of COL2A1 in chondrocytes (×100). *P<0.05, ** P<0.01 compared to the IL-1β group.
Figure 10.
The Impact of Nrf2 Inhibition on UA-Treated Chondrocytes regarding Oxidative Markers and COL2A1. (A–G) Effects on the protein expression and mRNA transcription of INOS, COX-2, and COL2A1. (H) IF detection of the inhibition of COL2A1 in chondrocytes (×100). *P<0.05, ** P<0.01 compared to the IL-1β group.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).