Submitted:
30 December 2023
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Plants as a potential source of new anticancer drugs
Anticancer effects of C. trifoliata
Cissus trifoliata compounds and their role in plant physiology
Mechanisms implicated in the cytotoxicity of C. trifoliata compounds
Antitumoral and antimetastatic activities of C. trifoliata compounds
Conclusion and perspectives
References
- Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. , Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 2021, 71 (3), 209-249.
- Mohar-Betancourt, A.; Reynoso-Noverón, N.; Armas-Texta, D.; Gutiérrez-Delgado, C.; Torres-Domínguez, J. A. , Cancer trends in Mexico: essential data for the creation and follow-up of public policies. Journal of Global Oncology 2017, 3(6), 740–748. [Google Scholar] [CrossRef] [PubMed]
- Méndez-López, L. F. , Revisiting epithelial carcinogenesis. International Journal of Molecular Sciences 2022, 23(13), 7437. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R. A. , Hallmarks of cancer: the next generation. Cell 2011, 144(5), 646–674. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. , EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010, 29(34), 4741–4751. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. , The different mechanisms of cancer drug resistance: a brief review. Advanced Pharmaceutical Bulletin 2017, 7(3), 339. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Kaldor, J. M. , Secondary malignancies following cancer chemotherapy. Acta Oncologica 1994, 33(6), 591–598. [Google Scholar] [CrossRef]
- Cutler, D. M. , Are we finally winning the war on cancer? The Journal of Economic Perspectives 2008, 22(4), 3–26. [Google Scholar] [CrossRef]
- Manders, D. B.; Kehoe, S. M.; Miller, D. S.; Lea, J. S.; Richardson, D. L. , Third-line salvage chemotherapy for recurrent carcinoma of the cervix is associated with minimal response rate and high toxicity. American Journal of Clinical Oncology 2017. [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D. B.; Johnston, P. G. , Cancer drug resistance: an evolving paradigm. Nature Reviews Cancer 2013, 13(10), 714–726. [Google Scholar] [CrossRef]
- Siddiqui, A. J.; Jahan, S.; Singh, R.; Saxena, J.; Ashraf, S. A.; Khan, A.; Choudhary, R. K.; Balakrishnan, S.; Badraoui, R.; Bardakci, F. , Plants in anticancer drug discovery: from molecular mechanism to chemoprevention. BioMed Research International 2022, 2022. [Google Scholar] [CrossRef]
- Oberlies, N. H.; Kroll, D. J. , Camptothecin and taxol: historic achievements in natural products research. Journal of Natural Products 2004, 67(2), 129–135. [Google Scholar] [CrossRef]
- Grever, M. R.; Schepartz, S. A.; Chabner, B. A. In The National Cancer Institute: cancer drug discovery and development program, Seminars in Oncology, Elsevier: 1992; pp 622-638.
- Suffness, M.; Pezzuto, J. , Assays related to cancer drug discovery. Academic Press: London, 1990; Vol. 6, p 360.
- Méndez-López, L. F.; Garza-González, E.; Ríos, M. Y.; Ramírez-Cisneros, M. Á.; Alvarez, L.; González-Maya, L.; Sánchez-Carranza, J. N.; Camacho-Corona, M. d. R. , Metabolic profile and evaluation of biological activities of extracts from the stems of Cissus trifoliata. International Journal of Molecular Sciences 2020, 21(3), 930. [Google Scholar] [CrossRef]
- Wen, J.; Lu, L. M.; Nie, Z. L.; Liu, X. Q.; Zhang, N.; Ickert-Bond, S.; Gerrath, J.; Manchester, S. R.; Boggan, J.; Chen, Z. , A new phylogenetic tribal classification of the grape family (Vitaceae). Journal of Systematics and Evolution 2018. [CrossRef]
- Banu, J. , Medicinal properties of plants from the genus Cissus: A review. Journal of Medicinal Plants Research 2012, 6(16), 3080–3086. [Google Scholar]
- McCartney, P. , SEINet: metadata-mediated access to distributed ecological data. LTER. DataBits Spring 2003, 1. [Google Scholar]
- Standley, P. C. , Trees and shrubs of Mexico. Smithsonian Institution: 1967; Vol. 1.
- Adams, N. F.; Collinson, M. E.; Smith, S. Y.; Bamford, M. K.; Forest, F.; Malakasi, P.; Marone, F.; Sykes, D. , X-rays and virtual taphonomy resolve the first Cissus (Vitaceae) macrofossils from Africa as early-diverging members of the genus. American Journal of Botany 2016, 103(9), 1657–1677. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Ankli, A.; Frei, B.; Weimann, C.; Sticher, O. , Medicinal plants in Mexico: Healers' consensus and cultural importance. Social Science and Medicine 1998, 47(11), 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- de las Mercedes Rodríguez, L. , Etnobotánica maya: Algunas plantas de uso medicinal en estomatología. Revista ADM, 2015, 72 (1).
- Alejandro, M.; Alberto, M.; Gama Campillo, L. M.; Mariaca Méndez, R. , El uso de las plantas medicinales en las comunidades Maya-Chontales de Nacajuca, Tabasco, México. Polibotánica, 2010, (29), 213-262.
- Mendieta, R. M.; Rodríguez, A. Plantas medicinales del estado de Yucatán. Boletín de la Sociedad Botánica de México, (43), 94-95.
- Estrada-Castillón, E.; Soto-Mata, B. E.; Garza-López, M.; Villarreal-Quintanilla, J. Á.; Jiménez-Pérez, J.; Pando-Moreno, M.; Sánchez-Salas, J.; Scott-Morales, L.; Cotera-Correa, M. , Medicinal plants in the southern region of the State of Nuevo León, México. Journal of Ethnobiology and Ethnomedicine 2012, 8(1), 45. [Google Scholar] [CrossRef] [PubMed]
- Atjanasuppat, K.; Wongkham, W.; Meepowpan, P.; Kittakoop, P.; Sobhon, P.; Bartlett, A.; Whitfield, P. J. , In vitro screening for anthelmintic and antitumour activity of ethnomedicinal plants from Thailand. Journal of Ethnopharmacology 2009, 123(3), 475–482. [Google Scholar] [CrossRef]
- Bhujade, A.; Gupta, G.; Talmale, S.; Das, S.; Patil, M. , Induction of apoptosis in A431 skin cancer cells by Cissus quadrangularis Linn stem extract by altering Bax-Bcl2 ratio, release of cytochrome c from mitochondria and PARP cleavage. Food and Function 2013, 4(2), 338–346. [Google Scholar] [CrossRef]
- Opoku, A.; Geheeb-Keller, M.; Lin, J.; Terblanche, S.; Hutchings, A.; Chuturgoon, A.; Pillay, D. , Preliminary screening of some traditional Zulu medicinal plants for antineoplastic activities versus the HepG2 cell line. Phytotherapy Research 2000, 14(7), 534–537. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, M.; Garcia, M.; Quilez, A.; Ahumada, M. , Cytotoxic activity of Agave intermixta L.(Agavaceae) and Cissus sicyoides L.(Vitaceae). Phytotherapy Research, 2000, 14 (7), 552-554.
- Line-Edwige, M.; Raymond, F. G.; François, E.; Edouard, N. , Antiproliferative effect of alcoholic extracts of some Gabonese medicinal plants on human colonic cancer cells. African Journal of Traditional, Complementary and Alternative Medicines, 2009, 6 (2).
- Adesanya, S. A.; Nia, R.; Martin, M.-T.; Boukamcha, N.; Montagnac, A.; Païs, M. , Stilbene derivatives from Cissus quadrangularis. Journal of Natural Products 1999, 62(12), 1694–1695. [Google Scholar] [CrossRef]
- Billet, K.; Houillé, B.; de Bernonville, T. D.; Besseau, S.; Oudin, A.; Courdavault, V.; Delanoue, G.; Guérin, L.; Clastre, M.; Giglioli-Guivarc'h, N. , Field-based metabolomics of vitis vinifera l. Stems provides new insights for genotype discrimination and polyphenol metabolism structuring. Frontiers in Plant Science 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Gniwotta, F.; Vogg, G.; Gartmann, V.; Carver, T. L.; Riederer, M.; Jetter, R. J. , What do microbes encounter at the plant surface? Chemical composition of pea leaf cuticular waxes. Plant Physiology 2005, 139(1), 519–530. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Ali, M.; Singh, V.; Singla, R. K. , Isolation and characterization of phytoconstituents from the stems of Ichnocarpus frutescens. Chinese Journal of Natural Medicines 2010, 8(6), 401–404. [Google Scholar] [CrossRef]
- Hannoufa, A.; McNevin, J.; Lemieux, B. , Epicuticular waxes of eceriferum mutants of Arabidopsis thaliana. Phytochemistry 1993, 33(4), 851–855. [Google Scholar] [CrossRef]
- Nyemb, J. N.; Magnibou, L. M.; Talla, E.; Tchinda, A. T.; Tchuenguem, R. T.; Henoumont, C.; Laurent, S.; Mbafor, J. , Lipids constituents from gardenia aqualla stapf & hutch. Open Chemistry 2018, 16(1), 371–376. [Google Scholar]
- Kunst, L.; Samuels, A. , Biosynthesis and secretion of plant cuticular wax. Progress in Lipid Research 2003, 42(1), 51–80. [Google Scholar] [CrossRef] [PubMed]
- Lara, I.; Belge, B.; Goulao, L. F. , A focus on the biosynthesis and composition of cuticle in fruits. Journal of Agricultural and Food Chemistry 2015, 63(16), 4005–4019. [Google Scholar] [CrossRef]
- Koonce, S. D.; Brown, J. , A study of the alcohols of carnauba wax. Journal of the American Oil Chemists' Society, 1944, 21 (8), 231-234.
- Heldt, H. , Plant biochemistry. Elsevier Academic Press. XXIV: 2005.
- Zhukov, A. , Palmitic acid and its role in the structure and functions of plant cell membranes. Russian Journal of Plant Physiology 2015, 62(5), 706–713. [Google Scholar] [CrossRef]
- Gunstone, F. D.; Harwood, J. L.; Dijkstra, A. J. , The lipid handbook with CD-ROM. CRC press: 2007.
- Piironen, V.; Lindsay, D. G.; Miettinen, T. A.; Toivo, J.; Lampi, A. M. , Plant sterols: biosynthesis, biological function and their importance to human nutrition. Journal of the Science of Food and Agriculture 2000, 80(7), 939–966. [Google Scholar] [CrossRef]
- Griebel, T.; Zeier, J. , A role for β-sitosterol to stigmasterol conversion in plant-pathogen interactions. The Plant Journal 2010, 63(2), 254–268. [Google Scholar] [CrossRef]
- Jing, X.; Grebenok, R. J.; Behmer, S. T. , Plant sterols and host plant suitability for generalist and specialist caterpillars. Journal of Insect Physiology 2012, 58(2), 235–244. [Google Scholar] [CrossRef]
- Santos Júnior, H. M.; Lopes, K. C.; Alves, D. S.; Carvalho, G. A.; Oliveira, D. F. , Ursolic acid and cis-tilroside produced by Merremia tomentosa affect oviposition of Leucoptera coffeella on coffee plants. Química Nova, 2018, 41 (3), 302-309.
- Wal, P.; Wal, A.; Sharma, G.; Rai, A. , Biological activities of lupeol. Systematic Reviews in Pharmacy 2011, 2(2), 96. [Google Scholar] [CrossRef]
- Lozano-Grande, M. A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A. L. , Plant sources, extraction methods, and uses of squalene. International Journal of Agronomy 2018. [CrossRef]
- Hiebert-Giesbrecht, M. R.; Escalante-Erosa, F.; García-Sosa, K.; Dzib, G. R.; Calvo-Irabien, L. M.; Peña-Rodríguez, L. M. , Spatio-temporal variation of terpenoids in wild plants of Pentalinon andrieuxii. Chemistry and Biodiversity 2016, 13(11), 1521–1526. [Google Scholar] [CrossRef]
- Rosy, B. A.; Rosakutty, P. , GC-MS analysis of methanol wild plant and callus extracts from three Cissus species, Family Vitaceae. Journal of Chemical and Pharmaceutical Research 2012, 4(7), 3420–3426. [Google Scholar]
- Chipiti, T.; Ibrahim, M. A.; Koorbanally, N. A.; Islam, M. S. , In vitro antioxidant activity and GC-MS analysis of the ethanol and aqueous extracts of Cissus cornifolia (Baker) Splanch (Vitaceae) parts. ActaPoloniae Pharmaceutica Drug Research 2015, 72(1), 119–127. [Google Scholar]
- Vishnuthari., N.; Sripathi., S. K. 52. Vishnuthari., N.; Sripathi., S. K., GC-MS Analysis of hexane extract of stems and roots of the ethnomedicinal plant Cissus quadrangularis Linn. Journal of Chemical, Biological and Physical Sciences, 2015, 5 (4), 3954-3963.
- Kumar, S.; Anandan, A.; Jegadeesan, M. , Identification of chemical compounds in Cissus quadrangularis L. Variant I of different samples using GC-MS analysis. Archives of Applied Science Research, 2012, 4 (4), 1782-1787.
- Eswaran, R.; Anandan, A.; Doss, A.; Sangeetha, G.; Anand, S. , Analysis of chemical composition of Cissus quadrangularis linn by GC-MS. Asian Journal of Pharmaceutical and Clinical Research 2012, 2, 139–40. [Google Scholar]
- Xie, Y.; Deng, P.; Zhang, Y.; Yu, W. , Studies on the chemical constituents from Cissus assamica. Journal of Chinese Medicinal Materials 2009, 32(2), 210–213. [Google Scholar]
- Sani, Y.; Musa, A.; Tajuddeen, N.; Abdullahi, S.; Abdullahi, M.; Pateh, U.; Idris, A. , Isoliquiritigenin and sitosterol from Cissus polyantha Tuber Glig and Brandt. Journal of Medicinal Plants Research 2015, 9(35), 918–921. [Google Scholar]
- Saifah, E.; Vaisiriroj, V.; Kelley, C. J.; Higuchi, Y. , Constituents of the roots of Cissus rheifolia. Journal of Natural Products 1987, 50(2), 328–328. [Google Scholar] [CrossRef]
- Pan, G.; Li, W.; Luo, P.; Qin, J.; Su, G. , Study on steroidal and triterpenoid constituents from Cissus pteroclada. Journal of Chinese Medicinal Materials 2013, 36(8), 1274–1277. [Google Scholar] [PubMed]
- Chanda, S.; Baravalia, Y.; Nagani, K. , Spectral analysis of methanol extract of Cissus quadrangularis L. stem and its fractions. Journal of Pharmacognosy and Phytochemistry, 2013, 2 (4).
- Pathomwichaiwat, T.; Ochareon, P.; Soonthornchareonnon, N.; Ali, Z.; Khan, I. A.; Prathanturarug, S. , Alkaline phosphatase activity-guided isolation of active compounds and new dammarane-type triterpenes from Cissus quadrangularis hexane extract. Journal of Ethnopharmacology 2015, 160, 52–60. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; He, H.; Gao, S.; Kong, N.; Ding, M.; Hao, X. , Lignans and triterpenoids from Cissus repens (Vitaceae). Acta Botanica Yunnanica 2006, 28(4), 433–437. [Google Scholar]
- Chan, Y. Y.; Wang, C. Y.; Hwang, T. L.; Juang, S. H.; Hung, H. Y.; Kuo, P. C.; Chen, P. J.; Wu, T. S. , The constituents of the stems of cissus assamica and their bioactivities. Molecules 2018, 23(11), 2799. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Guerrero, J. A.; Benítez, J. J.; Domínguez, E.; Bayer, I. S.; Cingolani, R.; Athanassiou, A.; Heredia, A. , Infrared and Raman spectroscopic features of plant cuticles: a review. Frontiers in Plant Science 2014, 5, 305. [Google Scholar] [CrossRef]
- Choubey, S.; Varughese, L. R.; Kumar, V.; Beniwal, V. J. , Medicinal importance of gallic acid and its ester derivatives: a patent review. Pharmaceutical Patent Analyst 2015, 4(4), 305–315. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T. E. , Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Critical Reviews in Biotechnology, 2004, 24 (2-3), 59-83.
- Liu, S.; Jiang, J.; Ma, Z.; Xiao, M.; Yang, L.; Tian, B.; Yu, Y.; Bi, C.; Fang, A.; Yang, Y. , The role of hydroxycinnamic acid amide pathway in plant immunity. Frontiers in Plant Science 2022, 13, 922119. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sharma, A. K. , Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech 2013, 3(6), 439–459. [Google Scholar] [CrossRef]
- Mekawy, A. M. M.; Abdelaziz, M. N.; Ueda, A. , Apigenin pretreatment enhances growth and salinity tolerance of rice seedlings. Plant Physiology Biochemistry 2018, 130, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ruttanaphan, T.; Thitathan, W.; Piyasaengthong, N.; Nobsathian, S.; Bullangpoti, V. , Chrysoeriol isolated from Melientha suavis Pierre with activity against the agricultural pest Spodoptera litura. Chemical Biological Technologies in Agriculture 2022, 9(1), 1–7. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C. M. , Anthocyanins: Biosynthesis, distribution, ecological role, and use of biostimulants to increase their content in plant foods A review. Agriculture 2021, 11(3), 212. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S. ; Kim, K. M., Bioactivity and therapeutic potential of kaempferol and quercetin: New insights for plant and human health. Plants 2022, 11(19), 2623. [Google Scholar] [CrossRef] [PubMed]
- Manan, F. A.; Mamat, D. D.; Samad, A. A.; Ong, Y. S.; Ooh, K. F.; Chai, T. T. , Heavy metal accumulation and antioxidant properties of Nephrolepis biserrata growing in heavy metal-contaminated soil. Global NEST 2015.
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D. K.; Hasanuzzaman, M. , Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants 2022, 11(22), 3158. [Google Scholar] [CrossRef] [PubMed]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M.; Turkan, I. , Flavonoid naringenin alleviates short-term osmotic and salinity stresses through regulating photosynthetic machinery and chloroplastic antioxidant metabolism in Phaseolus vulgaris. Frontiers in Plant Science 2020, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Samkumar, A.; Karppinen, K.; McGhie, T. K.; Espley, R. V.; Martinussen, I.; Jaakola, L. , Flavonoid biosynthesis is differentially altered in detached and attached ripening bilberries in response to spectral light quality. Frontiers in Plant Science 2022, 13, 969934. [Google Scholar] [CrossRef] [PubMed]
- Valletta, A.; Iozia, L. M.; Leonelli, F. , Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10(1), 90. [Google Scholar] [CrossRef]
- Keller, M.; Viret, O.; Cole, F. M. , Botrytis cinerea infection in grape flowers: defense reaction, latency, and disease expression. Phytopathology 2003, 93(3), 316–322. [Google Scholar] [CrossRef]
- Laitonjam, W. S.; Yumnam, R. S.; Kongbrailatpam, B. D. , Study on isolation and comparison of the chemical compositions of Cissus adnata Roxb. leaves and Smilax lanceaefolia Roxb. roots and their free radical scavenging activities. International Research Journal of Pure and Applied Chemistry 2011, 1(1), 1. [Google Scholar] [CrossRef]
- Ahmadu, A.; Onanuga, A.; Aquino, R. , Flavonoid glycosides from the leaves of Cissus ibuensis hook (vitaceae). African Journal of Traditional, Complementary and Alternative Medicines, 2010, 7 (3).
- Al-Said, M.; Khalifa, A.; Al-Azizi, M. , Flavonoids from Cissus digitata. International Journal of Pharmacognosy 1991, 29(4), 281–283. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhang, Z.-K.; He, H.-P.; Wang, J.-S.; Zhou, H.; Ding, M.; Hao, X.-J. , Stilbene C-glucosides from Cissus repens. Journal of Asian Natural Products Research 2007, 9(7), 631–636. [Google Scholar] [CrossRef]
- Barbosa, W.; Vincieri, F.; Gallori, S.; Pinto, L.; Silva, A.; Anunciacao, J.; di Scienze Farmaceutiche, D.; Barbosa, W. L. R. , Characterisation of flavonoid glycosides in pharmacopoeial preparation of Cissus verticillata (l) nicolson & ce jarvis) using HPLC-DAD and HPLC-MS. International Journal of Pharmaceutical Sciences and Research 2013, 4(10), 3871. [Google Scholar]
- Vijayalakshmi, A.; Kumar, P.; Sakthi Priyadarsini, S.; Meenaxshi, C. , In vitro antioxidant and anticancer activity of flavonoid fraction from the aerial parts of Cissus quadrangularis linn against human breast carcinoma cell lines. Journal of Chemistry 2013, 2013. [Google Scholar]
- Thakur, A.; Jain, V.; Hingorani, L.; Laddha, K. , Improved high-performance liquid chromatography-DAD method for the simultaneous analysis of quercetin and kaempferol in the stems of Cissus quadrangularis Linn. Acta Chromatographica 2009, 21(1), 95–103. [Google Scholar] [CrossRef]
- Beltrame, F. L.; Sartoretto, J. L.; Bazotte, R. B.; Cuman, R. N.; Cortez, D. A. G.; Fernandes, L. C.; Tchaikovski, O. , Phytochemical study and evaluation of the antidiabetic potential of Cissus sicyoides L.(Vitaceae). Química Nova, 2001, 24 (6), 783-785.
- Kaur, J.; Dhiman, V.; Bhadada, S.; Katare, O.; Ghoshal, G. , LC/MS guided identification of metabolites of different extracts of Cissus quadrangularis. Food Chemistry Advances 2022, 1, 100084. [Google Scholar] [CrossRef]
- Toledo, M.; Reyes, F.; Iaderoza, F.; Francis, F.; Draettao, S. , Anthocyanins from anil trepador Cissus sicyoides. Journal of Food Science 1983, 48. [Google Scholar] [CrossRef]
- Onyeweaku, G.; Nyananyo, B.; Ozimede, C. , Taxonomic studies on the genus Cissus L.(Vitaceae) present in Obio/Akpor local government area of Rivers State, Nigeria. Journal of Applied Sciences Environmental Management, 2020, 24 (1), 139-145.
- Mohan, S.; Prabhakaran, V.-S.; Narayanaswamy, R. , In silico analysis of cissus rotundifolia constituents as human neutrophil elastase (HNE), matrix metalloproteinases (MMP 2 and MMP 9), and tyrosinase inhibitors. Applied Biochemistry Biotechnology 2022, 1–14. [Google Scholar]
- De Rosso, M.; Tonidandel, L.; Larcher, R.; Nicolini, G.; Dalla Vedova, A.; De Marchi, F.; Gardiman, M.; Giust, M.; Flamini, R. , Identification of new flavonols in hybrid grapes by combined liquid chromatography–mass spectrometry approaches. Food Chemistry 2014, 163, 244–251. [Google Scholar] [CrossRef]
- Khan, M. A.; Nabi, S. G.; Prakash, S.; Zaman, A. , Pallidol, a resveratrol dimer from Cissus pallida. Phytochemistry 1986, 25(8), 1945–1948. [Google Scholar] [CrossRef]
- Strasser, A.; O'Connor, L.; Dixit, V. M. , Apoptosis signaling. Annual Review of Biochemistry 2000, 69(1), 217–245. [Google Scholar] [CrossRef] [PubMed]
- Hickman, J. A. , Apoptosis induced by anticancer drugs. Cancer Metastasis Reviews 1992, 11, 121–139. [Google Scholar] [CrossRef]
- Grattan, B. , Plant sterols as anticancer nutrients: evidence for their role in breast cancer. Nutrients 2013, 5(2), 359–387. [Google Scholar] [CrossRef]
- Tahsin, T.; Wansi, J. D.; Al-Groshi, A.; Evans, A.; Nahar, L.; Martin, C.; Sarker, S. D. , Cytotoxic properties of the stem bark of citrus reticulata blanco (Rutaceae). Phytotherapy Research 2017, 31(8), 1215–1219. [Google Scholar] [CrossRef]
- Samita, F.; Ochieng, C. O.; Owuor, P. O.; Manguro, L. O. A.; Midiwo, J. O. , Isolation of a new β-carboline alkaloid from aerial parts of Triclisia sacleuxii and its antibacterial and cytotoxicity effects. Natural Product Research 2017, 31(5), 529–536. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. , Chemical composition and in vitroevaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chemistry Central Journal 2014, 8(1), 1. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Wadood, A.; Junaid, M.; Ullah, F.; Khan, N. Z. , Cytotoxicity and molecular docking studies on phytosterols isolated from Polygonum hydropiper L. Steroids 2019, 141, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Chinnam, M.; Fink, C.; Bradford, P. , β-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine 2007, 14(11), 747–754. [Google Scholar] [CrossRef]
- Awad, A. B.; Barta, S. L.; Fink, C. S.; Bradford, P. G. , β-Sitosterol enhances tamoxifen effectiveness on breast cancer cells by affecting ceramide metabolism. Molecular Nutrition and Food Research 2008, 52(4), 419–426. [Google Scholar] [CrossRef]
- Chen, Y. C.; Lee, H. Z.; Chen, H. C.; Wen, C. L.; Kuo, Y. H.; Wang, G. J. , Anti-inflammatory components from the root of Solanum erianthum. International Journal of Molecular Sciences 2013, 14(6), 12581–12592. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. S.; Li, X. F.; Kang, K. H.; Ryu, B.; Kim, S. K. , Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Reports 2014, 47(8), 433. [Google Scholar] [CrossRef] [PubMed]
- Gill, B. S.; Kumar, S. ; Navgeet, Triterpenes in cancer: significance and their influence. Molecular Biology Reports 2016, 43, 881–896. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, F.; Zhang, L.; Wu, Y.; Hu, B.; Zhang, Y.; Li, Y.; Liu, H. , Growth inhibition and apoptosis induced by lupeol, a dietary triterpene, in human hepatocellular carcinoma cells. Biological and Pharmaceutical Bulletin 2011, 34(4), 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kang, S. C.; Lim, S. Y.; Song, Y. J. , Lupeol is one of active components in the extract of Chrysanthemum indicum Linne that inhibits LMP1-induced NF-κB activation. PLoS One 2013, 8(11), e82688. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Nigam, N.; Kalra, N.; Shukla, Y. , Regulation of signaling pathways involved in lupeol induced inhibition of proliferation and induction of apoptosis in human prostate cancer cells. Molecular Carcinogenesis 2008, 47(12), 916–924. [Google Scholar] [PubMed]
- Siveen, K.; Nguyen, A.; Lee, J.; Li, F.; Singh, S.; Kumar, A. P.; Low, G.; Jha, S.; Tergaonkar, V.; Ahn, K. , Negative regulation of signal transducer and activator of transcription-3 signalling cascade by lupeol inhibits growth and induces apoptosis in hepatocellular carcinoma cells. British Journal of Cancer 2014, 111(7), 1327. [Google Scholar] [CrossRef] [PubMed]
- Król, S. K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. , Comprehensive review on betulin as a potent anticancer agent. Biomed Research International 2015, 2015. [Google Scholar] [CrossRef]
- Damle, A. A.; Pawar, Y. P.; Narkar, A. A. , Anticancer activity of betulinic acid on MCF-7 tumors in nude mice. Indian Journal of Experimental Biology 2013.
- Zhang, X.; Hu, J.; Chen, Y. , Betulinic acid and the pharmacological effects of tumor suppression. Molecular Medicine Reports 2016, 14(5), 4489–4495. [Google Scholar] [CrossRef]
- Hsu, Y. L.; Kuo, P. L.; Lin, C. C. , Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sciences 2004, 75(19), 2303–2316. [Google Scholar] [CrossRef]
- Liu, T.; Ma, H.; Shi, W.; Duan, J.; Wang, Y.; Zhang, C.; Li, C.; Lin, J.; Li, S.; Lv, J. , Inhibition of STAT3 signaling pathway by ursolic acid suppresses growth of hepatocellular carcinoma. International Journal of Oncology 2017, 51(2), 555–562. [Google Scholar] [CrossRef]
- Kassi, E.; Sourlingas, T.; Spiliotaki, M.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Moutsatsou, P. , Ursolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cells. Cancer Investigation 2009, 27(7), 723–733. [Google Scholar] [CrossRef]
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. , Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. Journal of Cancer Research and Clinical Oncology 2007, 133(7), 493–500. [Google Scholar] [CrossRef]
- Kubina, R.; Iriti, M.; Kabała-Dzik, A. , Anticancer potential of selected flavonols: Fisetin, kaempferol, and quercetin on head and neck cancers. Nutrients 2021, 13(3), 845. [Google Scholar] [CrossRef]
- Rhman, M. A.; Devnarain, N.; Khan, R.; Owira, P. M. , Synergism potentiates oxidative antiproliferative effects of naringenin and quercetin in MCF-7 breast cancer cells. Nutrients 2022, 14(16), 3437. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Wudtiwai, B.; Khawon, P. , Induction of human hepatocellular carcinoma HepG2 cell apoptosis by naringin. Asian Pacific Journal of Cancer Prevention 2016, 17(7), 3289–3294. [Google Scholar]
- Lim, W.; Park, S.; Bazer, F. W.; Song, G. , Naringenin-induced apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways. Journal of Cellular Biochemistry 2017, 118(5), 1118–1131. [Google Scholar] [CrossRef]
- Lu, W. L.; Yu, C. T. R.; Lien, H. M.; Sheu, G. T.; Cherng, S. H. , Cytotoxicity of naringenin induces Bax-mediated mitochondrial apoptosis in human lung adenocarcinoma A549 cells. Environmental toxicology 2020, 35(12), 1386–1394. [Google Scholar] [CrossRef]
- Larasati, L.; Kusharyanti, I.; Hermawan, A.; Susidarti, R. A.; Meiyanto, E. , Naringenin enhances the anti-tumor effect of doxorubicin on HeLa cervical cancer cells through cytotoxic activity and apoptosis induction. Indonesian Journal of Cancer Chemoprevention 2011, 2(3), 325–333. [Google Scholar] [CrossRef]
- Kaur, P.; Shukla, S.; Gupta, S. , Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: an in vitro and in vivo study. Carcinogenesis 2008, 29(11), 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Lu, H. F.; Chie, Y. J.; Yang, M. S.; Lee, C. S.; Fu, J. J.; Yang, J. S.; Tan, T. W.; Wu, S. H.; Ma, Y. S. ; Apigenin induces caspase-dependent apoptosis in human lung cancer A549 cells through Bax-and Bcl-2-triggered mitochondrial pathway. International Journal of Oncology 2010, 36(6), 1477–1484. [Google Scholar] [PubMed]
- Chiang, L. C.; Ng, L. T.; Lin, I. C.; Kuo, P. L.; Lin, C. C. , Anti-proliferative effect of apigenin and its apoptotic induction in human Hep G2 cells. Cancer Letters 2006, 237(2), 207–214. [Google Scholar] [CrossRef] [PubMed]
- Souza, R. P.; Bonfim-Mendonça, P. d. S.; Gimenes, F.; Ratti, B. A.; Kaplum, V.; Bruschi, M. L.; Nakamura, C. V.; Silva, S. O.; Maria-Engler, S. S.; Consolaro, M. E. , Oxidative stress triggered by Apigenin induces apoptosis in a comprehensive panel of human cervical cancer-derived cell lines. Oxidative Medicine and Cellular Longevity 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, L.; Orazizadeh, M.; Niazvand, F.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. , Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratislavske Lekarske Listy 2017, 118(2), 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bhat, F. A.; Sharmila, G.; Balakrishnan, S.; Singh, P. R.; Srinivasan, N.; Arunakaran, J. , Epidermal growth factor-induced prostate cancer (PC3) cell survival and proliferation is inhibited by quercetin, a plant flavonoid through apoptotic machinery. Biomedicine & Preventive Nutrition, 2014, 4 (4), 459-468.
- Haddad, A.; Venkateswaran, V.; Viswanathan, L.; Teahan, S.; Fleshner, N.; Klotz, L. , Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer and Prostatic Diseases 2006, 9(1), 68. [Google Scholar] [CrossRef]
- Granado-Serrano, A. B.; Martín, M. A.; Bravo, L.; Goya, L.; Ramos, S. , Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). The Journal of nutrition 2006, 136(11), 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Li, X. M.; Luo, X. G.; He, J. F.; Wang, N.; Zhou, H.; Yang, P. L.; Zhang, T. C. , Induction of apoptosis in human cervical carcinoma HeLa cells by active compounds from Hypericumáascyron L. Oncology Letters 2018, 15(3), 3944–3950. [Google Scholar]
- Li, X. M.; Liu, J.; Pan, F. F.; Shi, D. D.; Wen, Z. G.; Yang, P. L. , Quercetin and aconitine synergistically induces the human cervical carcinoma HeLa cell apoptosis via endoplasmic reticulum (ER) stress pathway. PloS one 2018, 13(1), e0191062. [Google Scholar] [CrossRef]
- Klimaszewska-Wiśniewska, A.; Hałas-Wiśniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, A.; Grzanka, D. , Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton. Acta Histochemica 2017, 119(2), 99–112. [Google Scholar] [CrossRef]
- Harrath, A. H.; Jalouli, M.; Oueslati, M. H.; Farah, M. A.; Feriani, A.; Aldahmash, W.; Aldawood, N.; Al-Anazi, K.; Falodah, F.; Swelum, A. , The flavonoid, kaempferol-3-O-apiofuranosyl-7-O-rhamnopyranosyl, as a potential therapeutic agent for breast cancer with a promoting effect on ovarian function. Phytotherapy Research 2021, 35(11), 6170–6180. [Google Scholar] [CrossRef]
- Nguyen, T.; Tran, E.; Ong, C.; Lee, S.; Do, P.; Huynh, T.; Nguyen, T.; Lee, J.; Tan, Y.; Ong, C. , Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. Journal of Cellular Physiology 2003, 197(1), 110–121. [Google Scholar] [CrossRef] [PubMed]
- Kashafi, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. , Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomedicine and Pharmacotherapy 2017, 89, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ren, F.; Zhang, L.; Zhang, X.; Yang, R.; Xie, B.; Li, Z.; Hu, Z.; Duan, Z.; Zhang, J. , Kaempferol induces apoptosis in HepG2 cells via activation of the endoplasmic reticulum stress pathway. Molecular Medicine Reports 2016, 13(3), 2791–2800. [Google Scholar] [CrossRef]
- Da, J.; Xu, M.; Wang, Y.; Li, W.; Lu, M.; Wang, Z. , Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer. Analytical Cellular Pathology 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Zhang, X. D. , Myricetin suppresses p21-activated kinase 1 in human breast cancer MCF-7 cells through downstream signaling of the β-catenin pathway. Oncology Reports 2016, 36(1), 342–348. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, C.; Huang, H.; Yang, B.; Xiao, G.; Kong, D.; Tian, Q.; Song, Q.; Song, Y.; Tan, H. , The natural compound myricetin effectively represses the malignant progression of prostate cancer by inhibiting Pim1 and disrupting the PIM1/CXCR4 interaction. Cellular Physiology and Biochemistry 2018, 48(3), 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.-L.; Shi, S.; Shen, Y.-L.; Wang, L.; Chen, H.-Y.; Zhu, J.; Ding, Y. , Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. International Journal of Clinical and Experimental Pathology 2015, 8(2), 1116. [Google Scholar] [PubMed]
- Shih, Y.-W.; Wu, P.-F.; Lee, Y.-C.; Shi, M.-D.; Chiang, T.-A. , Myricetin suppresses invasion and migration of human lung adenocarcinoma A549 cells: possible mediation by blocking the ERK signaling pathway. Journal of Agricultural and Food Chemistry 2009, 57(9), 3490–3499. [Google Scholar] [CrossRef]
- Zhang, X. H.; Chen, S. Y.; Tang, L.; Shen, Y. Z.; Luo, L.; Xu, C. W.; Liu, Q.; Li, D. , Myricetin induces apoptosis in HepG2 cells through Akt/p70S6K/bad signaling and mitochondrial apoptotic pathway. Anti-Cancer Agents in Medicinal Chemistry, 2013, 13 (10), 1575-1581.
- Zhang, X. H.; Zou, Z. Q.; Xu, C. W.; Shen, Y. Z.; Li, D. , Myricetin induces G2/M phase arrest in HepG2 cells by inhibiting the activity of the cyclin B/Cdc2 complex. Molecular Medicine Reports 2011, 4(2), 273–277. [Google Scholar]
- Xue, Y. Q.; Di, J. M.; Luo, Y.; Cheng, K. J.; Wei, X.; Shi, Z. , Resveratrol oligomers for the prevention and treatment of cancers. Oxidative Medicine Cellular Longevity 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Chen, Y.; Cheng, X.; Zhang, X.; He, Q. , Potentiation of resveratrol-induced apoptosis by matrine in human hepatoma HepG2 cells. Oncology Reports 2014, 32(6), 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Guisado, E.; Merino, J. M.; Mulero-Navarro, S.; Lorenzo-Benayas, M. J.; Centeno, F.; Alvarez-Barrientos, A.; Salguero, P. M. F. , Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-κB. International Journal of Cancer 2005, 115(1), 74–84. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, J.; Yang, Y.; Zhao, X.; Liu, Y.; Jiang, Y.; Zhou, L.; Feng, Y.; Yu, Y.; Cheng, Y. , Resveratrol modulates the apoptosis and autophagic death of human lung adenocarcinoma A549 cells via a p53-dependent pathway: Integrated bioinformatics analysis and experimental validation. International Journal of Oncology 2020, 57(4), 925–938. [Google Scholar] [CrossRef]
- Hu, S.; Li, X.; Xu, R.; Ye, L.; Kong, H.; Zeng, X.; Wang, H.; Xie, W. , The synergistic effect of resveratrol in combination with cisplatin on apoptosis via modulating autophagy in A549 cells. Acta Biochimica et Biophysica Sinica 2016, 48(6), 528–535. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S. C.; Marín-Hernández, Á.; Gallardo-Pérez, J. C.; Robledo-Cadena, D. X.; Hernández-Reséndiz, I.; García-García, J. D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L. , Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicology Applied Pharmacology 2019, 370, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Moradi, F.; Maddalena, L. A.; Ferreira-Tollstadius, B.; Selim, S.; Stuart, J. A. , Resveratrol integrates metabolic and growth effects in PC3 prostate cancer cells-involvement of prolyl hydroxylase and hypoxia inducible factor-1. Oncology Letters 2019, 17(1), 697–705. [Google Scholar] [CrossRef]
- Gogada, R.; Prabhu, V.; Amadori, M.; Scott, R.; Hashmi, S.; Chandra, D. , Resveratrol induces p53-independent, X-linked inhibitor of apoptosis protein (XIAP)-mediated Bax protein oligomerization on mitochondria to initiate cytochrome c release and caspase activation. Journal of Biological Chemistry 2011, 286(33), 28749–28760. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. , Natural products as anticancer agents: Current status and future perspectives. Molecules 2022, 27(23), 8367. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Y.; Mooney, S. M.; Yin, B.; Mizokami, A.; Namiki, M.; Getzenberg, R. H. , Resistance to paclitaxel increases the sensitivity to other microenvironmental stresses in prostate cancer cells. Journal of Cellular Biochemistry 2011, 112(8), 2125–2137. [Google Scholar] [CrossRef]
- Jeong, J. H.; An, J. Y.; Kwon, Y. T.; Rhee, J. G.; Lee, Y. J. , Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. Journal of Cellular Biochemistry 2009, 106(1), 73–82. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Wang, N.; Liu, R.; Wu, Q.; Pei, H.; Li, W. , β-sitosterol suppresses hepatocellular carcinoma growth and metastasis via FOXM1-regulated Wnt/β-catenin pathway. Authorea 2023. [CrossRef]
- Topcul, M.; Cetin, I. , Endpoint of cancer treatment: targeted therapies. Asian Pacific Journal of Cancer Prevention 2014, 15(11), 4395–4403. [Google Scholar] [CrossRef]
- Bordoloi, D.; Roy, N.; Monisha, J.; Kunnumakkara, A. , Multi-targeted agents in cancer cell chemosensitization: What we learnt from curcumin thus far. Recent Patents on Anti-Cancer Drug Discovery 2016, 11 (1); 67-97,
- Williams, G. H.; Stoeber, K. , The cell cycle and cancer. The Journal of Pathology 2012, 226(2), 352–364. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Moralli, S.; Tarrado-Castellarnau, M.; Miranda, A.; Cascante, M. , Targeting cell cycle regulation in cancer therapy. Pharmacology Therapeutics 2013, 138(2), 255–271. [Google Scholar] [CrossRef]
- Wang, W.-L.; Chen, S.-M.; Lee, Y.-C.; Chang, W.-W. , Stigmasterol inhibits cancer stem cell activity in endometrial cancer by repressing IGF1R/mTOR/AKT pathway. Journal of Functional Foods 2022, 99, 105338. [Google Scholar] [CrossRef]
- Prasad, N.; Sabarwal, A.; Yadav, U. C.; Singh, R. P. , Lupeol induces S-phase arrest and mitochondria-mediated apoptosis in cervical cancer cells. Journal of Biosciences 2018, 43, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Mishra, T.; Arya, R. K.; Meena, S.; Joshi, P.; Pal, M.; Meena, B.; Upreti, D.; Rana, T.; Datta, D. , Isolation, characterization and anticancer potential of cytotoxic triterpenes from Betula utilis bark. PloS one 2016, 11(7), e0159430. [Google Scholar] [CrossRef]
- Arul, D.; Subramanian, P. , Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathology Oncology Research 2013, 19, 763–770. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G. O.; Li, Z.; DePriest, L.; Chen, Y. C. , Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chemistry 2011, 128(2), 513–519. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Y.; Xia, J.; Liu, B.; Zhang, Q.; Liu, J.; Luo, L.; Peng, Z.; Song, Z.; Zhu, R. , Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Molecular Medicine Reports 2015, 11(4), 2459–2464. [Google Scholar] [CrossRef]
- Bhatt, M.; Patel, M.; Adnan, M.; Reddy, M. N. , Anti-metastatic effects of lupeol via the inhibition of MAPK/ERK pathway in lung cancer. Anti-Cancer Agents in Medicinal Chemistry, 2021, 21 (2), 201-206.
- Chen, Y.; Wu, X.; Liu, C.; Zhou, Y. , Betulinic acid triggers apoptosis and inhibits migration and invasion of gastric cancer cells by impairing EMT progress. Cell Biochemistry Function 2020, 38(6), 702–709. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, C.; Shen, L. , Ursolic acid inhibits epithelial-mesenchymal transition through the Axl/NF-B pathway in gastric cancer cells. Evidence-Based Complementary Alternative Medicine, 2019, 2019.
- Lou, C.; Zhang, F.; Yang, M.; Zhao, J.; Zeng, W.; Fang, X.; Zhang, Y.; Zhang, C.; Liang, W. , Naringenin decreases invasiveness and metastasis by inhibiting TGF-β-induced epithelial to mesenchymal transition in pancreatic cancer cells. PloS one 2012, 7(12), e50956. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhao, D.; Zhou, H.; Wang, X.; Zhong, W. l.; Chen, S.; Gu, W.; Wang, W.; Zhang, C.; Liu, Y. , Apigenin inhibits NF-κB and snail signaling, EMT and metastasis in human hepatocellular carcinoma. Oncotarget 2016, 7(27), 41421. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Park, S. J.; Choi, Y. S.; Jeon, W.-K.; Kim, B.-C. , Kaempferol suppresses transforming growth factor-β1–induced epithelial-to-mesenchymal transition and migration of A549 lung cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3 at threonine-179. Neoplasia 2015, 17(7), 525–537. [Google Scholar] [CrossRef] [PubMed]
- Bhat, F. A.; Sharmila, G.; Balakrishnan, S.; Arunkumar, R.; Elumalai, P.; Suganya, S.; Singh, P. R.; Srinivasan, N.; Arunakaran, J. , Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. The Journal of Nutritional Biochemistry 2014, 25(11), 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N. D. , Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PloS one 2015, 10(10), e0141370. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Q.; Zeng, L.; Xie, L.; Zhao, Q.; Xu, H.; Wang, X.; Jiang, N.; Fu, P.; Sang, M. , Resveratrol suppresses the growth and metastatic potential of cervical cancer by inhibiting STAT3Tyr705 phosphorylation. Cancer Medicine 2020, 9(22), 8685–8700. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A. S.; Mandave, P. C.; Deshpande, M.; Ranjekar, P.; Prakash, O. , Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Frontiers in Pharmacology 2020, 10, 1614. [Google Scholar] [CrossRef]
- Méndez-López, L. F.; Caboni, P.; Arredondo-Espinoza, E.; Carrizales-Castillo, J. J.; Balderas-Rentería, I.; Camacho-Corona, M. d. R. , Bioassay-guided identification of the antiproliferative compounds of Cissus trifoliata and the transcriptomic effect of resveratrol in prostate cancer PC3 cells. Molecules 2021, 26(8), 2200. [Google Scholar] [CrossRef]
- Amico, V.; Barresi, V.; Chillemi, R.; Tringali, C. , Bioassay-guided isolation of antiproliferative compounds from grape (Vitis vinifera) stems. Natural Product Communications 2008, 4(1), 27–34. [Google Scholar] [CrossRef]
- Shanmugam, M. K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A. P.; Ho, P. C. , Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. Journal of Molecular Medicine 2011, 89(7), 713. [Google Scholar] [CrossRef] [PubMed]
- Damle, A. A.; Pawar, Y. P.; Narkar, A. A. , Anticancer activity of betulinic acid on MCF-7 tumors in nude mice. Indian Journal of Experimental Biology 2013, 51, 485–491. [Google Scholar] [PubMed]
- Qin, L.; Jin, L.; Lu, L.; Lu, X.; Zhang, C.; Zhang, F.; Liang, W. , Naringenin reduces lung metastasis in a breast cancer resection model. Protein Cell 2011, 2(6), 507–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. H.; Hwang, K. A.; Choi, K. C. , Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. The Journal of Nutritional Biochemistry 2016, 28, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Harper, C. E.; Patel, B. B.; Wang, J.; Arabshahi, A.; Eltoum, I. A.; Lamartiniere, C. A. , Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis 2007, 28(9), 1946–1953. [Google Scholar] [CrossRef]
- Caesar, L. K.; Cech, N. B. , Synergy and antagonism in natural product extracts: when 1+ 1 does not equal 2. Natural Product Reports 2019, 36(6), 869–888. [Google Scholar] [CrossRef]
- Lucena, F. R.; Almeida, E. R.; Aguiar, J. S.; Silva, T. G.; Souza, V. M.; Nascimento, S. C. , Cytotoxic, antitumor and leukocyte migration activities of resveratrol and sitosterol present in the hidroalcoholic extract of Cissus sicyoides L., Vitaceae, leaves. Revista Brasileira de Farmacognosia, 2010, 20 (5), 729-733.
| Cell line | Hexane | CHCl3-MeOH | Aqueous |
|---|---|---|---|
| Lung cancer A549 | 51 | 85 | 94 |
| Liver cancer Hep3B | 24 | 81 | 81 |
| Breast cancer MCF7 | 30 | 78 | 30 |
| Cervical cancer HeLa | 35 | 82 | 90 |
| Prostate cancer PC3 | 62 | 61 | 58 |
| Function in plants | Compound | Presence in Cissus plants |
|---|---|---|
| Components of cuticular wax | Triacontanediol | C. trifoliata |
| Hentriacontane | C. quadrangularis | |
| Nonacosane | C. cornifolia | |
| Octacosane | C. trifoliata | |
| Structural role in cellular membranes | Arachidic acid | C. quadrangularis |
| Stearic acid | C. quadrangularis | |
| Palmitic acid | C. quadrangularis, C. vitiginea | |
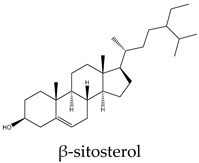
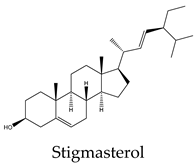
| β-sitosterol | C. quadrangularis, C. assamica, C. polyantha, C. rheoifolia, C. pteroclada | |
| Campesterol | C. quadrangularis, C. rheifolia | |
| Stigmasterol | C. quadrangularis, C. assamica, C. polyantha, C. rheoifolia, C. pteroclada | |
| Squalene | C. quadrangularis | |
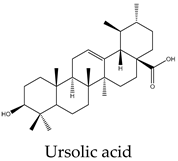
| Defensive role, acting as antifeedant, and antimicrobial | Ursolic acid | C. assamica, C. repens |
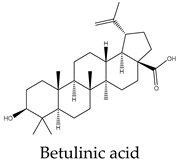
| Betulinic acid | C. assamica | |
| Lupeol | C. quadrangularis, C. assamica, C. repens. |
| Function in plants | Compound | Presence in Cissus plants |
|---|---|---|
| Structural component in the cell wall, and serves to enhance its rigidity and strength | Isoferulic acid | C. trifoliata |
| Regulatory role in inducing abiotic stress tolerance and enhancing the direct defense against insects | Protocatechuic acid | C. trifoliata |
| Antimicrobial properties and protection against oxidative stress | Trigallic acid | C. trifoliata |
| Vital role in response to pathogenic infections | Trans-p-coumaric acid | C. trifoliata |
| Protect photosynthetic tissues from oxidative damage, and confer enhanced salinity tolerance and growth | Apigenin | C. adnata, C. digitata, C. verticillata and C.quadrangularis |
| Protect from oxidative stress induced by heat, water, nutrients, or mechanical damage due to insects, or fungal infections | Cyanidin | C. sicyoides, C. quadrangularis |
| Promote plant defense systems to environmental and pathogenic stress | Delphinidin | C. sicyoides, C. quadrangularis |
| Inhibits bacterial or fungal infections | Myricetin | C. quadrangularis |
| Involved in auxin-regulated cell division as a signaling molecule. Increases grown in heavy metal-contaminated soils | Kaempferol | C. ibuensis, C. sicyoides, C. quadrangularis |
| Regulate response to salt stress tolerance | Dihydrokaempferol | C. quadrangularis |
| Allelochemical, impairing auxin transport and alleviates short-term osmotic and salinity stresses | Naringenin | C. rotundifolia |
| Antifeedant activity and toxicity against insects | Chrysoeriol | C. aralioides, C. lageniflora and C. petiolata |
| Protect epidermal tissue from oxidative stress in response to high light intensity. Regulates the antioxidant enzymes at the transcriptional level | Quercetin | C. ibuensis, C. digitata and C.quadrangularis |
| Reduce the oxidative damage and susceptibility to fungal infections | Syringetin | C. trifoliata |
| Antimicrobial, nematocidal, and insecticidal activities, increase resistance to drought, thermal stress, ultraviolet radiation, mechanical stress, heavy metals, salts, and air pollutants | Resveratrol | C. ibuensis, C. sicyoides, C. quadrangularis |
| Contribute to resistance against fungal infection | ε-viniferin | C. repens, C. sicyoides |
| Display antifungal activities | Pallidol | C. quadrangularis, C. pallida |
| Produced in response to infection, has antimicrobial activity and increases the tolerance to stress | Piceatannol | C. quadrangularis |
| Compound | A549 | MCF7 | HeLa | PC3 | Hep* | Mechanisms underlying apoptosis |
 |
96 | 250 | 170 | 74 | 25 | Increases caspase-8 activity and impairs sphingolipid metabolism |
 |
21 | 9 | 170 | 8 | 12 | Upregulation of Bax and p53 expression and downregulation of Bcl-2 |
 |
21 | 32 | 38 | 30 | 48 | Triggers mitochondrial cell death pathway by downregulation of Bcl-2, Bcl-xL, and survivin expression with a negative modulation of STAT3 activities |
 |
15 | 9 | 10 | 10 | 10 | Cell death through intrinsic pathway promoting the activities of caspases 9, 3, and 7 and the cleavage PARP and inhibition of NF-κB pathway |
 |
18 | 24 | 5 | 15 | 22 | Upregulation of Fas/APO-1 and downregulation of JAK2/STAT3 and NF-κB pathways, Bcl-2, and Bcl-xL expression |
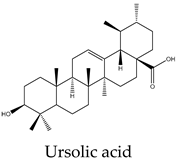 |
218 | 468 | 53 | 13 | 54 | Enhances TRAIL death receptor expression, the formation of the apoptosome complex, caspase-3 activation, and decreases Bcl-2 expression |
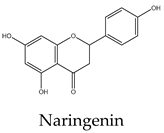 |
20 | 8 | 3 | 11 | 22 | Induces protein production of p53, Bid, and Bax while decreasing the levels of Bcl-2. It also causes the release of cytochrome c, caspase activation, and suppresses the phosphorylation of Akt, Bad, and glycogen synthase kinase-3 |
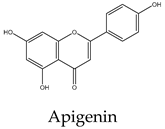 |
10 | 48 | 14 | 17 | 11 | Upregulates p21, p53, caspases 3, 4, 7, and downregulates of Bcl-2 and Bcl-xL |
 |
22 | 50 | 56 | 14 | 24 | Induces the expression of Bax, and caspase-3 with downregulation of NF-κB, mTOR, Bcl-2, and the PI3k/Akt and ERK pathways |
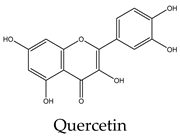 |
16 | 26 | 19 | 15 | 30 | Inhibits survivin, protein p21-activated kinase 1, PI3k/Akt, ERK, and the β-catenin pathway, with activation of caspases 3, 9 and increasing the expression of Bad and p53 |
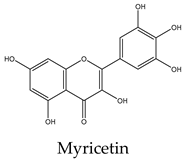 |
2 | 45 | 20 | 46 | 12 | Induces cytochrome c release and activation of caspases 3 and 9, while upregulates p53, Bax and downregulates Bcl-2 and NF-κB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





