1. Introduction
Many textile products in daily life are made with adhesives, such as protective clothing, swimwear, casual wear, sportswear, conveyor belts, and so on. Clothing and textiles have become a necessity in modern life. In the past, people wore clothes for warmth and survival. However, with the development of modern technology, people now not only demand beauty, eco-friendliness, specific functions, convenience, and intelligence, but also comfort when wearing clothing. For manufacturers, they hope to shorten production time, reduce labor demand, and have more extensive design flexibility. Traditional textile processing methods mostly use the sewing technique of needles and threads to piece together fabrics. During the processing, wrinkles, skipped stitches, uneven seams, broken threads, and other defects may occur. Even the combination of some functional clothing can be harmful. For example, if needles and threads are used to stitch together functional clothing such as diving suits, firefighting clothing, and medical protective clothing, it will damage the integrity and performance of these garments [
1].
Therefore, the use of seamless bonding technology in textiles can not only strengthen the bond between fabrics but also shorten the processing time and avoid gaps. The use of adhesive technology involves using adhesive materials to quickly and tightly bond two fabrics together, which can replace the seams and fasteners commonly used in clothing. For the general consumer, it can reduce discomfort caused by friction between the seams and skin, while for designers and producers, it can offer a wider range of design options for the appearance of clothing. Therefore, in recent years, a seam-free processing technology has emerged that uses adhesives widely used in various industries. The use of adhesives is more suitable than traditional joining methods in many ways, such as fatigue resistance, design flexibility, environmental durability, and the ability to use adhesive methods. Therefore, it has been used in various fields such as aviation, electronics, automotive, sports, ships, petroleum, construction, shoe and clothing adhesion [
2,
3,
4].
Currently, there are still some challenges in the industry regarding seamless bonding technology. This study summarizes the common defects in seamless bonding provided by textile factories into the following four points: (i) The hot melt adhesive cannot bond tightly with the substrate, resulting in insufficient adhesion and curling. (ii) During the process of melt pressing and lamination, the base material may produce visible defects. (iii) After the process of melt and pressure lamination, the fabric may shrink and have a poor appearance. (iv) The melting temperature of the hot melt adhesive is too high, resulting in severe color differences in the fabric. Based on the above issues, it is clear that the defects are all caused by the high melting temperature of the hot melt adhesive. Therefore, the softening processing temperature of the hot melt adhesive will be one of the important breakthroughs in seamless bonding technology.
Polyurethane is widely used in coatings, sealants, adhesives, insulators, footwear, rigid foam, flexible foam, textiles, medical, marine industry, piping, and many other fields [
5,
6,
7,
8]. Polyurethane is frequently used in adhesives for flooring, roofing, doors and windows, footwear, soft packaging, glass, textiles, etc. [
9,
10,
11,
12,
13,
14,
15,
16]. Polyurethane formed by the polymerization reaction contains a large number of urethane bonds. Urethane bonds are formed by the reaction between the -NCO group of isocyanates and the hydroxyl group of polyols [
17,
18,
19,
20]. The skeleton of thermoplastic polyurethane is a linear copolymer composed of alternating flexible soft chain segments and rigid hard chain segments. The soft chain segments are composed of long-chain polyols, which can be polyether such as polyethylene glycol, polypropylene glycol, polytetrahydrofuran, polyester polyols [
21,
22,
23,
24]. The hard chain segments are composed of polyisocyanates and small molecule alcohols. Due to the thermodynamic incompatibility between the two types of chain segments during polymerization, micro-phase separation structure is formed, which makes thermoplastic polyurethane have unique mechanical properties, high durability, excellent chemical resistance, and easy processing and application [
25,
26,
27,
28]. The most common method of seamless processing for textiles is the use of adhesive hot-melt film/strips. Hot-melt adhesive is a 100% solid thermoplastic material that can become sticky when heated. The process involves heating and melting the adhesive, which is then applied to the substrate to be bonded. The adhesive adheres to the substrate and the two materials are bonded together, and then it cools and solidifies to complete the bonding process. Kuo [
29] found in his study that the hydroxyl group of polyurethane hot melt pressure-sensitive adhesive can form a chemical bond with the nylon fiber substrate having polar functional groups, further improving the adhesion between the adhesive and the substrate. It also exhibited strong adhesion in peel strength and shear strength.
Seamless bonding technology can effectively reduce the weaknesses in functional clothing caused by sewing areas and the discomfort caused by friction between the body and the seams when wearing tight-fitting clothing [
30]. Compared with traditional hot melt adhesives, polyurethane hot melt adhesives are block or multi-block copolymers formed by alternating flexible and rigid chain segments. Its characteristic lies in its structure containing a high content of urethane and urethane ester bonds. Polyurethane hot melt adhesives have many advantages, such as being environmentally friendly, easy to use and process, effectively wetting the surface of many substrates, diffusing through porous substrates, having good toughness and water resistance, impact resistance, chemical resistance, and forming covalent bonds with substrates containing active hydrogen. [
31,
32,
33].
In this study, it is focused on the raw materials and curing methods of polyurethane hot melt adhesives to overcome different problems. Polyurethane hot melt adhesives were synthesized by using three different types of polyols. 2-HEA and ODA form hydrogen bonding interactions through C=C groups with the functional groups on the nylon fiber, which can enhance the adhesion performance as well as the microphase separation degree which ultimately strengthen the adhesive. In addition to this, the alkyl chain group in the photoinitiator ODA is used to enhance the resistance of water towards adhesives. Different techniques i.e., FTIR, APC, TGA and moisture content have been used to analyze the results. By defining desired objectives for significant characteristics, the study intends to meet the requirements of seamless bonding in textile manufacturers. The NCO/OH ratio, three different categories of polyols, and the amount of photocurable monomer added are selected as control factors. According to the studies of Gogoi [
34] and Somani [
35], the NCO/OH ratio is mostly between 3:1 and 1:1. The three types of polyols used in the soft segment of this study are traditional polyester, polyether, and the newer polycarbonate. The aim is to determine which type of polyol provides excellent adhesion to nylon fabric. The use of n-Octyl Acrylate (ODA) is to achieve crosslinking through the C=C groups shared by 2-HEA and ODA under UV light stimulation. Additionally, the longer alkyl chain of ODA can increase the water resistance of the adhesive and improve its wetting properties on substrates. Lastly, the grey relational analysis method was used to perform multi-quality optimization parameter design for the dual-cure polyurethane hot-melt adhesive to optimize mechanical properties such as peel strength and shear strength of the adhesive
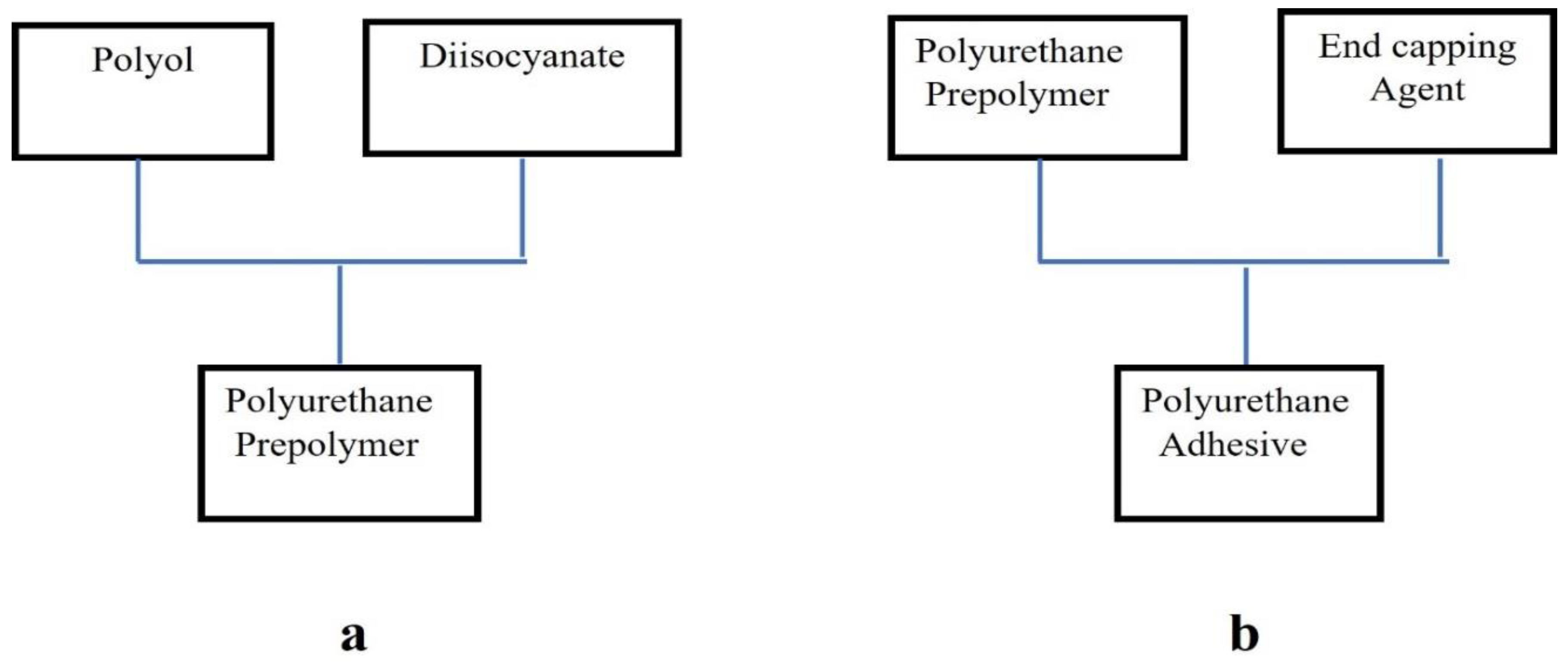
Figure 1.
Synthesis of polyurethane hot melt adhesive (a) combination of diisocyanate and polyol (b) combination of polyurethane prepolymer end-capping agent.
Figure 1.
Synthesis of polyurethane hot melt adhesive (a) combination of diisocyanate and polyol (b) combination of polyurethane prepolymer end-capping agent.
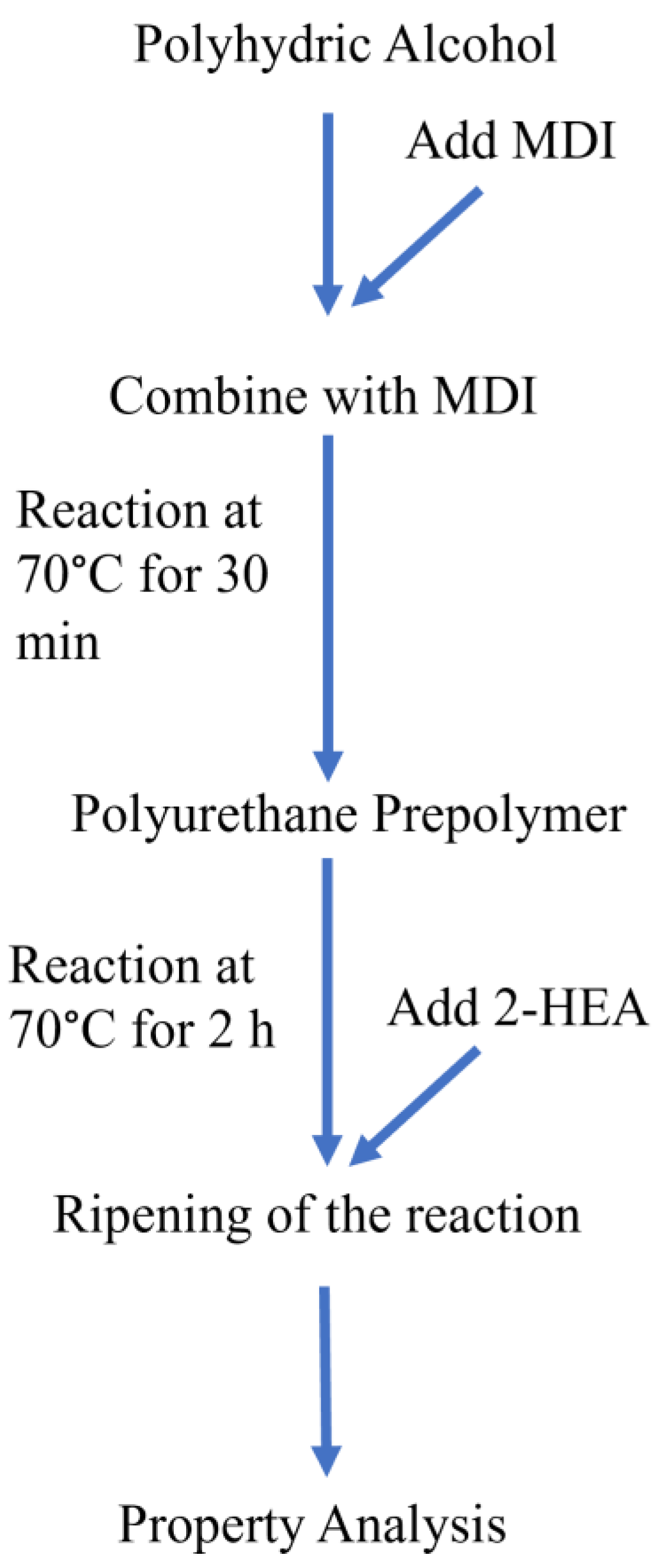
Figure 2.
Experimental flowchart of polyurethane prepolymer synthesis and cured dual-curing polyurethane hot-melt adhesive.
Figure 2.
Experimental flowchart of polyurethane prepolymer synthesis and cured dual-curing polyurethane hot-melt adhesive.
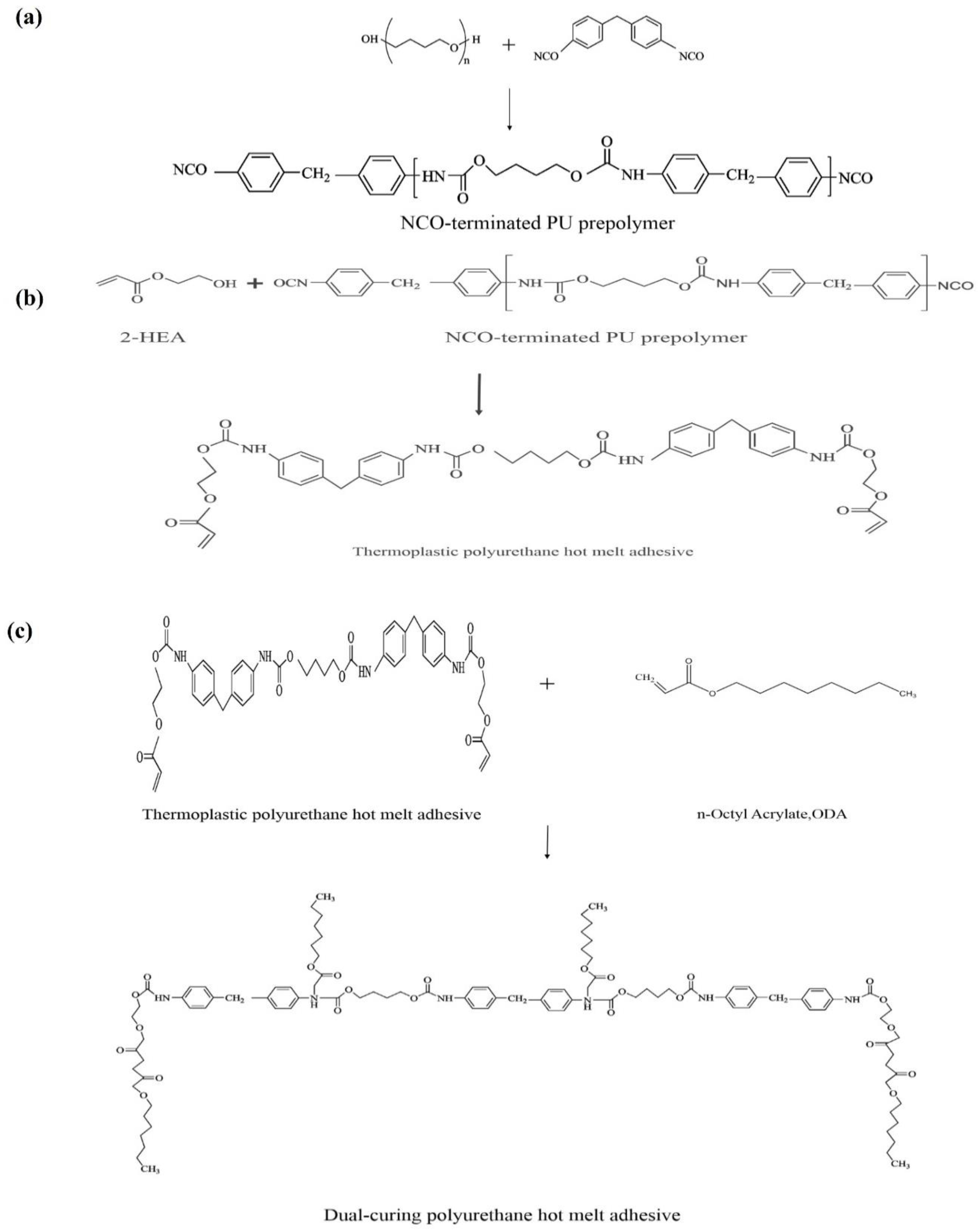
Figure 3.
Chemical reaction of synthesis of polyurethane hot-melt adhesive (a) Reaction synthesis of NCO-terminated PU prepolymer (b) Reaction synthesis of thermoplastic polyurethane hot melt adhesive (c) Reaction synthesis of dual-cure polyurethane hot melt adhesive.
Figure 3.
Chemical reaction of synthesis of polyurethane hot-melt adhesive (a) Reaction synthesis of NCO-terminated PU prepolymer (b) Reaction synthesis of thermoplastic polyurethane hot melt adhesive (c) Reaction synthesis of dual-cure polyurethane hot melt adhesive.
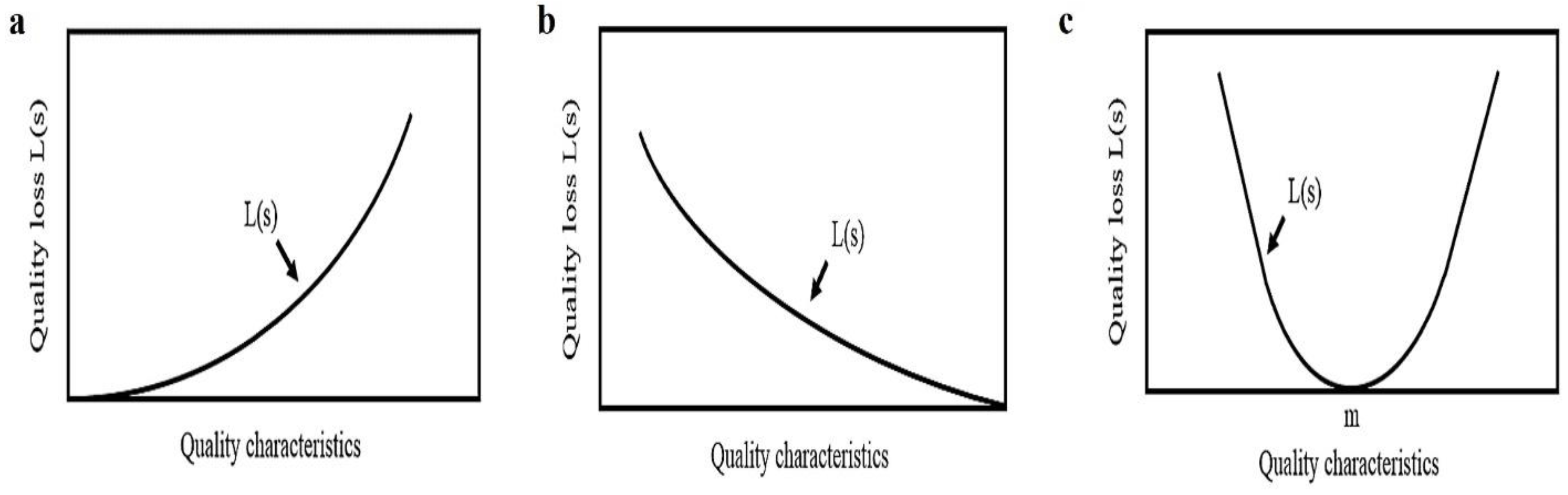
Figure 4.
Quality Loss function of STB, LTB, and NTB characteristic.
Figure 4.
Quality Loss function of STB, LTB, and NTB characteristic.
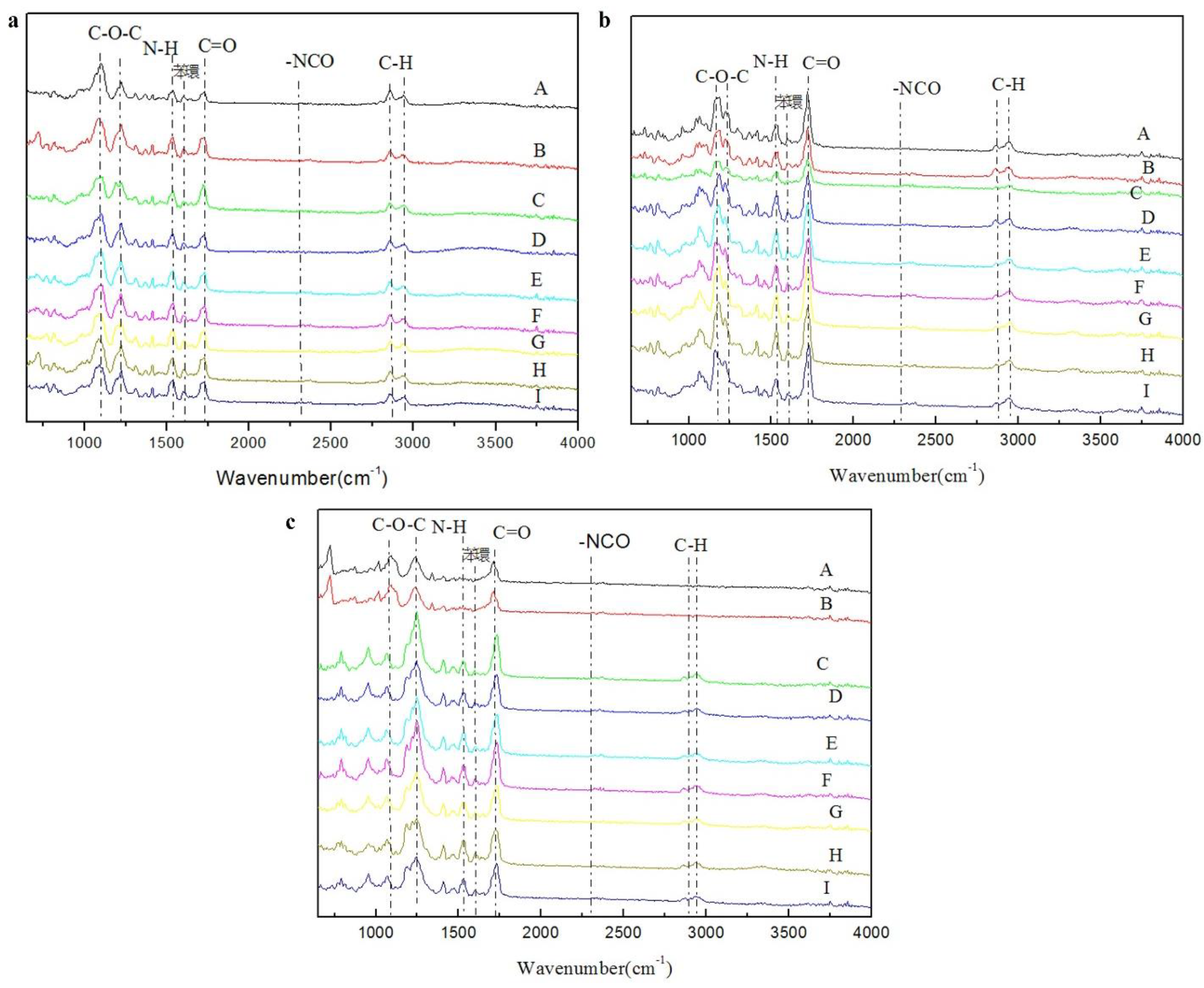
Figure 5.
shows the FTIR spectra of dual-cure polyurethane prepared using (a) PTMG polyol (b) PCL diol and (c) PHCD polyol.
Figure 5.
shows the FTIR spectra of dual-cure polyurethane prepared using (a) PTMG polyol (b) PCL diol and (c) PHCD polyol.
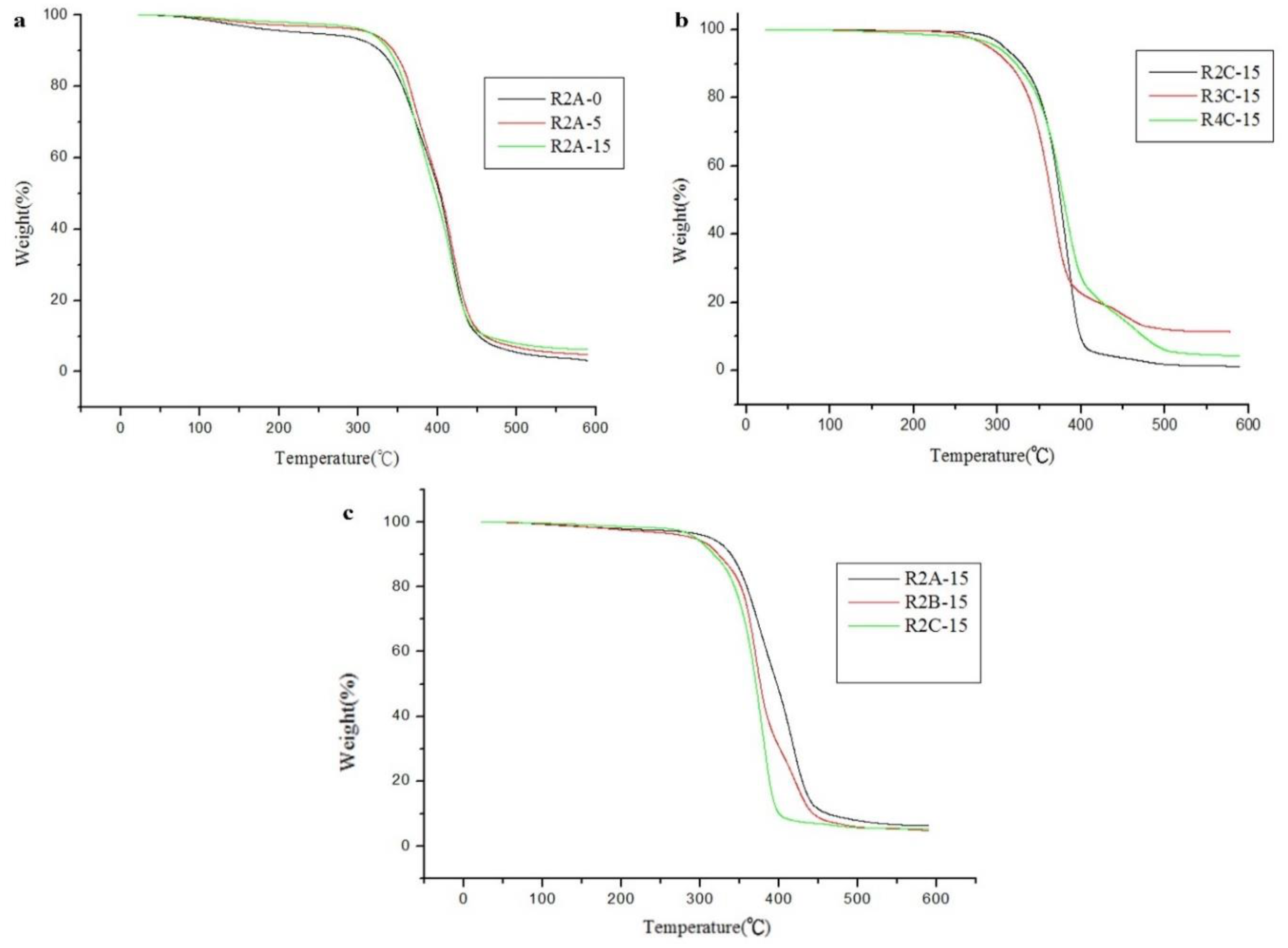
Figure 6.
TGA curves of polyurethane hot melt adhesives with the (a) same PTMG polyol and NCO:OH ratio (b) same PHCD polyol and ODA at different molar ratios and (c) same NCO:OH ratio and different ODA contents.
Figure 6.
TGA curves of polyurethane hot melt adhesives with the (a) same PTMG polyol and NCO:OH ratio (b) same PHCD polyol and ODA at different molar ratios and (c) same NCO:OH ratio and different ODA contents.
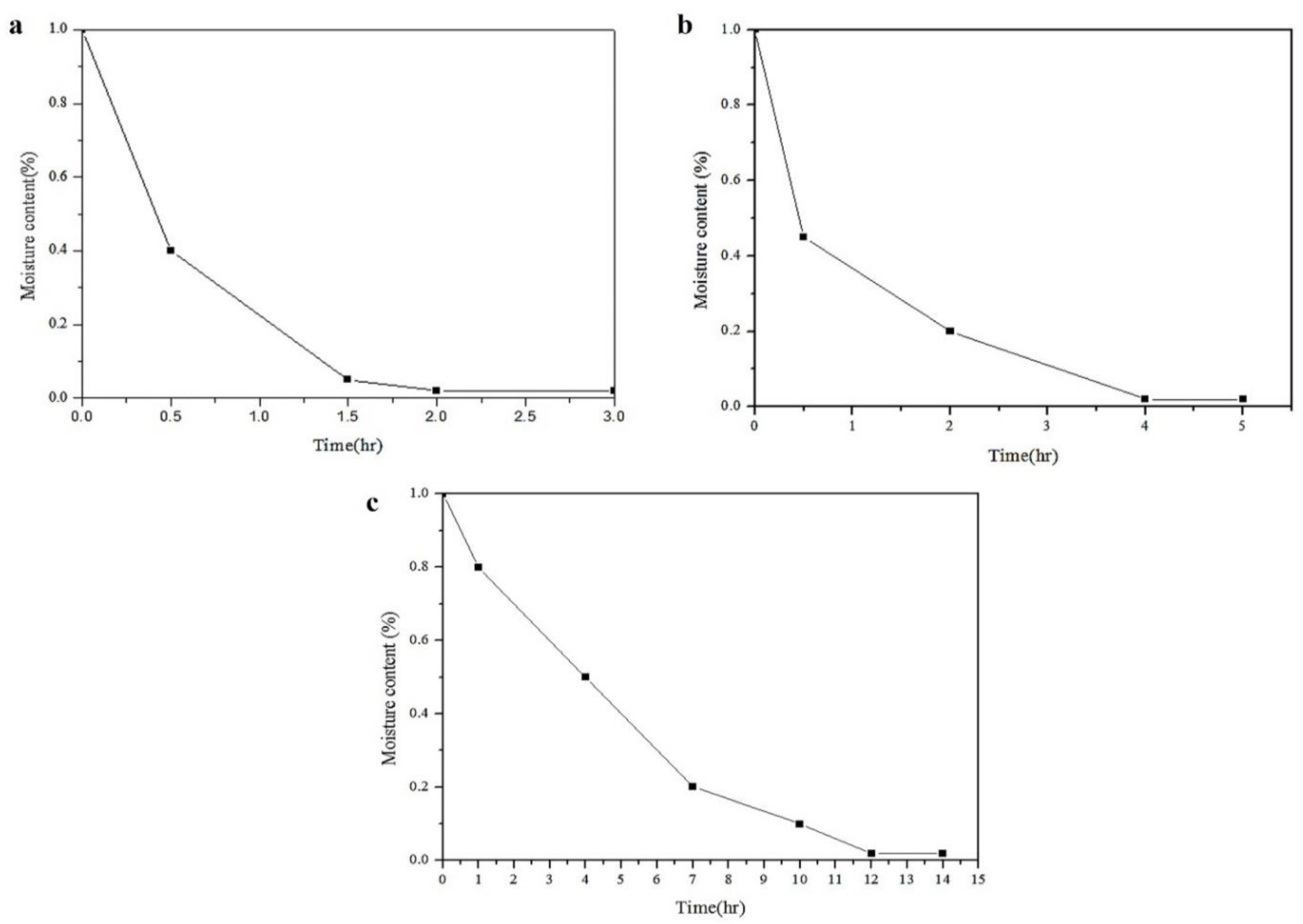
Figure 7.
Moisture content of (a) PTMG, (b) PCL and (c) PHCD at different drying times.
Figure 7.
Moisture content of (a) PTMG, (b) PCL and (c) PHCD at different drying times.
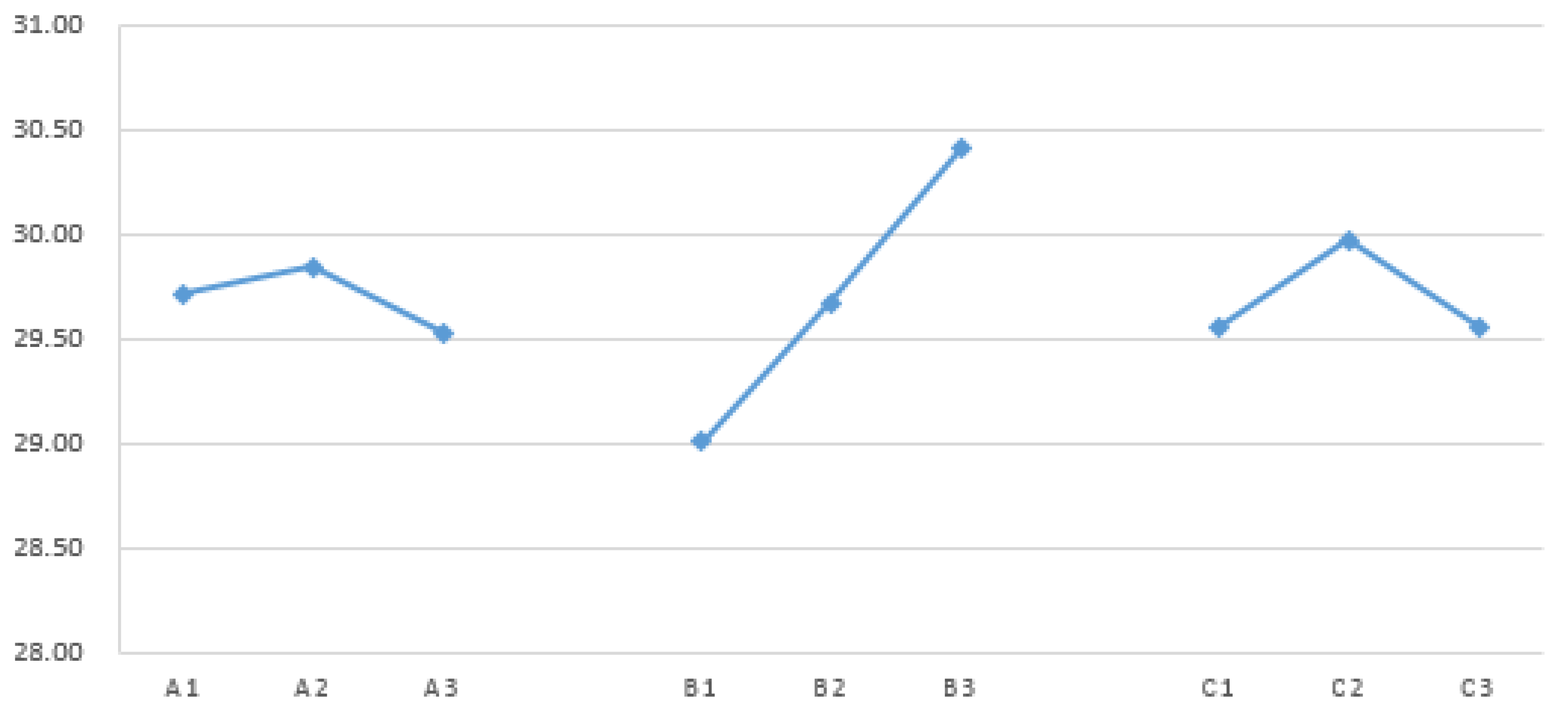
Figure 8.
Factor response graph for shear strength of dual-cure polyurethane hot melt adhesive.
Figure 8.
Factor response graph for shear strength of dual-cure polyurethane hot melt adhesive.
Figure 9.
Factor response chart for peel strength of dual-cured polyurethane hot-melt adhesive.
Figure 9.
Factor response chart for peel strength of dual-cured polyurethane hot-melt adhesive.
Figure 10.
The main effect response plot of the dual-cure polyurethane hot melt adhesive.
Figure 10.
The main effect response plot of the dual-cure polyurethane hot melt adhesive.
Table 1.
Polymerization Parameters of Dual-Cure Polyurethane Hot Melt Adhesive.
Table 1.
Polymerization Parameters of Dual-Cure Polyurethane Hot Melt Adhesive.
| Control factors |
level value 1 |
level value 2 |
level value 3 |
| A |
Polyol |
PTMG |
PCL |
PHCD |
| B |
NCO:OH ratio |
2:1 |
3:1 |
4:1 |
| C |
ODA molar ratio |
0 |
0.5 |
0.15 |
Table 2.
Detailed Formulation of Samples for Dual-Curing Polyurethane Hot-Melt Adhesive.
Table 2.
Detailed Formulation of Samples for Dual-Curing Polyurethane Hot-Melt Adhesive.
Materials
Names |
MDI (moles) |
PTMG
(moles) |
PCL
(moles) |
PHCD
(moles) |
2-HEA
(moles) |
ODA (moles) |
| R2-0ODA |
4 |
2 |
0 |
0 |
4 |
0 |
| R2-5ODA |
4 |
2 |
0 |
0 |
4 |
5 |
| R2-15ODA |
4 |
2 |
0 |
0 |
4 |
15 |
| R3-0ODA |
6 |
0 |
2 |
0 |
8 |
0 |
| R3-5ODA |
6 |
0 |
2 |
0 |
8 |
5 |
| R3-15ODA |
6 |
0 |
2 |
0 |
8 |
15 |
| R4-0ODA |
8 |
0 |
0 |
2 |
12 |
0 |
| R4-5ODA |
8 |
0 |
0 |
2 |
12 |
5 |
| R4-15ODA |
8 |
0 |
0 |
2 |
12 |
15 |
Table 3.
Taguchi L9 Orthogonal Array.
Table 3.
Taguchi L9 Orthogonal Array.
| |
factors |
A
(Polyol category) |
B
(NCO:OH ratio) |
C
(ODA molar ratio) |
| Experiment |
|
| 1 |
1 |
1 |
1 |
| 2 |
1 |
2 |
2 |
| 3 |
1 |
3 |
3 |
| 4 |
2 |
1 |
2 |
| 5 |
2 |
2 |
3 |
| 6 |
2 |
3 |
1 |
| 7 |
3 |
1 |
3 |
| 8 |
3 |
2 |
1 |
| 9 |
3 |
3 |
2 |
Table 4.
Basic Orthogonal Table for Taguchi Method.
Table 4.
Basic Orthogonal Table for Taguchi Method.
| Orthogonal table |
number of columns |
Maximum number of factors |
Maximum number of level rows |
| 2 level number |
3 level number |
4 level number |
5 level number |
| L4
|
4 |
3 |
3 |
|
|
|
| L8
|
8 |
7 |
7 |
|
|
|
| L9
|
9 |
4 |
|
4 |
|
|
| L12
|
12 |
11 |
11 |
|
|
|
| L16
|
16 |
15 |
15 |
|
|
|
| L16
|
16 |
5 |
|
|
5 |
|
| L18
|
18 |
8 |
1 |
7 |
|
|
| L25
|
25 |
6 |
|
|
|
6 |
| L27
|
27 |
13 |
|
12 |
|
|
| L32
|
32 |
31 |
31 |
|
|
|
| L32
|
32 |
10 |
1 |
|
9 |
|
| L36
|
36 |
23 |
11 |
12 |
|
|
| L36
|
36 |
16 |
3 |
13 |
|
|
| L50
|
50 |
12 |
1 |
|
|
11 |
| L54
|
54 |
26 |
1 |
25 |
|
|
| L64
|
64 |
63 |
63 |
|
|
|
| L64
|
64 |
21 |
|
|
21 |
|
| L81
|
81 |
40 |
|
40 |
|
|
Table 5.
Taguchi L9 (34) Orthogonal Array.
Table 5.
Taguchi L9 (34) Orthogonal Array.
|
Factor |
A |
B |
C |
| Experiment |
|
| 1 |
1 |
1 |
1 |
| 2 |
1 |
2 |
2 |
| 3 |
1 |
3 |
3 |
| 4 |
2 |
1 |
2 |
| 5 |
2 |
2 |
3 |
| 6 |
2 |
3 |
1 |
| 7 |
3 |
1 |
3 |
| 8 |
3 |
2 |
1 |
| 9 |
3 |
3 |
2 |
Table 6.
Factor Effecting the response.
Table 6.
Factor Effecting the response.
| |
I |
J |
K |
L |
M |
N |
| Level 1 |
I1
|
J1
|
K1
|
L1
|
M1
|
N1
|
| Level 2 |
I2
|
J2
|
K2
|
L2
|
M2
|
N2
|
| Level 3 |
I3
|
J3
|
K3
|
L3
|
M3
|
N3
|
| MAX |
IMAX
|
JMAX
|
KMAX
|
LMAX
|
MMAX
|
NMAX
|
| MIN |
IMIN
|
JMIN
|
KMIN
|
LMIN
|
MMIN
|
NMIN
|
| Difference |
△I
|
△J
|
△K
|
△L
|
△M
|
△N
|
Table 7.
Illustration of Analysis of variance Table.
Table 7.
Illustration of Analysis of variance Table.
| Factor |
SS |
DOF |
Var |
F |
ρ |
P |
| A |
SSA
|
DOFA
|
VarA
|
FA
|
ρA
|
|
| B |
SSB
|
DOFB
|
VarB
|
FB
|
ρB
|
|
| C |
SSC
|
DOFC
|
VarC
|
FC
|
ρC
|
|
| …… |
…… |
…… |
…… |
…… |
…… |
…… |
| H |
SSH
|
DOFH
|
VarH
|
FH
|
|
|
| error |
SSe
|
DOFe
|
Vare
|
|
|
|
| total |
SST
|
DOFT
|
|
|
|
|
Table 8.
F-value Table for 95% Confidence Level.
Table 8.
F-value Table for 95% Confidence Level.
| DOFe
|
1 |
2 |
3 |
4 |
5 |
| 1 |
161.5 |
199.5 |
215.7 |
224.6 |
230.2 |
| 2 |
18.51 |
19.00 |
19.16 |
19.25 |
19.30 |
| 3 |
10.13 |
9.95 |
9.28 |
9.12 |
9.01 |
| 4 |
7.71 |
6.94 |
6.59 |
6.39 |
6.26 |
| 5 |
6.61 |
5.79 |
5.41 |
5.19 |
5.05 |
| 6 |
5.99 |
5.14 |
4.76 |
4.53 |
4.39 |
| 7 |
5.59 |
4.74 |
4.35 |
3.84 |
3.69 |
| 8 |
5.32 |
4.46 |
4.07 |
3.63 |
3.48 |
| 9 |
5.12 |
4.26 |
3.86 |
3.48 |
3.33 |
| 10 |
4.96 |
4.10 |
3.71 |
3.36 |
3.20 |
| 11 |
4.84 |
3.98 |
3.59 |
3.26 |
3.11 |
| 12 |
4.75 |
3.89 |
3.49 |
3.18 |
3.03 |
| 13 |
4.67 |
3.81 |
3.41 |
3.11 |
2.96 |
| 14 |
4.60 |
3.74 |
3.34 |
3.06 |
2.90 |
| 15 |
4.54 |
3.68 |
3.29 |
3.01 |
2.85 |
| 16 |
4.49 |
3.63 |
3.24 |
2.96 |
2.81 |
| 17 |
4.45 |
3.59 |
3.20 |
2.93 |
2.77 |
| 18 |
4.41 |
3.55 |
3.16 |
2.90 |
2.74 |
| 19 |
4.38 |
3.52 |
3.13 |
2.87 |
2.71 |
| 20 |
4.35 |
3.49 |
3.10 |
2.84 |
2.68 |
| 21 |
4.32 |
3.47 |
3.07 |
2.82 |
2.66 |
| 22 |
4.30 |
3.44 |
3.05 |
2.80 |
2.64 |
| 23 |
4.28 |
3.42 |
3.03 |
2.78 |
2.62 |
| 24 |
4.26 |
3.40 |
3.01 |
2.78 |
2.62 |
| 25 |
4.24 |
3.39 |
2.99 |
2.763 |
2.60 |
Table 9.
Corresponding functional groups and peak values in the FTIR spectra of polyurethane hot melt adhesives.
Table 9.
Corresponding functional groups and peak values in the FTIR spectra of polyurethane hot melt adhesives.
| Wavenumber (cm−1) |
Functional group |
Wavenumber (cm−1) |
Functional group |
| C-H |
2860-2950 cm−1
|
Benzene ring |
1590-1600 cm−1
|
| NCO |
2268 cm−1
|
N-H |
1535 cm−1
|
| C=O |
1720-1735 cm−1
|
C-O-C |
1100-1245 cm−1
|
Table 10.
Molecular weight distribution at different NCO: OH, ratios and mol ratios of photopolymerizable monomer in the same polyol analyzed by APC. The sample names are composed of two numbers representing the NCO: OH, ratio and the molar ratio of photopolymerizable monomer added.
Table 10.
Molecular weight distribution at different NCO: OH, ratios and mol ratios of photopolymerizable monomer in the same polyol analyzed by APC. The sample names are composed of two numbers representing the NCO: OH, ratio and the molar ratio of photopolymerizable monomer added.
| Sample name |
Mn |
Mw |
PDI |
| PTMG soft segment |
| R2-0 |
11026 |
14185 |
1.29 |
| R2-5 |
28454 |
28875 |
1.01 |
| R2-15 |
35090 |
35468 |
1.01 |
| R3-0 |
11494 |
13686 |
1.19 |
| R3-5 |
26624 |
29570 |
1.11 |
| R3-15 |
33007 |
38476 |
1.17 |
| R4-0 |
11888 |
18024 |
1.52 |
| R4-5 |
28367 |
31314 |
1.10 |
| R4-15 |
33086 |
36142 |
1.09 |
| PCL soft segment |
| R2-0 |
14391 |
17816 |
1.24 |
| R2-5 |
25799 |
28830 |
1.12 |
| R2-15 |
33265 |
36168 |
1.09 |
| R3-0 |
12241 |
14644 |
1.20 |
| R3-5 |
22979 |
24770 |
1.08 |
| R3-15 |
37487 |
38505 |
1.03 |
| R4-0 |
11804 |
13776 |
1.17 |
| R4-5 |
27537 |
29542 |
1.07 |
| R4-15 |
37801 |
39666 |
1.05 |
| PHCD soft segment |
| R2-0 |
15107 |
19495 |
1.29 |
| R2-5 |
29082 |
31632 |
1.09 |
| R2-15 |
31312 |
34107 |
1.09 |
| R3-0 |
10092 |
10966 |
1.09 |
| R3-5 |
26051 |
29382 |
1.13 |
| R3-15 |
39103 |
39695 |
1.02 |
| R4-0 |
12011 |
12011 |
1.00 |
| R4-5 |
22826 |
24233 |
1.06 |
| R4-15 |
36702 |
38403 |
1.05 |
Table 11.
TGA data of dual-cure polyurethane hot melt adhesives in nitrogen atmosphere.
Table 11.
TGA data of dual-cure polyurethane hot melt adhesives in nitrogen atmosphere.
| Sample name |
T5(℃) |
T10(℃) |
Sample name |
T5(℃) |
T10(℃) |
|
PTMG soft segment
|
PCL soft segment |
|
R2-0
|
233.52 |
327.03 |
R3-5 |
295.54 |
322.80 |
|
R2-5
|
283.64 |
318.39 |
R3-15 |
302.22 |
324.53 |
|
R2-15
|
328.20 |
344.78 |
R4-0 |
285.29 |
312.87 |
|
R3-0
|
299.30 |
320.09 |
R4-5 |
295.29 |
321.67 |
|
R3-5
|
302.94 |
325.43 |
R4-15 |
304.68 |
330.38 |
|
R3-15
|
311.96 |
349.89 |
PHCD soft segment |
|
R4-0
|
263.84 |
313.42 |
R2-0 |
279.32 |
308.23 |
|
R4-5
|
293.07 |
320.89 |
R2-5 |
289.97 |
314.43 |
|
R4-15
|
295.29 |
321.67 |
R2-15 |
306.60 |
324.10 |
|
PCL soft segment
|
R3-0 |
292.82 |
318.23 |
|
R2-0
|
292.43 |
322.86 |
R3-5 |
293.08 |
311.31 |
|
R2-5
|
295.54 |
322.80 |
R3-15 |
299.66 |
323.98 |
|
R2-15
|
312.71 |
334.30 |
R4-0 |
291.03 |
318.32 |
|
R3-0
|
291.80 |
316.41 |
R4-5 |
302.78 |
322.98 |
|
R3-5
|
295.54 |
322.80 |
R4-15 |
304.46 |
326.62 |
Table 12.
Analysis of peel strength, shear strength, and softening point of dual-curing polyurethane hot melt adhesives.
Table 12.
Analysis of peel strength, shear strength, and softening point of dual-curing polyurethane hot melt adhesives.
| Sample name |
Softening point (℃) |
Peel strength
(kg/cm) |
Shear strength
(kg/cm2) |
|
Target value
|
> 100 |
> 0.39 |
> 13 |
|
PTMG
|
|
R2-0
|
75 |
0.15 |
28.50 |
|
R3-5
|
75 |
0.23 |
31.77 |
|
R4-15
|
80 |
0.52 |
32.46 |
|
PCL
|
|
R2-5
|
65 |
0.34 |
29.67 |
|
R3-15
|
65 |
0.52 |
30.43 |
|
R4-0
|
55 |
0.81 |
33.11 |
|
PHCD
|
|
R2-0
|
70 |
1.13 |
27.34 |
|
R3-5
|
80 |
1.33 |
29.68 |
|
R4-15
|
80 |
1.59 |
33.71 |
Table 13.
Analysis of peel strength, shear strength, and softening point of dual-curing polyurethane hot melt adhesives.
Table 13.
Analysis of peel strength, shear strength, and softening point of dual-curing polyurethane hot melt adhesives.
| Sample name |
softening point (℃) |
Peel strength
(kg/cm) |
shear strength
(kg/cm2) |
|
Target value
|
> 100 |
> 0.39 |
> 13 |
|
PTMG
|
|
R2-0
|
75 |
0.15 |
28.50 |
|
R3-5
|
75 |
0.23 |
31.77 |
|
R4-15
|
80 |
0.52 |
32.46 |
|
PCL
|
|
R2-5
|
65 |
0.34 |
29.67 |
|
R3-15
|
65 |
0.52 |
30.43 |
|
R4-0
|
55 |
0.81 |
33.11 |
|
PHCD
|
|
R2-0
|
70 |
1.13 |
27.34 |
|
R3-5
|
80 |
1.33 |
29.68 |
|
R4-15
|
80 |
1.59 |
33.71 |
Table 14.
Analysis Results of Shear Strength of Dual-cured Polyurethane Hot Melt Adhesive.
Table 14.
Analysis Results of Shear Strength of Dual-cured Polyurethane Hot Melt Adhesive.
| No. |
Factors |
| A |
B |
C |
Test 1
(MPa) |
Test 2
(MPa) |
Test 3
(MPa) |
Ave.
(MPa) |
St. dev. |
S/N
(dB) |
| 1 |
1 |
1 |
1 |
28.5 |
27.06 |
27.84 |
27.80 |
0.72 |
28.87 |
| 2 |
1 |
2 |
2 |
31.77 |
31.46 |
31.69 |
31.64 |
0.16 |
30.00 |
| 3 |
1 |
3 |
3 |
32.46 |
32.64 |
32.58 |
32.56 |
0.09 |
30.25 |
| 4 |
2 |
1 |
2 |
29.67 |
29.37 |
29.48 |
29.51 |
0.15 |
29.40 |
| 5 |
2 |
2 |
3 |
30.43 |
30.67 |
30.27 |
30.46 |
0.20 |
29.67 |
| 6 |
2 |
3 |
1 |
33.11 |
33.31 |
33.48 |
33.30 |
0.19 |
30.45 |
| 7 |
3 |
1 |
3 |
27.34 |
27.02 |
27.8 |
27.39 |
0.39 |
28.75 |
| 8 |
3 |
2 |
1 |
29.68 |
29.19 |
29.03 |
29.30 |
0.34 |
29.34 |
| 9 |
3 |
3 |
2 |
33.71 |
33.21 |
33.62 |
33.51 |
0.27 |
30.50 |
Table 15.
Factor response table for shear strength of dual-cure polyurethane hot melt adhesive.
Table 15.
Factor response table for shear strength of dual-cure polyurethane hot melt adhesive.
| |
A |
B |
C |
| Level 1 |
29.71 |
29.01 |
29.55 |
| Level 2 |
29.84 |
29.67 |
29.97 |
| Level 3 |
29.53 |
30.40 |
29.56 |
| Max |
29.84 |
30.40 |
29.97 |
| Min |
29.53 |
29.01 |
29.55 |
| effect |
0.31 |
1.40 |
0.42 |
| Sort |
3 |
1 |
2 |
Table 16.
The ANOVA analysis table for shear strength of dual-curing polyurethane hot melt adhesive.
Table 16.
The ANOVA analysis table for shear strength of dual-curing polyurethane hot melt adhesive.
| Control factor |
SS |
DOF |
Var |
F |
Contribution |
| A |
0.15 |
2 |
0.07 |
18.11 |
4.27% |
| B |
2.92 |
2 |
1.46 |
362.33 |
85.49% |
| C |
0.34 |
2 |
0.17 |
42.37 |
10.00% |
| Error |
0.01 |
2 |
0.00 |
|
|
| Combined Error |
0.01 |
2 |
0.00 |
|
|
| Total |
3.42 |
8 |
|
|
|
Table 17.
Experimentation of shear strength for Dual-Cure Polyurethane Hot Melt Adhesive.
Table 17.
Experimentation of shear strength for Dual-Cure Polyurethane Hot Melt Adhesive.
| Major significant factor |
Test 1 |
Test 2 |
Test 3 |
Mean value |
S/N ratio |
| A2, B3, C2 |
33.91 |
33.94 |
34.02 |
33.96 |
30.62 |
Table 18.
Results of Peel Strength Analysis for Dual-Cure Polyurethane Hot Melt Adhesive.
Table 18.
Results of Peel Strength Analysis for Dual-Cure Polyurethane Hot Melt Adhesive.
| No. |
|
S/N
(dB) |
| A |
B |
C |
Test 1 |
Test 2 |
Test 3 |
Average |
Std. |
| 1 |
1 |
1 |
1 |
0.15 |
0.13 |
0.18 |
0.15 |
0.03 |
-16.62 |
| 2 |
1 |
2 |
2 |
0.23 |
0.27 |
0.24 |
0.25 |
0.02 |
-12.25 |
| 3 |
1 |
3 |
3 |
0.52 |
0.54 |
0.55 |
0.54 |
0.02 |
-5.42 |
| 4 |
2 |
1 |
2 |
0.34 |
0.28 |
0.32 |
0.31 |
0.03 |
-10.20 |
| 5 |
2 |
2 |
3 |
0.52 |
0.61 |
0.57 |
0.57 |
0.05 |
-5.02 |
| 6 |
2 |
3 |
1 |
0.81 |
0.85 |
0.88 |
0.85 |
0.04 |
-1.47 |
| 7 |
3 |
1 |
3 |
1.13 |
1.17 |
1.17 |
1.16 |
0.02 |
1.26 |
| 8 |
3 |
2 |
1 |
1.33 |
1.38 |
1.36 |
1.36 |
0.03 |
2.64 |
| 9 |
3 |
3 |
2 |
1.59 |
1.57 |
1.61 |
1.59 |
0.02 |
4.03 |
Table 19.
Factor Response Table for Peel Strength of Dual-cure Polyurethane Hot-melt Adhesive.
Table 19.
Factor Response Table for Peel Strength of Dual-cure Polyurethane Hot-melt Adhesive.
| |
A |
B |
C |
| Level 1 |
-11.43 |
-8.52 |
-5.15 |
| Level 2 |
-5.56 |
-4.87 |
-6.14 |
| Level 3 |
2.64 |
-0.95 |
-3.06 |
| Max |
2.64 |
-0.95 |
-3.06 |
| Min |
-11.43 |
-8.52 |
-6.14 |
| effect |
14.07 |
7.57 |
3.08 |
| Ranking |
1 |
2 |
3 |
Table 20.
ANOVA Analysis Table of Peel Strength for Dual-curing Polyurethane Hot Melt Adhesive.
Table 20.
ANOVA Analysis Table of Peel Strength for Dual-curing Polyurethane Hot Melt Adhesive.
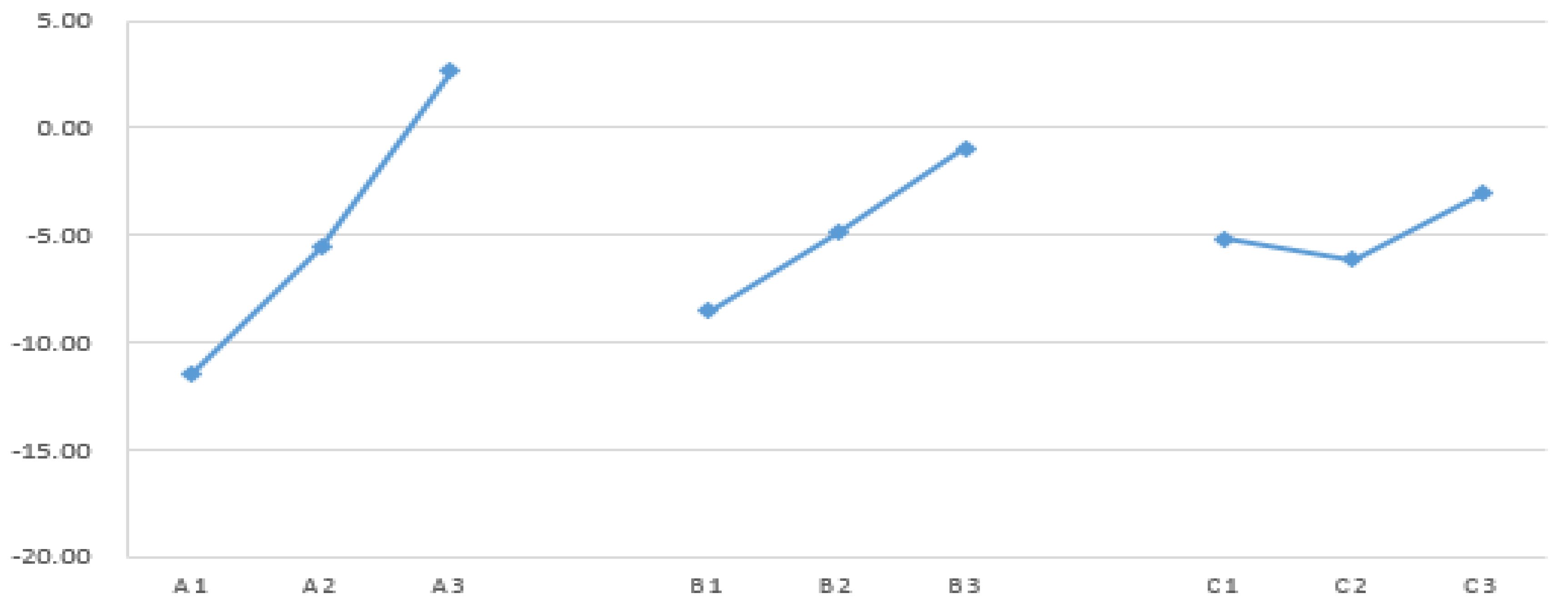
| Control Factor |
Sum of Squares |
Degrees of Freedom |
Variance |
F Distribution |
Contribution |
| A |
299.83 |
2 |
149.91 |
55.78 |
73.84% |
| B |
85.99 |
2 |
42.99 |
16.00 |
21.18% |
| C |
14.87 |
2 |
7.44 |
2.77 |
3.66% |
| error |
5.38 |
2 |
2.69 |
- |
- |
| Combined Error |
5.38 |
2 |
2.69 |
- |
- |
| Total |
406.06 |
8 |
- |
- |
- |
Table 21.
Confirmation Experiment for Dual-Cure Polyurethane Hot Melt Adhesive Peel Strength.
Table 21.
Confirmation Experiment for Dual-Cure Polyurethane Hot Melt Adhesive Peel Strength.
| Primary significant factor |
Test1 |
Test2 |
Test3 |
mean value |
S/N ratio |
| A3B3C3 |
1.63 |
1.62 |
1.62 |
1.62 |
4.21 |
Table 22.
Signal-to-Noise Ratio Sequence Table for Peel Strength and Shear Strength.
Table 22.
Signal-to-Noise Ratio Sequence Table for Peel Strength and Shear Strength.
| |
Peel strength S/N ratio |
Shear strength S/N ratio |
| 1 |
-16.62 |
28.87 |
| 2 |
-12.25 |
30.00 |
| 3 |
-5.42 |
30.25 |
| 4 |
-10.20 |
29.40 |
| 5 |
-5.02 |
29.67 |
| 6 |
-1.47 |
30.45 |
| 7 |
1.26 |
28.75 |
| 8 |
2.64 |
29.34 |
| 9 |
4.03 |
30.50 |
| Maximum value |
4.03 |
30.50 |
| Minimum value |
-16.62 |
28.75 |
| Difference |
20.65 |
1.76 |
Table 23.
Grey correlation generation table for peel strength and shear strength.
Table 23.
Grey correlation generation table for peel strength and shear strength.
| |
Peel strength S/N ratio |
Shear strength S/N ratio |
| X0
|
1 |
1 |
| X1
|
0.070656 |
0 |
| X2
|
0.715659 |
0.211866 |
| X3
|
0.857615 |
0.542759 |
| X4
|
0.370251 |
0.311021 |
| X5
|
0.526921 |
0.56219 |
| X6
|
0.968642 |
0.733956 |
| X7
|
0 |
0.866014 |
| X8
|
0.334678 |
0.93313 |
| X9
|
1 |
1 |
| Maximum value |
1 |
1 |
| Minimum value |
0 |
0 |
Table 24.
Difference Sequence for Peel Strength and Shear Strength.
Table 24.
Difference Sequence for Peel Strength and Shear Strength.
| |
Peel strength S/N ratio |
Shear strength S/N ratio |
| X0
|
0 |
0 |
| X1
|
1 |
0.929344 |
| X2
|
0.788134 |
0.284341 |
| X3
|
0.457241 |
0.142385 |
| X4
|
0.688979 |
0.629749 |
| X5
|
0.43781 |
0.473079 |
| X6
|
0.266044 |
0.031358 |
| X7
|
0.133986 |
1 |
| X8
|
0.06687 |
0.665322 |
| X9
|
0 |
0 |
Table 25.
Grey correlation degree table for peel strength and shear strength.
Table 25.
Grey correlation degree table for peel strength and shear strength.
| |
Peel strength S/N ratio |
Shear strength S/N ratio |
Average |
| X0
|
1 |
1 |
1 |
| X1
|
0.333333 |
0.349811 |
0.341572 |
| X2
|
0.388158 |
0.637478 |
0.512818 |
| X3
|
0.522334 |
0.77835 |
0.650342 |
| X4
|
0.420529 |
0.442576 |
0.431553 |
| X5
|
0.533157 |
0.513833 |
0.523495 |
| X6
|
0.652704 |
0.940985 |
0.796844 |
| X7
|
0.78866 |
0.333333 |
0.560997 |
| X8
|
0.882037 |
0.429066 |
0.655552 |
| X9
|
1 |
1 |
1 |
Table 26.
Main Effects Response Table for Dual-Cured Polyurethane Hot Melt Adhesive.
Table 26.
Main Effects Response Table for Dual-Cured Polyurethane Hot Melt Adhesive.
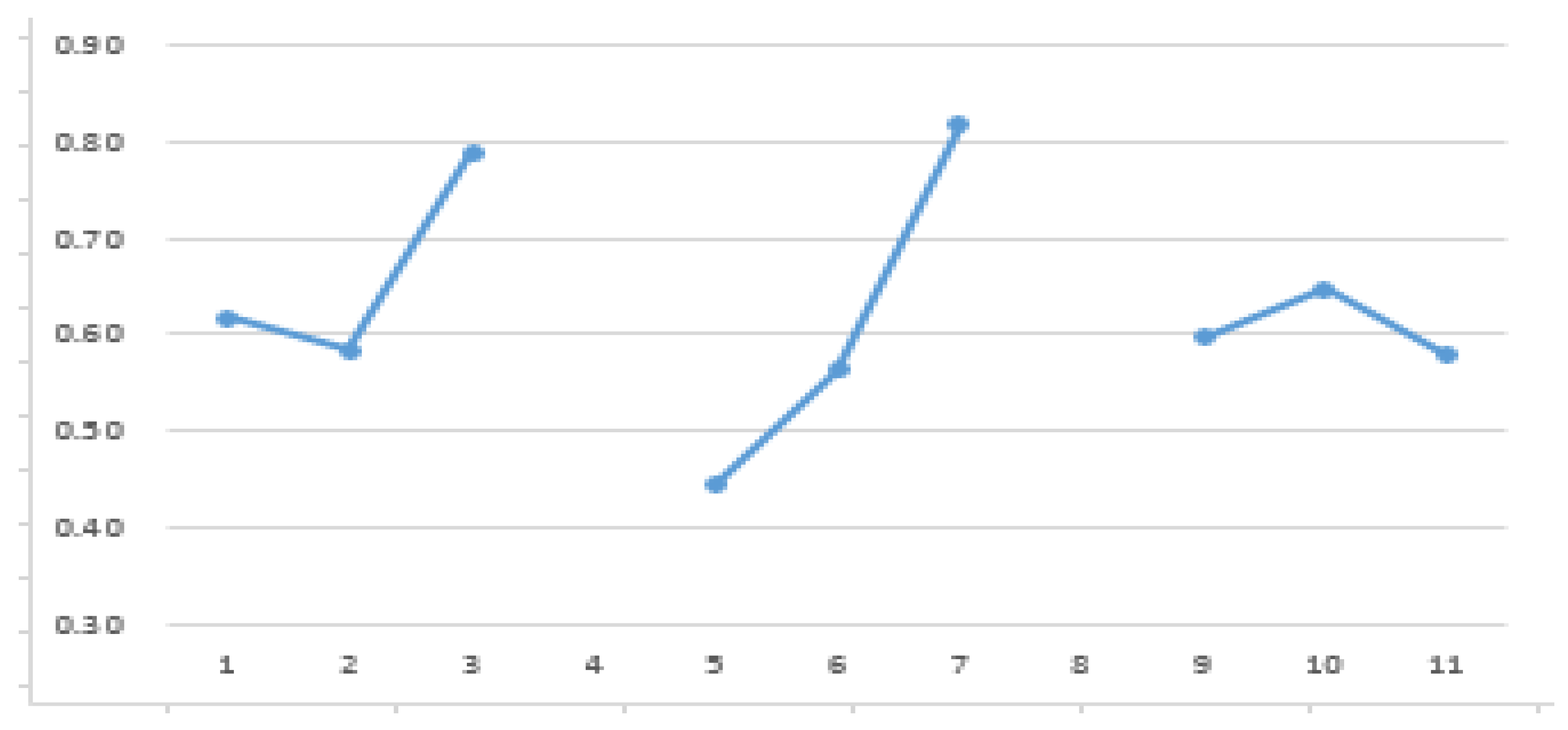
| |
A |
B |
C |
| Polyol |
NCO:OH ratio |
ODA molar ratio |
| Level 1 |
0.62 |
0.44 |
0.60 |
| Level 2 |
0.58 |
0.56 |
0.65 |
| Level 3 |
0.74 |
0.82 |
0.58 |
| Max |
0.74 |
0.82 |
0.65 |
| Min |
0.58 |
0.44 |
0.58 |
| effect |
0.15 |
0.37 |
0.07 |
| ranking |
2 |
1 |
3 |
Table 27.
Confirmation experiment output table (peel strength) for dual-cure polyurethane hot melt adhesive.
Table 27.
Confirmation experiment output table (peel strength) for dual-cure polyurethane hot melt adhesive.
| Significant controlling factors |
Test1 |
Test2 |
Test3 |
mean value |
S/N ratio |
| A3B3C2 |
1.67 |
1.69 |
1.68 |
1.68 |
4.51 |
Table 28.
Confirmation experiment output table (shear strength) for dual-cure polyurethane hot melt adhesive.
Table 28.
Confirmation experiment output table (shear strength) for dual-cure polyurethane hot melt adhesive.
| Significant controlling factors |
Test1 |
Test2 |
Test3 |
mean value |
S/N ratio |
| A3B3C2 |
35.62 |
34.52 |
34.67 |
34.94 |
30.86 |
Table 29.
Comparison of properties between multi-quality experiment and commercial hot melt adhesive.
Table 29.
Comparison of properties between multi-quality experiment and commercial hot melt adhesive.
| Analysis Item |
Target Value |
Commercial Hot Melt Adhesive |
Unoptimized Experiment |
Multi-quality Optimization Experiment |
Gain (%) |
| Peel strength |
0.39 kg/cm above |
0.73 |
1.57 |
1.69 |
7.64 |
| Shear strength |
13kg/cm2 above |
19.36 |
33.21 |
35.62 |
7.25 |
Table 30.
Comparison of processing conditions between optimized experiment and commercial hot melt adhesive.
Table 30.
Comparison of processing conditions between optimized experiment and commercial hot melt adhesive.
| Analysis Item |
Commercial Hot Melt Adhesive |
Multi-quality Optimization Experiment |
| Processing Temperature (℃) |
150-170 |
100 |
| Processing Time (s) |
20-30 |
20 |
| Hot Pressing Pressure (psi) |
40-60 |
15 |