1. Introduction
Eli Lilly and Company launched tirzepatide (Manjaro🄬) in the United States on June 7, 2022. Tirzepatide was initially released in Japan on April 18, 2023, including two initiation and maintenance doses (2.5 and 5 mg), followed by four higher doses (7.5, 10, 12.5, and 15 mg) on June 12. Tirzepatide, a new medication based on a natural gastric inhibitory peptide (GIP) sequence, has been structurally modified to bind to glucagon-like peptide 1 (GLP-1) receptors. It is designed based on amino acid sequence of GIP but is modified to also bind to the GLP-1 receptor, thereby stimulating insulin secretion in a glucose concentration-dependent manner [
1,
2]. The insulin-secreting effect of GIP is significantly reduced in patients with chronic hyperglycemic conditions. Additionally, GIP enhances energy accumulation in adipocytes, thereby potentially causing obesity [
3]. However, GIP has recently been reported to suppress appetite and induce weight loss effects at concentrations far above physiological levels. A completely new once-weekly injectable formulation has been developed to act on GIP receptors with the accumulation of new results.
The SURPASS J-mono study [
4], a domestic phase III clinical trial, was conducted to assess the superiority of weekly tirzepatide doses (5, 10, or 15 mg) over dulaglutide of 0.75 mg in 636 Japanese patients with inadequately controlled type 2 diabetes mellitus (T2DM). Participants were randomized to the tirzepatide or dulaglutide groups, and tirzepatide exhibited a significant reduction in glycated hemoglobin (HbA1c) compared with dulaglutide. Adverse events did not significantly differ between the groups. The focus on obese subjects and minimal representation of older individuals raises questions about generalizability. Additionally, the first-line use of incretin modulator should be acknowledged.
The Japanese population demonstrates lower insulin secretion capacity than Westerners. Impaired insulin secretion has been suggested to play a more crucial role than insulin resistance in diabetes development in the Japanese population. The average body mass index (BMI) of registered patients with T2DM in 2018 was 24.79 kg/m
2 [
5]. The BMI of Japanese population with diabetes is increasing, but it remains significantly lower than that of their Western counterparts. The continuous glucose monitoring (CGM) substudy in the SURPASS-3 evaluated the association of tirzepatide with hyperglycemic or hypoglycemic time and glycemic variability compared with insulin deluded in 243 individuals over 52 weeks [
6]. The study focused on titration in the SURPASS-3 subpopulation with diabetes (mean BMI: 33.9 kg/m
2) and revealed that tirzepatide reduced daily glycemic variability and was closely aligned with target ranges. This multicenter, randomized, open-label study predominantly included obese participants, which differs from the Japanese population with T2DM. The limited representation of individuals aged ≥75 years raises uncertainty regarding the applicability of the study to this older population.
The HbA1c is the gold standard for the glycemic control index, but it does not reflect the detailed daily fluctuations in blood glucose levels. The use of CGM provides vital data for “better glycemic management quality” in older populations prone to unconscious hypoglycemia. Furthermore, the simplification of diabetes drug therapy is essential in older patients with impaired cognitive function, visual function, and hand dexterity. Therefore, we investigated the short-term advantages and disadvantages of tirzepatide onset evaluated by CGM in older patients with T2DM in Japan.
2. Materials and Methods
Study design
This observational pilot study investigated short-term change of glycemic control induced by the tirzepatide onset using CGM in Japanese older patinets with T2DM.
Participants
Patients with diabetes in our department underwent CGM, specifically when notable blood glucose fluctuations and/or hypoglycemia unawareness were suspected. This study focuses on tirzepatide administration in patients with T2DM who actively manage their condition through a combination of dietary control, exercise, and pharmacological treatment, yet continue to suffer insufficient glycemic control. Tirzepatide initiation was well explained and performed. The local ethics committee of the National Centre for Geriatrics and Gerontology approved this exploratory observational study aimed at building evidence on diabetes in older individuals (reception No. 1724).
Continuous glucose monitoring
The CGM was performed using a continuous glucose monitoring system (FreeStyle Libre Pro) worn on the upper arm of the nondominant hand for up to 14 days. The data were averaged over the measurement period. Furthermore, according to a consensus report [
7], Furthermore, CGM measured the appropriate glucose range (70–180 mg/dL, time in range [TIR]), hyperglycemic range (>180 mg/dL, time above range [TAR]), and hypoglycemic range (<70 mg/dL, time below range [TBR]) in addition to sensor glucose and coefficient of variation (%CV). Participants signed written informed consent during the FreeStyle Libre Pro sensor attachment.
Category Classification
A joint committee of the Japan Diabetes Society and Japan Geriatrics Society published “Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS)” to provide safe and effective diabetes care tailored to individual patient conditions [
8,
9]. Patients were categorized into three classifications based on health status, age, use of medications that can cause hypoglycemia and comorbidities. The more advanced the category, the worse the patient’s daily function. Patients were classified following Daily Functional Scale-8 questionnaire (DAFS-8) questionnaire [
10].
Statistical Analysis
Mean CGM metrics included normally distributed data and were therefore compared before and after tirzepatide administration using a paired t-test. The IBM Statistical Package for the Social Sciences version 28 (Armonk, NY, USA) was used for statistical analyses.
3. Case Presentations
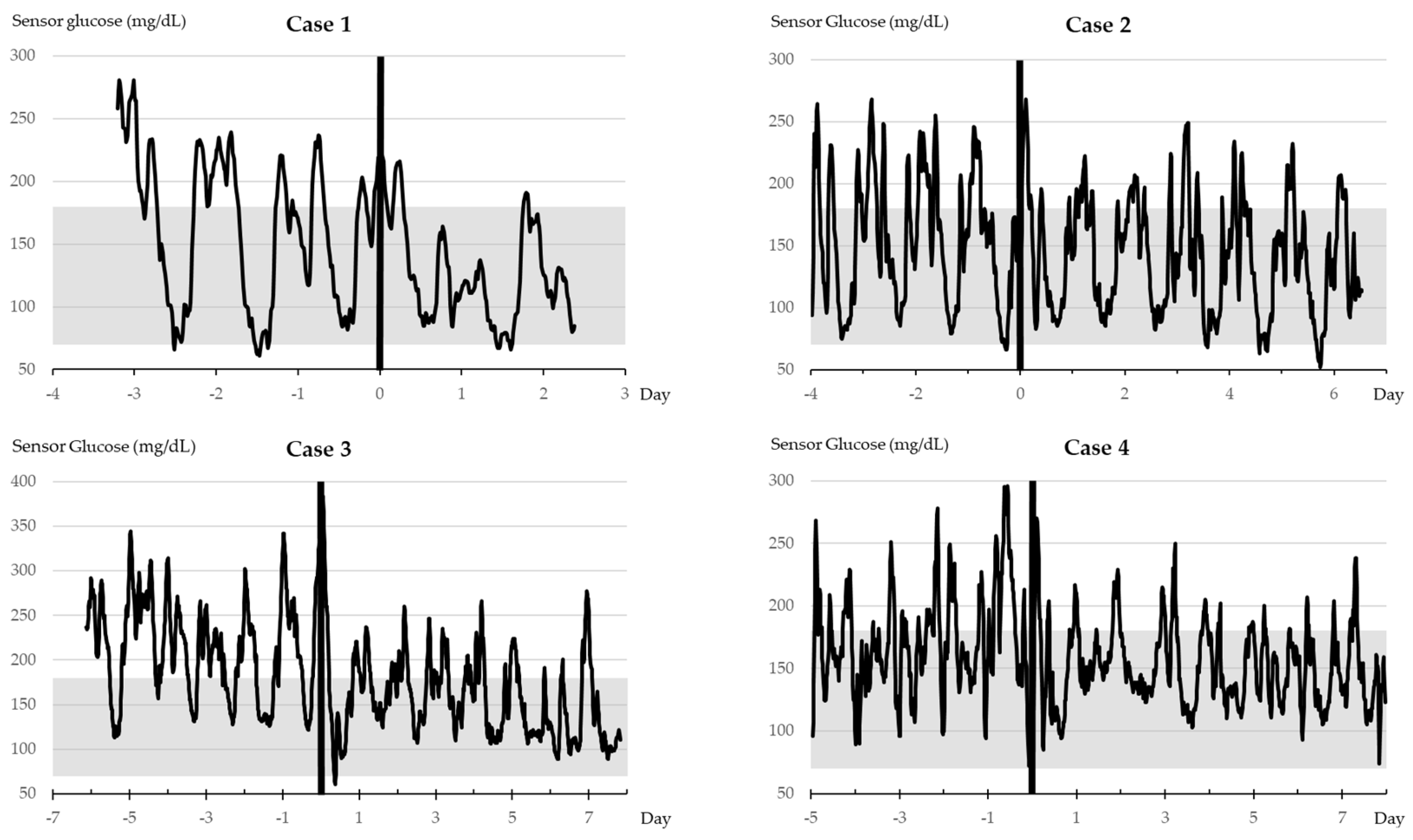
(Case 1) An 81-year-old female patient with an 18-year diabetes duration and Category II diabetes reported a history of hypertension, dyslipidemia, hyperuricemia, and osteoporosis. She is currently treated with glimepiride at 1 mg/day and acarbose at 300 mg/day, with the recent addition of tirzepatide. The glimepiride dose enabled to be reduced to 0.5 mg/day after 36 days and was discontinued after 64 days.
Table 1.
Treatment course of case 1.
Table 1.
Treatment course of case 1.
| Administration |
Before 7 days |
After 23 days |
| HbA1c (%) |
12.1 |
- |
| LDL/HDL/TG (mg/dL) |
84/52/171 |
78/53/147 |
| eGFRcr (mL/min/1.73m2) |
40.2 |
34.9 |
| AST/ALT (IU/L) |
38/44 |
24/44 |
| Body weight (kg) |
75.6 |
73.9 |
(Case 2) An 87-year-old male patient with a 13-year diabetes duration and Category I disease reported a history of angina pectoris and polymyalgia rheumatica for which he takes prednisolone at 3 mg/day. His diabetes is currently managed with glimepiride at 0.5 mg/day, empagliflozin at 10 mg/day, imeglimine at 2000 mg/day, and dulaglutide at 0.75 mg/week. The dulaglutide supply was limited. Thus, tirzepatide replaced dulaglutide. Imeglimine was enabled to be free after 38 days. Furthermore, tirzepatide was also discontinued due to weight loss.
Table 2.
Treatment course of case 2.
Table 2.
Treatment course of case 2.
| Administration |
Before 4 days |
After 24 days |
| HbA1c (%) |
7.8 |
- |
| LDL/HDL/TG (mg/dL) |
82/51/99 |
- |
| eGFRcr (mL/min/1.73m2) |
70.7 |
- |
| AST/ALT (IU/L) |
17/10 |
- |
| Body weight (kg) |
54.0 |
52.7 |
(Case 3) A 74-year-old male patient with an 11-year diabetes duration and Category II cancer reported a preexisting nonfunctioning adrenal tumor, along with arteriosclerosis obliterans, hypertension, and hyperuricemia. His medications included dapagliflozin (5 mg/day), voglibose (0.6 mg/day), pioglitazone 15 mg/day), and insulin degludec/insulin injections (twice a day). Voglibose was discontinued. The frequency of insulin injections was reduced from twice a day to once a day after 22 days.
Table 3.
Treatment course of case 3.
Table 3.
Treatment course of case 3.
| Administration |
Before 6 days |
After 22 days |
| HbA1c (%) |
8.8 |
7.8 |
| LDL/HDL/TG (mg/dL) |
98/64/150 |
73/50/101 |
| eGFRcr (mL/min/1.73m2) |
50.8 |
54.6 |
| AST/ALT (IU/L) |
16/11 |
15/8 |
| Body weight (kg) |
71.2 |
71.0 |
(Case 4) A 76-year-old male patient with approximately 26-year diabetes duration and Category II reported a history of angina pectoris. The patient received insulin injections four times a day. Insulin aspart injections three times a day were discontinued, and the amount of insulin injections was considerably reduced after the tirzepatide initiation.
Table 4.
Treatment course of case 4.
Table 4.
Treatment course of case 4.
| Administration |
Before 5 days |
After 15 days |
| HbA1c (%) |
7.7 |
7.5 |
| LDL/HDL/TG (mg/dL) |
89/56/71 |
85/46/69 |
| eGFRcr (mL/min/1.73m2) |
70.5 |
63.6 |
| AST/ALT (IU/L) |
21/21 |
18/18 |
| Body weight (kg) |
53.0 |
- |
Vertical bold lines indicate the timing of tirzepatide administration. The grayscale denotes the TIR range.
4. Results
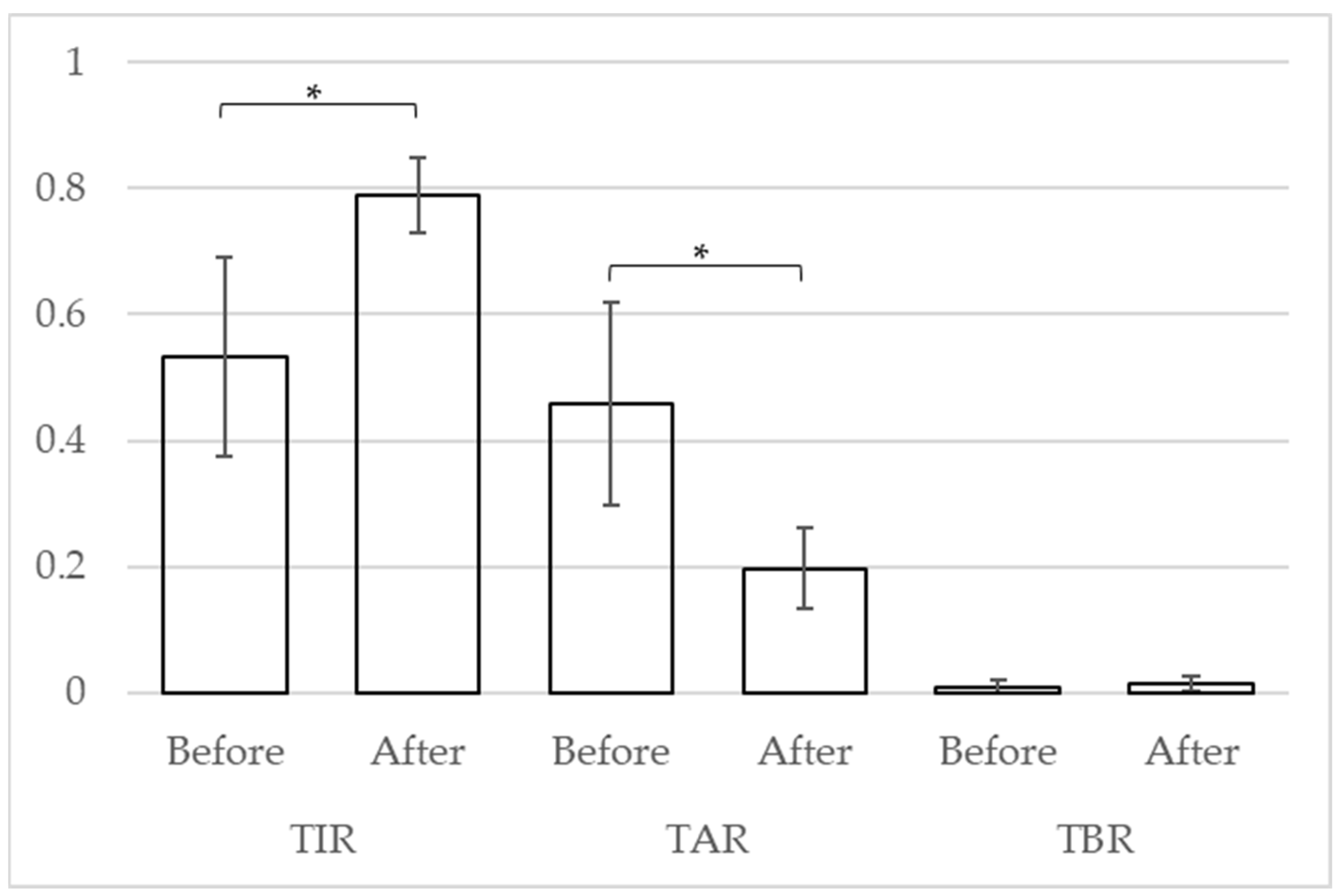
A t-test was used to compare the CGM indices of the four cases. After the tirzepatide treatment, the mean of TIR, TAR, and TBR improved from 53.2% (standard deviation [SD]: 18.2) to 78.9% (SD: 6.8) (p = 0.041), from 45.8% (SD: 18.6) to 19.7% (SD: 7.4) (p = 0.038), and from 1.0% (SD: 1.4) to 1.5% (SD: 1.5) (p = 0.206), respectively.
Figure 2.
Change in CGM Indicators. The vertical axis indicates the proportion of each indicator. * p < 0.05.
Figure 2.
Change in CGM Indicators. The vertical axis indicates the proportion of each indicator. * p < 0.05.
4. Discussion
Herein, we presented four cases of CGM recorded by older Japanese patients with T2DM who received the tirzepatide onset. Immediately after the tirzepatide administration, hyperglycemia improved and TIR increased in older patients with T2DM. Furthermore, the mean TBR increased, but this short-term observation of some patients did not demonstrate significant differences. Three of the four patients were Category II (mild cognitive impairment to mild dementia or instrumental activity of daily living [ADL] decline and basic ADL independence) [
8,
9]. This analysis focused on older patients who were prefrail. The early and potent hypoglycemic effect reduced the number of daily oral hypoglycemic medications and insulin injections. Correcting hyperglycemia while avoiding hypoglycemia is particularly important in older patients with T2DM [
11]. Therefore, tirzepatide administration could be a useful treatment option for older patients with T2DM.
Conversely, a strong weight loss effect of tirzepatide has been reported [
12,
13,
14], including rapid weight loss in three of four patients. Correction of obesity may be beneficial in older patients with T2DM with obesity, but weight loss may be a risk factor for frailty/sarcopenia. One patient discontinued tirzepatide because of weight loss. An increase in the tirzepatide dose from the initial dose is expected to improve the weight loss effect. However, whether nonobese older patients with T2DM will benefit only from tirzepatide if they continue to receive it remains unknown. Additionally, developing and implementing diabetes treatments that can extend healthy life expectancy is a real need, as the life expectancy of people with T2DM increases. Therefore, longer-term observation in the Japanese patient population, with a large proportion of older and nonobese patients, is essential.
This study has some limitations due to the small number of cases analyzed and the short duration of CGM use. However, the experience gained from its application in older patients was deemed valuable.
5. Conclusions
The CGM index immediately improved after the tirzepatide administration. In the short term, tirzepatide may potentially decrease the requirement for multiple medications and streamline treatment regimens. The long-term effects of tirzepatide in a population that includes the older and nonobese individuals will be investigated in future studies.
Author Contributions
T.O. and H.T. conceptualized the study. T.O. and A.I. collected the clinical data. T.O. and H.T. drafted the manuscript. All authors have reviewed and approved the final version for publication.
Funding
This study was supported by JSPS MEXT KAKENHI (JP23K16812) from the Japan Society for the Promotion of Science, Chukyo Medical Research Grant (2023.3.7.9) from Chukyo longevity medical research and promotion foundation, and Research Funding for Longevity Science (21-1) from the National Center for Geriatrics and Gerontology. The funders played no role in the preparation of the manuscript.
Institutional Review Board Statement
This trial was conducted in accordance with the 1964 Declaration of Helsinki ethical standards and later amendments. This study was approved by the local ethics committee of the National Centre for Geriatrics and Gerontology.
Informed Consent Statement
Data Availability Statement
Anonymized data will be available on request to any qualified investigator after approval by the Ethics Committee.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akihiro Hamazaki, Nobuya Inagaki. "Manglaro(R) Subcutaneous Injection (Tirzepatide)." Diagnosis and Treatment 111(7): 983-987, 2023. [in Japanese].
- Hiroaki Satoh, Daisuke Yabe. "Differentiation and Positioning of Sustained GIP/GLP-1 Receptor Agonists." Japan Medical Journal, No. 5183, issued on August 26, 2023, p. 53. [in Japanese].
- Seino Y, Yabe D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J Diabetes Investig. 2013 Mar 18;4(2):108-30. [CrossRef]
- Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022 Sep;10(9):623-633. [CrossRef]
- Chisato Kusunoki-Tsuji, Hiroshi Maegawa. Impact of Obesity on Medical Care in Japanese Patients With Diabetes Mellitus. Journal of the Japan Diabetes Society. Vol. 63, No. 7, pp. 422-426, July 30, 2020. [in Japanese].
- Battelino T, Bergenstal RM, Rodríguez A, Fernández Landó L, Bray R, Tong Z, Brown K. Efficacy of once-weekly tirzepatide versus once-daily insulin degludec on glycaemic control measured by continuous glucose monitoring in adults with type 2 diabetes (SURPASS-3 CGM): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022 Jun;10(6):407-417. [CrossRef]
- Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, Cobelli C, Dassau E, DeVries JH, Donaghue KC, Dovc K, Doyle FJ 3rd, Garg S, Grunberger G, Heller S, Heinemann L, Hirsch IB, Hovorka R, Jia W, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Levine B, Mayorov A, Mathieu C, Murphy HR, Nimri R, Nørgaard K, Parkin CG, Renard E, Rodbard D, Saboo B, Schatz D, Stoner K, Urakami T, Weinzimer SA, Phillip M. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019 Aug;42(8):1593-1603. [CrossRef]
- Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes; Haneda M, Ito H. Glycemic targets for elderly patients with diabetes. Diabetol Int. 2016 Nov 29;7(4):331-333. [CrossRef]
- Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes. Glycemic Targets for Elderly Patients with Diabetes. Geriatr Gerontol Int. 2016 Dec;16(12):1243-1245. [CrossRef]
- Omura T, Tamura Y, Sakurai T, Umegaki H, Iimuro S, Ohashi Y, Ito H, Araki A; Japanese Elderly Diabetes Intervention Trial Research Group. Functional categories based on cognition and activities of daily living predict all-cause mortality in older adults with diabetes mellitus: The Japanese Elderly Diabetes Intervention Trial. Geriatr Gerontol Int. 2021 Jun;21(6):512-518. [CrossRef]
- Omura T, Araki A. Skeletal muscle as a treatment target for older adults with diabetes mellitus: The importance of a multimodal intervention based on functional category. Geriatr Gerontol Int. 2022 Feb;22(2):110-120. Epub 2022 Jan 5. [CrossRef] [PubMed]
- Aronne LJ, Sattar N, Horn DB, Bays HE, Wharton S, Lin WY, Ahmad NN, Zhang S, Liao R, Bunck MC, Jouravskaya I, Murphy MA; SURMOUNT-4 Investigators. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA. 2023 Dec 11:e2324945. [CrossRef]
- Tsukamoto S, Tanaka S, Yamada T, Uneda K, Azushima K, Kinguchi S, Wakui H, Tamura K. Effect of tirzepatide on glycaemic control and weight loss compared with other glucagon-like peptide-1 receptor agonists in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2024 Jan;26(1):262-274. [CrossRef]
- Nauck MA, D’Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc Diabetol. 2022 Sep 1;21(1):169. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).