Submitted:
26 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
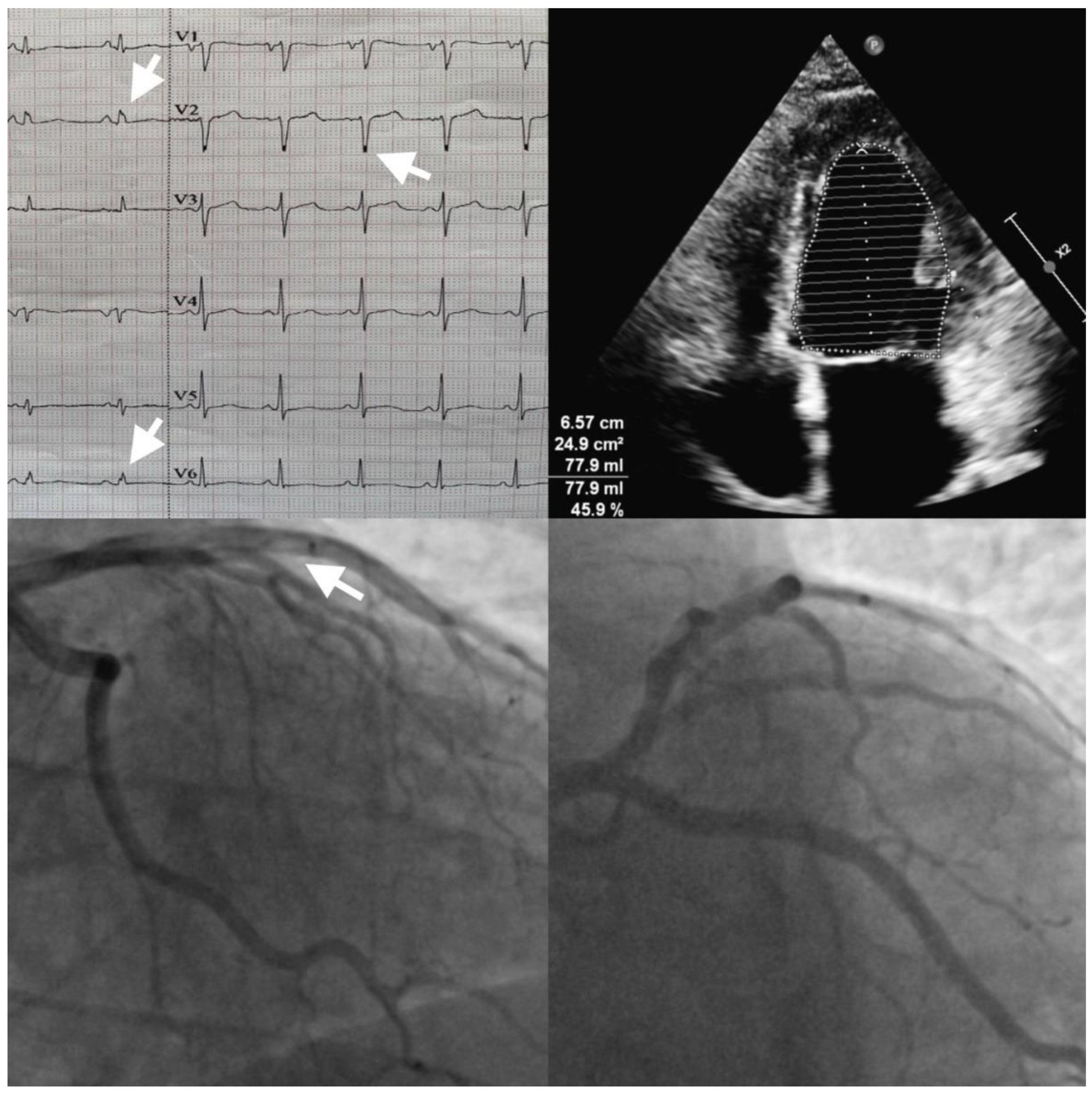
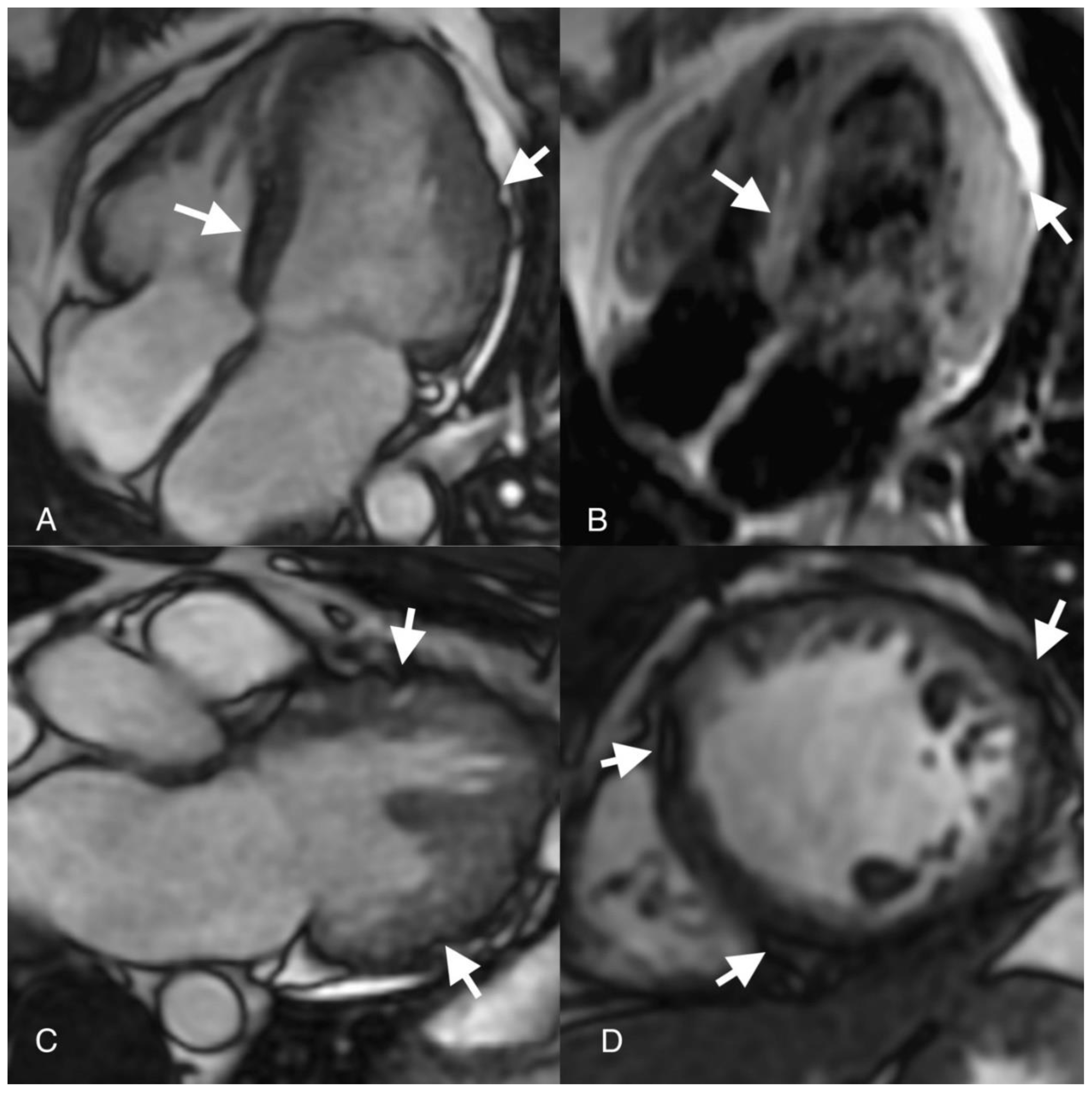
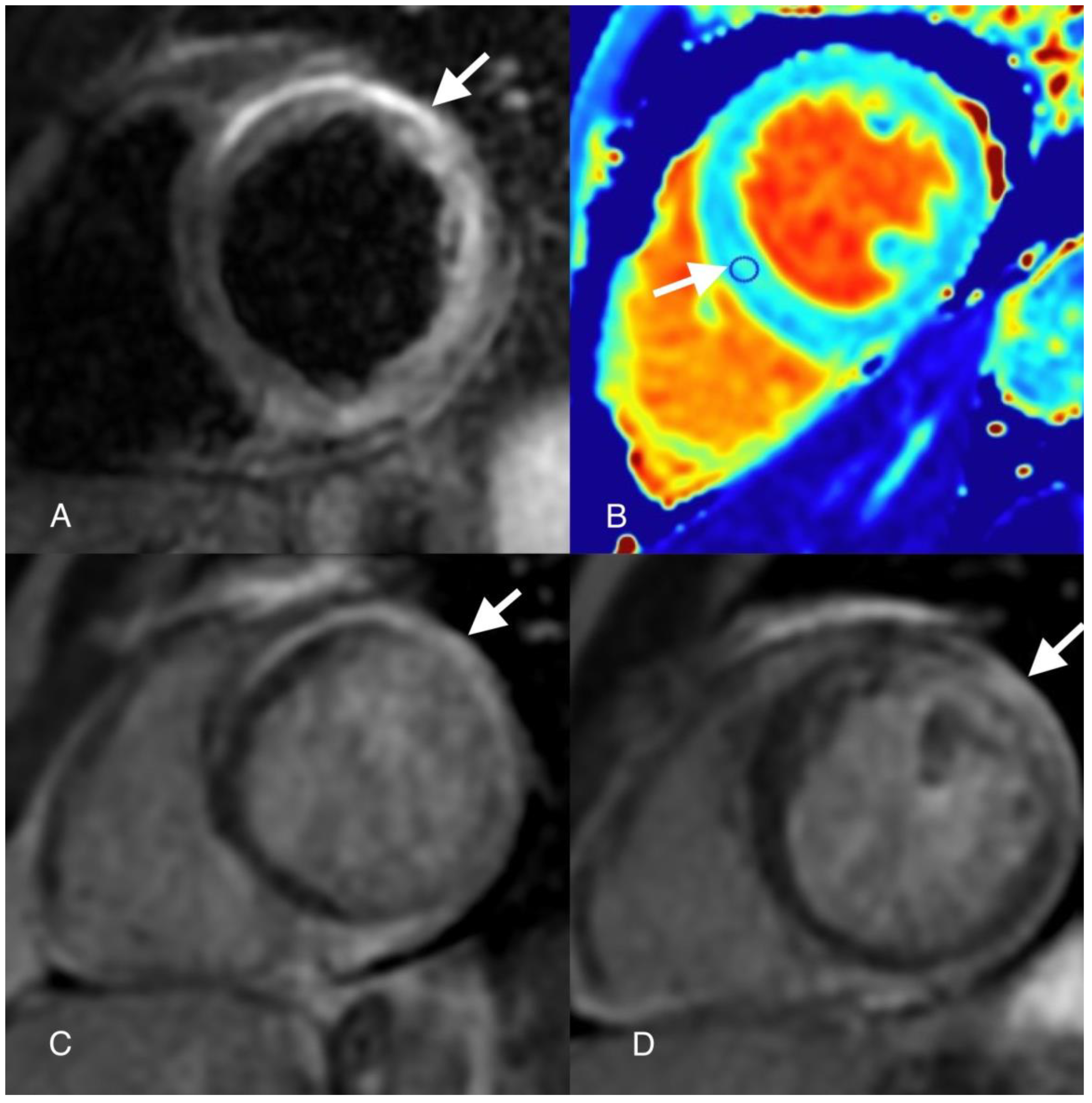
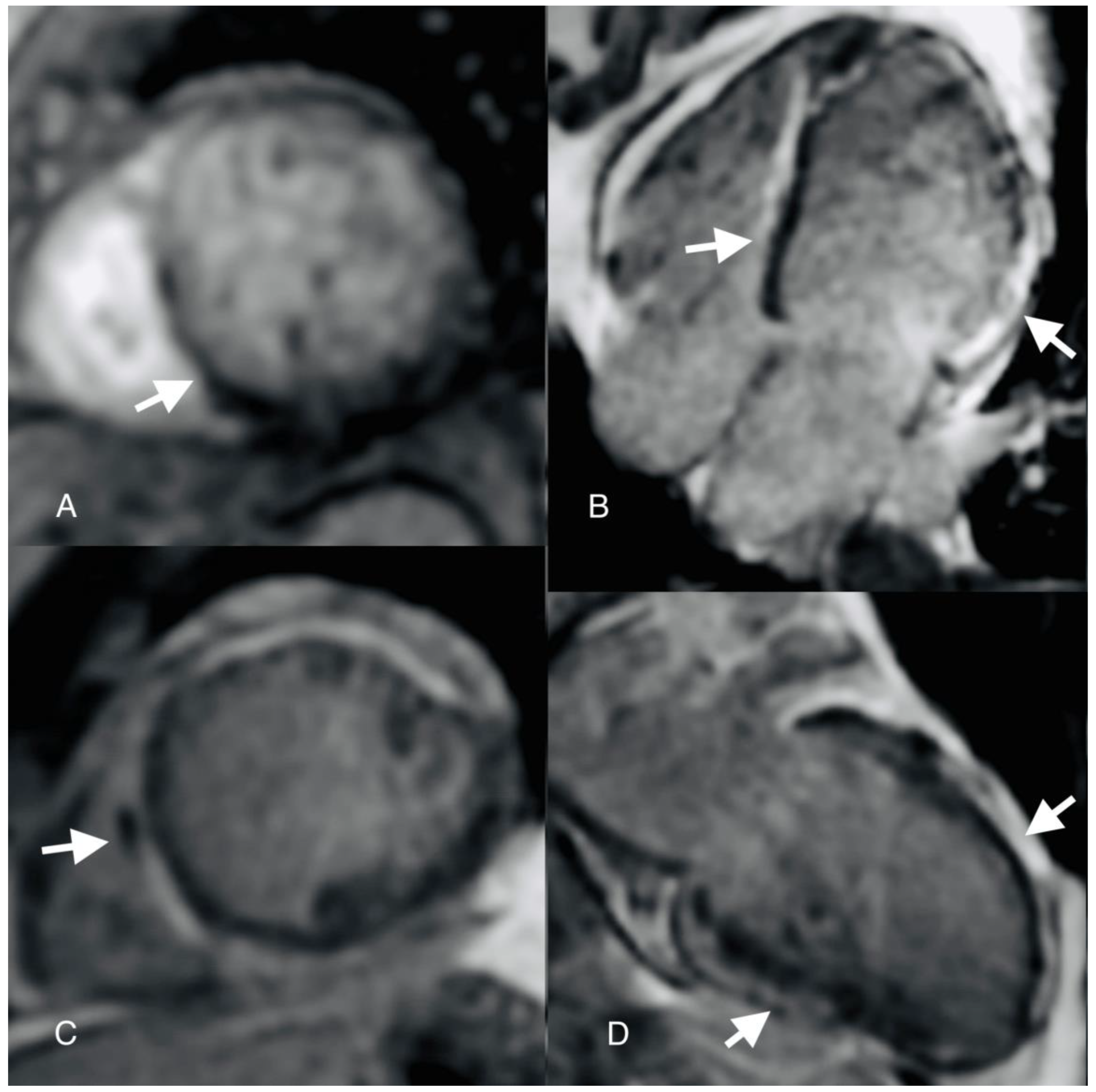
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari M, Migliore F, Pilichou K, Rampazzo A, Rigato I, Rizzo S, Thiene G, Anastasakis A, Asimaki A, Bucciarelli-Ducci C, Haugaa KH, Marchlinski FE, Mazzanti A, McKenna WJ, Pantazis A, Pelliccia A, Schmied C, Sharma S, Wichter T, Bauce B, Basso C. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int J Cardiol. 2020 Nov 15;319:106-114. [CrossRef]
- Asatryan B, Asimaki A, Landstrom AP, Khanji MY, Odening KE, Cooper LT, Marchlinski FE, Gelzer AR, Semsarian C, Reichlin T, Owens AT, Chahal CAA. Inflammation and Immune Response in Arrhythmogenic Cardiomyopathy: State-of-the-Art Review. Circulation. 2021 Nov 16;144(20):1646-1655. [CrossRef]
- Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmo- genic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983–991. [CrossRef]
- Reichl K, Kreykes SE, Martin CM, Shenoy C. Desmoplakin variant-as- sociated arrhythmogenic cardiomyopathy presenting as acute myo- carditis. Circ Genom Precis Med. 2018;11:e002373. [CrossRef]
- Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. (1997) 30:1512–20. [CrossRef]
- Campuzano O, Alcalde M, Iglesias A, Barahona-Dussault C, Sarquella-Brugada G, Benito B, et al. Arrhythmogenic right ventricular cardiomyopathy: severe structural alterations are associated with inflammation. J Clin Pathol. (2012) 65:1077–83. [CrossRef]
- Bariani R, Rigato I, Cipriani A, Bueno Marinas M, Celeghin R, Basso C, Corrado D, Pilichou K, Bauce B. Myocarditis-like Episodes in Patients with Arrhythmogenic Cardiomyopathy: A Systematic Review on the So-Called Hot-Phase of the Disease. Biomolecules. 2022 Sep 19;12(9):1324. [CrossRef]
- Martins D, Ovaert C, Khraiche D, Boddaert N, Bonnet D, Raimondi F. Myocardial inflammation detected by cardiac MRI in Arrhythmogenic right ventricular cardiomyopathy: A paediatric case series. Int J Cardiol. 2018 Nov 15;271:81-86. [CrossRef]
- Bowles NE, Ni J, Marcus F, Towbin JA. The detection of cardiotropic viruses in the myocardium of patients with arrhythmogenic right ventriculardysplasia/cardiomyopathy. J Am Coll Cardiol. (2002) 39:892–5.
- Campian ME, Verberne HJ, Hardziyenka M, de Groot EA, van Moerkerken AF, van Eck-Smit BL, Tan HL. Assessment of inflammation in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Nucl Med Mol Imaging. 2010;37:2079–2085. [CrossRef]
- Protonotarios A, Wicks E, Ashworth M, Stephenson E, Guttmann O, Savvatis K, Sekhri N, Mohiddin SA, Syrris P, Menezes L, et al. Prevalence of 18F- fluorodeoxyglucose positron emission tomography abnormalities in pa- tients with arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol. 2019;284:99–104. [CrossRef]
- Poller W, Haas J, Klingel K, Kühnisch J, Gast M, Kaya Z, Escher F, Kayvanpour E, Degener F, Opgen-Rhein B, et al. Familial recurrent myocarditis triggered by exercise in patients with a truncating variant of the desmoplakin gene. J Am Heart Assoc. 2020;9:e015289. [CrossRef]
- Mavrogeni S, Protonotarios N, Tsatsopoulou A, Papachristou P, Sfendouraki E, Papadopoulos G. Naxos disease evolution mimicking acute myocarditis: the role of cardiovascular magnetic resonance imaging. Int J Cardiol. (2013) 166:e14–5. [CrossRef]
- Zhu Q, Wang S, Huang X, Zhao C, Wang Y, Li X, Jia D, Ma C. Understanding the pathogenesis of coronary slow flow: Recent advances. Trends Cardiovasc Med. 2022 Dec 11:S1050-1738(22)00142-6. [CrossRef]
- Woudstra L, Juffermans LJM, van Rossum AC, Niessen HWM, Krijnen PAJ. Infectious myocarditis: the role of the cardiac vasculature. Heart Fail Rev. 2018 Jul;23(4):583-595. [CrossRef]
- Paul M, Rahbar K, Gerss J, Kies P, Schober O, Schäfers K, Breithardt G, Schulze-Bahr E, Wichter T, Schäfers M. Microvascular dysfunction in nonfailing arrhythmogenic right ventricular cardiomyopathy. Eur J Nucl Med Mol Imaging. 2012 Mar;39(3):416-20. [CrossRef]
- Gerull, B.; Brodehl, A. Insights Into Genetics and Pathophysiology of Arrhythmogenic Cardiomyopathy. Curr. Heart Fail. Rep. 2021, 18, 378–390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




