1. Introduction
PE is a complex pregnancy-specific syndrome characterized by multiple disorders including hypertension, proteinuria, thrombocytopenia, impaired liver function, renal insufficiency, pulmonary, etc. The only effective treatment currently for PE is delivering the fetus and placenta, and finding new therapies is extremely urgent [
1,
2,
3]. Although the pathogenesis of PE is still largely unknown, plenty of studies have indicated that immune mechanisms and the renin-angiotensin system are implicated in PE[
4,
5]. Women with PE have elevated concentrations of AT1-AA, antiangiogenic factors (such as sFlt1 and s-endoglin), inflammation, oxidative stress, endothelial dysfunction, and increased sensitivity to Angiotensin II(ANG II)[
6,
7,
8,
9]. AT1-AA was first characterized in 1999 by a group of researchers in Germany[
10]. They isolated AT1-AA from the PE serum, and these antibodies could stimulate the number of beats per minute of rat cardiomyocytes, which had a similar function to ANGII[
11,
12]. Recent studies have shown that AT1-AA can enhance the binding with AT1R and stimulate the downstream effects of ANG II, such as the expression of sFlt-1, sEng, and ET-1 [
6,
13]. These suggest that AT1-AA may play a critical role in the pathogenesis of PE, but previous research is still unable to clarify the mechanisms for AT1-AA production.
In healthy individuals, necrosis and apoptotic cells are promptly and effectively removed by professional phagocytes like macrophages, and dendritic cells [
14]. This process is composed of recognition and engulfment, which is important to prevent autoimmune response and inflammation against intracellular antigens[
15]. In PE there is a well-documented increase in the amount of trophoblast excessive apoptosis and autophagy[
16]. Accelerated cell death can induce abnormal autoantigen generation, meanwhile, defective phagocytosis of macrophages will attenuate the ability of autoantigen removal, and all above will stimulate autoantibody accumulation[
17].
Milk fat globule epidermal growth factor 8 (MFG-E8) is a glycoprotein produced by thioglycolate-elicited macrophages. It can bind to apoptotic cells by recognizing phosphatidylserine, which plays a critical role in maintaining homeostasis[
18]. When MFG-E8 is engaged by phospholipids, it can bind to cells via the Arg-Gly-Asp motif, particularly strongly to cells expressing the αvβ3 integrin[
19].MFG-E8 works as a bridge between apoptotic cells and phagocytes and promotes engulfment of apoptotic cells by phagocytes[
19,
20,
21,
22]. There are several reports indicating that MFG-E8 deficiency exacerbates the induction of autoantibody, furthermore, the differentiated macrophages show decreased phagocytosis of apoptotic neutrophils because of the lower levels of MFG-E8 expression[
23,
24]. However, neither the relationship between MFG-E8 and AT1-AA in PE nor the expression and function of MFG-E8 in PE has been elucidated.
In this study, we first found that the expression of placental MFG-E8 decreased in PE patients, while the expression of placental AT1-AA increased. This result shows that there may be a certain relationship between MFG-E8 and AT1-AA.In order to verify our hypothesis, we investigated the direct effect of MFG-E8 on LPS-induced PE rats. Furthermore, we utilized the trophoblast cell line co-culture with macrophage to detect the possible mechanism of MFG-E8. Our findings might explain the therapeutic effect of MFG-E8 on PE.
2. Materials and Methods
2.1. Materials and reagents
MFG-E8 antibody and ATR1 antibody were purchased from Affinity (USA);LPS and Amobarbital Sodium were purchased from Sigma-Aldrich(USA); MFG-E8 was purchased from Sino Biological(China);Mouse Monoclonal Anti-β-actin, HRP-labeled Goat Anti-Mouse IgG and HRP-labeled Goat Anti-Rabbit IgG were purchased from ZSGB-BIO(USA); DAB Substrate Kit,TUNEL Apoptosis Detection Kit, Human Milk Fat Globule EGF Factor 8 (MFGE8)ELISA kit and Human AT1-AA kit were purchased from R&D (USA);The BCA protein assay kit was purchased from Pierce (USA). Rat TNF-α ELISA kit was purchased from Elabscience(USA); Rat sLFt-1 ELISA kit were purchased from Shenyan Biology(China); Annexin V-FITC cell apoptosis detection kit was manufactured by BD Biosciences (USA).
2.2. Study Cohort
Blood samples and placenta samples were obtained from 40 women. Pregnant women with PE(n=20) and healthy pregnant women(n=20) with similar characteristics including age, body mass index, gestational ages were selected from the first affiliated hospital of Wenzhou medical university between June 2020 to June 2021.PE was diagnosed according to the International Federation of Gynecology and Obstetrics. Women with multiple pregnant, diabetes mellitus, chronic hypertension, and kidney disease, autoimmune disease, infectious disease and other complications of pregnancy were excluded. Blood sample were collected for routine prenatal examinations. After centrifugation, serum were collected and stored at -80℃.Both blood and placenta samplings were obtained after informed consent and approved by the Ethical Committee of the first affiliated hospital of Wenzhou medical university.
2.3. Animals
Female Sprague-Dawley rats (10-12weeks old, weighing 220-250g) were purchased from the Experimental Animal Center of Wenzhou medical university. They were housed individually in plastic cages with wood chips as bedding under pathogen-free conditions, in a controlled environment of temperature at 20–25 ◦ C and 12 h cycles of light and dark. Rats were fed a standard laboratory diet and water ad libitum. The female rats were caged with fertile male rats overnight. A positive vaginal smear for spermatozoa was defined as day 1 of pregnancy (duration of gestation is 21 days). All of the animal processes were conducted according to relevant national and international guidelines. All studies involving rats were approved by the committee for experimental animals of Wenzhou medical university.
2.4. Experimental protocol
Rats were randomly divided into control group (n=8), LPS group (n=8),LPS+MFG-E8(n=6).Experimental PE was induced by infusion of LPS (1μg/kg body weight) in 2 ml of sterile saline through an infusion pump into the tail vein (infusion rate, 2 ml/h) on day 14 of pregnancy[
16]. 1mg/kg MFG-E8 in 2ml saline was administrated intraperitoneally 30min prior to LPS on day 14 and once daily through day 19 of pregnancy. Normal pregnant control rats were infused with equal saline alone.
2.5. Measurement of systolic blood pressure
The systolic blood pressures (SBPs) were determined in conscious, restrained pregnant rat once every 2 days in the indicated time from day 10 through 20 of pregnancy. We use a non-invasive sphygmomanometer designed for rats to measure blood pressure[
13]. The pregnant rat being tested is placed in a quiet and warm room for half an hour to allow it to acclimate to the environment. Afterward, the pregnant rat is securely restrained for the test. When the pregnant rat is calm, the cuff is placed at the base of the tail. Measure 5 times consecutively and calculate the average value.
2.6. Determination of urinary albumin excretion
For 24h urine collection, on gestational day 12 and 19, the rats were placed in metabolic cages. To avoid contaminating the collected urine, rats were restricted from food rather than water. Rats were fed in other cages for 30 min every 6 h to avoid the adverse effects of fasting. Urine samples were centrifuged at 2000 rpm for 15 min at room temperature, and supernatant was stored at −80 ◦ C for urinary albumin analysis. Urine protein concentrations were determined with a BCA protein assay kit using bovine serum albumin as standard.
2.7. Specimen collection
On day 14 of pregnancy before saline or LPS administration, about 0.5 ml blood specimen was drawn from tail vein. On day 20 of pregnancy, after the rats were anes- thetized, about 1.5 ml blood specimen was drawn by heart punctio. Serum was stored at −80 ◦ C for further assessment. Fetal pups and placentae were removed and weighed. Three placentae were randomly selected from each rat and fixed with 10% neutral-buffered formalin for histological evaluation. Three placentae from each rat were stored at −80 ◦ C for subsequent examination and analysis.
2.8. TUNEL assay
Apoptosis was detected by TUNEL assay. The sections were treated according to the cell death detection kit manufacturer’s instructions. Apoptosis was determined by TUNEL assay. The sections were examined according to the cell death detection kit manufacturer’s instructions. More than 1,000 trophoblast cell nuclei were counted in each experimental group, and the average percentage of trophoblast tunel-positive nuclei was determined [
17].
2.9. H&E, IHC of tissue
For histological evaluation, placentas were fixed in 4% neutral-buffered polyformaldehyde overnight at room temperature. Tissues were infiltrated and embedded in paraffin. H&E staining was performed by standard techniques on 4-μm paraffin sections of placenta specimens for conventional morphological evaluation with light microscopy (Nikon Eclipse 90i ,Japan).
For the assessment of AT1-AA and MFG-E8 expressions in human, we performed routine immunohistochemistry experiments. Concisely, the sections were deparaffinized and subjected to antigen retrieval in a sodium citrate solution (pH 6.0). Then incubated with different primary antibodies at 4 °C overnight. Sections were then washed and incubated with the secondary antibody for 45 minutes at room temperature. The sections were subsequently incubated with DAB substrate, lightly counterstained with hematoxylin, dehydrated, and mounted. The prepared sections of IHC staining were examined with light microscopy (Nikon Eclipse 90i ,Japan).
2.10. MFG-E8 siRNA transfection experiments
To study trophoblast MFG-E8 RNA silencing, HTR-8/SVneo cells were cultured in 6-well plates to 70% confluency. The MFG-E8 sequence siRNA vector (Has-MFGE8-siRNA-3, 5’- CCUGGAGAAUGGGAACAUUTT-3’) was designed. The siRNA vector of the recombinant sequence (5’- UUCUCCGAACGUGUCACGUTT-3’) was used as a negative control. According to the routine methods established in the laboratory, Lipofectamine-3000 reagent was used to transfect HTR-8/SVneo cells constructed with each siRNA, and different detections were performed 48 hours later.
2.11. Cell culture and treatment
Human first-trimester trophoblast cell line HTR-8/ SVneo and human monocytic cell line THP-1 were purchased from BeNa Culture Collection (China). Cells were cultured in 1640 medium with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin G and 100 U/mL streptomycin in a humidified atmosphere of 37°C with 5% CO2. To prepare dead trophoblast cells, HTR-8/SVneo cells were primed with 1 μg/ml LPS for 24 h.
2.12. Phagocytosis assay of dead trophoblasts
To obtain the effective phagocytic ability of macrophages, THP-1 cells were differentiated into macrophages by incubating with 100 ng/ml PMA for 48 h in the culture plate, then washing twice with a PMA-free medium and resting for 24h. Then nonadherent macrophages, and added apoptotic trophoblast cells into the macrophage culture at a ratio of 1:3 (macrophages: trophoblasts) for co-culture.After 4h cultivation,the fixed macrophages were subjected to CD68 staining and TUNEL assay and observed by light microscopy.phagocytosis was detected by phase-contrast light microscopy and quantified as a phagocytic index(the number of engulf RBCs per 100 macrosphages).
2.13. FACS analysis of apoptosis
Annexin V-FITC and PI apoptosis detection kits were used according to the manufacturer’s instructions to measure cell apoptosis. The trophoblast cell apoptotic rate was analyzed with a FACScan flow cytometer (BD, Biosciences).
2.14. Western blot
Total protein, nuclear protein, and cytoplasmic protein were extracted using ProteoJETTM and NE-PER nuclear and cytoplasmic extraction reagents respectively. Determine protein concentration using a BCA protein assay kit. Equal amounts (40μg) of protein were subjected to 12% SDS-PAGE electrophoresis and transferred to nitrocellulose membranes. Different primary antibodies were incubated with the membrane overnight at 4°C. β -actin served as an internal control. The bound antibody was detected by an enhanced chemiluminescence on an X-ray film.
2.15. Enzyme-linked immunosorbent assay (ELISA)
We determined the concentrations of MFG-E8,AT1-AA,TNF-α,sFlt-1 in human, mouse serum, and cell culture with commercial ELISA kits according to the manufacturer’s instructions.
2.16. Statistical analysis
All statistical analyses were done using the SPSS 19.0 software. Total values were expressed as mean± S.E.M and analyzed by a 1-way ANOVA. Multiple comparison between the groups was performed using S-N-K method. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Clinical characteristics of the study participants
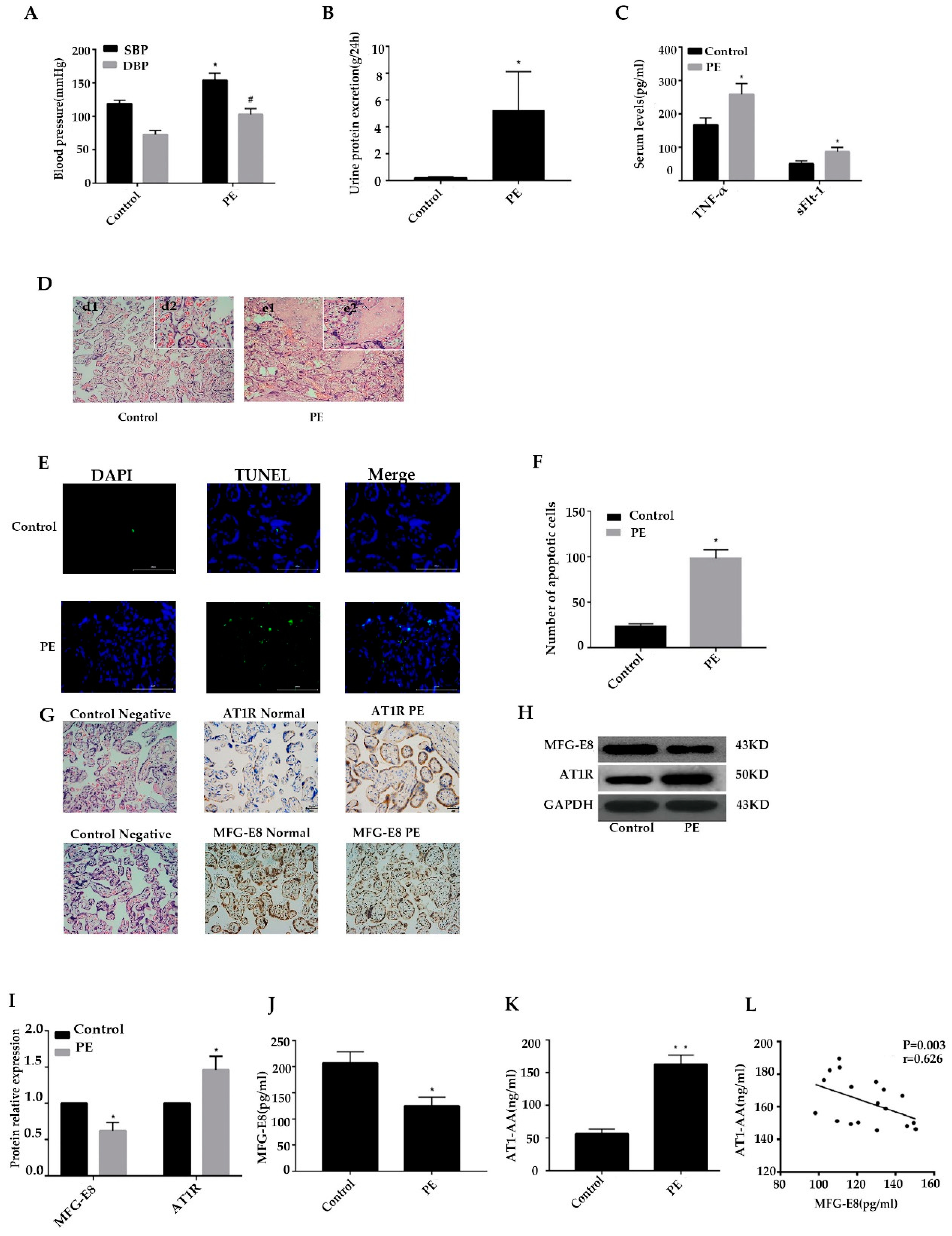
20 cases with PE and 20 healthy pregnant controls were included in the study. PE patients presented with hypertension, proteinuria, hypoproteinemia, renal insufficiency, preterm delivery, and low birth weight. There were statistically significant differences in systolic blood pressure, diastolic blood pressure,24h urinary protein, serum TNF-α and sLFt-1 levels conpared to the control(p <0.05). (
Figure 1A-C).
3.2. Placental morphological changes in PE patients vs healthy controls
Normal placenta presented with abundant and clarified structured villi, which could promote the transportation of gases and nutrients between mother and fetus. The placenta of the PE group has dense villous structure, narrow spaces, increased syncytial nodules, degeneration and necrosis of villi, and deposition of fibrinoid material (
Figure 1D).
3.3. PE induced placental trophoblast apoptosis
We used the TUNEL method to detect the apoptosis of placental trophoblast cells in the two groups. The number of apoptosis of placental trophoblast cells in the preeclampsia group was significantly greater than that in the control group (p<0.05) (
Figure 1E,F).
3.4. MFG-E8 and AT1R expressed differently in the PE placenta and peripheral blood
We compared MFG-E8 and AT1R expressions in the placenta of two groups by IHC and western blotting. As shown in
Figure 1G, we found MFG-E8 and AT1R expressed differently in the PE placenta, and then we compared the differentiated expressions by western blotting. IHC and WB results showed that placental MFG-E8 expressions downregulated significantly in PE, while placental AT1R expression was shown obviously increased in
Figure 1H, I(p <0.05).
The level of MFG-E8 and AT1R in the peripheral blood of the two groups was detected by ELISA. In
Figure 1J-L the mean level of MFG-E8 in the blood of the PE group was 124.5±16.6pg/ml, lower than that of the control group (206.5±21.7pg/ml) (p<0.05). The AT1-AA level in the PE group was 163.09±13.5ng/ml, higher than that in the control group (56.3±7.5ng/ml) (p<0.01). Interestingly, there were negative correlations between MFG-E8 and AT1-AA expression in PE peripheral blood.
3.5. MFG-E8 alleviated the symptoms of PE in rats
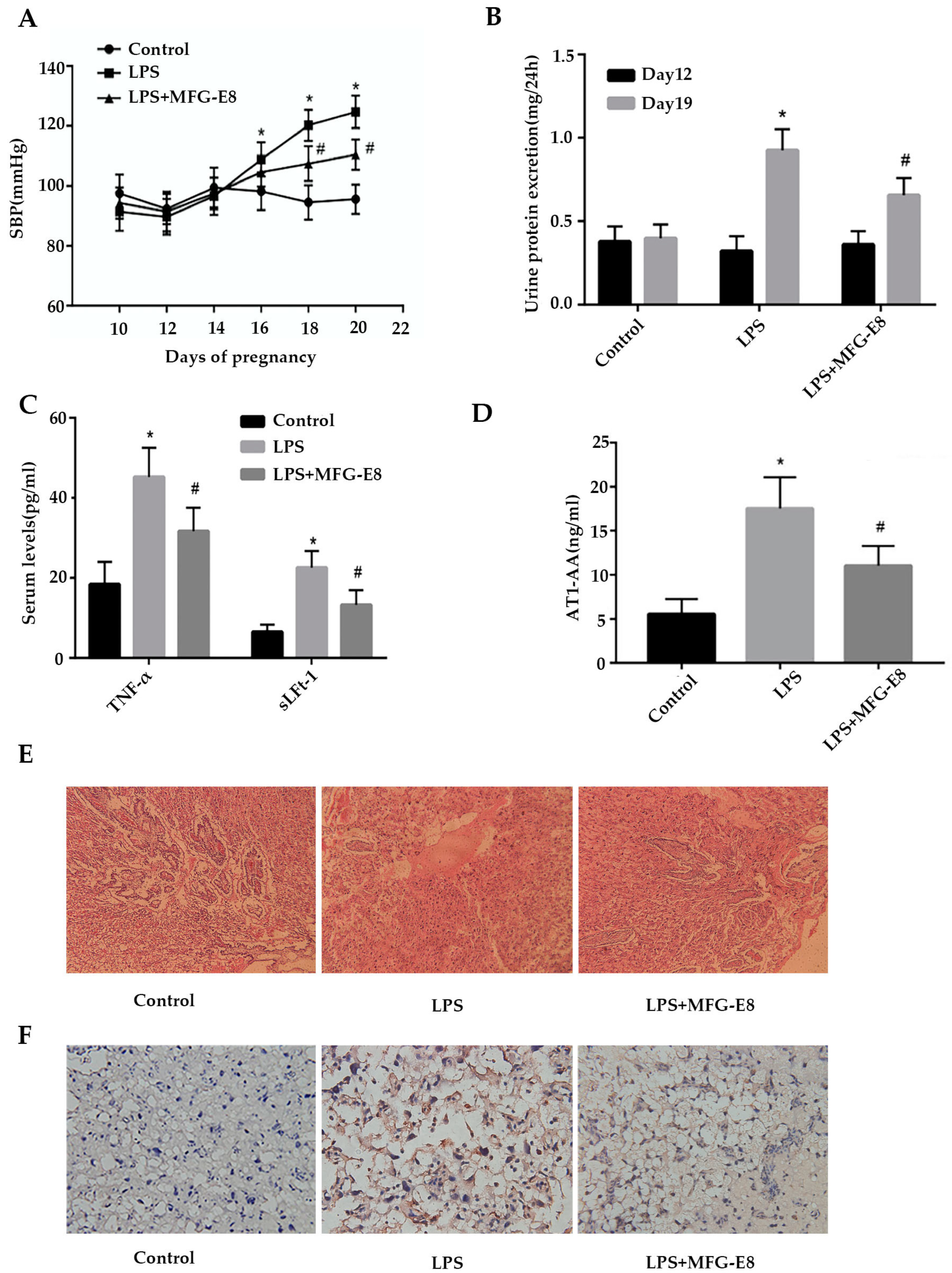
To verify the above clinical data in animal models of PE, we constructed PE model in female Sprague-Dawley rats by injecting an ultra-low-dose LPS. In the current study, none rats that were exposed to low-dose LPS infusion developed any sign of behavior changes or loss of appetite.No fetal growth restricted and demise were observed in any groups. It had been demonstrated that the systolic blood pressure in the control group did not change significantly at day 20 of gestation, but in the LPS group, systolic blood pressure increased significantly after 1 µg/kg LPS was injected at day 14 of gestation. At day 16,18,20 of gestation, systolic blood pressure increased dramatically in the LPS group and LPS + MFG-E8 group than the control group at the corresponding time (p<0.05). Moreover, in rats treated with 1 mg/kg MFG-E8 every day from the 14th day of gestation for 6 consecutive days, the systolic blood pressure in the LPS+MFG-E8 group was lower than that at the corresponding time in the LPS group at the 18th and 20th days of gestation (p<0.05
Figure 2A). On the 12th day of gestation, there was no significant difference in the mean 24h urinary protein between the control group and the LPS group before injected saline and LPS (p>0.05,
Figure 2B). However, at day 19 of gestation, 24 h albuminuria level in LPS group was significantly higher than that in the control group (p<0.05,). The level of albuminuria at 24 h on day 19 of gestation in the LPS+MFG-E8 group was significantly lower than that in the LPS group (p<0.05
Figure 2B).
3.6. MFG-E8 reduces the levels of TNF-α, sLFt-1, and AT1-AA in the blood of LPS-induced PE rats.
We detected the levels of inflammatory cytokines TNF-α and sLFt-1 in blood of each group by ELISA. As shown in
Figure 2C, serum concentrations of TNF-α and sLFt-1 in LPS group were 45.2±7.34pg/ml and 22.6±4.2pg/ml respectively, which were significantly higher than those in control group (18.4±5.61pg/ml and 6.46.6±1.89pg/ml) (p<0.05). After treated with MFG-E8, serum concentrations of TNF-α and sLFt-1 in LPS+MFG-E8 group were reduced to 36.7±5.90pg/ml and 13.3±3.67pg/ml respectively, compared with LPS group (p<0.05).
Figure 2D showed the level of AT1-AA in each group. The serum AT1-AA concentration in LPS group was 17.5±3.61ng/ml, which was higher than that in control group (5.56±1.71pg/ml) (p<0.05). The concentration of AT1-AA in LPS+MFG-E8 group decreased to 11.1±2.23pg/ml, compared with LPS group (p<0.05).
3.7. MFG-E8 reduced the pathological damage of placenta in LPS-induced PE rats
Compared with the control group, the placental villus capillary wall in the LPS group was thickened and accompanied by fibrinoid necrosis, while the pathological damage of the placenta in the LPS+MFG-E8 group was reduced(
Figure 2E).
Figure 2F was TUNEL pictures of placental tissue. Compared with the control group, placental trophoblast apoptosis increased significantly in the LPS group, while placental trophoblast apoptosis decreased in the LPS+MFG-E8 group.
3.8. Dysfunction of MFG-E8 expression inhibits phagocytosis of dead trophoblast cells by macrophages
Macrophages were transfected with MFG-E8 siRNA and then interfered with by fluorescent quantitative PCR and western blotting. According to the interference efficiency SiRNA-2 group was selected for subsequent experiments (Supplementary Figure1).
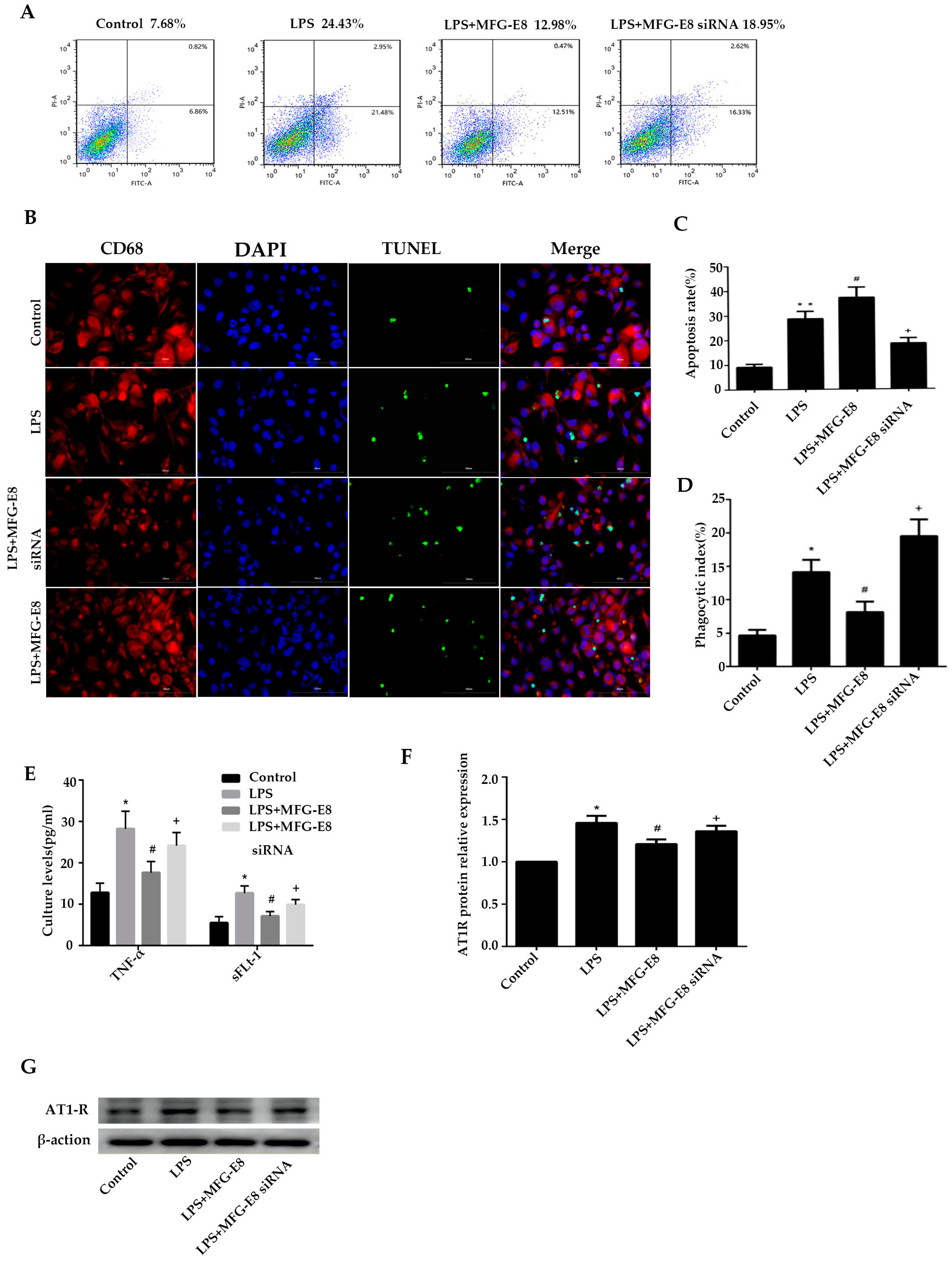
Flow cytometry and immunofluorescence staining were used to detect the phagocytotic efficiency of macrophages in the co-culture model of trophoblast cells and macrophages, as shown in
Figure 3A-B. After HTR8/Svneo cells were stimulated with 100ng/mL LPS for 24 h, the apoptosis rate of cells in the LPS group increased to 28.6±3.0%. The apoptosis rate of macrophages in the LPS+MFG-E8 SiRNA group was further increased to 37.2±4.2% after the MFG gene was silenced (p<0.05, compared with the LPS group).
Figure 3C-D showed the phagocytosis of apoptotic trophoblast cells by macrophages. After stimulating trophoblast cells with LPS, the phagocytosis rate of macrophages increased to 14.1±1.84%. The phagocytosis rate in the LPS+MFG-E8 SiRNA group decreased to 8.12±1.58% after silencing the MFG gene (p<0.05, compared with the LPS group), which was significantly higher than that in the control group (4.65±0.85%). In conclusion, dysfunction of MFG-E8 expression inhibited macrophages from clearing apoptotic trophoblast and increased apoptosis and necrosis of trophoblast.
3.9. MFG-E8 promotes phagocytosis of apoptotic trophoblast cells of macrophages
As shown in
Figure 3A,B. After HTR8/Svneo cells were stimulated with 100ng/mL LPS for 24 h, the apoptosis rate of cells in the LPS group increased to 28.6±3.0%. It was significantly higher than that in the control group (9.1±1.3%, p<0.05), and the apoptosis rate in the LPS+MFG-E8 group decreased to 18.5±2.3% after being treated with 500ng/mL concentration of MFG-E8 (p<0.05, compared with LPS group).
Figure 3C,D showed the phagocytosis of apoptotic trophoblast cells by macrophages. After stimulating trophoblast cells with LPS, the phagocytosis rate of macrophages increased to 14.1±1.84%, which was significantly higher than that in the control group (4.65±0.85%, p<0.05). Moreover, the phagocytosis rate in the LPS+MFG-E8 group increased to 19.5±2.51% after MGG-E8 stimulation (p<0.05, compared with the LPS group). In conclusion, MFG-E8 could promote macrophages to clear apoptotic trophoblast.
3.10. MFG-E8 regulated the production of trophoblastic inflammatory cytokines.
As shown in
Figure 3E, TNF-α and sLFt-1 in the control group were 12.8±2.3pg/ml and 5.5.8±1.5pg/ml respectively, and in the LPS group were increased to 24.2 ±3.2pg/ml and 9.9±1.2pg/ml, respectively. They were significantly higher than that in the control group (p<0.05). After the MFG gene silenced in macrophages, TNF-α and sLFt-1 in the LPS+MFG-E8 siRNA group were dramatically increased to 28.2.±4.2pg/ml and 12.7±1.7pg/ml, respectively, which were significantly higher than those in LPS group (p<0.05). The concentrations of LPS+MFG-E8 were decreased to 17.6±2.7pg/ml and 7.1±1.1pg/ml after being treated with MFG-E8 respectively, which were significantly higher than those of the LPS group (p<0.05). In conclusion, dysfunction of MFG-E8 gene expression in macrophages led to increase in the levels of TNF-α and sLFt-1, while MFG-E8 could decrease both of them in the LPS-stimulated trophoblast-macrophage co-culture model.
3.11. MFG-E8 regulated phagocytosis of AT1R antigen by macrophages
Western blotting was used to detect the AT1R antigen level in the co-culture model of trophoblast and macrophage. As shown in
Figure 3F,G, the AT1R antigen level in the LPS group was significantly higher than that in the control group after LPS stimulated(p<0.05). Meanwhile, it was obviously higher in the LPS+MFG-E8 siRNA group compared with the LPS group (p<0.05). After being treated with MFG-E8, the AT1R antigen level in the LPS+MFG-E8 group was decreased than that in the LPS group (p<0.05). In conclusion, in the co-culture model of trophoblast and macrophage stimulated by LPS, the dysfunction of MFG-E8 gene expression in macrophages inhibited phagocytosis of AT1R antigen, while MFG-E8 could promote phagocytosis of AT1R antigen in macrophages.
4. Discussion
This study revealed several novel finding regarding the relationship between MFG-E8 and AT1-AA. Foremost,PE patients and rat model developed AT1-AA associated with the lack of MFG-E8 expression. Furthermore, MFG-E8 could reduce AT1-AA production and enhance phagocytosis of macrophages via mediating trophoblast apoptosis and AT1R exposure. Our data indicate that MFG-E8 may serve as a potential key therapeutic target for attenuating AT1-AA production in PE.
Considerable clinical evidence has accumulated that PE is caused by inflammation, endothelial dysfunction, and an imbalance between angiogenic and angiostatic factors. Placental ischemia is associated with increased levels of sFlt-1, sEng, TNF-α, and AT1-AA in plasma [
25]. TNF-α is slightly elevated in normal pregnancy but increases twofold in preeclamptic pregnancy [
26,
27]. One mechanism by which TNF-α appears to activate AT1R to mediate AT1-AA production is through the induction of gestational hypertension [
28]. Our data have verified that placental cellulosic degeneration and necrosis are more pronounced in PE compared to normal pregnancy. The peripheral levels of TNF-α and sFlt-1 were significantly higher in our study compared to normal pregnancies. Besides, the apoptosis of trophoblast cells, the level of AT1-AA in plasma, and the expression of AT1R in PE placentas are significantly higher than normal. Previous reports have showed that MFG-E8 can suppress pro-inflammatory cytokines transcription and plays a protective role in inflammation-induced tissue injury in the lungs, liver, colon and periodontium by using mouse models [
29,
30,
31,
32,
33,
34,
35]. In the present study, the levels of MFG-E8 in PE placentas and serum were significantly lower than normal pregnancies. As expected, we found higher expression of AT1R in PE placenta and higher serum levels of AT1-AA in PE compared to normal, the same with previous studies [
11,
36,
37]. This result shows that there may be a certain relationship between MFG-E8 and AT1-AA.
To further validate the clinical data in the animal model, we constructed PE model in rats. These rats exhibited overt symptoms of human PE, such as hypertension, proteinuria, and placental oxidative damage. In addition, we found higher expressions of sFlt-1, AT1-AA, and TNF-α in the serum from PE rats. Furthermore, treatment with MFG-E8 abrogated the upregulation of sFlt-1, AT1-AA, and TNF-α. The result above supports the important role of MFG-E8 in attenuating the PE symptoms. By employing the PE model, we found that placental trophoblast death was enhanced in the PE model. However, MFG-E8 treatment inhibited trophoblast death, suggesting the MFG-E8 could mediate trophoblast apoptosis.
To elucidate the mechanisms of MFG-E8 in AT1-AA production in vitro, we utilized LPS as PAMP. As expected, LPS could induce trophoblast cell apoptosis, elevate the levels of TNF-α and sLFt-1, and stimulate the release of AT1R exposure in vitro. Macrophages can engulf apoptotic cells, while defective phagocytosis of apoptotic cells can lead to an excessive expression of autoantigens and stimulate the production of autoantibodies [
4,
38,
39]. It is still unknown whether MFG-E8 can enhance the phagocytosis of macrophages to clear apoptotic trophoblast cells in PE. In this study, we co-cultured macrophages with trophoblast cells and divided into 4 groups. A marked increase in TUNEL-stained dead cells was observed in the LPS group and MFG-E8 siRNA group compared to the control and MFG-E8 group. The aggravated accumulation of dead cells was partially caused by increased apoptosis and partially induced by inefficient phagocytosis of apoptotic cells by macrophages. Moreover, MFG-E8 siRNA could also induce apoptosis in trophoblast cells and increase AT1R exposure. On the contrary, pretreatment with MFG-E8 could enhance the phagocytosis of macrophages, resulting in the clearance of apoptotic trophoblast cells and a decrease in the expression of TNF-a, sLFt-1, and AT1R. According to these results, our study provides evidence supporting the notion that MFG-E8 can enhance the macrophages' ability to clear apoptotic trophoblast cells, decrease AT1R exposure, and reduce AT1-AA production. Suggesting that MFG-E8 plays an important role in the production of AT1-AA and the pathogenesis of PE.
In conclusion, there is a lack of studies demonstrating the relevance of MFG-E8 to AT1-AA in PE. Relying on the results gathered from clinical data, rat model, and trophoblast model, the study confirmed that MFG-E8 mediates AT1-AA production in PE. By using animal and trophoblast models, we further detected that MFG-E8 could suppress AT1-AA production by enhancing the apoptotic-phagocytosis of macrophages. The study proposes a common mechanism underlying the production of AT1-AA and provides new insights into the understanding of PE.
Author Contributions
Conceptualization, Z.S.; Methodology, F.L.; Data curation, F.X; Writing—original draft, F.X.; Writing—review & editing, F.X., F.L., C.L., and J.Z.; Supervision, F.L.; Funding acquisition, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: This research was funded by Zhejiang Provincial Natural Science, grant number LY20H040004.
Institutional Review Board Statement
The animal experiment protocol was approved by the Experimental Animal Ethics Committee of the Institute of Zoology, Chinese Academy of Sciences (approval no.WYDW2019-0524). All animal experiments were conducted in accordance with the National Law on the Use of Experimental Animals (China). Appropriate measures were taken to minimize the use of animals and reduce their suffering.
Informed Consent Statement
Note applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Feng Lin for his experimental and theoretical guidance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chaiworapongsa, T. , et al., Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 2014, 10, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Spradley, F.T., A. C. Palei, and J.P. Granger, Immune Mechanisms Linking Obesity and Preeclampsia. Biomolecules 2015, 5, 3142–3176. [Google Scholar] [CrossRef] [PubMed]
- Costantine, M.M. , et al., Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016, 214, 720–e1. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. , et al., Lipoxin A4 suppresses angiotensin II type 1 receptor autoantibody in preeclampsia via modulating caspase-1. Cell Death Dis 2020, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.H. , et al., Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 2010, 55, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N., B. LaMarca, and M.W. Cunningham, Jr., The Role of Agonistic Autoantibodies to the Angiotensin II Type 1 Receptor (AT1-AA) in Pathophysiology of Preeclampsia. Curr Pharm Biotechnol 2018, 19, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J. , et al., Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension 2013, 62, 886–892. [Google Scholar] [CrossRef]
- Cunningham, M.W., Jr. , et al., Agonistic Autoantibodies to the Angiotensin II Type 1 Receptor Enhance Angiotensin II-Induced Renal Vascular Sensitivity and Reduce Renal Function During Pregnancy. Hypertension 2016, 68, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.C. , et al., The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond) 2016, 130, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G. , et al., Agonistic autoantibodies directed against the angiotensin II AT1 receptor in patients with preeclampsia. Can J Physiol Pharmacol 2003, 81, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G. , et al., Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 1999, 103, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. , et al., Cadmium-induced immune abnormality is a key pathogenic event in human and rat models of preeclampsia. Environ Pollut 2016, 218, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Herse, F. and B. LaMarca, Angiotensin II type 1 receptor autoantibody (AT1-AA)-mediated pregnancy hypertension. Am J Reprod Immunol 2013, 69, 413–418. [Google Scholar] [CrossRef]
- Mahajan, A., M. Herrmann, and L.E. Munoz, Clearance Deficiency and Cell Death Pathways: A Model for the Pathogenesis of SLE. Front Immunol 2016, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Kimani, S.G. , et al., Contribution of Defective PS Recognition and Efferocytosis to Chronic Inflammation and Autoimmunity. Front Immunol 2014, 5, 566. [Google Scholar] [CrossRef] [PubMed]
- Shen, F. , et al., Trophoblast debris extruded from preeclamptic placentae activates endothelial cells: a mechanism by which the placenta communicates with the maternal endothelium. Placenta 2014, 35, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P. and M.J. Kaplan, Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin Immunol 2017, 185, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. , et al., MFG-E8, a clearance glycoprotein of apoptotic cells, as a new marker of disease severity in chronic obstructive pulmonary disease. Brazilian Journal of Medical and Biological Research 2015, 48, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R. , et al., Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R. , et al., Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol 2004, 172, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.D. and J.D. Pound, Cell death in the neighbourhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol 2011, 223, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y. , et al., Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem 2000, 127, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Majai, G. , et al., Decreased apopto-phagocytic gene expression in the macrophages of systemic lupus erythematosus patients. Lupus 2014, 23, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. , et al., Milk fat globule-EGF factor 8 suppresses the aberrant immune response of systemic lupus erythematosus-derived neutrophils and associated tissue damage. Cell Death Differ 2017, 24, 263–275. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B.D., J. Gilbert, and J.P. Granger, Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension 2008, 51, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L. , et al., Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol 1991, 139, 327–335. [Google Scholar] [PubMed]
- Conrad, K.P., T. M. Miles, and D.F. Benyo, Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol 1998, 40, 102–111. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B. , et al., Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 2008, 52, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.M. , et al., MFG-E8 Attenuates Intestinal Inflammation in Murine Experimental Colitis by Modulating Osteopontin-Dependent αβIntegrin Signaling. Journal of Immunology 2009, 182, 7222–7232. [Google Scholar] [CrossRef] [PubMed]
- Abe, T. , et al., Regulation of Osteoclast Homeostasis and Inflammatory Bone Loss by MFG-E8. Journal of Immunology 2014, 193, 1383–1391. [Google Scholar] [CrossRef]
- Aziz, M. , et al., Milk fat globule-epidermal growth factor-factor 8 attenuates neutrophil infiltration in acute lung injury via modulation of CXCR2. J Immunol 2012, 189, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A. , et al., Milk fat globule-EGF factor VIII ameliorates liver injury after hepatic ischemia-reperfusion. Journal of Surgical Research 2013, 180, E37–E46. [Google Scholar] [CrossRef] [PubMed]
- Dai, W. , et al., The roles of a novel anti-inflammatory factor, milk fat globule-epidermal growth factor 8, in patients with coronary atherosclerotic heart disease. Atherosclerosis 2014, 233, 661–665. [Google Scholar] [CrossRef]
- Uchiyama, A. , et al., MFG-E8 regulates angiogenesis in cutaneous wound healing. Am J Pathol 2014, 184, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A. , et al., Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol Med 2011, 17, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Herse, F. , et al., Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension 2007, 49, 604–611. [Google Scholar] [CrossRef]
- Sahay, A.S. , et al., A longitudinal study of circulating angiogenic and antiangiogenic factors and AT1-AA levels in preeclampsia. Hypertension Research 2014, 37, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Hanayama, R. , et al., Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 2004, 304, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S., R. Hanayama, and K. Kawane, Autoimmunity and the clearance of dead cells. Cell 2010, 140, 619–630. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).