Introduction
Tuberculosis is one of the most serious infectious diseases requiring long-term intensive treatment. One third of the world's population is thought to be infected with Mycobacterium tuberculosis; in rural areas, as well as in meat processing plants and cattle farms, M. bovis infection may be prevalent, as this pathogen affects humans and cattle alike [
1,
2]. Tuberculosis induced by
Mycobacterium tuberculosis and
M. bovis are clinically, radiologically, and pathologically indistinguishable in individual patients, except that M. bovis bacteria are inherently resistant to pyrazinamide, the first-line drug of treatment [
2,
3]. The emergence of multidrug-resistant strains forces researchers to pay close attention to the mechanisms regulating the immune response against mycobacteria, including those involving innate immune cells [
4,
5]. Mycobacteria are aerobic bacteria that have adapted to survive inside cells, advancing them in avoiding the host organism's immune response and obtaining essential elements for growth and proliferation [
3]. Moreover, they have adapted to exist in the lungs and have developed mechanisms of protection against the action of exogenous oxidants: the expression of methionine sulfoxide reductases, truncated hemoglobins trHbN and trHbO, capable of binding oxygen, the redox-sensitive DosR/S/T system, consisting of membrane and cytosolic proteins, as well as proteins of the WhiB family (discussed in the review [
6]). Some secreted and membrane proteins of mycobacteria are able to "disarm" immune cells by suppressing the fusion of phagosomes and lysosomes and recruiting membrane proteins such as V-ATPase and NADPH oxidase to the phagosome, as well as by blocking antigen presentation by the MHC II complex on the surface of the plasma membrane [
1,
2,
3,
4,
7].
In the lungs,
M. tuberculosis and
M. bovis primarily affect resident alveolar macrophages and recruited neutrophils [
8,
9]. It has been observed that only 5-10% of those infected develop the disease, and in the remaining 90% of cases, host immunity probably restrains or destroys the mycobacteria naturally [
10]. Just few people (less than 1%) who had direct and prolonged contact with patients with open forms of tuberculosis did not develop either active or latent tuberculosis [
11]. This means that the human immune system copes or eliminates the infection using mechanisms of innate immunity without triggering adaptive immunity with subsequent generation of memory T cells. This mechanism of innate defense before the development of an adaptive response has been called "early clearance" [
12]. However, this concept has not yet received convincing confirmation, since the tests used are not complete indicators of the T-cell response, as they do not detect either T cells recognizing non-protein mycobacterial antigens no IFN-γ-independent responses [
6].
Alveolar macrophages, which are the first to encounter mycobacteria in the lungs, play an important role in the mechanisms of early clearance. However, their ability to kill mycobacteria is still questionable. Increasing evidence suggests that peripheral blood neutrophils capable of releasing reactive oxygen species (ROS) are required. An inverse correlation has been shown between the number of neutrophils in peripheral blood and the risk of infection with
M. tuberculosis when in contact with a tuberculosis patient [
7]. Patients who appeared capable of early clearance of
M. tuberculosis had higher levels of neutrophil activation compared to individuals who developed the infection [
13]. Recruited neutrophils, as well as macrophages, are able to phagocyte of mycobacteria, however, the main function of neutrophils is the release of antimicrobial peptides, hydrolyzing enzymes and oxygen radicals into the site of inflammation [
4]. The latter function is realized by activation of the membrane enzyme NADPH oxidase upon stimulation of surface receptors by soluble factors, such as formylated peptides of bacterial origin, or upon phagocytosis of pathogenic particles. The ability to trigger the respiratory response of granulocytes is intrinsic for several types of receptors: G-protein coupled receptors (FPR formylated peptide receptors; angiotensin, purine, lysophosphatidic acid receptors), TNF-alpha receptors, lipopolysaccharide receptors, Toll-like receptors, and others [
14]. Activation of granulocyte NADPH oxidase occurs upon phosphorylation of oxidase subunits by protein kinase C (PKC) and their assembly on the plasma membrane; PKC is an effector molecule at the stimulation of both FPR and Fc/CR receptors [
15,
16]. Stimulation of FPR and C3aR/C5aR results in Gβγ/PLCβ-dependent activation of PKC, whereas stimulation of FcR, CR1/CR2 triggers the slower pathway of Src/PI3K/PLCγ-dependent activation of PKC. Possible targets of the mycobacterial proteins could be FPRs, lectin and scavenger receptors capable of recognizing glycoproteins, as well as TLRs recognizing pathogen-associated patterns.
The properties of mycobacterial proteins have been studied for a long time; however, the function of almost a third of the proteins remains unclear, and in particular their virulence properties [
17]. We have isolated serologically active proteins from
Mycobacterium bovis and studied their cytotoxicity and the effect on the oxidase activity of mouse granulocytes.
Materials and methods
Materials. Antigen isolation and immunoblotting: Acrylamide (99.9% pure, Bio-Rad, USA), Methylenebisacrylamide (ultra-pure grade, Helicon), Temed (Bio-Rad, USA), Ammonium persulfate (BioChemica), Diaminobenzidine tetrahydrochloride (Sigma-Aldrich, USA), HRP Color Development Reagent 4 CN (Bio-Rad, USA), Tris (ultra-pure, Sigma-Aldrich, USA), Glycine (99% pure, Sigma-Aldrich, USA), Suppoted Nitrocellulose Membrane, 0.45µm, 30 cm x 3 m (Bio-Rad, USA), Anti-rabbit IgG (whole molecule)-peroxidase antibody conjugate produced in goat (Sigma-Aldrich, Sigma-Aldrich, USA), 2 ml Lysing Matrix B (Blue lysing matrix) tubes, silicon carbide beads MP Biomedicals (USA).
Mouse granulocytes isolation. Percoll (Merck, USA); phosphate-salt buffer (PBS) (Paneco, Russia); RPMI-1640 culture medium (Paneco, Russia); 0.9% NaCl solution (Paneco, Russia).
Chemiluminescence (CL) assay: Hanks' solution (138 mM NaCl, 6 mM KCl, 1 mM MgSO4, 1 mM Na2HPO4, 5 mM NaHCO3, 5.5 mM glucose, 10 mM HEPES; 1 mM CaCl2). Before use, the pH of the solution was adjusted to 7.2-7.4 and sterilized by filtration through a 0.22 μm pore membrane filter.
Solution for CL registration: Hanks' solution with 0.35 mM luminol, 5 units/mL horseradish peroxidase, 0.1 mM NaN3. Substances were dissolved in bidistilled water, adjusted pH to 7.2-7.4, and sterilized by filtration through a 0.22 μm pore size membrane filter. (Luminol (Merck, USA); horseradish peroxidase (Merck, USA); HCl (Reakhim, Russia); NaOH (Reakhim, Russia), NaN3 (Merck, USA).
The synthetic agonist of formylated peptide receptors fMLF (N-Formyl-L-methionyl-L-leucyl-L-phenylalanine) (Merck, USA) was used to stimulate granulocytes; at a concentration of 10-5 M, the synthetic DAG analog phorbol-12-myristate-13-acetate (PMA, phorbol-12 mirystate 13-acetate) (Merck, USA) at a concentration of 10-6 M, and the respiratory burst inducer opsonized zymosan from E. coli (Merck, USA) at a concentration of 0,05 mg/mL.
Cell culture: THP-1 and A549 cell lines; DMEM medium, RPMI-1640 medium, 10% fetal bovine serum, L-glutamine, 1% penicillin/streptomycin (all-Paneko, Russia), propidium iodide (PI) (Thermo-Fisher Scientific, USA), MTT - reagent (Paneko, Russia), trypsin-EDTA solution (Paneko, Russia), Dulbecco's phosphate-salt solution (DPBS) (Paneko, Russia)
Granulocyte isolation
Granulocytes were isolated from the bone marrow of male BALB/c mice (8-12 weeks old, 20-25 g) obtained from the Stolbovaya branch vivarium of the Federal State Budgetary Institution Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency of Russia. The conditions of work with animals were in accordance with the regulatory legal act of the Ministry of Health of the Russian Federation No. 199-n "On Approval of the Rules of Good Laboratory Practice, international legal norms specified in the European Convention ETS No. 123 "On the Protection of Vertebrate Animals Used for Experiments or Other Scientific Purposes" and the manual on work with laboratory animals IBC RAS No. 57.30.12.2011.
Isolation of granulocytes from bone marrow (BM-granulocytes) was performed according to a previously described technique [
18]. Briefly, tibia, femur, and humerus were washed using a syringe with RPMI-1640 medium to obtain a cell suspension, which was fractionated by centrifugation in a 55%, 62.5%, 78% Percoll density gradient in phosphate-salt buffer (PBS, v/v) at 500g for 40 minutes. Granulocytes were selected at the 62.5-78% gradient boundary. They were washed with RPMI-1640 followed by PBS and transferred to Ca
2+-free Hanks' solution in which cells were stored for 1 h at 4°C before the experiment. Cell viability was determined by trypan blue staining, and mature granulocytes were identified by the shape of the nucleus when cells were stained with acridine orange. The isolated cells with viability and the proportion of mature granulocytes not less than 95% were used for experiments [
19].
Extraction, purification and characterization of mycobacterial antigens
Antigens were isolated from
M. bovis Bovinus-8 strain 700201 (obtained from the Scientific Center for Drug Expertise of the Ministry of Health of the Russian Federation) according to the previously described methodology [
20]. Cells were cultured on solid Lowenstein-Jensen medium for 28-30 days at 37°C, then destroyed using FastPrep-24 homogenizer with Lysing MatrixB (MP Biomedicals, USA). Clarified homogenates were prepared on a Mini prep Cell Bio-Rad (USA), fractionation control was performed using a UV-1 uvicord (Pharmacia LKB) and REC-102 recorder (Pharmacia LKB) with further collection of fractions on a Pharmacia Frac 100 collector. Each fraction was separated by electrophoresis in 12.5% PAGE under denaturing conditions, then transferred to a nitrocellulose membrane for detection of serologic activity by immunoblot. To determine serologic activity, hyperimmune rabbit serum from the collection of the State Autonomous Establishment of the Russian Center for Disease Control and Prevention (Kazan) was used.
Evaluation of cytotoxicity of mycobacterial antigens
To assess the effect of mycobacterial antigens on cellular viability and function, human cell cultures were used: THP-1 (cells of monocytic origin from a patient with acute monocytic leukemia) [
21]; A549 (derivatives of alveolar basal epithelial cells isolated from a patient with lung adenocarcinoma) [
22]. Cell lines were purchased from ATCC (USA) and received as a gift from Dr. J. Persson (Lund University, Lund, Sweden).
THP-1 and A549 cells were cultured in a humidified atmosphere of 5% CO2 and 37 °C in DMEM (A549) or RPMI-1640 (THP-1) culture medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 1% penicillin/streptomycin (Paneco, Russia). The cytotoxicity of antigens was assessed by propidium iodide (PI) staining. Antigens were added to growth medium, cells were incubated for 20 min, then A549 dissociation was performed using trypsin-EDTA solution and cell suspension was collected. Cells were washed with PBS, precipitated at 300 x g for 5 min, then stained with PI and fluorescence was analyzed using a CytoFlex flow cytofluorimeter (Beckman Coulter, USA). THP-1 cells were treated similarly except for the dissociation step because undifferentiated cells grow in suspension. The effect of antigen (cytotoxicity) was assessed by the proportion of dead cells calculated as the difference between the proportion of PI-positive cells in the control and antigen-treated samples. To evaluate the effect of mycobacterial antigens on the proliferation and viability of A549 and THP-1 cells under prolonged exposure, we used the MTT test (MTT reagent from Paneco, Russia), which allows us to assess the metabolic activity of cultured adherent cells. This colorimetric method allows measuring the activity of mitochondrial reductases in living cells that hydrolyze membrane-permeable tetrazolium salts of MTT with the formation of violet formazan crystals by recording the optical absorption of the sample at a wavelength of 570 nm (spectrophotometer Tecan Infinite 200 PRO, Switzerland). THP-1 cells were pre-differentiated with 10 nM PMA for 48 hr. Cells were seeded in 96-well plates at density of 2000 cells/well in complete growth medium and incubated with antigens (to the control cells an appropriate volume of 10 mM Tris, a solvent of the isolated proteins, was added); A549 cell viability was analyzed after 48 and 72 h of incubation with antigens. The viability of differentiated TNR-1 cells was assessed after 48 h of incubation with antigens. The metabolic activity of cells was assessed and the results were recorded according to the previously described methodology [
23].
Assessment of ROS production
ROS production was assessed by luminol-dependent chemiluminescence (CL) intensity using the chemiluminometer CHEMILUM-12 (IBC RAS, Russia) [
24]. BM-granulocytes were incubated for 20 min at 37°C, baseline CL level was recorded, then they were stimulated and cell response was recorded (to the control cells an appropriate volume of 10 mM Tris, a solvent of the isolated proteins, was added). The baseline level of ROS production was measured for 250 s, then a stimulus was added and CL was recorded for 5-40 min depending on the stimulus. Baseline, amplitude, response rate, and total ROS production were evaluated. Total ROS production was calculated for 2000 s after addition of PMA, for 375 s after addition of fMLF, and for 2250 s after addition of opsonized zymosan. Measurements has been done in three technical and at least three biological repeats at 37°C. To assess the effect of antigens on ROS generation, antigens were added to the cell medium 20 min before the start of the CL measurement, then baseline CL levels were recorded and cells were stimulated either with chemotactic peptide (fMLF, 10 µM), phorbol-12-myristate-13-acetate (PMA, 1 µM) or opsonized zymosan (OZ, 0,05 mg/ml). Normalized ROS production was calculated as the ratio of ROS production by antigen-treated cells to ROS production by control cells taken as 100%.
Statistical processing of the results was performed using GraphPad Prism 8.0 software (GraphPad software, USA).
Results
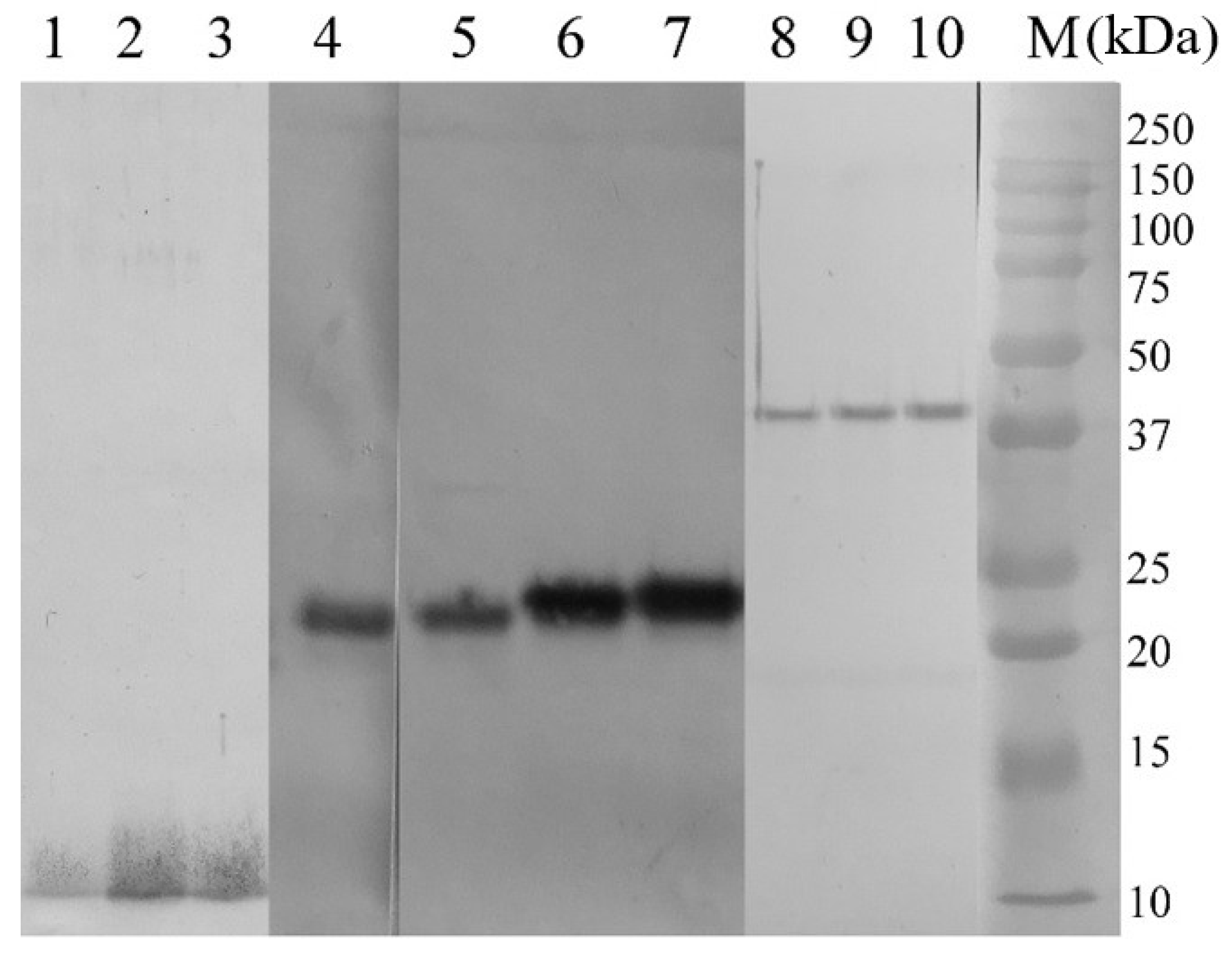
Characteristics of the proteins. M. bovis lysates were obtained through a homogenization process. As a result of preparative fractionation, 70 protein fractions were obtained, each of which was analyzed in an immunoblotting reaction with rabbit hyperimmune serum for specific serological activity.
Based on the results of immunoblotting, fractions with the highest serological activity were selected, which corresponded to polypeptides with a molecular weight of 10, 22, 24, 40 kDa (AG1-4, respectively).
Figure 1 demonstrates the results of immunoblotting of proteins of the corresponding fractions when interacting with hyperimmune serum and treated with secondary antibodies. Consistent with our previous findings, lipid and oligosaccharide groups are present in AG1 and AG4 antigens, whereas AG2 and AG3 proteins do not carry such groups (
Table 1, unpublished data). Due to the assumption that the AG2 and AG3 peptides we obtained might be the degradation products of MPB70 protein, synthetic polypeptides containing immunogenic epitopes of
M. bovis MPB 70 antigen (Immune Epitope Database and Analysis Resource (IEDB)) were used as a comparison group. The peptides were kindly provided by Associate Professor E. A. Shuralev (Kazan Federal University, Russian Federation) and are designated as antigens AG5 and AG6. The amino acid sequences within AG5 peptide that carries B cell response activation epitopes are AEYAAANP, GMSQDPV and KTNSSLLT; AG6 peptide epitopes NNPELTTL and NVVGTRQ are important in the formation of B cell response, and YMIDSVL is important in the formation of T cell response (IEDB).
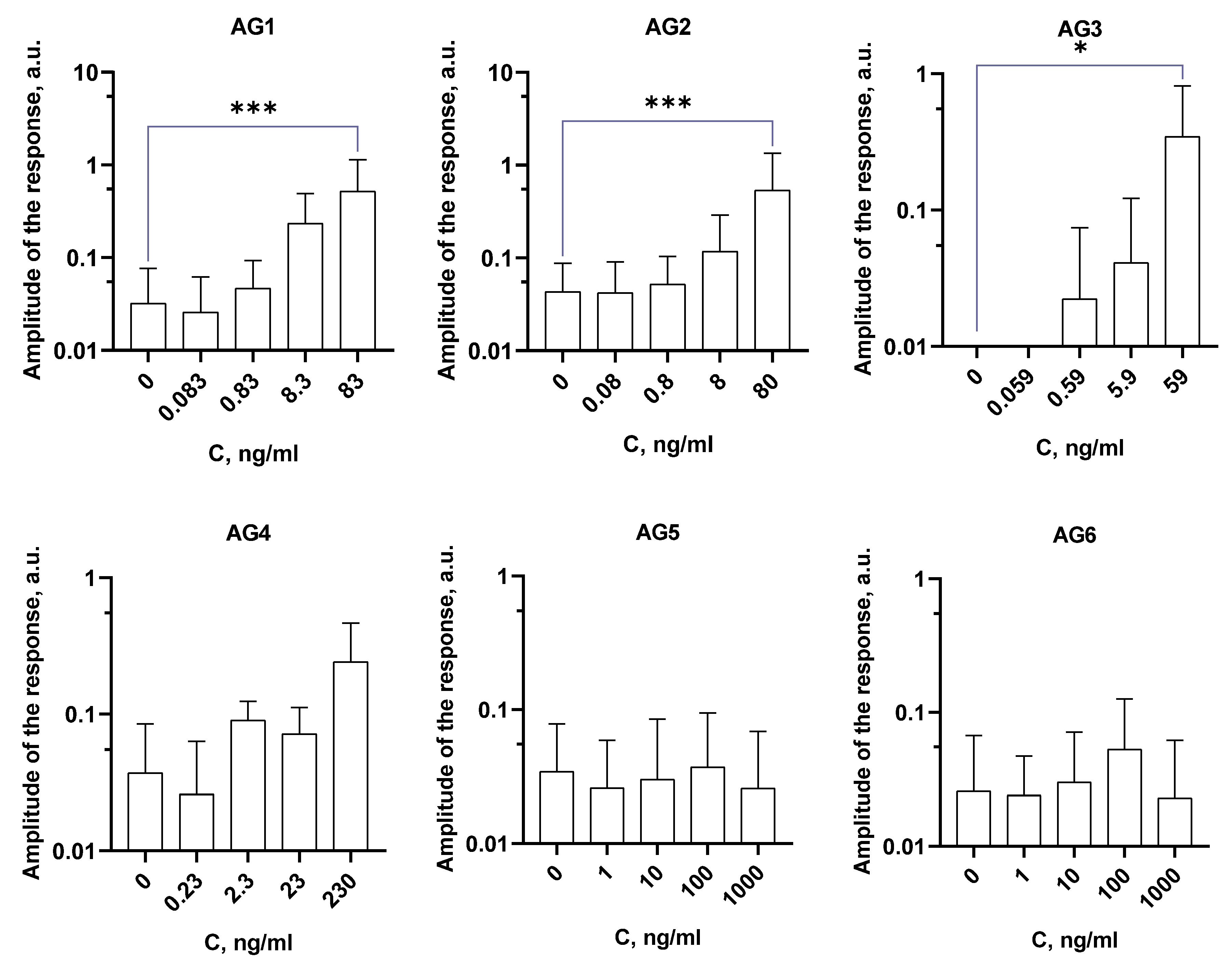
Activation of the granulocyte respiratory response. Initially, we have tested if mycobacterial antigens are able to stimulate ROS generation in BM-granulocytes. AG1 (0.083-83 ng/mL), AG2 (0.08-80 ng/mL), and AG3 (0.059-59 ng/mL) were found to induce a dose-dependent increase in CL (
Figure 2), indicating activation of ROS generation upon their addition. AG4 (0.23-230 ng/mL) was not able to activate ROS production. Also, the synthetic peptides AG5 and AG6 (1-1000 ng/mL) did not affect the respiratory activity of mouse DM-granulocytes. AG5 and AG6 peptides carry immunogenic epitopes of T and B cell response activation in their structure, they are able to interact with T and B cell receptors but might not with phagocyte receptors.
Modulation of stimulated respiratory response. Three stimuli were used to activate the respiratory response of granulocytes: 1) formyl peptide fMLF, an agonist of FPR’s; 2) opsonized zymosan (OZ), which could be bound by FcR’s and CR’s; 3) PMA, a synthetic analog of diacylglycerol capable of penetrating the cell membrane and activating PKC without the involvement of receptors and additional signaling components [
25]. We screened the isolated proteins at the concentrations shown in
Figure 2 for their effect on respiratory responses of granulocytes.
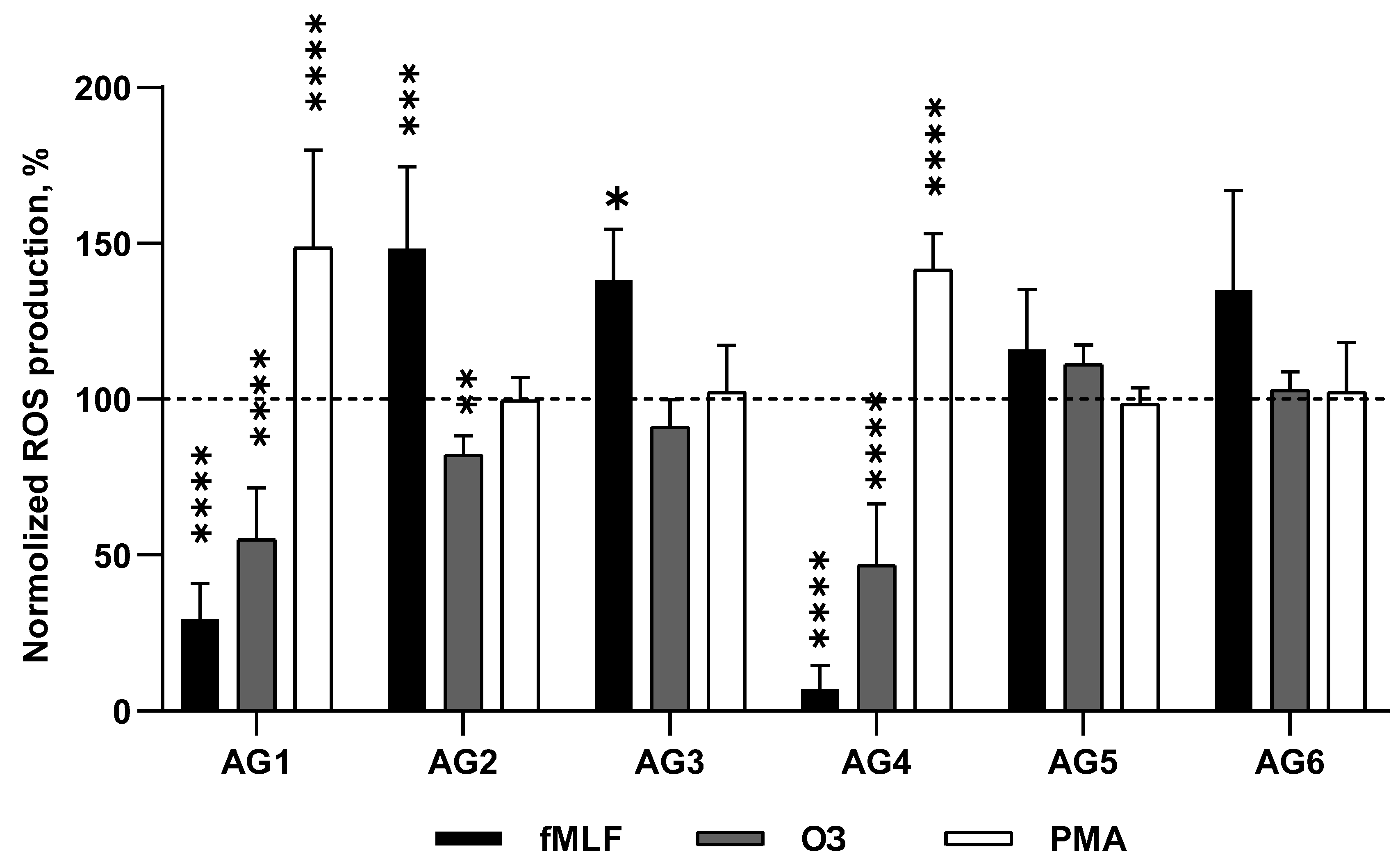
BM-granulocytes treated with mycobacterial proteins AG1 and AG4 at the concentrations of 83 and 230 ng/ml, respectively, showed significant inhibition of ROS production in response to fMLF (
Figure 3). A similar picture was observed upon stimulation of the cells by OZ: inhibition of ROS production in the presence of AG1 and AG4. Whereas, the response to PMA demonstrated a significant increase upon the pretreatment of the cells by AG1 and AG4. Proteins of the AG2 and AG3 fractions (80 and 59 ng/ml, respectively), on the contrary, potentiated the fMLF-induced respiratory response, AG2 suppressed the response of the cells to OZ, and AG3 did not affect it. There were no effects of any these proteins upon activation of the cells by PMA. Synthetic antigens AG5 and AG6 (both at 1000 ng/ml) also had no effect on ROS production stimulated by fMLF, PMA, or OZ (
Figure 3). It should be noted that at lower concentrations, the AG1-AG4 proteins had no effect on the respiratory responses activated by these stimuli (data not shown).
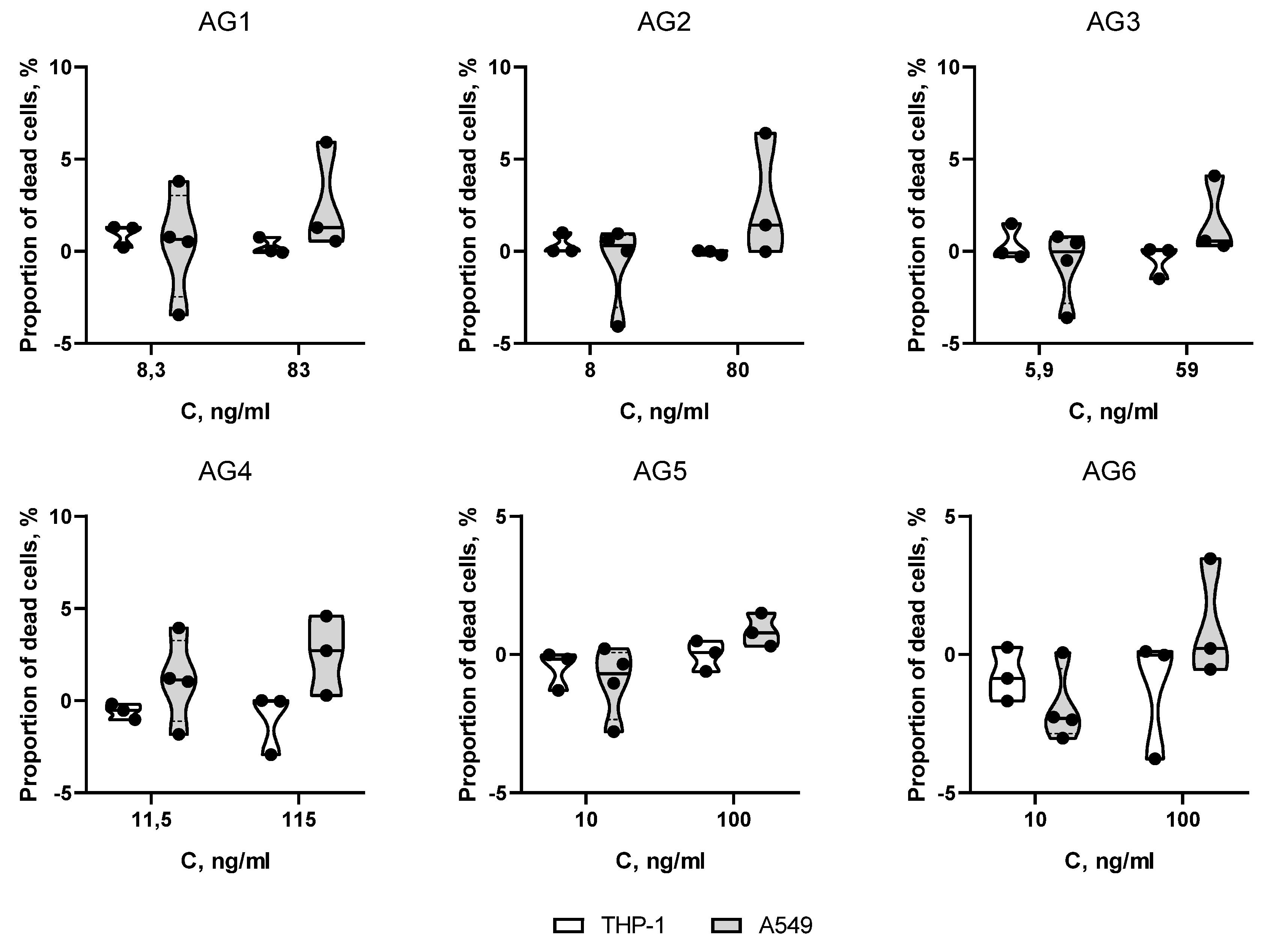
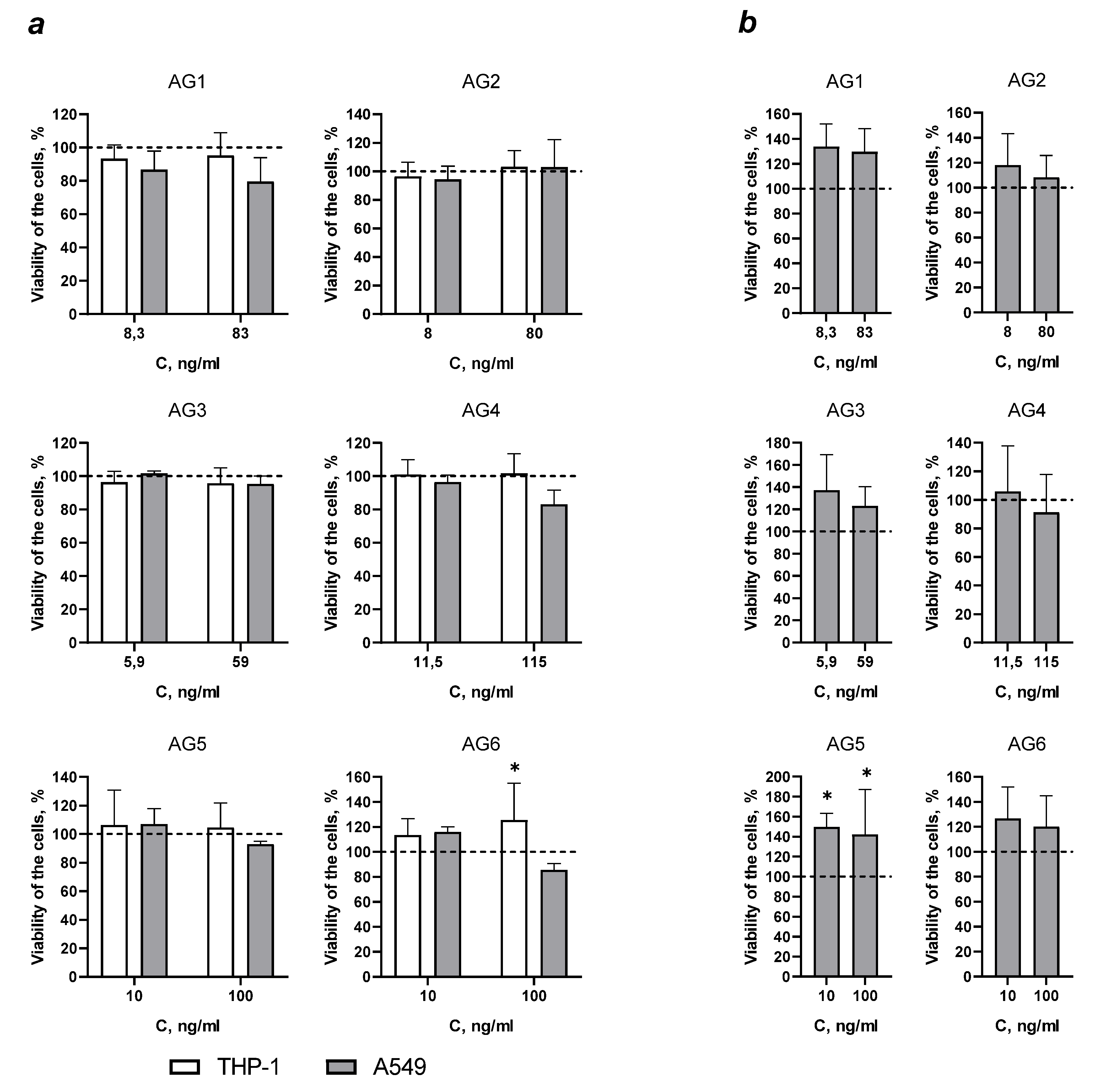
Evaluation of the effect of mycobacterial antigens on the viability and proliferation of THP-1 and A549 cells. To confirm the specificity of the inhibitory effect of antigens on ROS production, their cytotoxicity was assessed during short-term exposure (20 min) and long-term incubation (48 and 72 h).
During short-term incubation, none of the antigens significantly affected on the membrane integrity of the cells compared to the control cells, in both THP-1 and A549 cell line samples (
Figure 4).
Incubation of A549 cells and phorbol ester differentiated THP-1 cells with AG1-4 and AG5 antigens for 48 h had no significant effect on cell proliferation and viability. But AG6 caused a 25.6% increase in the number of viable THP-1 cells compared to control cells (
Figure 5, A). An increase in the number of viable A549 cells was also observed in the presence of 10 and 100 ng/ml of the synthetic AG5 peptide upon incubation for 72 hours.
Discussion
Phagocytes have a large arsenal of tools to kill bacteria: this is the capture and destruction of bacteria in phagolysosomes due to low pH values and the action of proteases, the production of reactive oxygen species, chlorinated oxidants and reactive nitrogen intermediates (in neutrophils), bactericidal peptides (defensins, lysozyme), the release of neutrophil or macrophage extracellular traps (NETs и METs) [
10,
26,
27]. There is still an ongoing debate about what is the key point in the destruction of bacteria. In our opinion, rapid generation of ROS in maximal proximity to the bacterium ensures its damage and prevents it from avoiding degradation within the phagosome. This was confirmed in studies of alveolar macrophages from mice knockout for the p47phox
-/- subunit of NADPH oxidase, which were unable to destroy phagocytosed
Pseudomonas aeruginosa bacteria, despite a decrease in pH and recruitment of hydrolyzing enzymes into the phagosome [
28]. Also, indirectly, this is confirmed by clinical data on the contribution of NADPH oxidase to innate immunity: patients with chronic granulomatous disease (CGD) have a higher susceptibility to mycobacterial infection, especially in countries with endemic areas for tuberculosis [
29,
30,
31,
32,
33]. In this case, only NADPH oxidase function is affected in CGD patients, due to abnormalities in genes encoding oxidase subunits, while other phagocyte functions are not impaired.
As an intracellular parasite,
M. tuberculosis tends to enter macrophages and modulates the bactericidal activity of immune cells. Entry of mycobacteria into phagocytes is realized by a variety of receptors of innate immune cells, including complement receptors CR3, CR1 and CR4, mannose receptor, surfactant protein A receptor, CD14, immunoglobulin fragment Fcγ receptors, scavenger receptors, macrophage and dendritic cell receptor DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin). [
34,
35]. The mycobacterial surface glycoprotein, mannose-capped lipoarabinomannan (Man-LAM), is recognized by C-type lectins and macrophage mannose receptors (MMRs). In addition, a large number of
M. tuberculosis surface proteins interact with TLR2 receptors. The interaction of mycobacterial proteins with those and other type of receptors can modulate the oxidase activity of immune cells. We found that the antigens AG1, AG2, AG3 and AG4 were able to induce a respiratory response in mouse granulocytes, probably through interaction with some of the aforementioned receptors (
Figure 2). The presence of lipid and oligosaccharide groups in the structure of AG1 and AG4 antigens increases the probability of interaction with additional receptors or incorporation into the plasma membrane and interference with the functioning of membrane enzymes. For example, it was shown that
M. tuberculosis phenolic glycolipids containing tri- and tetra-saccharides stimulated ROS production in neutrophils [
36]. Interestingly, the synthetic peptides AG5 and AG6, despite the presence of immunogenic epitopes, did not activate the respiratory response of granulocytes (
Figure 2). This may be due to the fact that the epitopes are designed to activate T- and B-cell responses through interaction with T- and B-cell receptors, and yet they apparently do not bind to any of the phagocyte receptors that activate ROS generation.
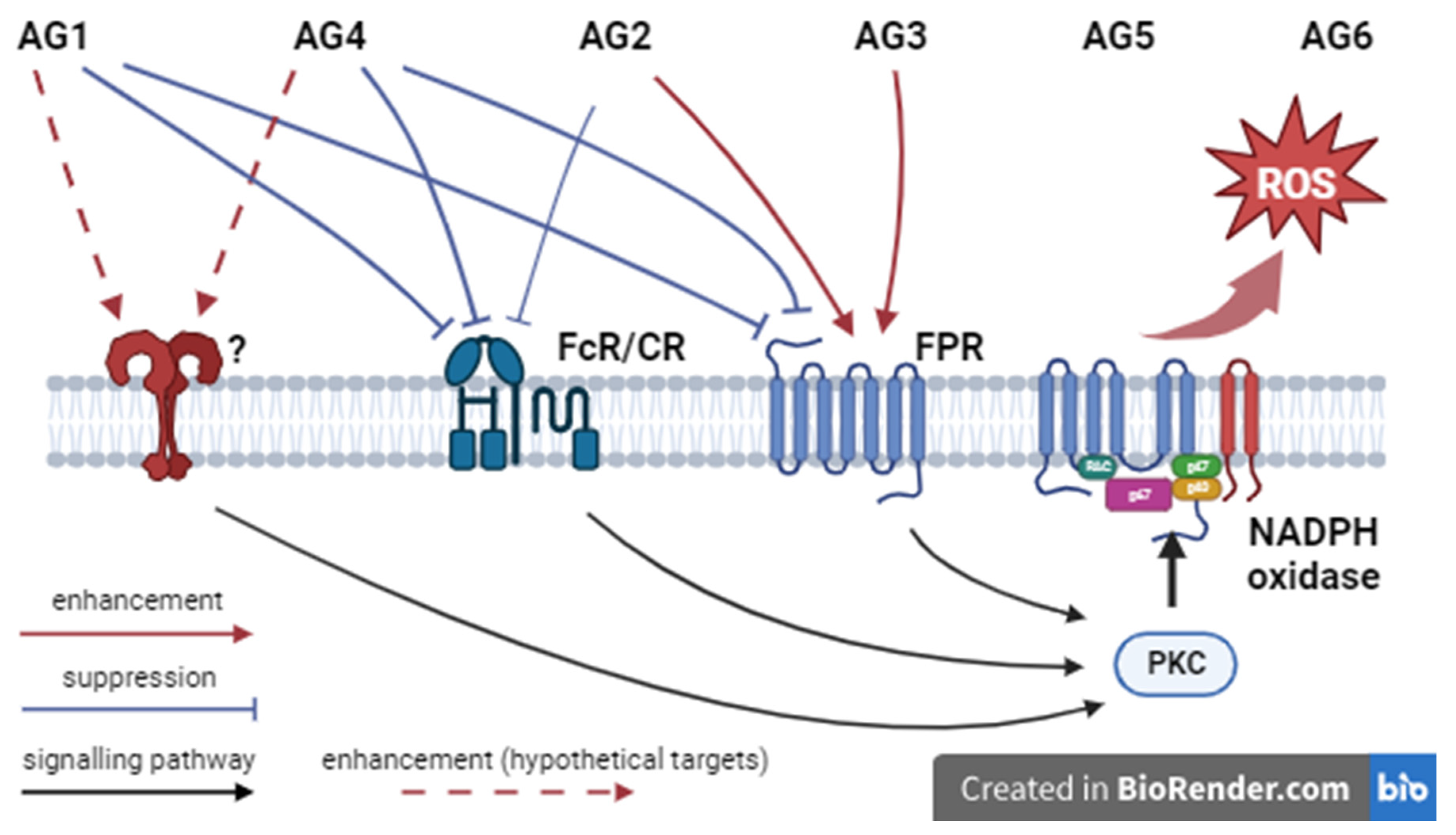
The isolated mycobacterial antigens can be divided into two groups according to their effect on the stimulated production of AFC by granulocytes. Incubation with antigens AG1 and AG4, which have lipid and oligosaccharide groups in their structure (
Table 1), significantly inhibited the respiratory responses of mouse BM-granulocyte to the stimulation of formyl peptide receptors FPR’s and opsonin receptors FcR’s/CR’s, possibly due to desensitization of these receptors (
Figure 3, scheme of
Figure 6). We suggest that AG1 and AG4 inhibit the activation of ROS production by granulocytes acting at the level of surface receptors. The reasons could be: i) antigens are rather large molecules that cannot penetrate into the cell; ii) their action is not the result of cell damage, since they hadn’t cytotoxic effect during short-term incubation and did not affect the viability of A549 cells and differentiated THP-1 cells during long-term incubation (
Figure 4 and
Figure 5). And, iii) stimulation of AG1- or AG4-pretreated cells with phorbol ester, an increased in ROS production by mouse granulocytes compared with non-treated cells. The latter fact suggests that AG1 and AG4 may interact with receptors of a different type, activation of which leads to priming of PMA-stimulated response (dotted arrows,
Figure 6).
In contrast to the AG1 and AG4 antigens, the AG2 and AG3 antigens enhanced the respiratory response induced by FPR stimulation, but upon stimulation with phorbol ester the cell response did not differ from the response of control cells (
Figure 3). Apparently, their main action is realized in the modulation of signaling pathways before reaching PKC, without affecting the activity of the kinase. It can be assumed that AG2 and AG3 potentiate the signaling pathways of FPRs to NADPH oxidase, which does not interfere with the signaling pathways of FcR/CR receptors since AG2 suppressed the respiratory response activated by OZ, and AG3 had no that effect. The synthetic antigens AG5 and AG6 were not able to activate the production of ROS by granulocytes and did not affect the respiratory responses stimulated by fMLF, OZ and PMA. The peptide sequence contains immunogenic epitopes of the MBP70 protein of
M. bovis necessary for the activation of T- and B-cell response, and apparently these antigens do not interact with innate immune cells, particularly in this case they did not affect the oxidase activity of granulocytes. Antigens AG5 and AG6 also did not have a cytotoxic effect; moreover, they increased cell viability after incubation with AG6 for 48 hours (THP-1 cells,
Figure 5a) and with AG5 for 72 hours (A549 cells,
Figure 5b ).
Thus, the antigenic proteins AG1, AG2, AG3, and AG4 isolated from
M. bovis are capable of modulating the activity of mouse granulocytes, but with an opposite effect: while AG1 and AG4 inhibited the stimulated oxidase function, AG2 and AG3 enhanced it. This is consistent with the data that cells of the innate immune system are targets of mycobacteria [
4]. Neutrophils constitute the largest fraction in bronchoalveolar lavage obtained from patients with tuberculosis, [
37], in the late stages of the disease there is a decrease in the number of neutrophils in the peripheral blood. It is assumed that mycobacteria suppress most functions of immune cells and initiate their exhaustion [
38,
39]. A decrease in the strength of the adaptive response leads to a weakening of antibody/opsonin production. When non-opsonized mycobacteria is phagocytosed, the response is realized by activation of mannose receptors (MR) and CD18/CD11b integrin receptors mainly. It could lead to a higher risk of mycobacterial survival inside the cell due to insufficient activation of signaling pathways leading to the formation and maturation of phagolysosomes compared to FcR’s for example [
40].
Some M. tuberculosis proteins, besides those mentioned, are also able to affect oxidase activity: a 19 kDa lipoprotein enhanced the respiratory response induced by the formylated peptide (1 µM, 10 min) and also caused a decrease in the expression of CD62 ligand (L-selectin) and an increase in the expression of CR1 and CR3 [
41]. The target receptors for most mycobacterial antigens have not yet been elucidated. Potentially, such receptors include: 1) G-protein coupled formylated peptide receptors, platelet activating factor receptors, complement component 5a receptors, and chemokine receptors that regulate cell migration and protective functions; 2) FcR and CR receptors, which initiate the uptake and destruction of opsonized particles, viruses and bacteria; 3) pattern recognition receptors, including TLRs, C-type lectin receptors CLRs, scavenger receptors and NOD-like receptors, which recognize structurally conserved fragments of microorganisms and promote particle uptake, and also activate signaling cascades that regulate immune/inflammatory responses [
42,
43,
44].
Suppression of OZ-induced ROS production by mycobacterial proteins may be due to several factors as suppression of the uptake of opsonized zymosan particles and phagosome maturation, and interference with signaling pathways for NADPH oxidase activation. In addition, mycobacteria can suppress the oxidase function of phagocytes through the production of enzymes with antioxidant activity, such as KatG with dual activity (catalase and peroxidase) and alkyl hydroperoxide reductase (ahpC), which allows inactivation of ROS, while the protein complex SodA, DoxX and SseA reduces oxidative damage, maintaining thiol homeostasis [
5,
45,
46]. In the peripheral blood of patients with tuberculosis, populations of low-density granulocytes were found, which are characterized by increased spontaneous production of ROS and increased release of NETs [
47]. It is assumed that mycobacteria activate the hyperproduction of ROS by neutrophils, provoking necrosis of immune cells, which ultimately weakens antimicrobial protection [
48]. When triggering
M. tuberculosis-infected neutrophil necrosis, ESAT-6 and CFP-10 proteins are responsible for stimulating the release of NETs [
49]. Some authors consider the ability of
M. tuberculosis to activate ROS-independent formation of METs by macrophages [
49], in particular, the mycobacterial protein ESAT6 stimulated the formation of METs by human macrophages in
in vitro studies [
50]. The mycobacterial protein nucleoside diphosphate kinase (Ndk), which activates macrophage GTPases Rab5 and Rab7, reduced the formation of phagolysosomes by blocking the fusion of phagosomes and lysosomes [
51]. The mechanism of action was that Ndk interacted with and inactivated the Rac1 protein, resulted in the inhibition of the assembly of the NADPH oxidase complex [
52]. Accordingly, Ndk knockdown showed a significant decrease in mycobacterial survival in vitro
in vitro and
in vivo. The mycobacterial virulence factor CpsA disrupted the assembly of NADPH oxidase, inhibited the production of ROS and, as a consequence, LC3 (microtubule-associated protein 1A/1B-light chain 3)-associated phagocytosis [
53]. Thus, inhibition of ROS production has been shown for ESAT-6 and EIS proteins; PPE2 and Ndk proteins inhibited the generation of ROS by blocking the assembly of the NADPH oxidase complex on the phagosome membrane. This has been associated with increased intracellular survival of
M. tuberculosis (discussed in review [
54]). There is no much data on the effects of
M. bovis proteins on the phagocyte functions. We have demonstrated that antigens with molecular weights of 10, 22, 24 and 40 kDa isolated from
M. bovis have serological activity and could be potential candidates for the development of diagnostic test systems. An assessment of the ability of antigens to modulate the oxidase activity of mouse granulocytes showed that the antigens activated the respiratory response and also influenced the stimulated production of ROS by BM-granulocytes. The presence of lipid and oligosaccharide groups in the structure of the antigen with a molecular weight of 10 and 40 kDa provided an inhibitory effect on fMLF- and OZ-stimulated ROS generation. We can speculate that
M. bovis antigens could find practical application for modifying existing tuberculosis vaccines and other drugs to enhance the bactericidal activity of phagocytes. Thus, further studies are needed to identify the isolated
M. bovis antigens and elucidate the molecular mechanisms of their action on the oxidase activity of phagocytes.