1. Introduction
As an interface between body and environment, human skin is exposed to interrelated internal and external factors, described by the term “exposome”, which contribute to skin aging process [
1]. Intrinsic or chronological aging describes the genetically determined process naturally occurring during life span that can be accelerated by environmental influences, what is called extrinsic aging [
2]. Skin is particularly exposed to extrinsic aging, with UV and air pollution being major players of the skin aging exposome [
1]. From 2009, Burke and Wei gathered evidence of synergistic damage caused by UVA and pollutants, increasing the risk of skin cancer [
3]. Recent works reported the interplay between air pollutants and UV in the disturbance of skin redox homeostasis, inflammation and barrier function alteration [
4,
5,
6]. Ozone or particulate matter combined to UV demonstrates additive effect in increasing markers of oxidation (lipid peroxidation, heme oxygenase-1 HO-1) and inflammation (transcription factor NFκB, cyclooxygenase 2 COX2), and in decreasing barrier associated proteins (filaggrin, involucrin). Polycyclic aromatic hydrocarbons (PAHs) or particulate matter and UVA triggers greater phototoxic effect than UV alone, and are associated with reactive oxygen species (ROS) generation and mitochondrial impairments.
ROS are continuously generated in living cells to ensure metabolism and act as intracellular messenger for cell and tissue homeostasis [
2]. Maintenance of physiological redox status is controlled by endogenous regulators, notably nuclear factor erythroid 2-related factor 2 (Nrf2) which targets an array of antioxidant and detoxifying genes as HO-1 and NAD(P)H quinone dehydrogenase 1 (NQO1) [
7]. Nrf2 induces those genes by binding a DNA motif called antioxidant response element (ARE).
UV, PAHs, volatile organic compounds, oxides, particulate matter, ozone and cigarette smoke induce oxidative stress in skin and combined pollution exposure has additive effect in the activation of Nrf2 [
8,
9]. Alteration of oxidative balance is the main driver of skin senescence especially in the epidermis where ROS load is high [
10,
11]. ROS impair proteins, lipids and DNA by direct oxidation but also induce biological responses: degradation of dermal structural components (collagen, elastin), secretion of inflammatory mediators through NFκB signaling. This direct relationship between oxidative stress and inflammation which in return generate a pro-oxidative environment, can represent a chronic challenge for the organism exposed to daily environmental insults, called oxinflammation [
12].
Aryl hydrocarbon receptor (AHR) is a xenobiotic chemical sensor, expressed in keratinocytes, fibroblasts, melanocytes and T-cell. AHR mediates oxidative response as well as antioxidant pathway in a fine-tuned cross talk with Nrf2 [
13]. AHR exogenous ligands include ozone, particulate matter and PAHs [
14]. After ligand binding in the cytosol, AHR is translocated to the nucleus, dimerizes with AHR nuclear translocator (ARNT) to bind xenobiotic-responsive element (XRE) of several genes, such as cytochrome P450 1A1 monooxygenase (CYP1A1) which can generate ROS and proinflammatory mediators. On the opposite, natural compounds as flavonoids bind AHR and activate Nrf2-NQO1 pathway which protects cells from ROS-induced oxidative damage. Another role of AHR signaling is the promotion of epidermal barrier function by upregulating genes involved in cornified cell envelop formation such as filaggrin (FLG) through the transcription factor OVO-like 1 (OVOL1) in keratinocytes [
15].
Recent findings pointed out the interaction between Nrf2 signaling pathway and cannabinoid receptor type 2 (CB2R) in different biological models and concluded that Nrf2 signaling pathway activation potentiates the effects of CB2R agonists [
16,
17,
18]. CB2R activation reduces inflammatory cytokines and lesions in psoriasis models through Nrf2 pathway and induces β-endorphin release in keratinocytes so decreasing nociception [
19,
20].
Topical application of cosmeceutical containing antioxidants appears an efficient strategy to prevent skin barrier damage induced by environmental stressors exposure [
5,
10,
21]. Silymarin (SM) is a seed extract of
Silybum marianum (L.) Gaertn. (Asteraceae) commonly named milk thistle and renowned for its hepatoprotective properties. SM contains specific flavonoids called flavonolignans, mixing taxifolin and coniferyl alcohol moieties. Dermatological applications of SM are based on its antioxidant properties and protective effects against UV-induced skin damages [
22]. Indeed, topical treatment containing SM inhibits photocarcinogenesis in murine model and protects human keratinocytes from UVB induced apoptosis and DNA damages [
23]. SM components also has anti-inflammatory properties and decrease the production of LPS-induced pro-inflammatory cytokines in keratinocytes [
24]. SM and silibinin, its main constituent (50-70%), exert antioxidant properties at different levels: direct free radical scavenging; inhibition of specific enzymes responsible for free radical production; maintenance of mitochondrial integrity in stress conditions; and activation of antioxidant enzymes and non-enzymatic antioxidants, mainly via transcription factors including NF-κB and Nrf2 [
25,
26].
The aim of this study was to explore the antioxidant and anti-inflammatory properties of SM in the context of skin daily exposure to UV and urban air pollution. SM decreased ROS and IL-1a in RHE exposed to UV and urban dust. A gene expression study suggested activation of Nrf2 and AHR, with antioxidant effector NQO1, and barrier function associated proteins filaggrin and keratin 16. SM induced CB2R and β-endorphin release. A clinical study confirmed SM to improve homogeneity and radiance of skin exposed to UV and air pollution and its positive psychological influence on volunteer’s mood through self-evaluation, outperforming cannabidiol.
2. Materials and Methods
2.1. SM composition
SM is a brownish-yellow powder obtained by ethyl-acetate extraction of Silybum marianum (L.) Gaertn. seeds. Quantitation of SM extract in silibinin equivalents was achieved by HPLC according to European Pharmacopeia quality control standards. Silibinin A and B, silicristin and silidianin and isosilibinin A and B were quantified. Analysis was performed on Lichrospher RP-18 125x 4mm, 5 µm (Merck) maintained at 25°C. Mobile phases were orthophosphoric acid 85% / methanol / water (0.5/ 35/65, v/v) and orthophosphoric acid 85% / methanol / water (0.5/50/50, v/v). Flow rate was 0.8 ml/min and injection volume 10 µL. Detection was performed in UV at 288 nm.
2.2. Impact of SM on induced oxidative stress in NHEKs
The cell culture was done on primary cells freshly isolated from biopsies. Normal human skin samples were collected from donor who had given informed consent. Normal Human Epidermal Keratinocytes (NHEKs) were seeded in a black plate with a glass bottom at 20 000 cells per well in 96-wells plates with a type I collagen pre-coating in quadruplicate. The cells were incubated for 24 hours in complete medium (Dermalife medium supplemented with factors) at 37°C with 5% CO2. Then, the cells were treated in complete medium supplemented with resveratrol (positive control) at 50 µM, or SM at 0.001, 0.005 and 0.01 mg/mL. Skin cells cultivated in complete medium without supplementation were used as negative control. After 24h of incubation at 37°C and 5% CO2, the 2′,7′-Dichlorofluorescin diacetate (DCFH-DA) probe has been added to the wells at 50 µM for at least 30-45 minutes at 37°C. Cells were then washed two times with phosphate buffered saline (PBS) and treated with the oxidative stress inducer, tert-Butyl hydroperoxide solution (TBP), at 5mM in PBS. The untreated cells remained in PBS. Finally, the emitted fluorescence was measured in darkness by excitation wavelength at 488 nm and emission wavelength at 525 nm with microplate reader (TECAN). The study was carried out in quadruplicate (n=4).
2.3. Impact of urban dust and UV exposure on RHE treated with SM
An 8-day-old reconstructed human epidermis (RHE-Lb) of 0.6 cm² was used for this test (Synelvia). Two products were tested: a formulation containing SM 1% (w/w) and the placebo cream (without SM). RHE were treated or not (negative control) with formulations, by topical application, and incubated for 24 hours.
INCI formula: AQUA/WATER, CETYL ALCOHOL, GLYCERYL STEARATE, PEG-75 STEARATE, CETETH-20, STEARETH-20, ISODECYL NEOPENTANOATE, PENTYLENE GLYCOL, PHENOXYETHANOL.
At day 9, the product residue was removed with a swab. RHE were then placed in irradiation medium containing DCFH-DA and incubated for 1 hour. After a medium change to remove DCFH-DA and a topical rinse, RHE were exposed to UVA/B stress (UVA 2.5 J/cm² + UVB 100 mJ/cm²) with or without urban dust (100 μg/ml, topical application, 50 μl/RHE) using BIO-SUN (Vilber). Urban dust corresponds to particulates matter of characterized composition: heavy metals (cadmium, lead, mercury, nickel, vanadium), aluminium, copper, chromium, manganese, chlorinated pesticides, polychlorinated Biphenyl (PCB) congeners, PAHs, nitro-PAHs, dibenzo-p-dioxin and dibenzofuran congeners. Immediately after the stress, RHE were transferred to fresh assay medium (Epilife optimized by Synelvia), and incubated for 6h applying placebo, SM 1% or no treatment, before being processed. Each RHE was cut in two parts. A half RHE was processed for histology and a half RHE was processed for ROS quantification. RHE media (subnatants) were collected and stored at -20°C before IL-1a quantification by ELISA or treatment of NHEKs for gene expression analysis. All experimental conditions were performed in triplicate (n=3).
Histological analysis was conducted on fixed tissues, dehydrated in multiple baths with increasing concentrations of ethanol, and then embedded in paraffin. Transversal sections were performed with a microtome (5 μm thickness, 1 slide per labeling) and maintained at room temperature. The sections were then deparaffinized and stained according to the standard protocol of HE (hematoxylin eosin) staining. Briefly, sections were stained with hematoxylin, rinsed and stained with eosin. The sections were then rinsed and mounted in aqueous medium.
To achieve ROS quantification, RHE were embedded in OCT (optimal cutting temperature) medium and frozen at -80°C until blocks were processed for sectioning in a cryostat and mounted on slides. Tissue sections were observed using a ZEISS 710 confocal microscope. Images were captured and processed with ZEN software (objective lens x20). The fluorescence intensity was quantified using ImageJ software and normalized to the living layer area of the epidermis (i.e., excluding the stratum corneum) identified by DAPI staining of nuclei. Three image per replicate were captured.
Quantification of IL-1a in RHE subnatants was performed according to the kit manufacturer’s instructions (R&D Systems # DY200-05, standard curve range: 7.8-500 pg/ml).
2.4. Gene expression study on NHEKs treated with stressed RHE conditioned medium
Keratinocytes were seeded in a 24-well plate and cultured for 24 hours in culture medium and was then replaced by assay medium containing, or not (control), the conditioned media of RHE for further 24 hours of incubation. Conditioned media were tested at 50%. The following experimental conditions were tested: stressed conditioned medium from non-treated RHE stressed with UV and urban dust and stressed conditioned medium from RHE stressed with UV and urban dust and treated with SM 1%. At the end of incubation, the cells were washed in PBS solution and immediately frozen at -80°C. All experimental conditions were performed in triplicate (n=3).
Gene expression analysis was realized by RT-qPCR method on total RNA extracted from the cell monolayers of each experimental condition. Total RNA was extracted from each sample using TriPure Isolation Reagent® according to the supplier’s instructions. The quality of RNA was evaluated using capillary electrophoresis (Bioanalyzer 2100, Agilent technologies). The quantity of RNA was evaluated using a spectrophotometer (Synergy H1, BioTek Instruments). The complementary DNA (cDNA) was synthetized by reverse transcription of total RNA in presence of oligo(dT) and « Transcriptor Reverse Transcriptase » (Roche). The cDNA quantities were then adjusted before qPCR step. The qPCR was performed using a LightCycler® system (Roche Molecular Systems Inc.) according to the supplier’s instructions. Reaction mix (10 µL) was prepared as follows: 2.5 µL of cDNA, primers (forward, reverse) and reagent mix (Ozyme) containing Taq DNA polymerase, SYBR Green I and MgCl2. Reference gene Actin Beta (ACTB) was used for data normalization.
2.5. Activation of human CB2R by CBD or SM: EC50 determination
The activation of the CB2R has been evaluated on human recombinant CB2R. Several concentrations of CBD or SM or 100 nM of WIN 55212-2, a CB2R agonist, or no treatment (control) have been applied during 10 minutes at 37°C and cAMP was measured by HTRF to evaluate the EC50. The EC50 values (concentration producing a half-maximal response) were determined by non-linear regression analysis of the concentration-response curves generated with mean replicate values using Hill equation curve fitting where Y = response, A = left asymptote of the curve, D = right asymptote of the curve, C = compound concentration, and C50= EC50 and
nH = slope factor.
This analysis was performed using software developed at Cerep (Hill software) and validated by comparison with data generated by the commercial software SigmaPlot® 4.0 for Windows® (© 1997 by SPSS Inc.). Cellular agonist effect was calculated as a percentage of control agonist response (WIN 55121-2) for each target, and cellular antagonist effect was calculated as a percentage of inhibition of control reference agonist response for each target.
2.6. Activation of human CB2R by CBD or SM in mast cells and β-endorphin release
Human Mast Cells (HMC1.1) have been isolated from fresh skin biopsies (SCC097, Sigma) and treated for 16 hours with CBD at 0.0025mg/mL or SM at 0.05mg/mL (w/v). Cells were then incubated at 37°C, 5% CO2. After 16 hours, supernatant was collected for further ELISA analysis and cells were trypsinised and counted with Luna cell counter. Release of cAMP and β-endorphin by mast cells was quantified using ELISA kits and following supplier recommendation (Abcam).
2.7. Clinical study: impact of SM on urban skins and comparison with cannabidiol
Double blind, placebo controlled clinical study has been carried on 68 women aged between 45 and 75 years old. All the subjects participating gave their informed consent signed at the beginning of the study. The study followed and was in compliance with the tenets of the Declaration of Helsinki. The volunteers are living in a dense urban environment in Thailand and are thus exposed both to UV rays and pollution (Spincontrol Asia). The volunteers applied twice a day for 56 days a placebo formula or formula with SM 1% or formula with CBD 0.02%.
The INCI formula composition was as follows: AQUA, CETHYL ALCOHOL, GLYCERYL STEARATE, PEG-75 STEARATE, CETETH-20, STEARETH-20, PENTYLENE GLYCOL, ISODECYL NEOPENTANOATE, +/- SILYBUM MARIANUM FRUIT EXTRACT, CAPRYLIC / CAPRIC TRIGLYCERIDE, CITRIC ACID, DIMETHICONE, SODIUM BENZOATE, AMMONIUM ACRYLOYDIMETHYLTAURATE/BEHENETH-25 METHACRYLATE CROSSPOLYMER, FRAGANCE, +/- CANNABIDIOL 99%
Inclusion criteria for the recruitment of panelists were: female subjects, 45-75 years of age, exposed to UV rays and pollution several times a day, all skin type at least 1 subject by skin type, having sensitive skin based in stinging test, having underneath eye wrinkles with a grade ≥ 4 according to the Skin Atlas (pp. 47) and having dull complexion according to the C.L.B.T. scale from Spincontrol Asia.
Digital photography using VISIA CR2.3® were taken to analyze skin homogeneity and radiance and the volunteer have completed a well-being questionnaire analyzed by Emospin. The Well-being Questionnaire (W-BQ) has been originally constructed to measure psychological well-being in people with a chronic somatic illness. Because of its versality and robustness, this tool is recommended by the World Health Organization for widespread use [
27].
2.8. Statistical analysis
For in vitro and ex vivo studies, a Shapiro-Wilk normality test has been performed to evaluate whether the data follow the Gaussian Law. If the results have a normal distribution, Student’s t-test statistical analysis was performed. If the results did not follow the Gaussian Low, a non-parametric statistical analysis was performed by Kruskal-Wallis ANOVA followed by Mann Whitney U test.
All raw data from in vivo experiments were analyzed by Shapiro-Wilk test in order to determine if the data followed Gaussian law or not. If yes, data were analysed using parametric paired or unpaired Student’s t-test. If not, data were analysed using non-parametric Wilcoxon test or non-parametric Mann-Whitney test. Self-assessment has been analysed by contingency method using Chi square test with ** p<0.01.
A result is considered as significant if p<0.1#, p<0.05*, p<0.01** and p<0.001***.
4. Discussion
Skin is particularly exposed to ultraviolet radiation (UV) and air pollution which are major players of the skin aging exposome [
1]. Recent works demonstrated the interplay between air pollutants and UV in the disturbance of skin redox homeostasis, inflammation and barrier function alteration resulting in oxinflammation [
4,
5,
6].
In our study, RHE stressed by a combination of UV and urban dust presented morphological alterations, increased ROS and IL-1a contents suggesting oxidative stress and inflammation. IL-1a is constitutively secreted as precursor protein in keratinocytes, and rapidly increases under UVB exposure, inducing the release of IL-6, IL-8 and inflammatory responses [
32,
33]. In double-stressed RHE, SM maintained a normal tissue organization, decreased ROS and and IL-1a, as well as the expression of
IL-6 and
IL-8. Frankova et al. suggested that anti-inflammatory properties of SM are at least supported by silibinin, a major flavonolignan of SM, which tend to decrease IL-1a, IL-8 and IL-6 during SDS induced inflammation in RHE.
Nrf2 is a master regulator of cellular antioxidant defenses and its activation appears an interesting strategy to prevent skin from damaged caused by combined exposure to pollution and sun [
40]. Indeed, Nrf2 mediates induction of a set of detoxifying and antioxidant enzymes such as NQO1 by binding a DNA sequence called antioxidant response element (ARE) [
7]. Moreover, oxidative stress is mediated by a fine-tuned cross talk between AHR and Nrf2 modulated by antioxidant phytochemicals divided in three groups: Nrf2 agonist with AHR agonistic activity, Nrf2 agonist with AHR antagonistic activity, NrF2 agonist with CYP1A1 inhibitor activity [
13]. According to our results, the antioxidant activity of SM was associated with an upregulation of Nrf2 and NQO1 together with genes involved in AHR activation (ARNT), suggesting agonistic activity on both pathways, and did not modify CYP1A1 expression. SM seems to belong to the group of Nrf2 agonist with AHR agonistic activity gathering soybean tar,
Opuntia ficus-indica extract,
Houttuynia cordata extract,
Bidens Pilosa extract, all containing high flavonoid content as SM. Flavonoids are present in several dietary sources and about 6000 structures have been identified. These phenolic compounds are clearly identified as Nrf2 activators [
41] and today attract attention for the prevention and treatment of neurodegenerative disorders, cancer or COVID-19 [
42,
43,
44]. Structure activity relationship study among flavonoids for Nrf2 induction would be of particular interest to determine the effect of the peculiar structure of SM flavonolignans combining taxifolin and coniferyl alcohol.
Under environmental stress, SM increased expression of genes encoding barrier function associated proteins, filaggrin and keratin 16. UV exposure decreases filaggrin content in the epidermis and this effect is aggravated and prolonged by combination to particulate matter exposure [
5]. Filaggrin is genetically mapped to the epidermal differentiation complex (EDC) locus, comprising genes encoding proteins involved in the terminal differentiation and cornification of keratinocytes [
45]. EDC genes expression, including filaggrin, is modulated by UV and pollutants themselves activating AHR and accelerating keratinocytes differentiation via OVO-like 1 transcription factor [
15]. Keratin 16 is transiently expressed in keratinocytes under Nrf2 activation, in the event of a barrier breach and regulates epithelial inflammation and keratinocyte proliferation serving wound repair [
30,
46]. Collectively these data support the hypothesis of AHR and Nrf2 activation by SM. Several studies report the direct crosstalk between AHR and Nrf2: AHR binding the xenobiotic-responsive element (XRE) of Nrf2, or the last interacting with cap’n’collar-small MAF binding element of AHR promoter. An indirect way involves AHR induced expression of cytochrome P450, releasing intermediate metabolites and then oxidative stress triggering NrF2 [
47].
Recent findings established evidence for CB2R and Nrf2 interaction. CB2R promoter region contains ARE element and is effectively regulated by Nrf2 in microglial cells [
16]. Agonists of CB2R induce Nrf2 in skeletal muscle [
17] and murine obesity model with anti-inflammatory effect [
48]. In diabetic mice, Nrf2 activation potentiates the effect of CB2R agonist [
18].
In skin, CB2R is mainly localized in cutaneous nerve fiber bundles, mast cells and in a lesser extent in epidermal keratinocytes [
36]. CB2R activation stimulates release from keratinocytes of β-endorphin, which acts at local neuronal µ-opioid receptors to inhibit nociception [
20]. CB2R deficiency exacerbates psoriasis symptoms and its agonists improves lesions, decreases inflammatory cytokines and oxidative stress through Nrf2 [
19,
49]. Anti-inflammatory properties of CB2R were also confirmed in skin wound healing process [
50]. CBD is a major compound of
Cannabis sativa L. which does not show psychoactivity but have potential to treat skin disorders due to its antioxidative and anti-inflammatory properties previously reviewed [
39,
51]. CBD reduces oxidative stress in normal human keratinocytes and induces CB2R that is also promoted by UVA and UVB [
52]. Moreover, UV stressed 2D and 3D skin models exhibited cytoprotective response when treated with CBD [
53].
We hypothesized that part of the anti-inflammatory activity of SM could be related to CB2R induction. In a first assay, SM was shown to be a weak agonist of human CB2R, similarly to CBD. Further tests were conducted on mast cells which appeared as an appropriate model as it play key role in immunity and microbiota management thanks to AHR [
34], in maintenance of epidermal barrier function [
35], and some evidence suggests the role of CB2R in immune modulatory effects during inflammation [
50,
54]. Very interestingly, SM was found to activate CB2R and induced release of β-endorphin from mast cell in a similar way as CBD. This opens a new area of research on the understanding anti-inflammatory and antioxidant properties of SM.
Solar radiation and air pollution cause premature ageing through several clinical signs: pigmentary changes including lentigines, wrinkles, roughness, skin pores, skin redness, rosaceae; altering skin homogeneity [
28]. A radiant complexion means healthy and young skin and influences facial attractiveness [
55,
56]. SM significantly improved skin homogeneity on 91% of volunteers who felt positive impact on their energy well-being, reflecting a more radiant and healthiest skin compared to volunteers applying CBD formula. Scientific literature was previously reviewed that establish CBD activities explaining its growing interest and use in the cosmetic industry: antioxidant, anti-inflammatory, β-endorphin inductor, skin redness reduction, mood enhancer [
57]. Finally, in the tested conditions, SM appeared as efficient as CBD to promote CB2R and β-endorphin in mast cells and better improved sensitive skin condition on women exposed to UV and air pollution.
Author Contributions
Conceptualization, Cloe Boira. Amandine Scandolera and Romain Reynaud; methodology, Cloe Boira, Emilie Chapuis, Amandine Scandolera and Romain Reynaud; validation, Cloe Boira, Emilie Chapuis, Amandine Scandolera, and Romain Reynaud; formal analysis, Cloe Boira and Emilie Chapuis; investigation, Cloe Boira; writing—original draft preparation, Cloe Boira; writing-review and editing, Cloe Boira, Emilie Chapuis, Amandine Scandolera and Romain Reynaud. All authors have read and agreed to the published version of the manuscript.
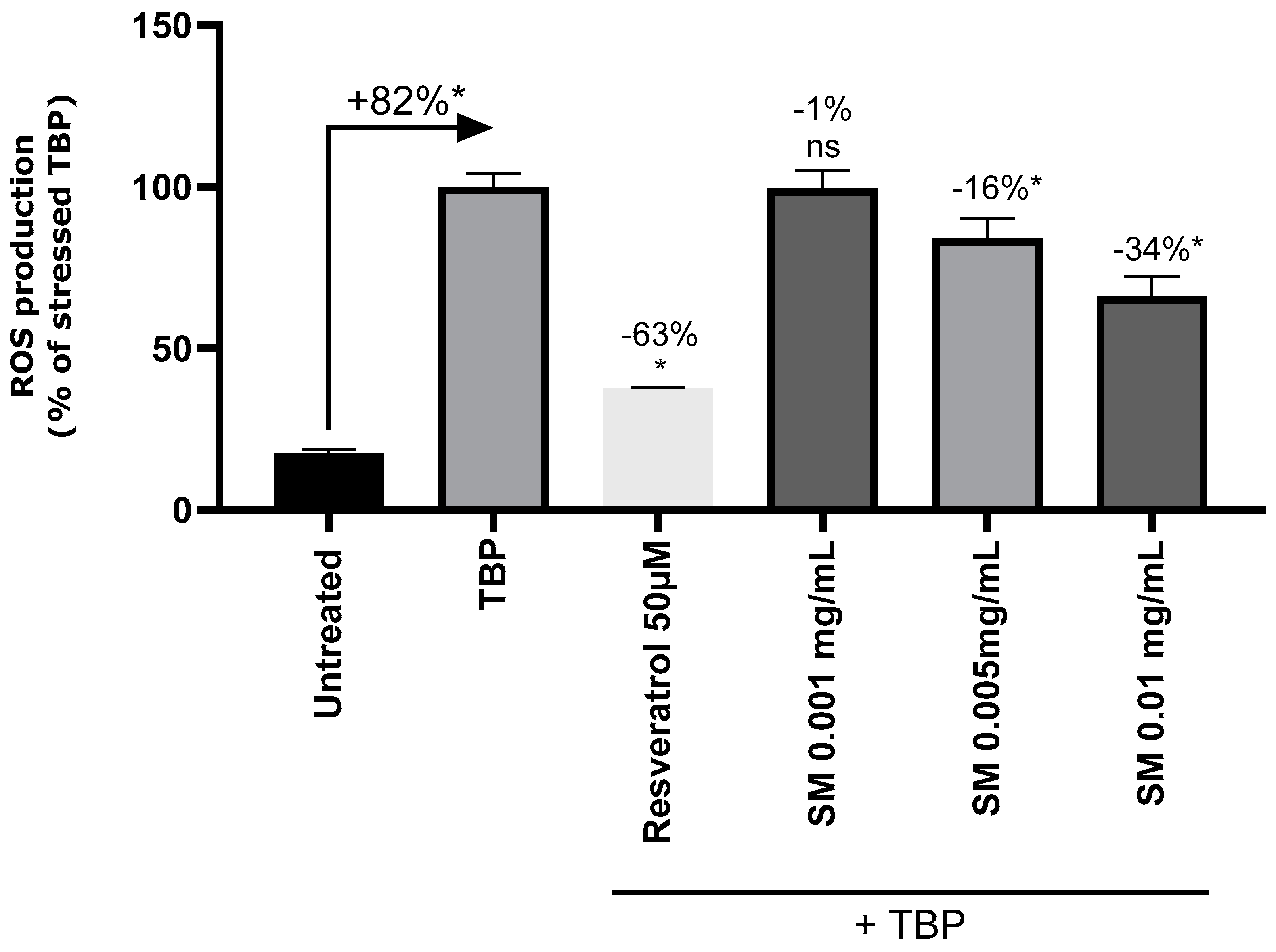
Figure 1.
Quantification of Reactive Oxygen species (ROS) in Normal Human Epidermal Keratinocytes (NHEKs) without treatment (untreated), treated with tert-Butyl hydroperoxide (TBP) 5 mM, and pretreated with resveratrol 50 µM (positive control) or Silymarin (SM) at 0.001 mg/mL, 0.005 mg/mL or 0.01 mg/mL before TBP application. Results are expressed in percentage of condition stressed with TBP. Mann-Whitney test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 1.
Quantification of Reactive Oxygen species (ROS) in Normal Human Epidermal Keratinocytes (NHEKs) without treatment (untreated), treated with tert-Butyl hydroperoxide (TBP) 5 mM, and pretreated with resveratrol 50 µM (positive control) or Silymarin (SM) at 0.001 mg/mL, 0.005 mg/mL or 0.01 mg/mL before TBP application. Results are expressed in percentage of condition stressed with TBP. Mann-Whitney test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
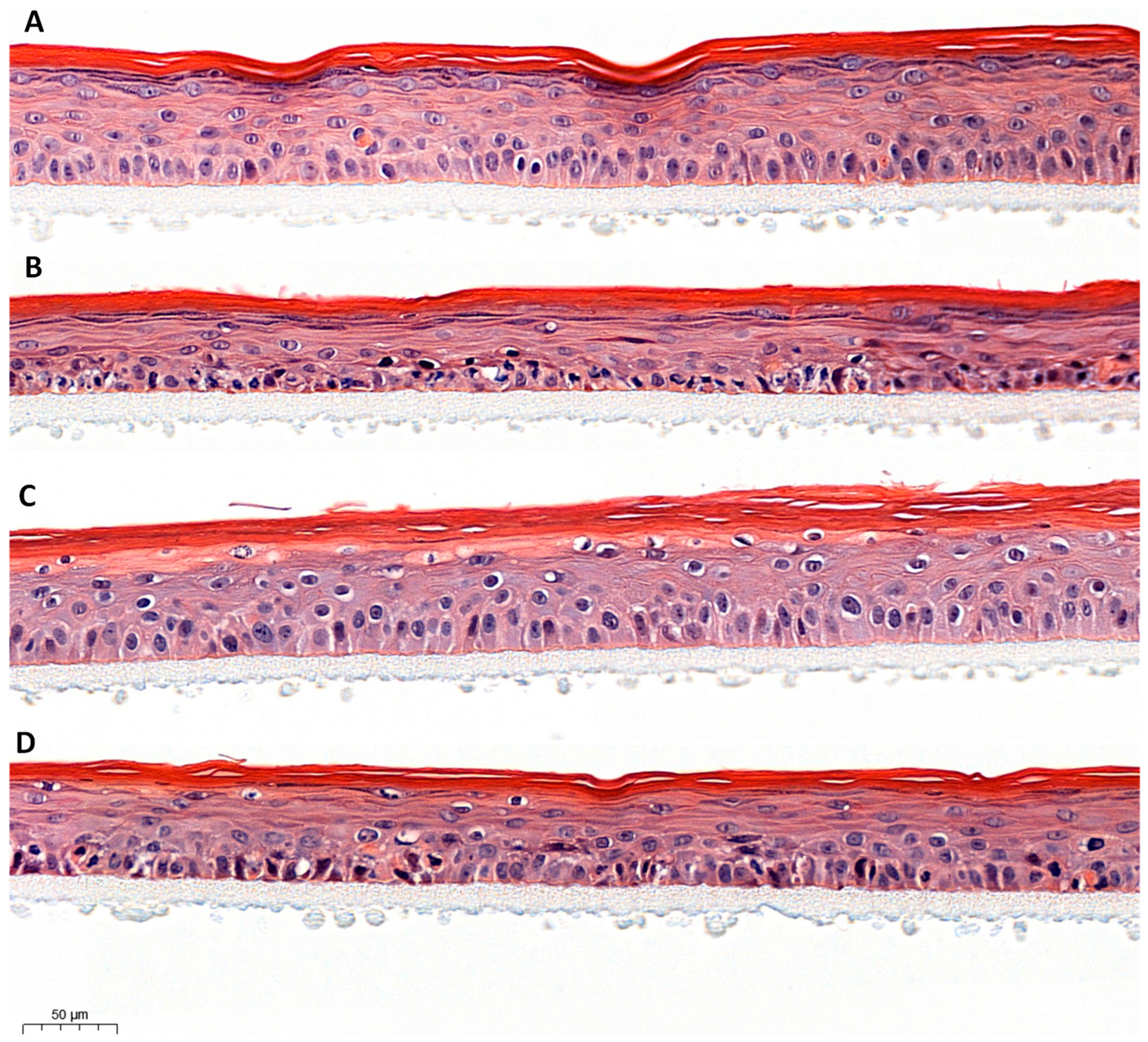
Figure 2.
Comparison of Reconstructed Human Epidermis (RHE) morphology: untreated control (A), exposed to UVA/B and urban dust (B), treated with Silymarin 1% (C) or placebo (D) by topical application before and after exposition to UVA/B and urban dust.
Figure 2.
Comparison of Reconstructed Human Epidermis (RHE) morphology: untreated control (A), exposed to UVA/B and urban dust (B), treated with Silymarin 1% (C) or placebo (D) by topical application before and after exposition to UVA/B and urban dust.
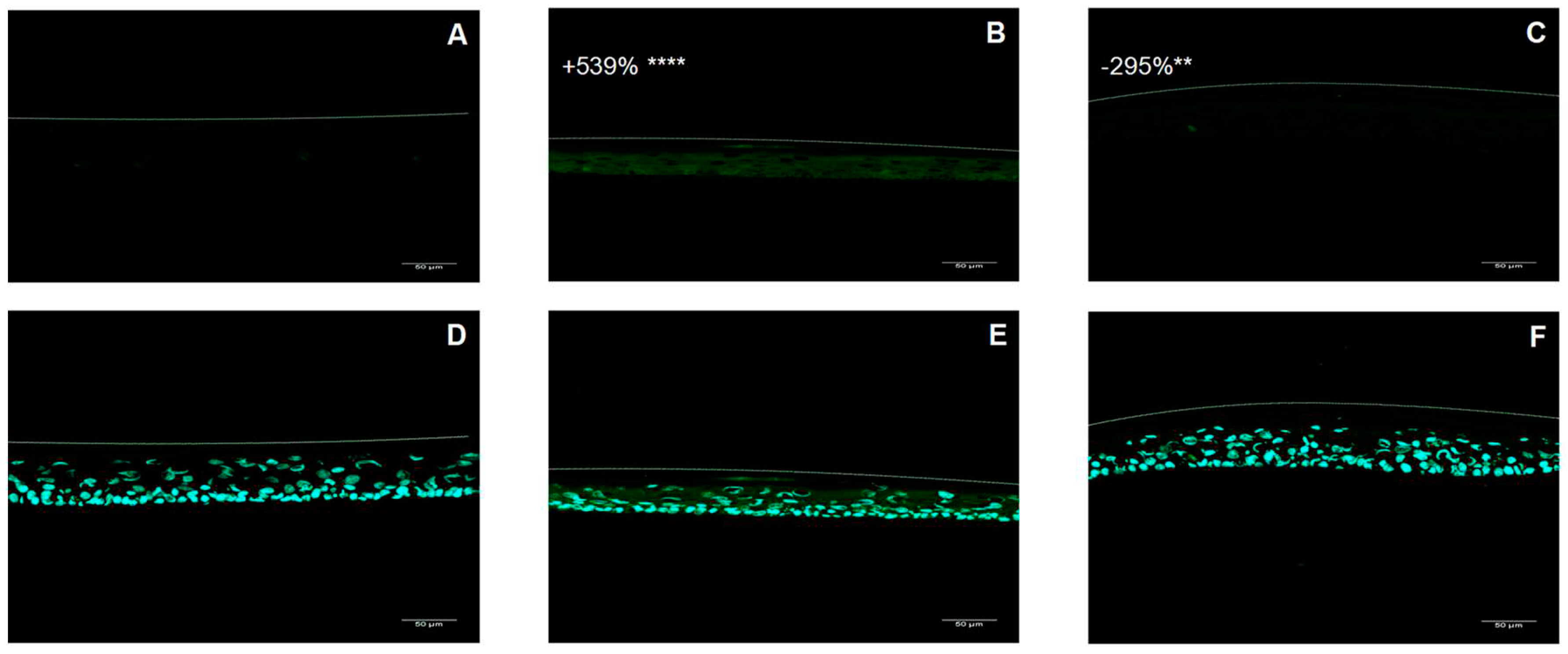
Figure 3.
Representative pictures of fluorescence confocal microscopy for reactive oxygen species detection by dichlorofluorescein (DCF) (A, B, C) and 4′,6-diamidino-2-phenylindole (DAPI) staining (D, E, F) in untreated Reconstructed Human Epidermis (RHE) (A, D), stressed with UVA/B and urban dust (B, E), and treated with formulation containing Silymarin 1% (C, F). Percentage is expressed in comparison to unstressed control except for Silymarin treated condition for which decrease is expressed in comparison to UVA/B and urban dust stressed condition. Mann Whitney U test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 3.
Representative pictures of fluorescence confocal microscopy for reactive oxygen species detection by dichlorofluorescein (DCF) (A, B, C) and 4′,6-diamidino-2-phenylindole (DAPI) staining (D, E, F) in untreated Reconstructed Human Epidermis (RHE) (A, D), stressed with UVA/B and urban dust (B, E), and treated with formulation containing Silymarin 1% (C, F). Percentage is expressed in comparison to unstressed control except for Silymarin treated condition for which decrease is expressed in comparison to UVA/B and urban dust stressed condition. Mann Whitney U test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
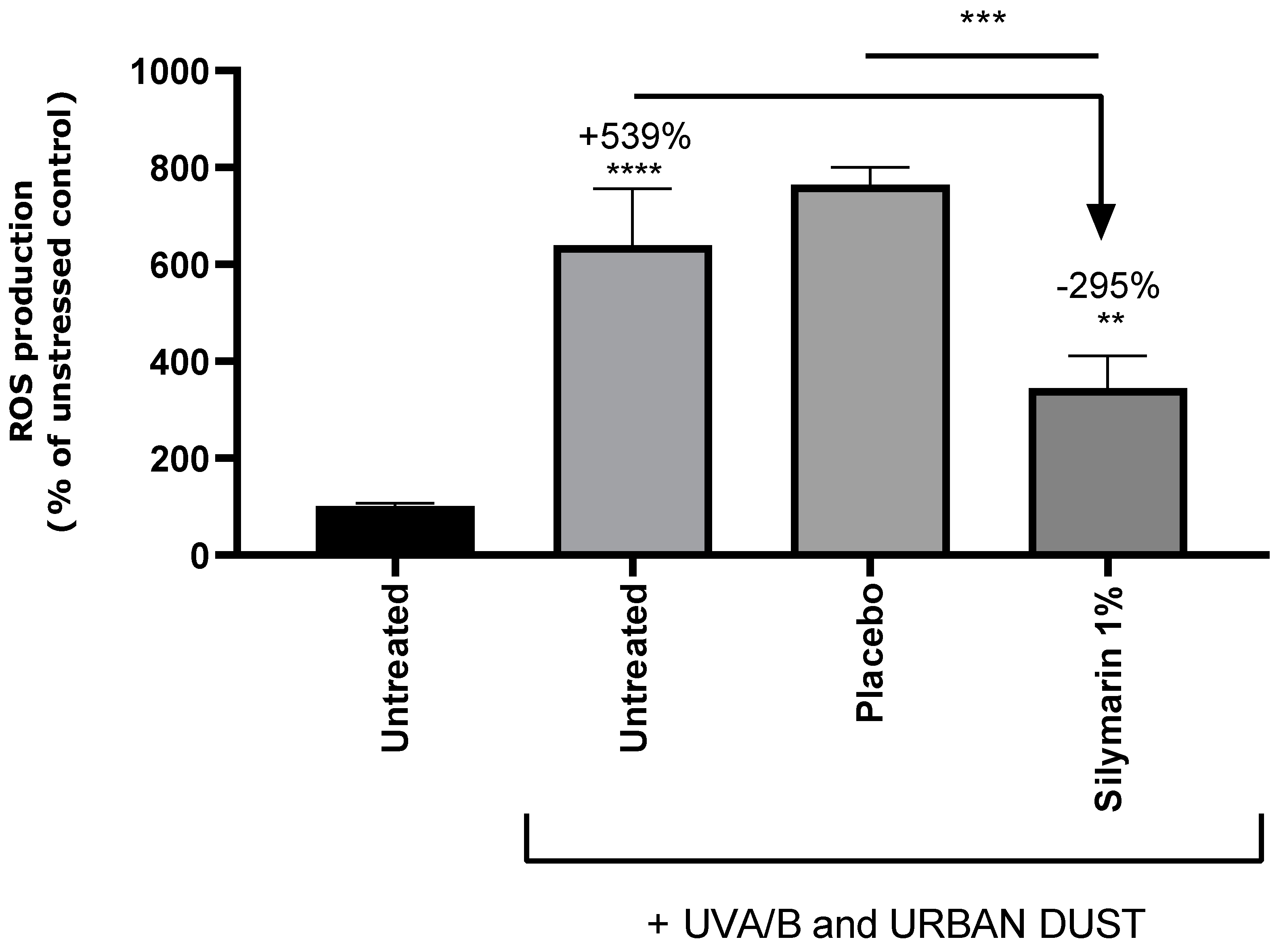
Figure 4.
Quantification of reactive oxygen species in untreated Reconstructed Human Epidermis, stressed with UVA/B and urban dust, and treated with placebo cream or formulation containing Silymarin 1%. Percentage is expressed in comparison to unstressed control except for Silymarin treated condition, for which decrease is expressed in comparison to UVA/B and urban dust stressed condition. Mann Whitney U test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 4.
Quantification of reactive oxygen species in untreated Reconstructed Human Epidermis, stressed with UVA/B and urban dust, and treated with placebo cream or formulation containing Silymarin 1%. Percentage is expressed in comparison to unstressed control except for Silymarin treated condition, for which decrease is expressed in comparison to UVA/B and urban dust stressed condition. Mann Whitney U test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 5.
Quantification of interleukin 1 alpha (IL-1a) by enzyme-linked immunosorbent assay (ELISA) in untreated Reconstructed Human Epidermis, stressed with UVA/B and urban dust, and treated with placebo cream or formulation containing Silymarin 1%. Percentage is expressed in comparison to unstressed control except for Silymarin treated condition for which decrease is expressed in comparison to UVA/B and urban dust stressed condition. Mann Whitney U test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 5.
Quantification of interleukin 1 alpha (IL-1a) by enzyme-linked immunosorbent assay (ELISA) in untreated Reconstructed Human Epidermis, stressed with UVA/B and urban dust, and treated with placebo cream or formulation containing Silymarin 1%. Percentage is expressed in comparison to unstressed control except for Silymarin treated condition for which decrease is expressed in comparison to UVA/B and urban dust stressed condition. Mann Whitney U test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
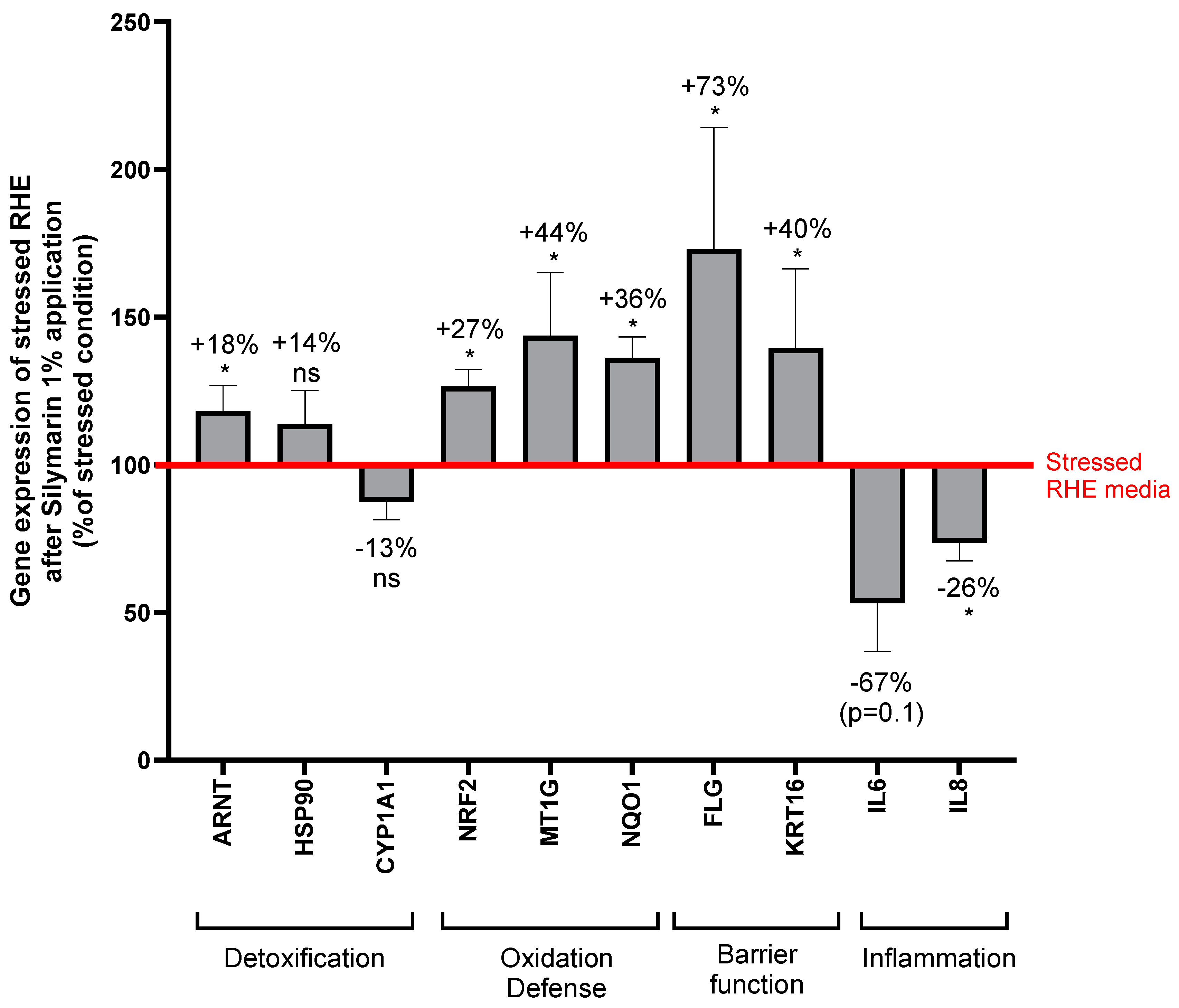
Figure 6.
Relative gene expression of Normal Human Keratinocytes treated with conditioned medium of stressed Reconstructed Human Epidermis (RHE) treated with Silymarin 1% before and after exposure to UVA/UVB and urban dust. Values are relative to the conditioned medium of stressed RHE without treatment. ARNT: AhR nuclear translocator, HSP90: heat shock protein 90, CYP1A1: cytochrome P450 1A1 monooxygenase, NRF2: nuclear factor erythroid 2-related factor 2, MT1G: metallothionein 1G, NQO1: NAD(P)H quinone dehydrogenase 1, FLG: filaggrin, KRT16: keratin 16, IL-8: interleukin 8, IL-6: interleukin 6. Mann Whitney U test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 6.
Relative gene expression of Normal Human Keratinocytes treated with conditioned medium of stressed Reconstructed Human Epidermis (RHE) treated with Silymarin 1% before and after exposure to UVA/UVB and urban dust. Values are relative to the conditioned medium of stressed RHE without treatment. ARNT: AhR nuclear translocator, HSP90: heat shock protein 90, CYP1A1: cytochrome P450 1A1 monooxygenase, NRF2: nuclear factor erythroid 2-related factor 2, MT1G: metallothionein 1G, NQO1: NAD(P)H quinone dehydrogenase 1, FLG: filaggrin, KRT16: keratin 16, IL-8: interleukin 8, IL-6: interleukin 6. Mann Whitney U test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
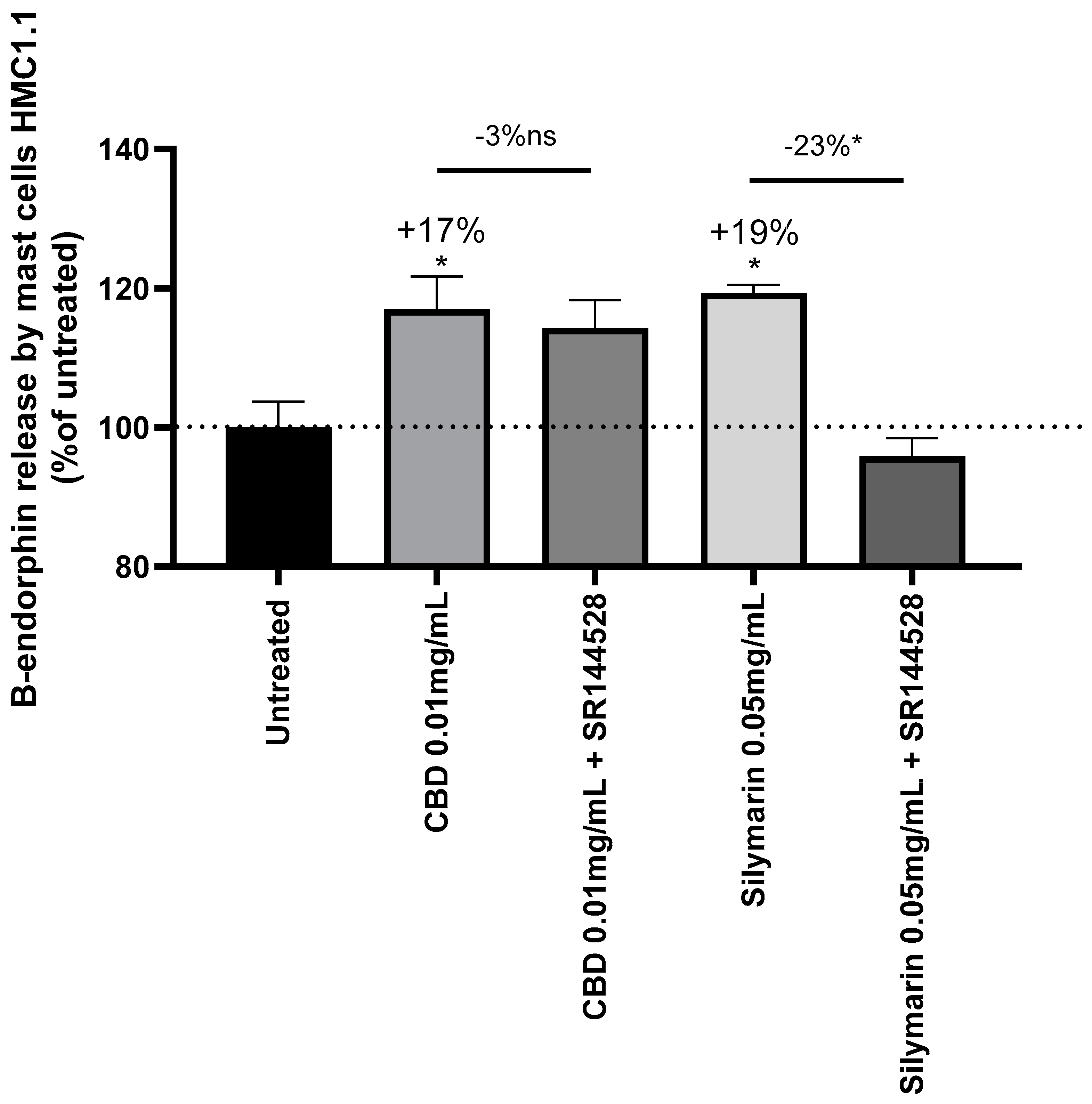
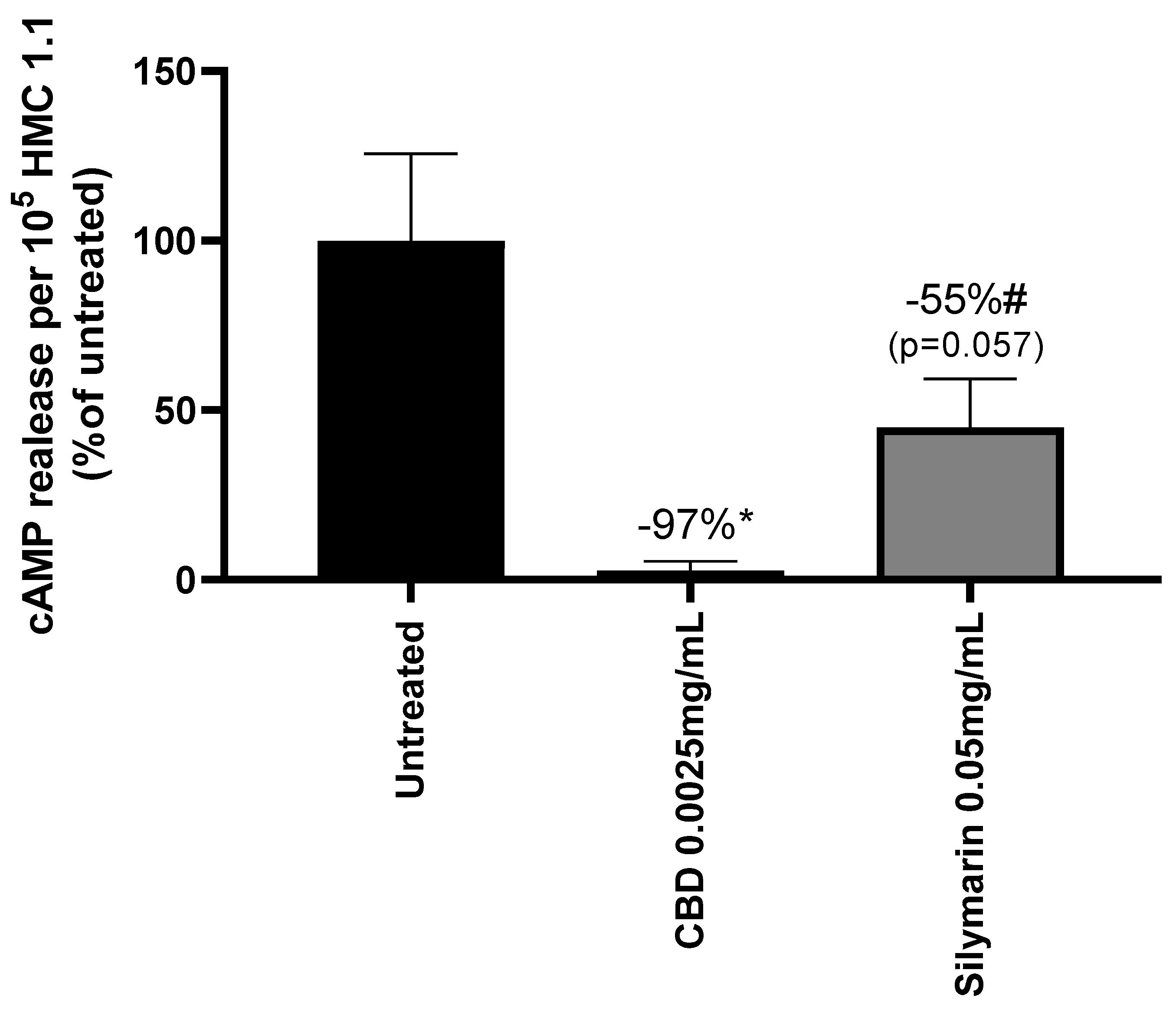
Figure 7.
Activation of cannabinoid receptor type 2 (CB2R) in mast cells (HMC1.1) measured through the inhibition of cAMP release by cannabidiol (CBD) and Silymarin. Results are expressed in percentage of the untreated cells. Mann-Whitney test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 7.
Activation of cannabinoid receptor type 2 (CB2R) in mast cells (HMC1.1) measured through the inhibition of cAMP release by cannabidiol (CBD) and Silymarin. Results are expressed in percentage of the untreated cells. Mann-Whitney test, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
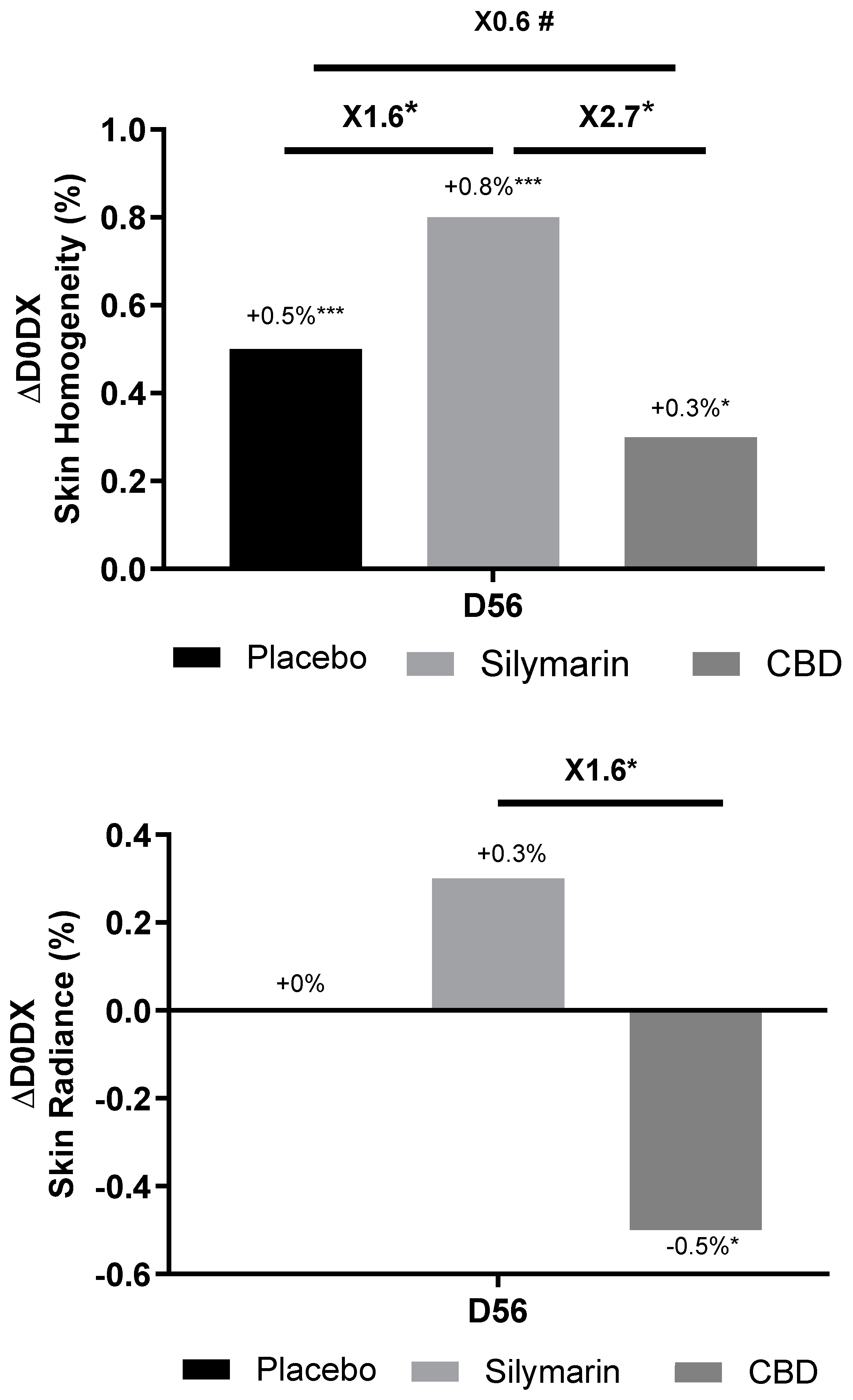
Figure 9.
Comparison of skin homogeneity (up) and radiance (down) at 56 (D56) vs day 0 (D0) between groups applying placebo cream or Silymarin 1% in formulation or cannabidiol (CBD) 0.02% in formulation. Wilcoxon test vs D0, Mann Whitney test vs placebo, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 9.
Comparison of skin homogeneity (up) and radiance (down) at 56 (D56) vs day 0 (D0) between groups applying placebo cream or Silymarin 1% in formulation or cannabidiol (CBD) 0.02% in formulation. Wilcoxon test vs D0, Mann Whitney test vs placebo, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 10.
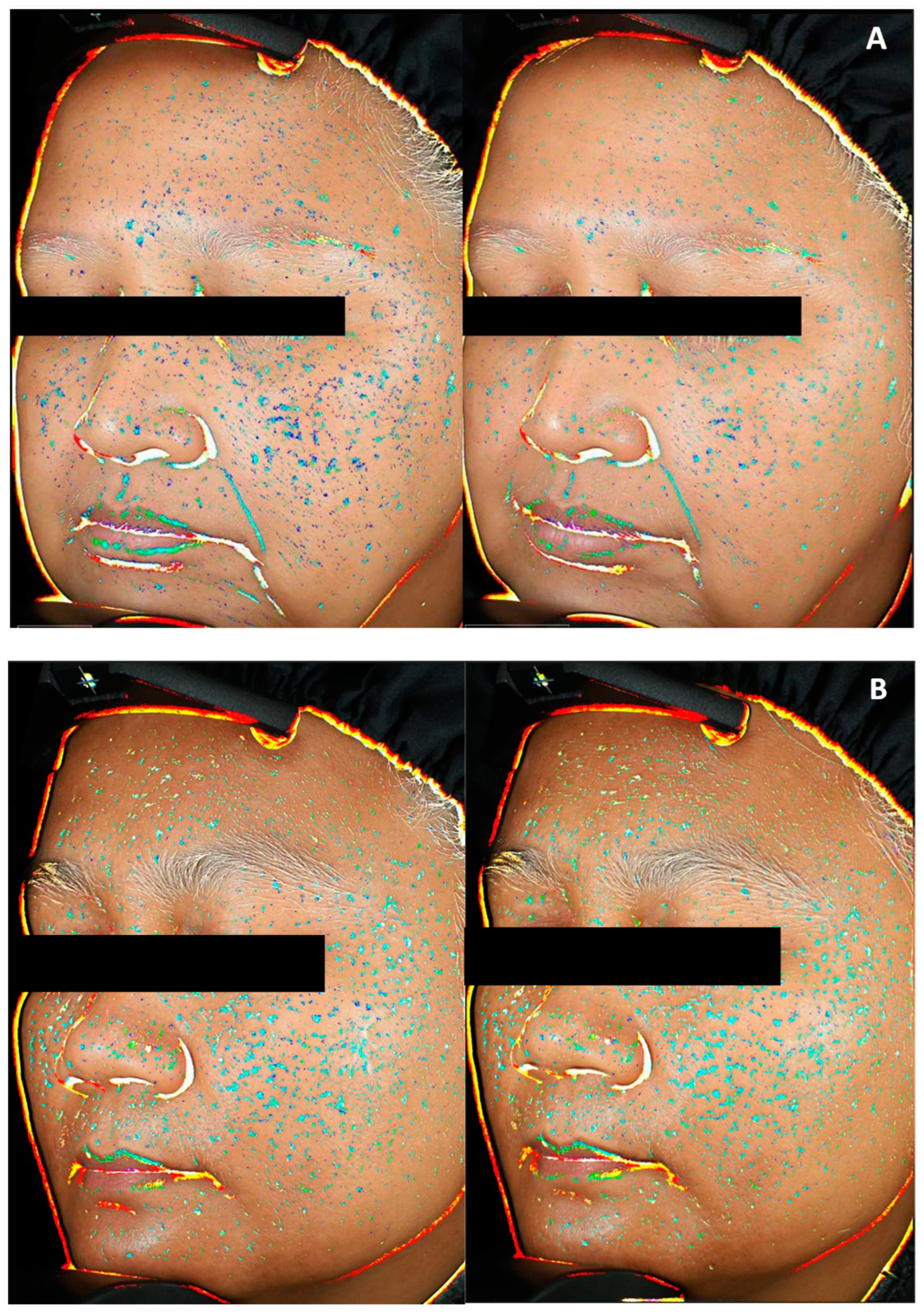
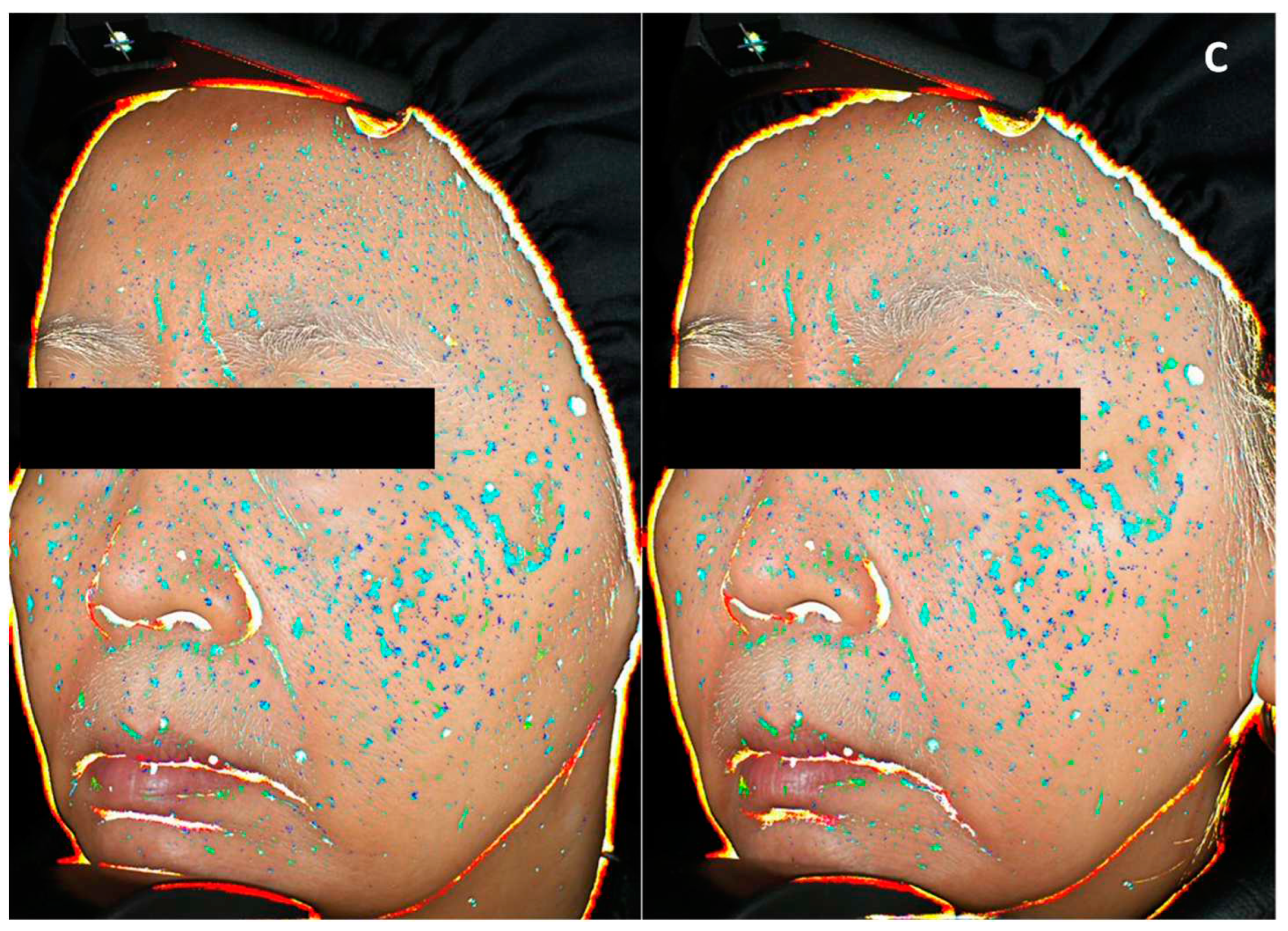
Representative pictures illustrating skin homogeneity changes at day 0 (left) and day 56 (right), of panelist applying Silymarin 1% in formulation (A) or cannabidiol 0.02% in formulation (B) or placebo cream (C). Heterogeneity is indicated by blue spots: light blue representing the highest contrast to dark blue representing the lowest contrast.
Figure 10.
Representative pictures illustrating skin homogeneity changes at day 0 (left) and day 56 (right), of panelist applying Silymarin 1% in formulation (A) or cannabidiol 0.02% in formulation (B) or placebo cream (C). Heterogeneity is indicated by blue spots: light blue representing the highest contrast to dark blue representing the lowest contrast.
Figure 11.
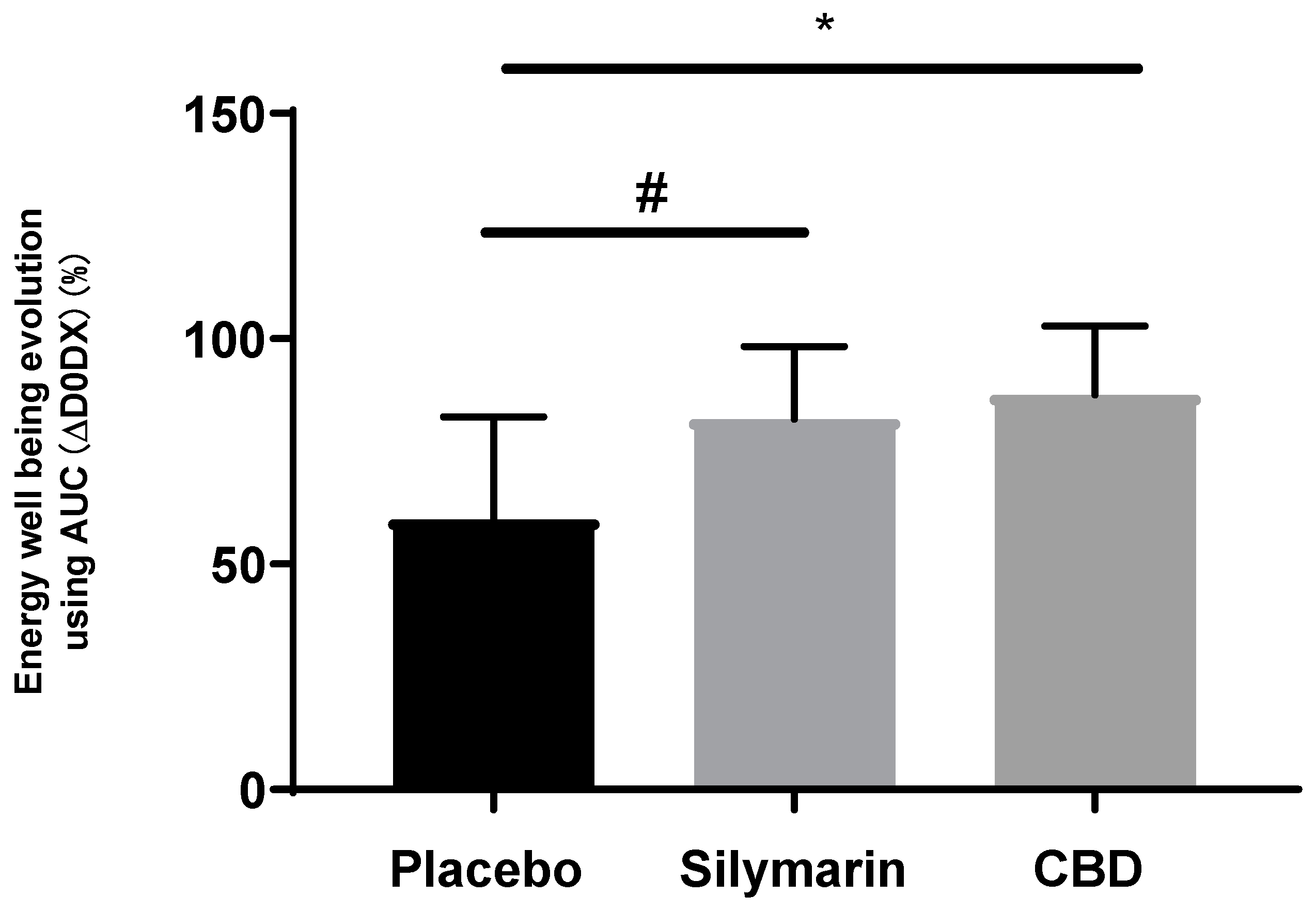
Quantitative data extracted from well-being questionnaire and comparing the evolution of volunteers’ self-evaluation of their skin energy. Mann Whitney test vs placebo, p<0.1#, p<0.05*, p<0.01** and p<0.001***.
Figure 11.
Quantitative data extracted from well-being questionnaire and comparing the evolution of volunteers’ self-evaluation of their skin energy. Mann Whitney test vs placebo, p<0.1#, p<0.05*, p<0.01** and p<0.001***.