1. Introduction
Cardiovascular diseases such as ischemic heart disease remains a major cause of death worldwide. Despite extensive research for pharmaceutical treatment, mortality rates remain high and therefore further investigations are needed (1-4). The first study of experimental induction of myocardial ischemia was reported in 1862. and since then, among various techniques for inducing myocardial infarction in experimental models, the ligation of left anterior descending artery (LAD) has become the most prominent (5-7).
Mesenchymal cells - stem cells are pluripotent cells which were firstly recognized in bone marrow of adults. Adipose Derived Stem Cells (ADSCs) transplantation is nowadays considered the ideal tissue choice due to decreased possibility of rejection. They are characterized by the ability of neovascularization and differentiation to multiple cell line including adipocytes, osteoblasts, chondroblasts. Furthermore, it is known that they can be differentiated to cardiomyocytes and can lead to newly formed cardiac tissue (7-9,29-30). Despite numerous current experimental trials, there are still many questions unanswered, such as the type of cells to be used, the number of cells necessary to be transplanted, the technique and route of administration, the confirmation of the improvement of the function of the target tissue such as the myocardium.
The molecular markers used in our study for evaluating the action of ADSCs in the myocardium were the GATA4, a zinc-finger transcription factor highly expressed in cardiomyocytes, which is a key to myocardial differentiation, and NKX2-5, a protein coding gene, which is involved in myocardial conduction and contractility. In vitro results have shown an actively proliferating stem cells’ population 72 hours after isolation, but it has been documented by immunochemical studies that the expression of stem cell markers needs approximately 7 days (8-13,23-25).
The goal of the study is to prove whether the ADSCs transplantation in the ischemic myocardium may produce a regeneration of the myocardial cells and increase the viable contractile tissue. For this purpose, we used a previously described animal model with many prototype features (1,14-16). Furthermore, for testing the heart function, we utilized a SPECT-CT evaluation before and after surgically induced ischemia. As shown previously, this non-invasive method evaluates both anatomical and physiological changes, offers a serial quantitative approach of the myocardial function and the myocardial metabolism in cellular level in the heart, and can easily correlate to modern approaches of cardiovascular diseases, in particularly when combined with molecular markers evaluation (1,8-10,17-20,26-28).
2. Methods
2.1. Animals and study protocol
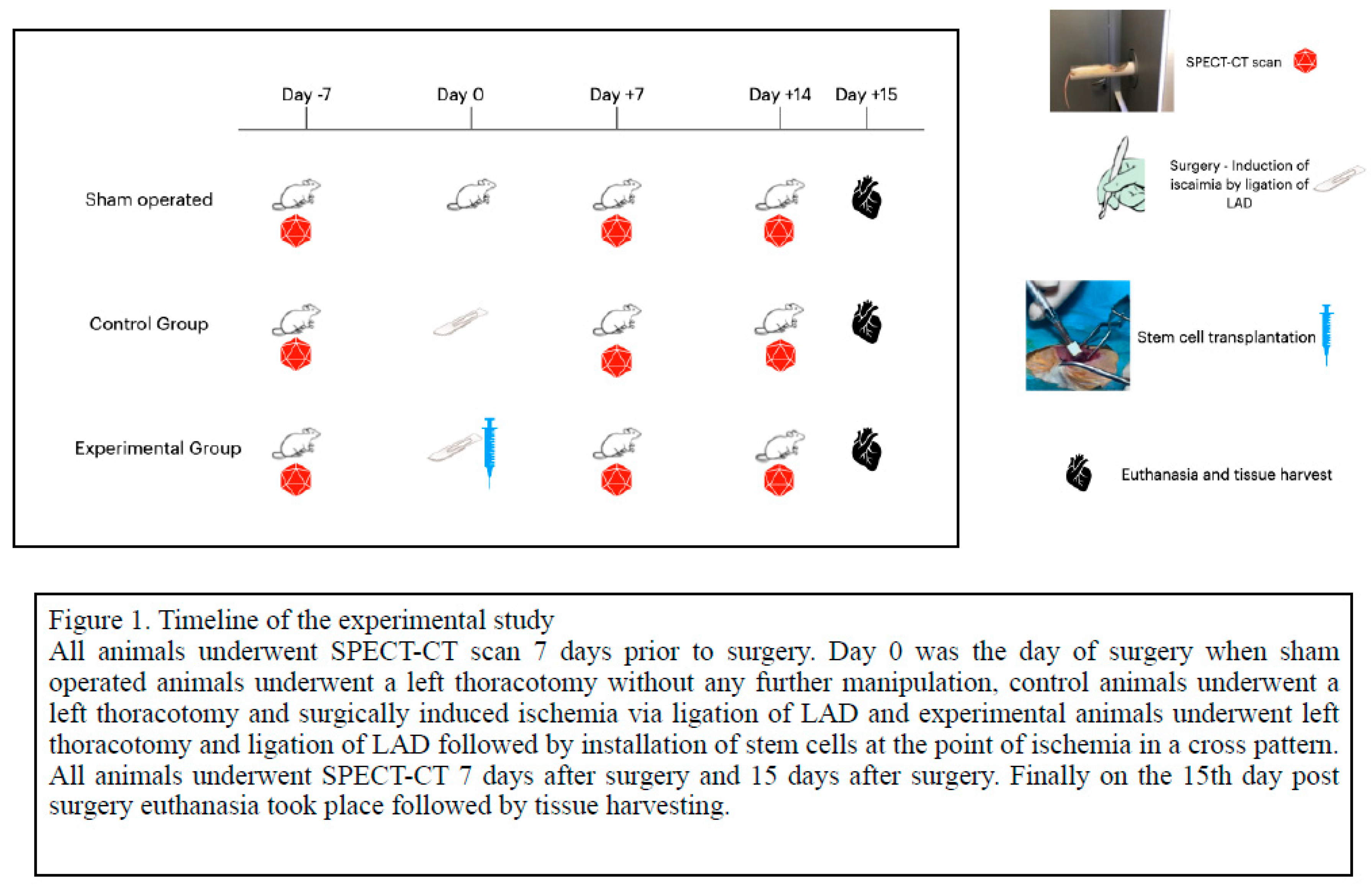
Our animal model of inducing myocardial ischemia, the documentation, monitoring and heart function as detected by SPECT-CT has been previously described in detailed (1). The ADSCs were retrieved from the abdominal adipose tissue of 6 male rats (donors), while 40 female rats were used for the control, sham, and experimental groups, all of them weighting 280-350 grams. The study was authorized by the national Animal Experiment Board of Greece and the Veterinary Association of Athens (License No: 1870/23-04-2018) and was carried out in compliance with EU legislation relating to the conduct of animal experimentation (21). The animals were hospitalized in a certified laboratory with the necessary equipment and veterinarian surveillance.
In accordance to the ARRIVE Guidelines and the 3Rs (22), animals were kept in 3 per cages with free access to water and food. The hygiene (waste, ventilation etc) and the pharmaceutical protocol were planned by the veterinarian committee according to the regulations of the authorized laboratory. After surgery, the animals were left to recover in cages alone or with other post- surgery animals in order to avoid cannibalism and other aggressive behaviors.
2.2. Surgical procedure
Sterile conditions resembling an operation room were utilized and the surgical field. Accordingly, all instruments were sterilized in dry sterilization chambers. The surgical bed, a mattress particularly made for rodents and other small animals weighting below one kilogram, was connected to special equipment allowing a continuous ECG recording, oximeter and thermometer, as well as heater and cooler. Nasopharyngeal intubation was achieved with a 17G tube and connected to an inhalator in a mixture of 30-40% sevoflurane. Analgesia was achieved with subcutaneous administration of Butorfanole (Dolorex) 10mg/ml at the beginning of the procedure and every 3-5 hours. Antibiotic coverage was done with intramuscular administration of oxytetracycline (Oxyvet 20%) 0,03ml. Thermometer was placed in the rectal orifice of the animal, an oximeter was placed on the left foot and non invasive measurement of the blood pressure was measured by cable placed on the left arm. A small thoracotomy was made in the 4th intercostal space and the pericardium was carefully entered. Except from the sham group, all others underwent LAD ligation at its first third after the 1
st diagonal branch using a 4-0 silk suture. The timeline of the experiment is graphically presented in
Figure 1. After 45 min of ischemia documented by ST elevation in the ECG, 0,4ml of liquid around the ischemic area in a cross pattern were administered. Control animals (n=10) received a buffer solution with heart friendly electrolytes, while experimental animals (n=10) received previously prepared ADSCs, following which the thoracotomy was closed. At the end of the experiment, (
Figure 1) via a small laparotomy, the inferior vena cava was used for blood sampling, following which administration of excessive KCl produced myocardial arrest. Subsequently, heart, lung and liver organs were harvested and placed in formaldehyde. Cadavers were destroyed in special biological material waste. There were 9 deaths: 2 deaths at the closure of the animal due to lung injury and pneumothorax, 2 deaths due to cardiac arrhythmias leading to ventricular fibrillation, 1 death from hyperthermia due to accidental removal of thermometer, 1 death due to dextrocardia and numerous manipulations for heart immobilization, 2 deaths in the first 24hours PO, and 1 due to cannibalism in the PO cage.
Drugs administered during the surgical procedure : 1) Tobrex drops for eye protection administered locally in the eyes at the beginning 2)Natural Saline 0,9% and Dextrose 5% for hydration administered subcutaneously (10-20mg/kg) at the beginning 3) Dolorex (Boutorfanole 10mg/ml) for analgesia administered subcutaneously (1-2mg/kg) at the beginning and every 3-5 hours 4) Oxyvet 20% (Oxytetracycline 200mg/ml) for antibiotic coverage administered intramuscularly (20mg/kg) at the beginning and 3 days post surgery.
Euthanasia: On the day of euthanasia (Day 15) the animals underwent the same procedure of anesthesia as at the day of surgery (described earlier) followed by laparotomy and identification and isolation of the Inferior Vena Cava in which administration of 3ml of KCl was established. This way the bradycardia was achieved and finally the heart stopped in diastole phase. Afterwards tissue harvesting was made and specifically removal of heart, lungs and liver which were placed in 10% neutral formaldehyde kits. The waste products were discharged and handled according to the European legislation from the authorized stuff of the experimental laboratory.
2.3. Isolation and culture of ADSCs
ADSCs were isolated from 3-month-old male Wistar rats obtained from the experimental facility of National Center for Scientific Research “Demokritos”, Athens, Greece, with official coding for breeding and provision of animals (EL 25 BIO 019 and EL 25 BIO 020, respectively). The cells were collected from subcutaneous layer of adipose tissue of the abdominal wall and were washed with PBS, minced using two scalpels and then digested in crude collagenase (1 mg/ml final concentration of collagenase; DMEM, Thermo Fisher Scientific, Inc.) for 30 min at 37°C. Later the material was centrifuged (200×g for 5 min) at 37°C to discard the supernatant, and the pellet was resuspended in DMEM, 10% FBS (Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin and then transferred to a culture flask. Overnight incubation at 37°C was followed by change of the medium order to remove the nonadherent cells, and the attached cells were further cultured in the same medium, under standard culture conditions. Novel DNA synthesis was measured with dual labeling with 5-bromo-2’-deoxyuridine (BrdU) and 4’, 6-diamino-2-phenylindole (DAPI) dihydrochloride (Sigma) (1). Furthermore, ADSCs cell surface markers were examined with, as described before (2, 3).
2.4. SPECT-CT acquisition and reconstruction
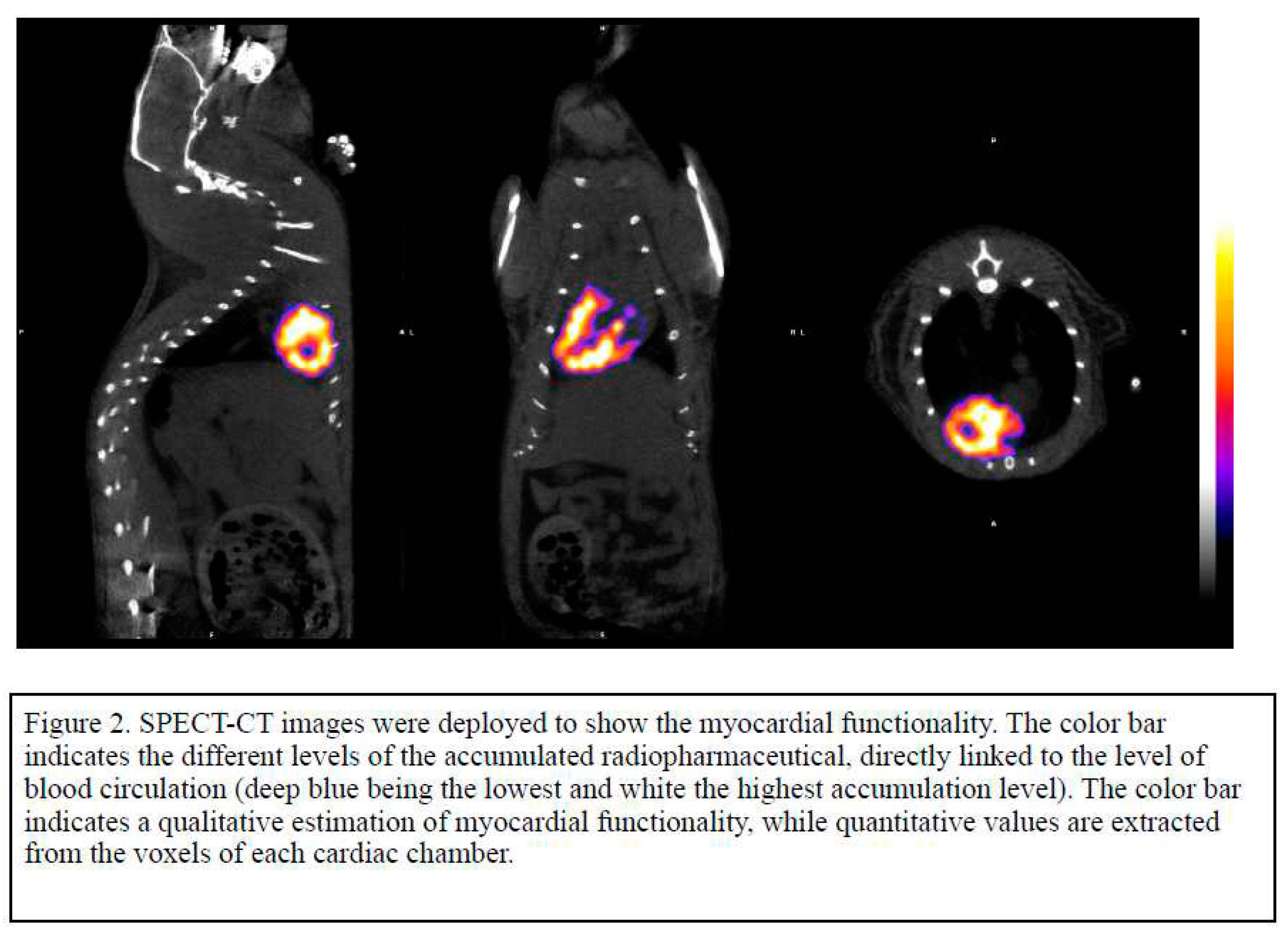
Our technique SPECT-CT imaging was especially created for small rodents and the use of a special chamber helped to focus on the thoracic cavity of the animal and has been published before (1). Rats were injected with 200ul of 1.5mCi -3mCi of 99mTc- Sestamibi, and imaging was created at 20minutes post injection. SPECT-CT imaging was made with x-Cube and γ-Cube. CT acquisition and post processing for whole body was accomplished using a general purpose protocol (50kVp) performing an ISRA reconstruction with 200um voxel size. All anatomical axes (short, long and horizontal) were created for each animal and 3D rendering with color qualitative indication scale was used to show the myocardial activity 7 and 14 days PO.
The measurement was done in voxels from each cardiac chamber and the ratio of left to right ventricle was evaluated in order to identify the changes of myocardial activity after established myocardial ischemia due to surgical ligation of LAD.
Figure 2
2.5. Histological and Immunohistochemical analysis
On day 15 PO, before euthanasia and under anesthesia, the heart was harvested and fixed in 10% neutral formaldehyde over night and was routinely processed.
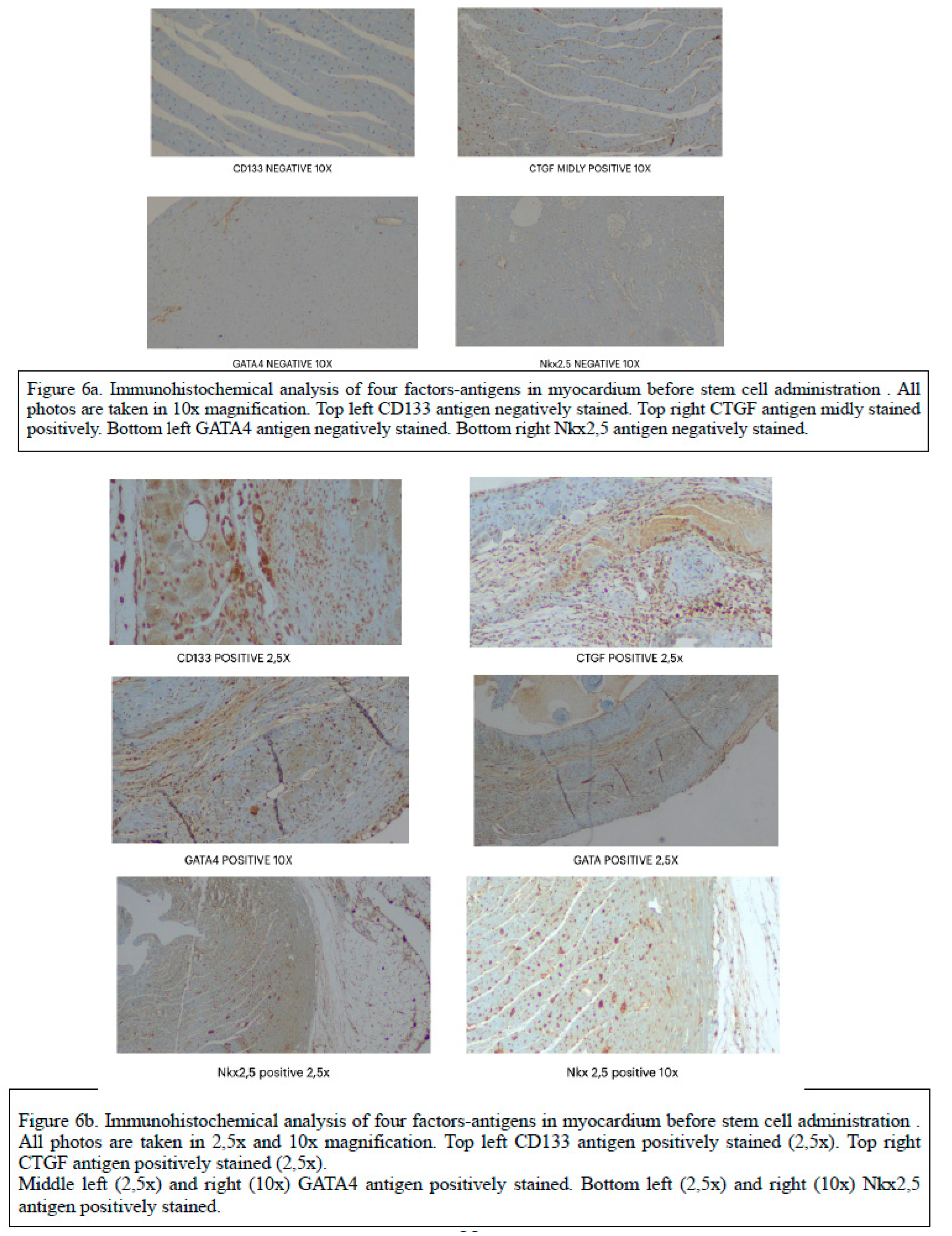
In our previous report of the experimental model, we have documented the histological proof of ischemia in the myocardium with decrease of tissue fibers (1). Furthermore, in this study, immunohistochemical staining for analyzing each antigen expression was performed. The expression of antigens was additionally performed in 4 μm thick tissue. Immunohistochemical staining allowed the visualization of antigens through the specific sequence of antibody-antigen bonding, then by a the bond of a secondary antibody to the primary and the creation of an enzymatic complex that makes a chromogenic reaction. The anti-rat antibodies used in this study after specific steps of clearance. The molecular markers used in our study for evaluating the action of ADSCs in the myocardium were directed against GATA4 and NKX2,5, CDB3 and CDGF (
Table 1). We used the polyclonal antibodies from the kits : 1) Origene TA354470 for CD133 2) Origene TA323092 for CTGF, 3) Origene AP20302PU-N for GATA4 and 4) Abcam ab214296. Diaminobenzidine was used as chrome agent and light hematoxylene as counter stain. Tissue sections, where the primary antibody was omitted, were used as negative controls.
2.6. Statistic evaluation and analysis
For quantitative variables the selected data were expressed as mean ± standard deviation (SD) while for qualitative variables as frequencies and percentages. In order to analyze the normality of the quantitive variables the Kolmogorov—Smirnov test was used. In order to compare the variables both qualitative and quantitative pairwise comparisons between experimental groups was performed using one way ANOVA with Bonferroni correction and the Chi-square test with Bonferroni correction, respectively. All tests were two-sided. Statistically significant difference was defined by a p-Value < 0.05. Statistical analysis was performed using the statistical package SPSS version 21.00 (IBM Corporation, Somers, NY, USA).
3. Results
3.1. SPECT-CT evaluation
The initial images were evaluated by performing a whole body spiral acquisition in a high resolution protocol. The visualization was firstly standardized by scanning all the animals before surgery and the distribution of the radioactive tracer 7 and 14 days after the surgical ligation of LAD proved the changes related to ischemia. (
Figure 2). The difference between healthy and ischemic myocardial tissue were easily recognizable after the axial and 3D reconstruction leading to the creation of a physiological and anatomical map of the rat’s heart (
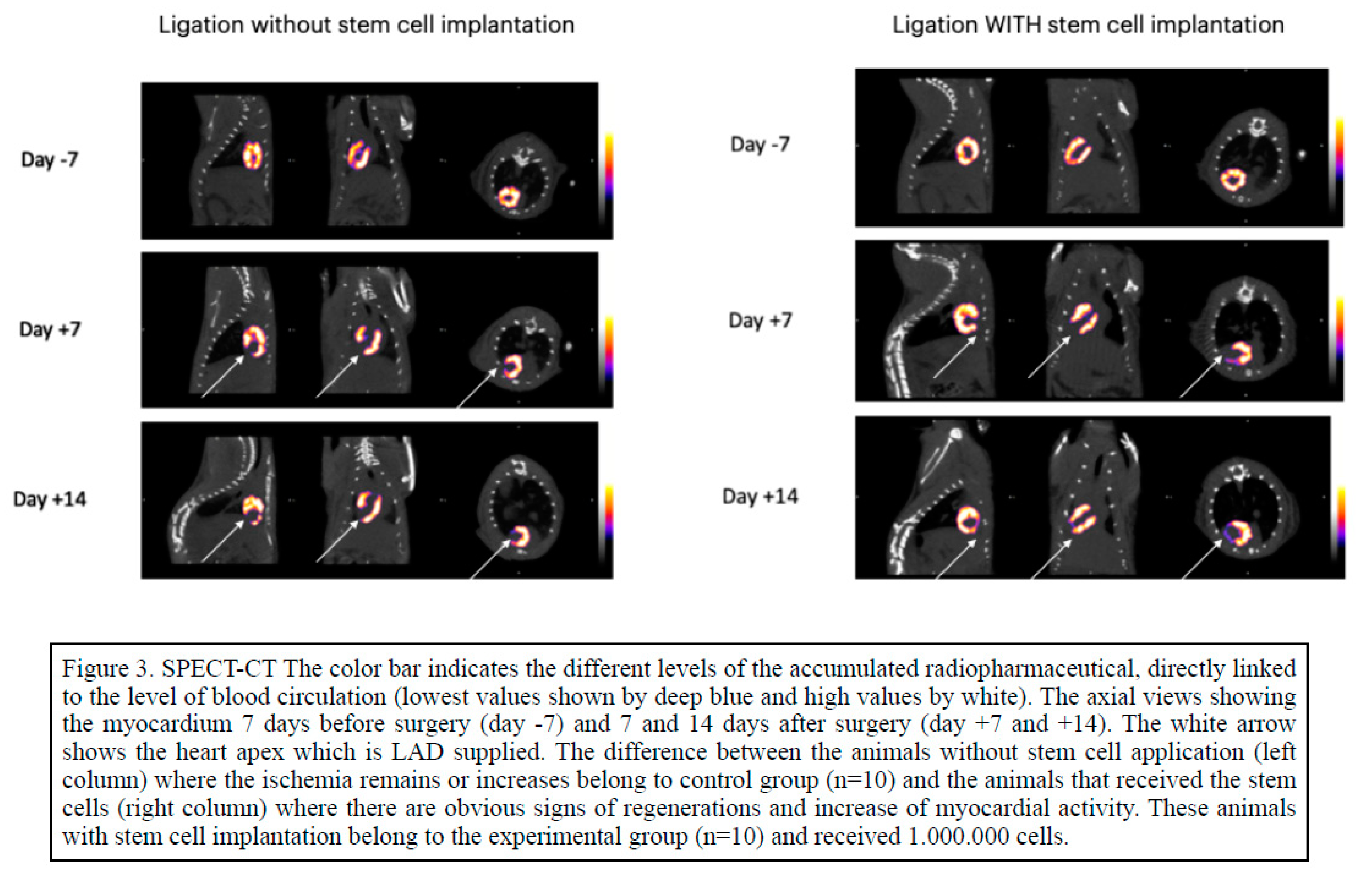
Figure 2). In healthy myocardial cells the radioactive absorbance was high as the cells are highly metabolic while in ischemic cells the absorbance was decreased or absent (
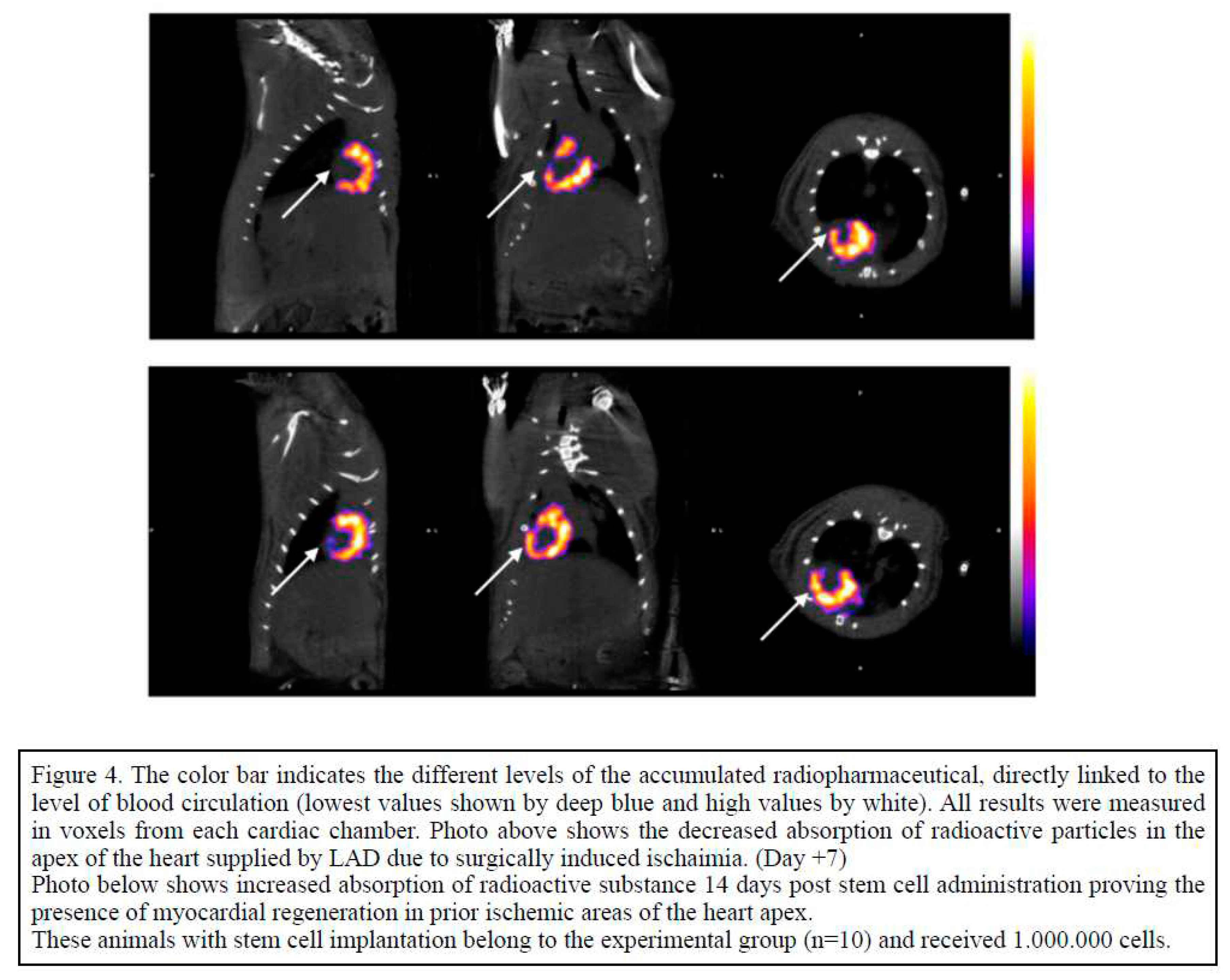
Figure 3, left column). It is notable that in the animals receiving stem cells transplantation, SPECT-CT evaluation revealed definite signs of regenerations and increase of myocardial activity (
Figure 3, right column,
Figure 4).
3.2. Left ventricle/right ventricle ratio (LV/RV ratio)
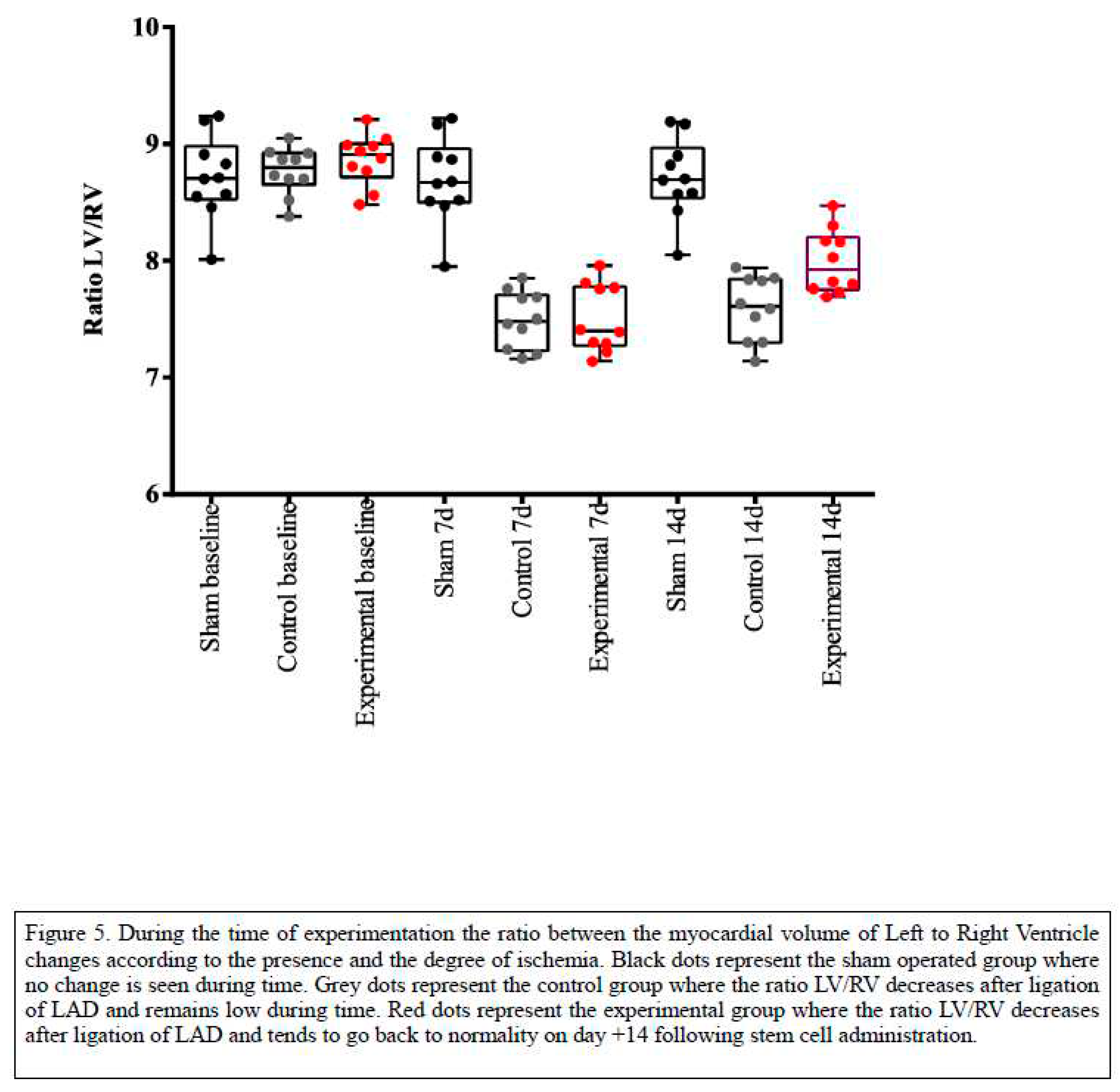
Moreover, the creation of the left ventricular to right ventricular area (LV/RV) ratio which is purely mathematical and not influenced by any other parameters of the experimental method, such as animal weight, dosage or radioactive substance, absolute number of voxels, led to a clear analysis of the distribution of the radioactive particle. Results are summarized in
Table 2. Due to the ligation of LAD the ischemia caused leads to decreased Left Ventricular area, therefore the LV/RV ratio changes pre and postoperatively. In more details, healthy individuals present a ratio LV/RV 7 days prior to surgery of about 8.7±0.3 (Day -7) and this remains stable in the animals which underwent only thoracotomy without ligation. However, there is statistically significant difference between groups with respect of ratio of variables at time 7 days (p<0.005), and pairwise comparisons show that Sham group presented higher values compared to control ( p<0.005) The animals that belong in control group had also significant decrease in the activity of myocardium with values of LV/RV ratio 7.5±0.2 post surgically. The animals of the sham operated group showed no increase in the myocardial activity fifteen days post surgery while the animals in the experimental group which received the mesenchymal stem cell showed an increase of functioning myocardial cells and specifically values of LV/RV ratio 8.00±0.2, demonstrating thus the beneficial effect of stem cell transplantation, as clearly depicted in
Figure 5
3.3. Immunohistochemical analysis , Evaluation of Immunohistochemistry
Strong immunostaining in myocardial cells was considered positive. Comparison and results are summarized in
Table 1 and immunohistochemical analysis results are Illustrated in
Figure 6A and
Figure 6B. There was a statistically significant difference between groups regarding the percent of positive result of
CTGF variable ( p=0.011), as well as with the CD133 variable. Pairwise comparisons showed that experimental group that received the stem cells, presented higher percent of positive result of CD133variable compared to Sham ( p<0.005) and control ( p<0.005) respectively. There was also a statistically significant difference between groups with respect of percent of positive result of
GATA4 variable ( p<0.005). Pairwise comparisons show that stem cells group presented higher percent of positive result of GAT4 variable compared to Sham ( p<0.005) and control ( p<0.005) respectively. There is statistically significant difference between groups with respect of percent of positive result of
Nkx2,5 variable ( p<0.005). Pairwise comparisons showed that the experimental group presented higher percent of positive result of NKX25 variable compared to Sham ( p=0.045) and control ( p<0.005) respectively. These favorable findings indicate without any doubt the beneficial effect of SCT in this rat model, in terms of regeneration of he ischemic myocardium. (
Figure 6a and
Figure 6b)
4. Discussion
Ischemic heart diseases are a leading cause of death worldwide, and thus, quick and accurate diagnosis and effective therapeutic protocols are needed. Numerous experimental protocols have been created in order to understand the mechanism of myocardial regeneration and develop guidelines for pre-clinical and clinical trials. (1-7,29-30)
The overall goal of such experimentation is the understanding of the ischemic mechanism in a way that the approach of regeneration is directed in the fastest and most effective pathway. The experimental model used in our study is translating the clinical symptoms of myocardial infarction into qualitative and quantitative presentation, while the therapy with stem cells acts in a long term regeneration clearly recognizable in vivo and post mortem. Therefore, we strongly believe that our findings can be helpful tool in the hands of researchers worldwide in order to understand, evaluate, present and cure the ischemic conditions of the myocardium. (2-6,14,16)
This study is important for two major reasons. Firstly, the ability to evaluate experimentally the degree of ischemia in living animals instead of the commonly used method of postmortem analysis. Secondly, the use of specific heart oriented mesenchymal stem cells is opening a new era of experimentation, which is organ-target specific and can take the stem cell transplantation in a new level of therapeutical goals. (1, 8-13,23-25)
The experimental model (rodents) was chosen due to its high compatibility to human physiology and anatomy concerning the heart. Therefore the knowledge we gain can be compared and repeated worldwide with great accuracy for in vivo evaluation. Establishment of myocardial ischemia and infarction by ligation of LAD is highly demanding due to increased mortality rates due to the stress of animals and waste products of the ischemic cells in circulation. By standardization of technique via left thoracotomy, we managed to decrease this mortality rate to less than 20% making it attractive for in vivo experimentation and real time results, in contrast to using pharmaceutical causes of infarction which were only resembling the actual heart attack (1-8, 14,16,29-30). Furthermore, SPECT-CT imaging of the living animals pre and post infarction made us understand and present the changes that occur in living myocardium both physiologically and anatomically with measurements which are of high qualitative and quantitative value. The use of SPECT creates a very recognizable pattern of living cells absorbing the radioactive substances, which can be measured and evaluated. The CT gives the exact anatomical structure of the heart and the changes in the left ventricle because of the infarction. Furthermore, the regeneration and increase in contractility of the heart tissue in animals which received the stem cell therapy was undeniable (17-20,26-28).
Mesenchymal Stem Cells have been intensely studied the past years, in an effort community to find new therapeutical methods in numerous diseases. Their use in myocardial tissue is not yet popular since there haven’t been any particular cells addressed to this tissue with comparable results. Our goal was to isolate myocardial specific cells and specifically GATA4 and Nkx2,5, and study their specific action in postinfarcted myocardium (8-13,23-25). Most researches have been using cell cultures with positive results but it was a big challenge to study their efficacy in living animals. In this study the administration of the cells was performed directly in the infarcted area in the beating heart and the positive-regenerative results were confirmed via the immunohistochemical analysis of the heart tissue. It was definitely proved that the animals which received the stem cells had increased volume of left ventricle and better contractility at 15 PO, as shown in
Table 2 and
Figure 5. Only in the experimental group of animals the stem cells were isolated and recognized postmortem. It is notable, that using this model and methodology, the great challenges of such an experiment were completed in a smooth and effective way.
5. Limitations of the study
Among the limitations of this study are: 1. The fact that only female rats were used as stem cell receivers and only male rats were used as stem cell donors 2. The rather short period of survival and monitoring before euthanasia. That was necessary for reasons related to the obtained license of this experiment. 3. The weight of the animals was not the same for all, but in a range corresponding to same age of adult rats, similarly the weight of their hearts was between a accepted range level.
In conclusion, this novel, valid and highly reproducible infraction model and the methodology used, gave a transparent vision of the myocardial activity, with both qualitative and quantitative parameters. Furthermore, verification by immunohistochemical analysis, demonstrated the therapeutic potential of ADSCs transplantation. In clinical perspective, therefore, concurrent ADSCs administration may applied in combination with coronary artery bypass grafting. However, additional studies are required to further clarify the regenerative effect of stem cell transplantation in the ischemic myocardium.
Institutional Review Board Statement
The study was authorized by the national Animal Experiment Board of Greece and the Veterinary Association of Athens (License No: 1870/23-04-2018) and was carried out in compliance with EU legislation conforming to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes relating to the conduct of animal experimentation.
Acknowledgments
Partial funding with a Scholarship by the Experimental, Educational and Research Center (EERC), ELPEN Pharmaceuticals, Athens, Greece. The contributions of Elpen Experimental center, Biomtech Laboratories and Democritus National Center for Scientific Research was clearly described in the experimental protocol with the required licenses and specific working hours.
Conflicts of Interest
The Authors declare no conflicts of interest about this study. Some authors work for Biomtech laboratories that produces the x-cube and γ-cube.
Abbreviations
| ADSCs: |
Adipose tissue Derived Stem Cells |
| ARRIVE: |
Animal Research Reporting of In Vivo Experiments |
| BrdU: |
5- bromo-2-deoxyuritidine |
| CD45, CD105, CD73, CD44, CD29, CD133: |
cell markers - Cluster of Differentiation (surface markers which are very useful for the identification and characterization of leukocytes and subpopulations of leukocytes) |
| CTGF: |
cell marker - Connective Tissue Growth Factor |
| DAPI: |
6-diamino-2-phenylindole |
| DNA: |
Deoxyribo Nucleic Acid |
| DMEM: |
Dulbecco’s Modified Eagle Medium |
| ECG: |
ElectroCardioGram |
| FBS: |
Fetal Bovine Serum |
| GATA4: |
mesenchymal factor - transcription factor located in nucleus |
| IVC: |
Inferior Vena Cava |
| ISRA: |
Image Space Reconstruction Algorithm |
| KCl: |
Potassium Chloride |
| LAD: |
Left Anterior Descending coronary artery |
| LCA: |
Left Coronary Artery |
| LV/RV: |
Left Ventricle / Right Ventricle (Volume) |
| MSCT: |
Mesenchymal Stem Cell Transplantation |
| Nkx2,5: |
mesenchymal factor - transcription factor located in cytoplasm |
| PBS: |
Phosphate Buffered Saline |
| PO: |
Post Operatively |
| SCT: |
Stem Cell Transplantation |
| SPECT/CT: |
Single Positron Emission Computed Tomography /Computed Tomography |
References
- Koutela A, Loudos G, Rouchota M, Kletsas D, Karameris A, Vilaras G, Zografos GC, Grypari IM, Dougenis D, Papalois AE. A Novel Experimental Rat Model for the In Vivo Assessment of Myocardial Ischemia Based on Single Photon Emission Computed Tomography. In Vivo 2023 Mar-Apr;37(2):649-654, PMID: 36881049; PMCID: PMC10026663. [CrossRef]
- Li H, Huang J, Liu C, Zhang Z, Song K, Ma K, Dennewitz CW and Wang S: A new model of heart failure post-myocardial infarction in the rat. J Vis Exp 172: e62540, 2021. [CrossRef] [PubMed]
- Goldman S and Raya TE: Rat infarct model of myocardial infarction and heart failure. J Card Fail 1(2): 169-177, 1995, 1995. [CrossRef] [PubMed]
- Krzemiński TF, Nozyński JK, Grzyb J and Porc M: Wide-spread myocardial remodeling after acute myocardial infarction in rat. Features for heart failure progression. Vascul Pharmacol 48(2-3): 100-1008, 2008, 2008. [CrossRef] [PubMed]
- Muthuramu I, Lox M, Jacobs F and De Geest B: Permanent ligation of the left anterior descending coronary artery in mice: a model of post-myocardial infarction remodelling and heart failure. J Vis Exp(94): 52206, 2014. [CrossRef] [PubMed]
- Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE and Heusch G: Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314(4): H812-H838, 2018, 2018. [CrossRef] [PubMed]
- Suzuki YJ, Nagase H, Day RM, Das DK. GATA-4 regulation of myocardial survival in the preconditioned heart. J Mol Cell Cardiol. 2004 Dec;37(6):1195-203. Erratum in: J Mol Cell Cardiol. 2016 Dec;101:25. [CrossRef] [PubMed]
- Välimäki MJ, Ruskoaho HJ. Targeting GATA4 for cardiac repair. IUBMB Life 2020 Jan;72(1):68-79, Epub 2019 Aug 16. PMID: 31419020; PMCID: PMC6973159. [CrossRef]
- Broderick TL, Jankowski M, Wang D, Danalache BA, Parrott CR, Gutkowska J. Downregulation in GATA4 and Downstream Structural and Contractile Genes in the db/db Mouse Heart. ISRN Endocrinol. 2012;2012:736860, Epub 2012 Mar 13. PMID: 22474596; PMCID: PMC3313578. [CrossRef]
- Kinnunen SM, Tölli M, Välimäki MJ, Gao E, Szabo Z, Rysä J, Ferreira MPA, Ohukainen P, Serpi R, Correia A, Mäkilä E, Salonen J, Hirvonen J, Santos HA, Ruskoaho H. Cardiac Actions of a Small Molecule Inhibitor Targeting GATA4-NKX2-5 Interaction. Sci Rep. 2018 Mar 15;8(1):4611, PMID: 29545582; PMCID: PMC5854571. [CrossRef]
- Katanasaka Y, Suzuki H, Sunagawa Y, Hasegawa K, Morimoto T. Regulation of Cardiac Transcription Factor GATA4 by Post-Translational Modification in Cardiomyocyte Hypertrophy and Heart Failure. Int Heart J. 2016 Dec 2;57(6):672-675, Epub 2016 Nov 4. [CrossRef] [PubMed]
- Välimäki MJ, Tölli MA, Kinnunen SM, Aro J, Serpi R, Pohjolainen L, Talman V, Poso A, Ruskoaho HJ. Discovery of Small Molecules Targeting the Synergy of Cardiac Transcription Factors GATA4 and NKX2-5. J Med Chem. 2017 Sep 28;60(18):7781-7798, 7781; Epub 2017 Sep 15. [CrossRef] [PubMed]
- George V, Colombo S, Targoff KL. An early requirement for nkx2.5 ensures the first and second heart field ventricular identity and cardiac function into adulthood. Dev Biol. 2015 Apr 1;400(1):10-22. Epub 2014 Dec 20. PMID: 25536398; PMCID: PMC4361364. [CrossRef]
- Alexopoulos P, Panoutsopoulou K, Vogiatzis G, Koletsis E, Dougenis D and Tsopanoglou NE: Combined treatment with exenatide and cyclosporine A or parstatin 1-26 results in enhanced reduction of infarct size in a rabbit model. J Cardiovasc Pharmacol 70(1): 34-41, 2017. 2017. [CrossRef] [PubMed]
- Heusch G: Critical issues for the translation of cardioprotection. Circ Res 120: 1477-1486, 2017, 2017. [CrossRef] [PubMed]
- Mitsos S, Katsanos K, Dougeni E, Koletsis EN and Dougenis D: A critical appraisal of open- and closed-chest models of experimental myocardial ischemia. Lab Anim 38(5): 167-77, 2009, 2009; -77. [CrossRef] [PubMed]
- Fukushima K, Momose M, Kondo C, Hagiwara N and Sakai S: Accelerated BMIPP uptake immediately after reperfused ischemia in the isolated rat heart model. Ann Nucl Med 25(8): 560-565, 2011, 2011. [CrossRef] [PubMed]
- Hirai T, Nohara R, Hosokawa R, Tanaka M, Inada H, Fujibayashi Y, Fujita M, Konishi J and Sasayama S: Evaluation of myocardial infarct size in rat heart by pinhole SPECT. J Nucl Cardiol 7(2): 107-111, 2000, 2000. [CrossRef] [PubMed]
- Liu Z, Zhao M, Zhu X, Furenlid LR, Chen YC and Barrett HH: In vivo dynamic imaging of myocardial cell death using 99mTc-labeled C2A domain of synaptotagmin I in a rat model of ischemia and reperfusion. Nucl Med Biol 34(8): 907-915, 2007. [CrossRef] [PubMed]
- Acton PD, Thomas D and Zhou R: Quantitative imaging of myocardial infarct in rats with high resolution pinhole SPECT. Int J Cardiovasc Imaging 22(3-4): 429-434, 2006, 2006. [CrossRef] [PubMed]
- Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Hurst V, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020 Jul 14;18(7):e3000411, 3000; PMID: 32663221; PMCID: PMC7360025. [CrossRef]
- David, I. Lewis. Animal experimentation: implementation and application of the 3Rs. Emerg Top Life Sci 2019; 3 (6): 675–679, 6: 3 (6). [CrossRef]
- Välimäki MJ, Ruskoaho HJ. Targeting GATA4 for cardiac repair. IUBMB Life. 2020 Jan;72(1):68-79. Epub 2019 Aug 16. PMID: 31419020; PMCID: PMC6973159. [CrossRef]
- Xin M, Olson EN, Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol. 2013 Aug;14(8):529-41, Epub 2013 Jul 10. PMID: 23839576; PMCID: PMC3757945. [CrossRef]
- Katanasaka Y, Suzuki H, Sunagawa Y, Hasegawa K, Morimoto T. Regulation of Cardiac Transcription Factor GATA4 by Post-Translational Modification in Cardiomyocyte Hypertrophy and Heart Failure. Int Heart J. 2016 Dec 2;57(6):672-675, Epub 2016 Nov 4. [CrossRef] [PubMed]
- Golestani R, Wu C, Tio RA, Zeebregts CJ, Petrov AD, Beekman FJ, Dierckx RA, Boersma HH, Slart RH. Small-animal SPECT and SPECT/CT: application in cardiovascular research. Eur J Nucl Med Mol Imaging 2010 Aug;37(9):1766-77, Epub 2010 Jan 13. PMID: 20069298; PMCID: PMC2918793. [CrossRef]
- Garikipati VN, Jadhav S, Pal L, Prakash P, Dikshit M, Nityanand S. Mesenchymal stem cells from fetal heart attenuate myocardial injury after infarction: an in vivo serial pinhole gated SPECT-CT study in rats. PLoS One. 2014 Jun 27;9(6):e100982, PMID: 24971627; PMCID: PMC4074116. [CrossRef]
- Strydhorst JH, Ruddy TD, Wells RG. Effects of CT-based attenuation correction of rat microSPECT images on relative myocardial perfusion and quantitative tracer uptake. Med Phys. 2015 Apr;42(4):1818-24. [CrossRef] [PubMed]
- Cheng K, Malliaras K, Li TS, Sun B, Houde C, Galang G, Smith J, Matsushita N, Marbán E. Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiacderived stem cells in a rat model of ischemia/reperfusion. Cell Transplant. 2012;21(6):1121-35. Epub 2012 Mar 8. PMID: 22405128; PMCID: PMC4323149. [CrossRef]
- Zhang Z, Tian H, Yang C, Liu J, Zhang H, Wang J, Hu S, Sun Z, He K, Chen G. Mesenchymal Stem Cells Promote the Resolution of Cardiac Inflammation After Ischemia Reperfusion Via Enhancing Efferocytosis of Neutrophils. J Am Heart Assoc. 2020 Mar 3;9(5):e014397, Epub 2020 Feb 21. PMID: 32079474; PMCID: PMC7335576. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).