Introduction
The area between the iris and the native or pseudophakic lens has been coined the posterior chamber, ciliary sulcus space or middle segment. The middle segment is an ideal location for delivery of novel optics, such as
STAAR® Surgical's phakic IOL, the EVO and Viva Impantable Collamer Lens (ICL) for treatment of myopia, astigmatism and with the Viva even presbyopia [
1]. In addition to optics, the middle segment may prove an ideal location for depot drug delivery therapeutics. To safely adopt Middle Space Operations (MiSO) and Middle Space Therapeutics (MiST) we must have a means of imaging the middle segment. The ophthalmologist
’s inherent visual approach to clinical medicine is a double-edged sword. Only secondary to the dermatologist, the ophthalmologist is offered direct visualization. For this reason, we lag behind other minimally invasive surgical fields in utilizing preoperative and intraoperative imaging guidance. Optical coherence tomography (OCT) provides excellent visualization for all ocular structures accessible by light [
2,
3,
4]. Imaging of the middle segment hidden by the iris requires non-light based imaging modalities such as ultrasound.
The ArcScan Insight 100 robotic ultrasound provides the highest-resolution images of the middle segment to date [
3,
4,
5]. Many clinicians utilize anterior segment OCT and even handheld UBM but very few currently have adopted the use of the ArcScan robotic ultrasound [
3,
4,
5]. Following the lead of our radiology colleagues, we must have repeatable scans that can be compared over time. Inherently this requires, a means of repeatedly imaging the same area. Through the use of robotic ultrasound with iris tracking, the end-user is provided with a high-resolution image of all structures, even those hidden from the light, from cornea to lens. Moreover, these scans can be overlaid over time to assess structural changes in the eye based on iris tracking. In the case of MiST, we will certainly want to place these therapeutics out of the visual axis and therefore out of the direct visualization with any light-based modalities ie slit lamp biomicroscopy or OCT.
Depot ocular drug delivery has the potential to revolutionize eye care. Serial administration of Durysta into the anterior chamber has proven problematic due to concerns about endothelial cell loss. In order to circumvent these challenges and enable serial delivery, the sulcus has been entertained as a potential means for Durysta delivery. Before this can be safely entertained, we must develop techniques to image the implant in the sulcus and better understand the impact of Durysta on the sulcus and potentially posterior structures if migration occurs posteriorly. Serial administration of Durysta to the middle segment may mitigate many TEAEs such as corneal endothelial cell loss, corneal edema, anterior chamber cell and iritis associated with repeated anterior administration.
DURYSTA has been associated with corneal adverse reactions and risks that increase with multiple implants. Due to possible corneal endothelial cell loss, administration of DURYSTA is currently limited to a single implant per eye without retreatment. Accordingly, Durysta has been approved for use as a single-application intracameral biodegradable sustained-release implant to lower intraocular pressure in open-angle glaucoma or ocular hypertension patients.
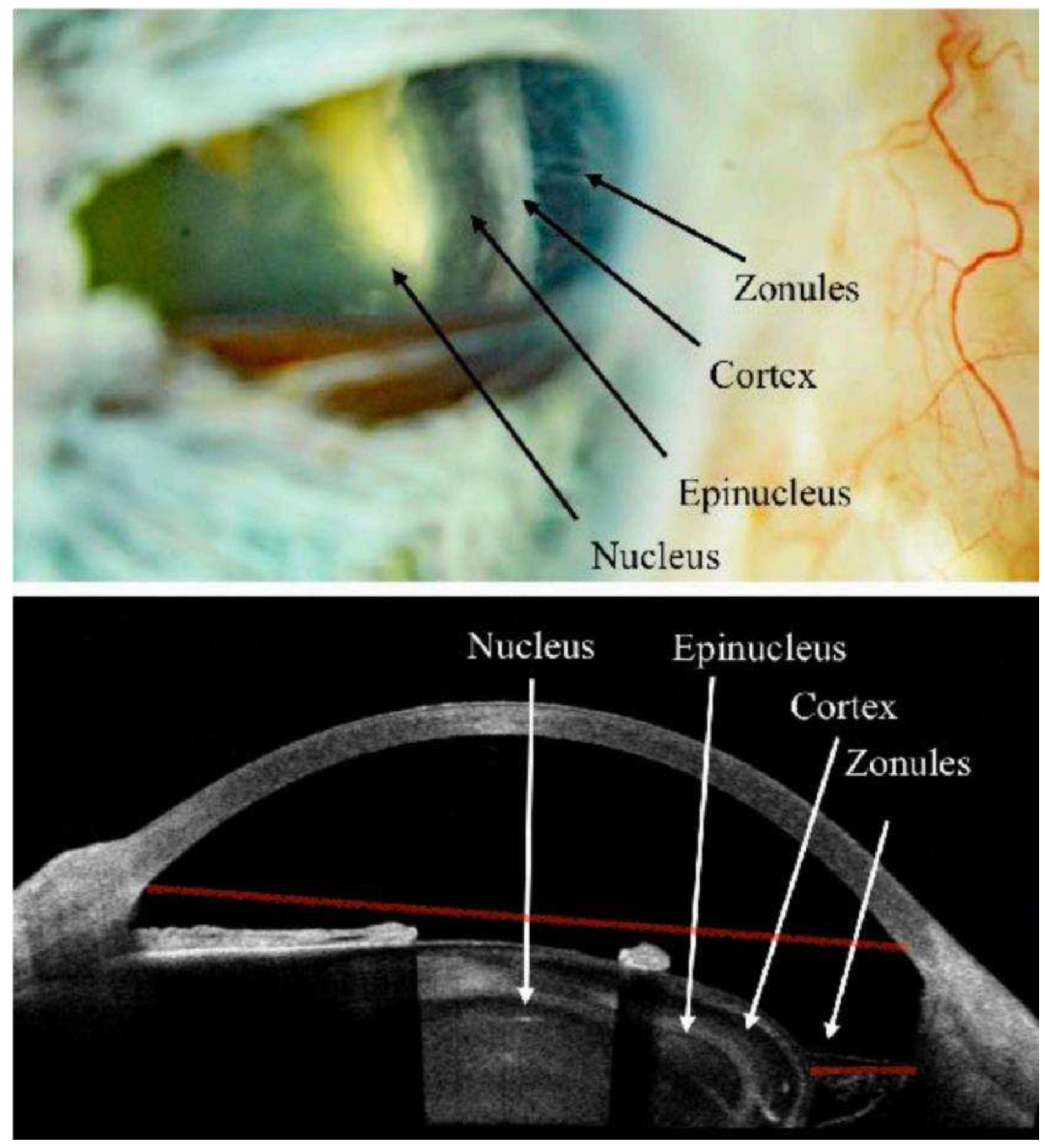
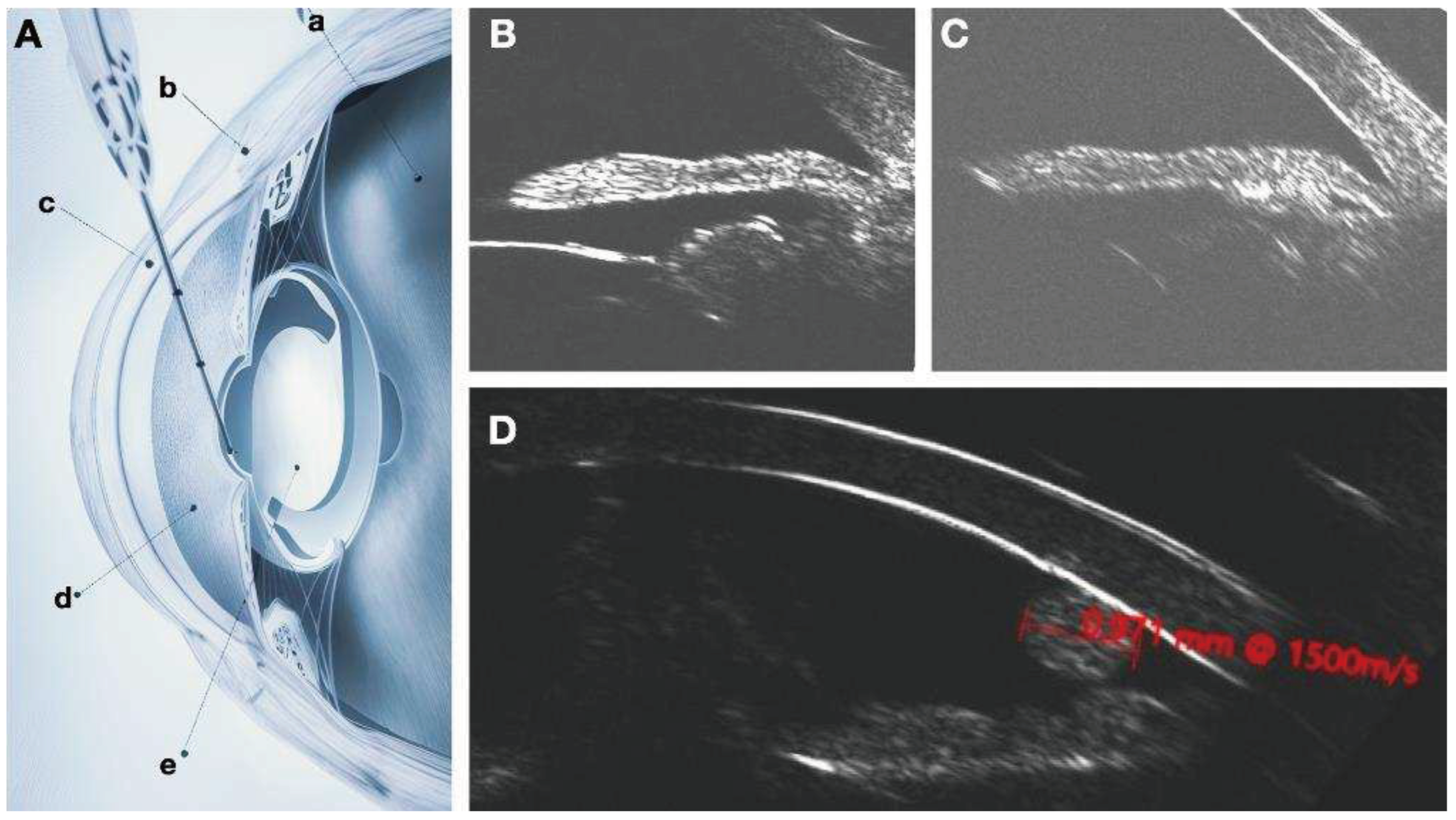
Given the documented efficacy of the DURYSTA implant and the chronic nature of glaucoma, an alternative to intracameral delivery is needed. Middle segment administration of the implant may overcome these contraindications for repeated administration. The ciliary sulcus space is poorly understood as it is not readily visualized by imaging techniques routinely employed. Fortunately, in previous research efforts I have demonstrated that hydrogels of micrometer size can be readily imaged with clinical ultrasound (
Figure 1). Robotic ultrasound imaging with the ArcScan Insight 100 will enable determination of location of the implant and its potential impact on anatomical structures of the eye (
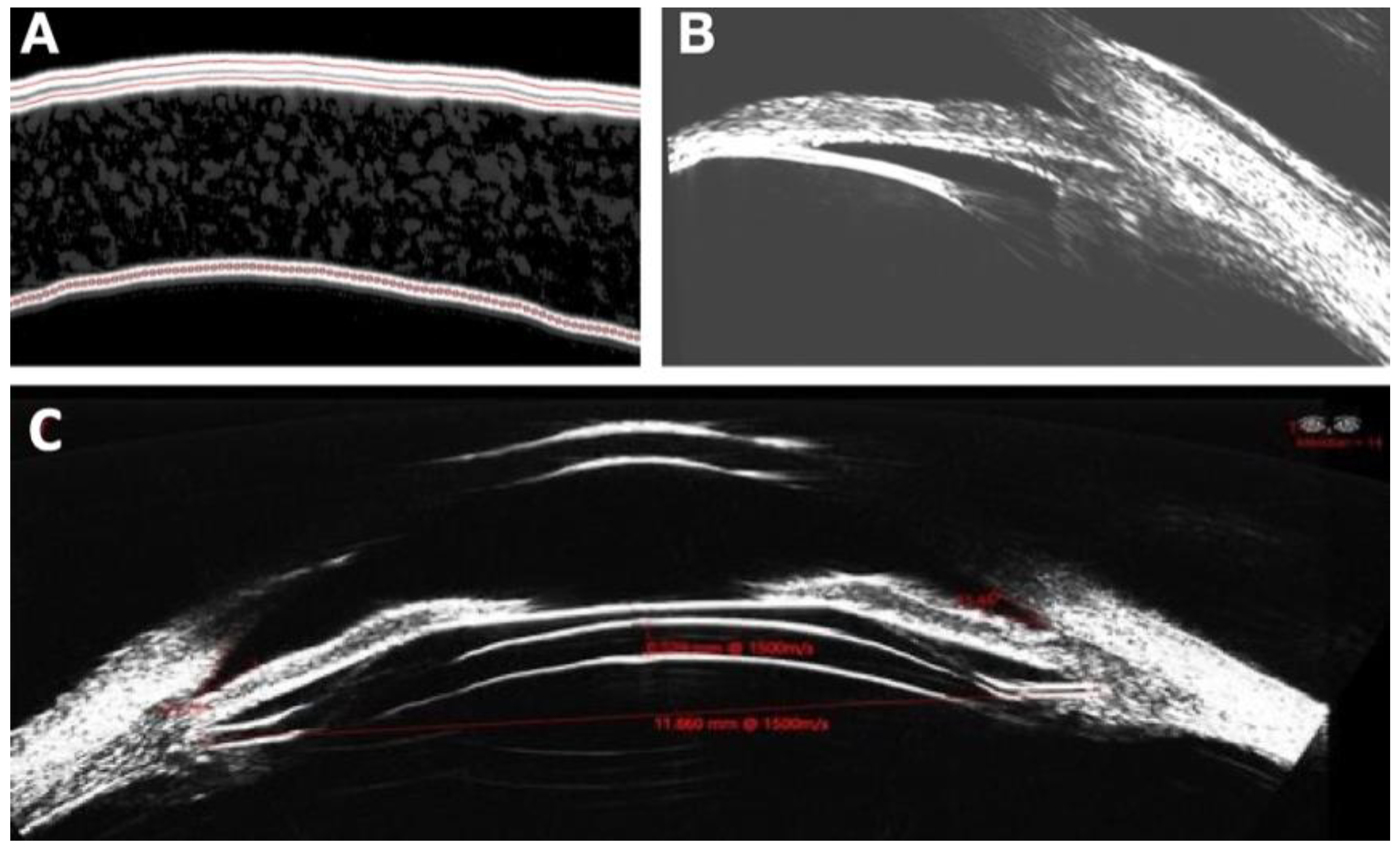
Figure 2). An understanding of these associated changes and determination of efficacy of the implant based upon localization will enable further refinement of Durysta administration protocols.
In the Artemis II trial, corneal treatment-emergent adverse events (TEAEs) were most commonly reported with serial administration. Although occurrence or worsening of corneal endothelial cell loss (CECL) was not reported after the first and second administrations, 3.8% (6/156) of patients after the third administration (through the end of the week 52 visit window), and 5.8% (9/155) of patients during the extended safety follow-up through month 20. CECL was reported in 0.6% (1/176) of patients after the first administration, 4.2% (7/165) of patients after the second administration, 11.6% (17/147) of patients after the third administration, and 14.6% (21/144) of patients during the extended safety follow-up through month 20.
In addition to CECL, serial Durysta administration increased the incidence of corneal edema, and corneal touch as well as inflammatory TEAEs such as anterior chamber cell and iritis. Phase I/II evaluation noted that 5-10% of patients showed indications of corneal endothelial cell loss, believed to be related to the build-up of the implant, leading to limits of one dose per eye of the implant. Since angle size and available space would be related to the risk of endothelial cell loss, being able to monitor if the implant has fully dissolved, where it is located, and if it has migrated in a manner that increases risk would empower the clinician to tailor dosage to the patient’s anatomy and disease state.
Unable to directly visualize a MiST, one is largely unaware if the implant migrated into the vitreous, if it is moving around the middle segment or if it is stationary. Without this information, it is nearly impossible to understand the cause of adverse events (AEs). For example, some have hypothesized that it is simply DURYSTA touching endothelial cells that causes the most corneal endothelial cell loss (CEC) loss [
6,
7,
8,
9,
10,
11,
12,
13]. Whereas, we hypothesize, it is not as much simply implant to endothelial touch that is the issue as much as implant migration causing it to variably strike and roll across anterior segment structures. With DURYSTA administered intracameral, one can easily address this issue by monitoring for migration of the implant and assessing correlation to CEC loss. The same cannot be said for the middle segment.
Middle segment chamber delivery is unlikely to lead to appreciable CEC loss. Indeed, this is why Dr. Nathan Radcliffe first proposed placing DURYSTA in this location. [
2,
7] With his promising preliminary work, we are now tasked with better understanding the best formulation and delivery strategy for MiST. Although CEC loss is less of a concern, it has been replaced with concern for Uveitic Glaucoma Hyphema (UGH) syndrome. A MisT oversized for a particular posterior chamber space will result in the MiST rubbing against the back side of the iris, causing iris chaffing, leading to loss of iris pigment and inflammation with microbleeding in the eye. The constellation of symptoms caused by a posterior chamber implant rubbing against the iris is coined the UGH syndrome—uveitis, glaucoma and hyphema.
Using light-based modalities, one would have to wait for transillumination defects to develop in the iris or for iris pigment to be found in the trabecular meshwork before one would be aware of UGH. Unless the MiST had been serially imaged, one would not know if UGH developed due to a mobile implant or a largely stationary implant. Moreover, one would not be able to assess if an implant seated adjacent to the ciliary body would be less likely to cause UGH than one closer to the pupillary axis and how individual anatomy contributes to the likelihood of UGH. In addition to UGH, one could envision a MiST implant integrating with zonules and causing stretching or tearing. Seldom are we hit with the bullet we are expecting, so although UGH and zonular loss are primary concerns a priori, MiST may have wholly larger hurdles to overcome that we are not even aware of. Certainly most, including authors of this manuscript, would not entertain placing MiST in a phakic individual due to fear of early cataract formation. These concerns are literal shots in the dark as the middle segment has largely been a black box prior to the ArcScan Insight 100.
As ophthalmologists and optometrists, we are well versed in choosing the correct sized polymeric implants for the punctal space by direct visualization. Such an approach is not possible for MiSO or MiST. Similar to the transformative nature of robotic ultrasound to match ICL size to individual anatomy, it will be central to the design of MiST treatment strategies. Most eyecare professionals are well versed in ocular ultrasound but few are familiar with robotic ultrasound. Robotic ultrasound, such as the ArcScan Insight 100, utilizes iris registration to enable serial overlay of images much like CT or MRI. The ArcScan device provides a precise anterior chamber depth and individual corneal layer thickness with arguably the best epithelial mapping of any instrument to date. It also provides a detailed image from cornea epithelium all the way to the lenticular posterior capsule. From this information we can serially measure sulcus-to-sulcus, angle-to-angle and inner diameter ciliary muscle width measurements within 120 micron precision. It is with this precision we can assess local tissue changes as they relate to MiST delivery strategy and location, implant size, material and implant migration. We are also able to assess in the case of Durysta if positioning closer to the cilliary body leads to greater therapeutic effect.
The ArcScan 100, through its contact-free 50MHz ultrasound probe, utilizes a water bath to achieve the highest resolution scan of the entire anterior segment possible. The use of immersion eliminates the variability induced by tear film instability when mapping epithelium [
3,
4,
5,
14]. With this data we may be able to more precisely recognize persons who are not LVC candidates as well as monitor outcomes from LVC [
3]. The high resolution imaging behind the iris has applications for monitoring depot drug delivery (ie Durysta sulcus administration) and ICL surgery, including sizing and verifying correct footplate positioning, While traditional ultrasound biomicroscopy can also visualize this space, the ArcScan resolution and repeatability (120 micron repeatability for measuring sulcus-to-sulcus), enables precise determination effective lens position as well as assessment of vault changes over the life of the patient. The ArcScan elevates ultrasound to a truly repeatable device by incorporating iris registration, that enables for us to obtain repeatable imaging of the entire anterior segment. This repeatability provides a yet unavailable diagnostic tool to effective lens position and ICL vault serially.
Materials and Methods
Posterior chamber implantation of the bimatoprost implant (DURYSTA) has recently been reported but limited efficacy and adverse event assessment has been performed to date [
6,
7]. Imaging and potential contact of the implant in the sulcus has not been studied [
1]. Assessment of Efficacy and Safety of the Bimatoprost Implant (DURYSTA) will be assessed based upon site of injection and assessment of migration as determined by longitudinal multimodal imaging. With an eye towards optimized sulcus imaging and implant monitoring, we will monitor migration of the bimatoprost implant and associated anatomic changes.
Ultrasound Biomicroscopy (UBM), utilizes high-frequency ultrasound (30 to 50Mhz) to facilitate high resolution anterior segment imaging. Although initially explored for middle segment Durysta imaging, standard handheld UBM lacked the precision and repeatability to effectively visualize Durysta in the sulcus.
The ArcScan Insight 100 is ideal for corneal imaging, glaucoma imaging, ICL sizing and for measurement of changes in phakic and pseduophakic effective lens position. [
1,
2,
3,
4,
5,
14,
15,
16] This is particularly for better understanding the degree of presbyopia as well as assessment of the efficacy of pseduophakic accommodating lenses. The Insight 100 obtains a comprehensive image of the entire anterior chamber, including lens position, tilt, and volume and imaging behind the scleral wall for trabecular meshwork,ciliary body, and choroidal space imaging. [
1,
2,
3,
4,
5,
14,
15,
16]
The ArcScan corneal imaging modules can be utilized for assessing Laser Vision Correction (LVC) candidacy as well as comprehensive cornea layer mapping for keratoconus screening and inlay evaluations. In addition to corneal measurements, ArcScan provides highly accurate, anterior chamber depth, angle-to-angle width, sulcus-to-sulcus width, and complete images of structures in the posterior segment.
In the study we utilized OCT, ArcScan Insight 100 robotic ultrasound and slit lamp biomicroscopy to image the Durysta intracameral implant in phakic and pseudophakic eyes. Robotic Ultrasound was utilized for posterior chamber imaging of the implant in pseudophakic eyes. Robotic ultrasound is uniquely poised to this application as the ciliary body and zonules are hidden behind the iris for direct visualization. Measurements were performed at California LASIK & Eye, Sacramento, California, in 2023. After receiving a full explanation of the study, all patients provided written informed consent.
Optical Coherence Tomography (OCT) measures the echo time delay and intensity of backscattered low-coherence light to provide high-resolution, cross-sectional images of tissues with micrometer-scale resolution. A variety of OCT types exist including Time-Domain OCT (TD-OCT), Spectral-Domain OCT (SD-OCT) , and Swept-Source OCT (SS-OCT). TD-OCT was the original type of OCT. It involves varying the length of the reference arm to obtain depth information. SD-OCT uses a spectrometer to measure the interference spectrum. It is faster and has become the dominant technology in OCT. SS-OCT is similar to SD-OCT but uses a tunable laser as a light source. It is particularly useful for imaging structures that are difficult to visualize with other types of OCT. Despite SS-OCT ability to image deeper tissue than other OCT techniques, it is incapable of effective visualization of the posterior chamber behind the iris. OCT can also be utilized for vascular visualization without contrast agent with OCT angiography (OCT-A).
Inclusion Criteria for the study were pseudophakic patients diagnosed with OAG or OHT already scheduled to receive a bimatoprost implant in at least one eye. Exclusion criteria included a history of any of the following ocular surgeries in the eye due to receive a bimatoprost intracameral implant:Ahmed Glaucoma Valve, Baerveldt shunt, Ex-Press glaucoma shunt implantation, Molteno shunt, Trabeculectomy, Vitrectomy, retinal surgery or CyPass Micro-Stent.
The primary outcome measure of the study is to assess the quality and repeatability of serial imaging modalities, slit-lamp biomicroscopy, OCT, UBM and ArcScan Insight 100 robotic ultrasound to visualize Durysta with intracameral and middle segment delivery.
In addition, we assessed the shape of the implant and associated migration and resulting anatomic changes.
A bimatoprost implant was inserted into intracamerally or in the sulcus space by a single surgeon (BB) at the slit lamp after standard preimplantation sterilization including 5% betadine to the ocular surface and eyelid speculum. Following implantation, the Maestro II OCT and camera (Topcon, Tokyo Japan) as well as the ArcScan Insight 100 (ArcScan, Golden CO USA) and also biomicroscopy recording on a BQ 900 (Haag Streit, Bern Switzerland) with a modified GoPro Hero5 (GoPro, San Mateo CA) for slit lamp recording was performed. Repeated images were captured at day 0 before implant as well as follow-up scans at week 1, month 1 and month 3.
Results
Precision for an ophthalmic surgeon is predicated as much by dexterity of the surgeon as it is by accurate preoperative imaging and predictive algorithms. For posterior chamber intraocular lenses, through use of A scan, optical biometry and more recently OCT, we are able to determine the ideal lens for capsular placement. These same imaging approaches do not provide information about the posterior chamber size beyond indirect information such as white to white and anterior chamber death. The ArcScan Insight®100—a very high frequency (VHF) ultrasound device— provides a detailed image of the posterior chamber including area behind the iris that would be otherwise invisible. This provides a yet unattainable image of the of the entire anterior segment ocular anatomy that is both highly precise and repeatable based upon iris registration.
Both OCT and ArcScan Insight 100 robotic ultrasound provided excellent corneal images including anterior chamber depth measurements and epithelial mapping. Unlike OCT, by using immersion, ArcScan robotic ultrasound provided greater epithelial mapping accuracy by negating the contribution of the tear film. Another significant advantage of ArcScan Insight 100 was the ability to image behind the iris. Although ultrasound biomicroscopy (UBM) provides imaging behind the iris, as it requires handheld probe placement, both resolution and repeatability was compromised as compared to the ArcScan which possesses 120 micron repeatability. The ArcScan enabled detailed imaging of the entire anterior segment and its structures with even zonular imaging possible.
When ultrasound waves encounter different tissues or materials with varying acoustic properties, they produce echoes that are used to generate images. Durysta is composed of a hydrogel. As the name would suggest, hydrogels are water-rich biomaterials. Due to the acoustic properties of hydrogels, and the impact of density of speed of sound propagation, hydrogels can be visualized as a distinct echogenicity as compared to native ocular structures. Although not explored in the study, the size of the implant combined with the global echogenicity of the implant may provide more definitive information about polymer degradation than simply size alone.
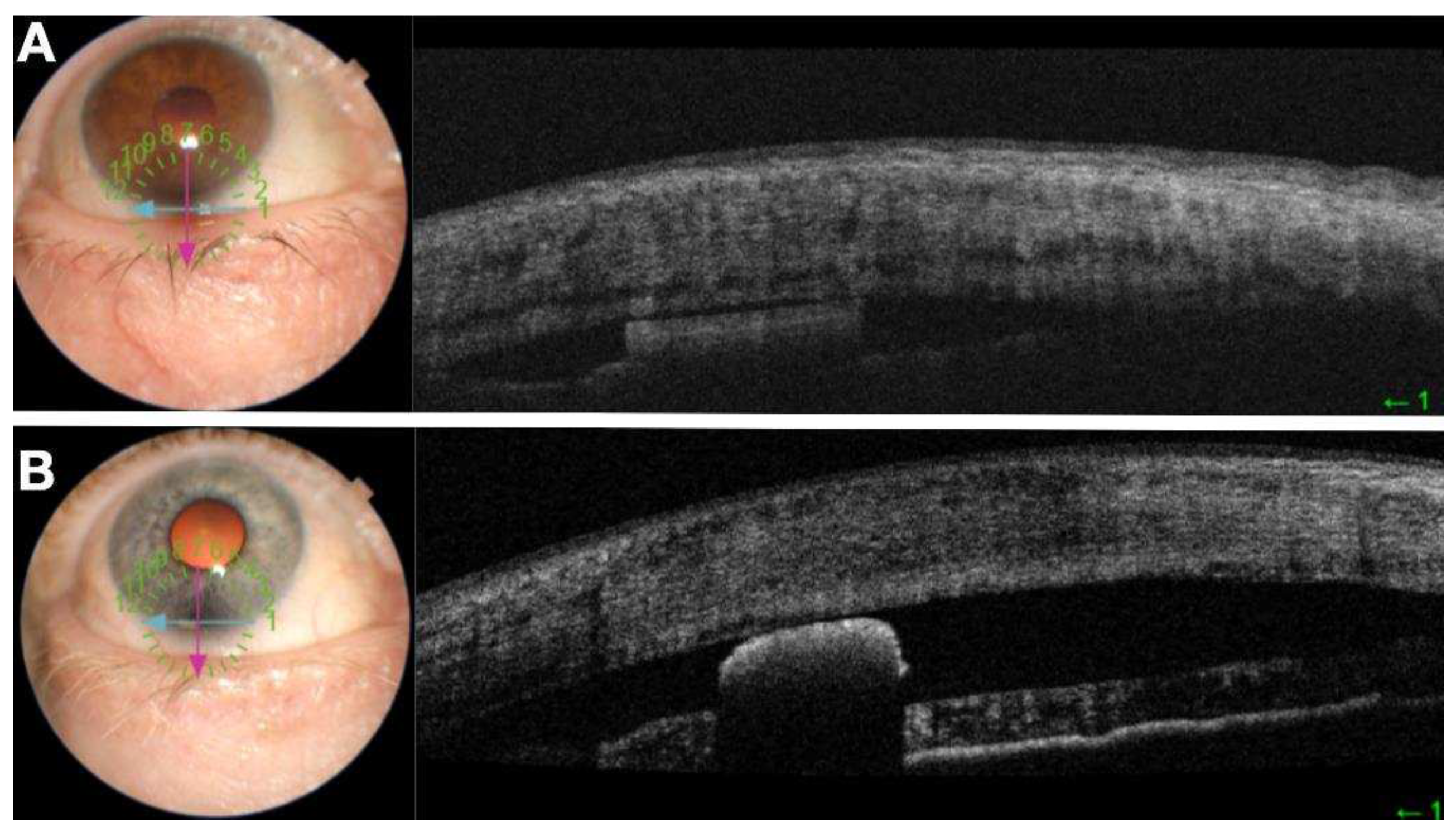
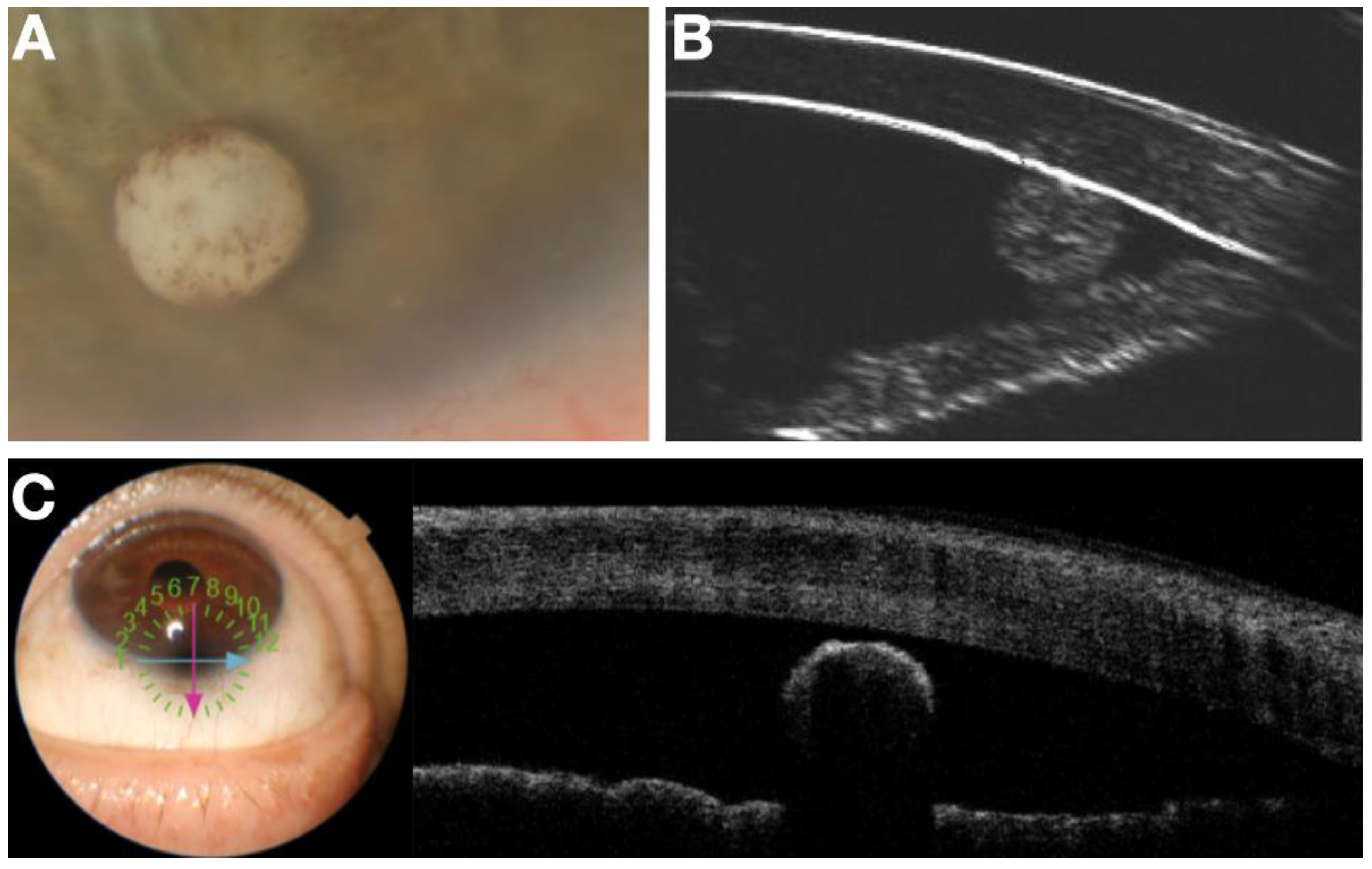
In these persons, measure the sulcus characteristics and estimate average opening distance between ciliary body and posterior iris. Measure the sulcus preimplantation. Wash subjects out of glaucoma medications and determine baseline washed out IOP. In thirty of these subjects, the Durysta will be placed in the ciliary sulcus. IOP will be measured at 1 week, 1 and 3 months. Measure sulcus implant position and size with ArcScan on week 1, 1 and 3 months. Validation of ArcScan imaging of the implant in the sulcus will be achieved by first implanting 10 of these 40 pseudophakic individuals with Durysta in the anterior chamber according to the standard approved protocol. Computerized Ultrasound Imaging, Anterior Segment OCT, corneal topography and slit lamp video capabilities will be used to understand the associated findings on ArcScan Ultrasound imaging in the anterior chamber (
Figure 3 and
Figure 4). With an ultimate goal of monitoring of sulcus administration, migration of the implant and associated tissue changes (
Figure 5), the validated findings from anterior chamber, multimodal imaging will be utilized to understand the Durysta implant with ArcScan imaging of the sulcus and the associated efficacy and toxicity as based upon localization. Migration of the bimatoprost implant was assessed by UBM and B-Scan. In the study we had one implant migrate into vitreous. We found with this patient although no AEs occurred IOP lowering impact of the implant was minimal suggesting the only viable intraocular locations for IOP lowering efficacy with Durysta are either intracameral or in the middle segment. Once placed, we found with the exception of one implant that migrated into vitreous, the other MiST Durysta implants remain localized to the clock hour of initial implantation. Much like well seated intracameral implants swelled to fill the potential space of surrounding ocular structures, the MiST Durysta remained more barrel shaped as opposed to round. One overwhelming observation for intracameral implants is a round and spotted implant is a mobile implant. Although CEC loss was not measured, it is likely in the same way that a round implant picks up iris pigment it is also causing death and loss of CECs.
Discussion
While depot drug delivery systems offer several advantages, they also have certain disadvantages and challenges. One challenge with depot drug delivery systems is achieving precise control over the release rate of the drug. In some cases, there may be an initial burst release of the drug, followed by a slower and sustained release. Achieving a consistent release profile can be challenging. Implantation of depot systems can trigger foreign body reactions, leading to inflammation or tissue responses at the site of administration. This may affect the biocompatibility of the system and influence its long-term efficacy. Implantable depot systems can pose a risk of infection, particularly if they remain in the body for an extended period. Infections at the implantation site can have serious consequences and may occur. Unintended movement of the delivery system can affect drug distribution and the intended therapeutic effect.
As compared to intracameral delivery, it stands to reason based upon proximity that the risk of CEC may be reduced but the potential for uveitis with inflammation of middle segment structures including the iris, ciliary body and uvea are potentially increased. One could envision a Durysta implant positioned between the intraocular lens and the iris could result in chaffing of the iris and uveitic glaucoma hyphema syndrome (UGH syndrome). If the mechanical interaction between the iris and the MiST causes repeated damage to the iris and microhyphema, we do not have an effective therapeutic to treat glaucoma as such chronic inflammation will act to exacerbate glaucoma.
For safe serial middle segment administration, we need a means of not only serial assessing implant position but also to serially image ocular structures to ensure no chronic changes occur. For example, MiST implants could integrate in zonules and cause stretching or tearing of zonular fibers. Over time this will lead to a change in effective lens position. Currently ArcScan is the only product on the market that will enable not only imaging of the implant and the effective lens position but also can visualize zonules directly. Moreover, ArcScan will enable focal assessment of local tissue changes occurring during and after polymer dissolution. Although still an area of active inquiry, it stands to reason that early changes in the iris, ciliary body and other structures MiST is likely to impact may have either changes in size and/or echogenicity resulting from MiST. We also may find that much like punctal plugs or ICL must be appropriately sized, implants may need to similarly be custom sized based upon preimplantation imaging. Alternatively, much like collagen punctal plugs swell to fill the potential space, it may be Durysta or other hydrogel MiST will somewhat conform to the space they are placed in.
Already in the pilot study we visualized some degree of focal elevation in the iris at the location of the Durysta implant which is not unsurprising as the iris has very little true structural integrity. Conversely, it may be exactly this ability to accommodate to different size implants and for changing implant size during degradation with iris bowing slightly anterior and then returning to primary position after implant fully degrades that may make the middle segment ideal for MiST. Quite simply it is too early to tell but one thing is certain, ArcScan Insight 100 imaging will be central to answering these questions and providing a potential path ahead for serial administration of Durysta and other depot MiST implants.
Depot ocular drug delivery provides extended controlled release of a therapeutic. This in turn enables drug to be delivered closer to the site of action, prevent therapeutic level peaks and troughs and also reduces the need for frequent administration. Taken together this reduces side-effects, maximizes efficacy while also increasing patient compliance. [
17,
18,
19] In regards to glaucoma specifically, all glaucoma drops have ocular surface toxicity but none are designed to act on the ocular surface. Moreover, many have come to hypothesize that IOP fluctuation may be more damaging than absolute IOP; therefore a means of maintaining nearly constant intraocular drug concentration and as corollary more controlled pressure, could be a game changer for glaucoma patients.
Ocular depot formulations include both degradable and non-biodegradable implants. [
2,
7] In an ideal design, biodegradable implants will release drug at a similar rate to polymeric degradation so no residual implant is retained after drug delivery is complete. Ideally for non-degradable implants, once drug release is complete, the implant can be easily and a-traumatically recovered. Early examples of non-degradable ocular implants have limited applications. For example, Vitrasert was a viable option for patients with AIDS who had CMV retinitis due to the unlikelihood of repeat administration secondary both to the infectious nature of the condition and the short life expectancy of patients with fulminant AIDS. Such an approach to treat a chronic condition like glaucoma would be highly problematic. Fortunately, we will soon have iDose from Glaukos, that similar to the Glaukos iStent is placed in the trabecular meshwork [
2,
7]. Unlike the iStent which facilitates aqeous outflow, the iDose will principally be a drug delivery vehicle designed to be recovered once drug has fully eluted and replaced with a new implant. [
2,
7]
Ocular surface disease induced by topical medical therapy has largely been viewed as a necessary evil. Efforts to eliminate preservative and drop volume are to be applauded. [
2,
7] Further, an interventional mindset in which SLT is typically first line and is serially employed in conjunction with MIGS interventions has been effective in reducing drop burden and therefore ocular toxicity. The addition of effective depot therapeutics will hopefully one day make glaucoma drops a thing of the past. It is lost upon most practitioners just how much collateral damage they are creating when they introduce a preservative containing prostaglandin analogue as a first line agent. With prostaglandin analogues, we are literally creating a controlled burn as they are inherently inflammatory molecules. Many eye care providers adopt the logic that one must bomb the village to save it and the use of inflammatory agent that creates debilitating ocular surface disease is the necessary path ahead. Quite simply, it is not and the lazy decision of the clinician is a burden the patient will later have to pay for dearly.
PGAs have a variety of topical side effects including conjunctival hyperemia, periocular and iris darkening, periocular fat atrophy. They also change eyelash color, length, and thickness. This latter side-effect has been utilized for therapeutic benefit with Latisse, which is a formulation of bimatoprost designed to apply to the lashes [
9,
20]. With Durysta we have the same drug bimatoprost but in a depot formulation [
9,
20] To use Durysta as a punctal plug for lash lengthening would be less elegant than Latisse being directly applied to the site of action with a lash brush. Similarly, applying bimatoprost topically when the therapeutic effect is intraocular, seems archaic when one considers we have depot formulations. Intraocular depot naysayers still have considerable ammunition against a purely depot based approach. One of the most compelling arguments against Durysta use, is single administration makes the therapy a non-starter. Therapeutic paradigm shifts require the necessary chair time for a patient to be re-educated that the drop they believe is saving their eye is also significantly damaging it. How does one have this conversation and in the same breath indicate to the patient after the first Durysta they will need to restart topical therapy?
The authors embarked on this project with the realization that repeated depot administration is the only path ahead. If we are to fully usher the paradigm shift that depot intraocular therapy is capable of, we must first ensure all the ophthalmologists serially prescribing a topical PGA will be comfortable with serial administration of a depot implant. To achieve widespread use, intraocular depot therapy will likely require elegant delivery mechanisms that can be performed by any surgeon with average dexterity at the slit lamp. A parallel example that we would be wise to emulate is intravitreal therapy such as anti-VEGF therapy that is serially administered in the clinic. If instead this therapy could only be administered in an operating room, we would not only not be able to meet the incredible disease burden but the cost of therapy would likely be prohibitive. The average eye doctor, if they can choose between taking a patient to an operating room to administer Durysta or initiate topical therapy, will elect for the latter.
Serial middle segment administration of Durysta and other MiST is poised to transform glaucoma therapy. This study introduces an alternate hypothesis that the major factor contributing to intracameral Durysta toxicity is motion of the implant. This study also provides for the first time a means of imaging MiST through the use of the ArcScan Insight 100. With further research efforts we may better understand the relevant anatomical measurements and best practices to ensure placement and visibility of the bimatropost implant that can lead practitioners to tailor treatment for individual eye anatomy, including sulcus to sulcus, anterior chamber depth and middle segment volume. With in-depth anatomical analysis, the location and migration of the implant can also be identified. Segmenting data based on age, ethnicity, gender, and angle architecture will add weight to treatment decisions based on patient profiles.
Conclusions
The ophthalmologist’s inherent visual approach to clinical medicine is a double-edged sword. Only secondary to the dermatologist, the ophthalmologist is offered direct visualization. For this reason, as a field we lag behind interventional radiology and other minimally invasive surgical fields in utilizing preoperative and intraoperative imaging guidance. Ophthalmologists have learned with the widespread adoption of optical coherence tomography (OCT), that with advanced imaging we can see more than meets the eye.
To safely adopt Minimally Invasive Sulcus Operations (MISO) we must have a means of imaging the sulcus. The ArcScan Insight 100 provides the highest-resolution images of the entire anterior segment to date. Many of us have anterior segment OCT and even handheld UBM but very few currently have adopted the use of the ArcScan. The ArcScan provides a precise anterior chamber depth and individual corneal layer thickness with arguably the best epithelial mapping of any instrument to date. It also provides a detailed image of the entire anterior segment providing precise sulcus-to-sulcus, angle-to-angle and inner diameter ciliary muscle width measurements within1-μm precision.
The ArcScan 100, through its contact-free 50MHz ultrasound probe, utilizes a water bath to achieve the highest resolution scan of the entire anterior segment possible. The use of immersion eliminates the variability induced by tear film instability when mapping epithelium. With this data we may be able to more precisely recognize persons that are not LVC candidates as well as monitor outcomes from LVC. The high resolution imaging behind the iris has applications for monitoring depot drug delivery (ie Durysta sulcus administration) and ICL surgery, including sizing and verifying correct footplate positioning, While traditional ultrasound biomicroscopy can also visualize this space, the ArcScan resolution and repeatability (120 micron repeatability for measuring sulcus-to-sulcus), enables precise determination effective lens position as well as assessment of vault changes over the life of the patient. The ArcScan elevates ultrasound to a truly repeatable device by incorporating iris registration that enables us to obtain repeatable imaging of the entire anterior segment. This repeatability provides a yet unavailable diagnostic to effective lens position and ICL vault serially.
Other clinical benefits of the precise imaging, mapping, and measurement tracking include: Early glaucoma management Improved ICL/IOL sizing, Keratoconus screening and Inlay evaluations. The ArcScan Insight 100 provides high-resolution images that depict the true anatomy of the entire anterior segment. The image results, says ArcScan, offer scanning types including glaucoma, cornea, anterior segment and capsule. ArcScan Insight 100 improvements include fine tuning the ultrasound transducers (one for cornea specific and the other for deeper scans) and the software that controls the transducer path along the arc, according to Barry Schafer, vice president of marketing and sales at ArcScan. Other enhancements include improvements in the EyeSeal design, upgrades to internal hardware components and the ability to do iris tracking between each meridian scan. Additional advances: automatic glaucoma and keratoconus report generation, a DICOM interface plus a new headrest design. The new iris-tracking capability allows for patient eye movement and improves image quality and measurements. When scanning multiple meridians, each scan references the iris, not the pupil.
A paradigm shift is underway. STAAR has demonstrated with the EVO ICL and soon the VIVA presbyopia correcting ICL, that the market is ripe for MiSO. In addition to phakic implantation, the Viva as well as the trifocal Sulfoclex lenses from Rayner, enable a premium refractive option for phakic individuals as well. In addition to optics, the middle segment is also poised to be an ideal location for MiST depot implants and undergo the same market explosion we saw with MIGS. Once the average ophthalmologist thinks of the middle segment as a safe space for primary surgery, we will see an explosion in technology as well. Already many refractive surgeons are exploring UBM and various ultrasound approaches to better understand the sulcus space and the changes that occur with ICL implantation. By nature of the iris precluding direct visualization, the middle segment has been in a way a black box for most ophthalmologists. Without visualization, the middle segment is approached in a ‘floor is lava” like approach.
We must get beyond our collective fear of sulcus intervention. ICLs are demonstrating, even in the phakic patient, MiSO can be safe and highly effective. Even more so in the pseudophakic, the middle segment is an obvious delivery site for secondary lenses and depot drug formulations alike. Corneal endothelial cells are arguably the Achilles heel of the anterior segment for serial intervention. Due to endothelial cell loss, Durysta has limited administrations with currently serial administration prohibited. Off-label delivery of Durysta to the sulcus space may prove an effective way to mitigate endothelial cell-loss thereby providing a safe approach to depot delivery for the life of the patient. Our preliminary work and that of Nathan Radcliffe that is yet to be published suggests that middle segment administration of Durysta and its visuzliation with ArcScan Insight 100 may become a central part of our clinical armaterium. Moreover, it lays the way for the development of novel MiST implants to release a variety of therapeutics for a variety of ocular conditions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Video 1- Movement of intracameral implant. Note rounded appearance and speckled appearance from uptake of iris pigment.
Author Contributions
The authors contributed to the study: conceptualization, BB, SG, SR, KL and BS.; Methodology, BB, SG, SR, KL and BS; Formal Analysis, BB, SG, SR, KL and BS.; Data Curation, BB, SG, SR, KL and BS.; Writing – Original Draft Preparation, BB; Writing – BB, SG, SR, KL and BS.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as standard imaging modalities were utilized for preliminary feasibility studies.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
BB is a consultant for ArcScan Inc. and Abbvie, SG and SR have no conflicts to report, KL and BS are employed by ArcScan Inc.
References
- Reinstein, D. Z.; Vida, R. S.; Archer, T. J. Visual Outcomes, Footplate Position and Vault Achieved with the Visian Implantable Collamer Lens for Myopic Astigmatism. Clin Ophthalmol 2021, 15, 4485-4497. [CrossRef]
- Chan, L.; Moster, M. R.; Bicket, A. K.; Sheybani, A.; Sarkisian, S. R.; Samuelson, T. W.; Ahmed, I. I. K.; Miller-Ellis, E.; Smith, O. U.; Cui, Q. N. New Devices in Glaucoma. Ophthalmol Ther 2023, 12 (5), 2381-2395. [CrossRef]
- Reinstein, D. Z.; Yap, T. E.; Archer, T. J.; Gobbe, M.; Silverman, R. H. Comparison of Corneal Epithelial Thickness Measurement Between Fourier-Domain OCT and Very High-Frequency Digital Ultrasound. J Refract Surg 2015, 31 (7), 438-445. [CrossRef]
- Ursea, R.; Feng, M.; Urs, R.; RoyChoudhury, A.; Silverman, R. H. Comparison of artemis 2 ultrasound and Visante optical coherence tomography corneal thickness profiles. J Refract Surg 2013, 29 (1), 36-41. [CrossRef]
- Urs, R.; Lloyd, H. O.; Reinstein, D. Z.; Silverman, R. H. Comparison of very-high-frequency ultrasound and spectral-domain optical coherence tomography corneal and epithelial thickness maps. J Cataract Refract Surg 2016, 42 (1), 95-101. [CrossRef]
- Bacharach, J.; Tatham, A.; Ferguson, G.; Belalcazar, S.; Thieme, H.; Goodkin, M. L.; Chen, M. Y.; Guo, Q.; Liu, J.; Robinson, M. R.; et al. Phase 3, Randomized, 20-Month Study of the Efficacy and Safety of Bimatoprost Implant in Patients with Open-Angle Glaucoma and Ocular Hypertension (ARTEMIS 2). Drugs 2021, 81 (17), 2017-2033. [CrossRef]
- Belamkar, A.; Harris, A.; Zukerman, R.; Siesky, B.; Oddone, F.; Verticchio Vercellin, A.; Ciulla, T. A. Sustained release glaucoma therapies: Novel modalities for overcoming key treatment barriers associated with topical medications. Ann Med 2022, 54 (1), 343-358. [CrossRef]
- Ghosn, C.; Rajagopalan, L.; Ugarte, S.; Mistry, S.; Orilla, W.; Goodkin, M. L.; Robinson, M. R.; Engles, M.; Dibas, M. Intraocular Pressure-Lowering Efficacy of a Sustained-Release Bimatoprost Implant in Dog Eyes Pretreated with Selective Laser Trabeculoplasty. J Ocul Pharmacol Ther 2022, 38 (4), 311-318. [CrossRef]
- Lee, S. S.; Dibas, M.; Almazan, A.; Robinson, M. R. Dose-Response of Intracameral Bimatoprost Sustained-Release Implant and Topical Bimatoprost in Lowering Intraocular Pressure. J Ocul Pharmacol Ther 2019, 35 (3), 138-144. [CrossRef]
- Lee, S. S.; Robinson, M. R.; Weinreb, R. N. Episcleral Venous Pressure and the Ocular Hypotensive Effects of Topical and Intracameral Prostaglandin Analogs. J Glaucoma 2019, 28 (9), 846-857. [CrossRef]
- Medeiros, F. A.; Walters, T. R.; Kolko, M.; Coote, M.; Bejanian, M.; Goodkin, M. L.; Guo, Q.; Zhang, J.; Robinson, M. R.; Weinreb, R. N.; Group, A. S. Phase 3, Randomized, 20-Month Study of Bimatoprost Implant in Open-Angle Glaucoma and Ocular Hypertension (ARTEMIS 1). Ophthalmology 2020, 127 (12), 1627-1641. [CrossRef]
- Shirley, M. Bimatoprost Implant: First Approval. Drugs Aging 2020, 37 (6), 457-462. [CrossRef]
- Weinreb, R. N.; Bacharach, J.; Brubaker, J. W.; Medeiros, F. A.; Bejanian, M.; Bernstein, P.; Robinson, M. R. Bimatoprost Implant Biodegradation in the Phase 3, Randomized, 20-Month ARTEMIS Studies. J Ocul Pharmacol Ther 2023, 39 (1), 55-62. [CrossRef]
- Silverman, R. H. Focused ultrasound in ophthalmology. Clin Ophthalmol 2016, 10, 1865-1875. [CrossRef]
- Reinstein, D. Z.; Archer, T. J.; Gobbe, M.; Silverman, R. H.; Coleman, D. J. Epithelial thickness after hyperopic LASIK: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg 2010, 26 (8), 555-564. [CrossRef]
- Reinstein, D. Z.; Gobbe, M.; Archer, T. J.; Silverman, R. H.; Coleman, D. J. Epithelial, stromal, and total corneal thickness in keratoconus: three-dimensional display with artemis very-high frequency digital ultrasound. J Refract Surg 2010, 26 (4), 259-271. [CrossRef]
- Blizzard, C.; Desai, A.; Driscoll, A. Pharmacokinetic Studies of Sustained-Release Depot of Dexamethasone in Beagle Dogs. J Ocul Pharmacol Ther 2016, 32 (9), 595-600. [CrossRef]
- Li, L.; Deng, F.; Qiu, H.; Li, Y.; Gong, Z.; Wang, L.; Wang, J.; Wu, W.; Nan, K. An adherent drug depot for retinal ganglion cell protection and regeneration in rat traumatic optic neuropathy models. RSC Adv 2021, 11 (37), 22761-22772. [CrossRef]
- Radhakrishnan, K.; Vincent, A.; Joseph, R. R.; Moreno, M.; Dickescheid, A.; Agrawal, R.; Venkatraman, S. Hollow Microcapsules as Periocular Drug Depot for Sustained Release of Anti-VEGF Protein. Pharmaceutics 2019, 11 (7). [CrossRef]
- Woodward, D. F.; Liang, Y.; Krauss, A. H. Prostamides (prostaglandin-ethanolamides) and their pharmacology. Br J Pharmacol 2008, 153 (3), 410-419. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).