Submitted:
18 December 2023
Posted:
20 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Design
2.2. Data Source and Variables
2.3. Data Analysis
- is the expected value of the response variable yi for subject i;
- is the model intercept and are the k independent explanatory variables with corresponding regression coefficients
- σεi is the disturbance term.
3. Results
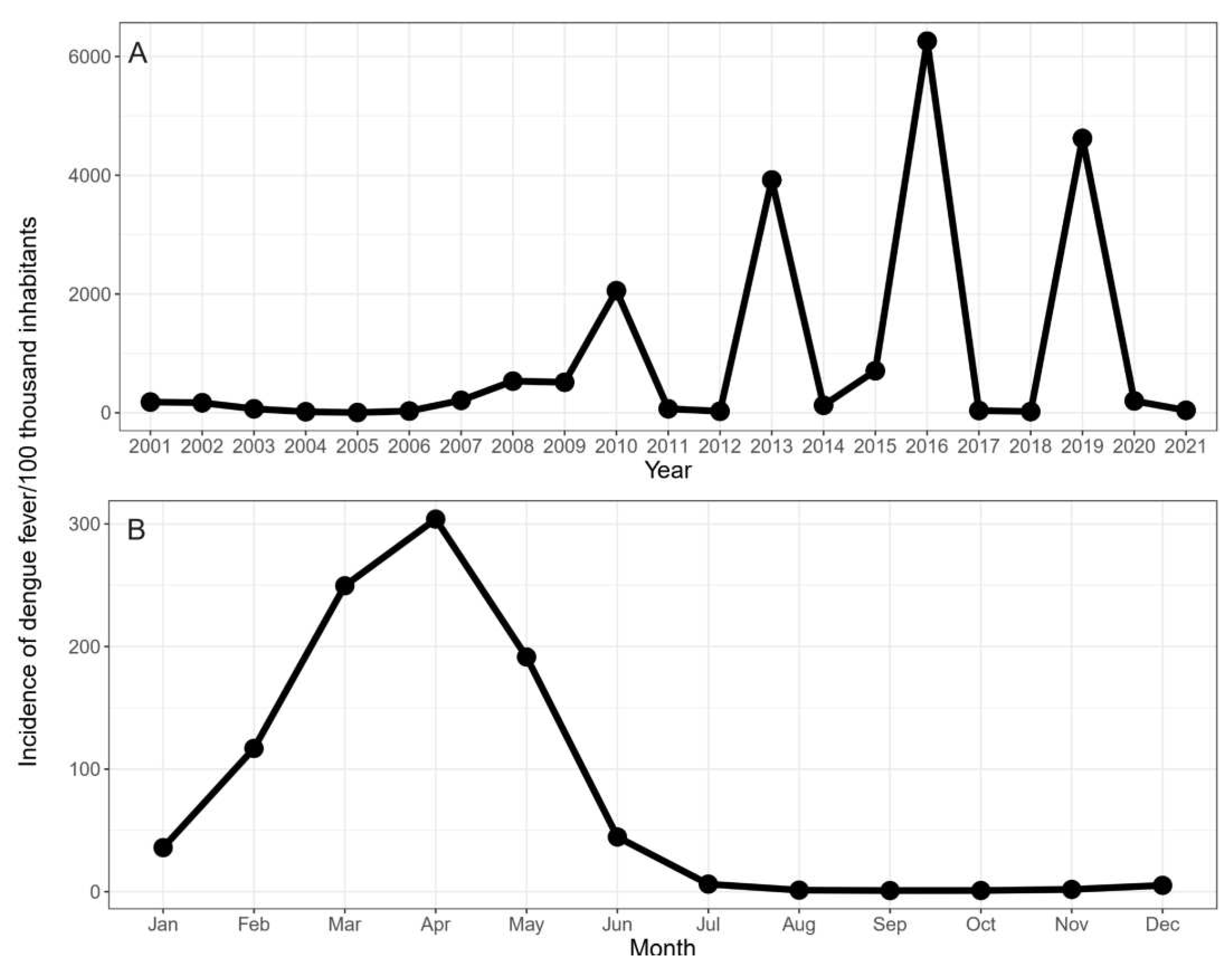
3.1. Annual and Monthly Incidence of Dengue Fever
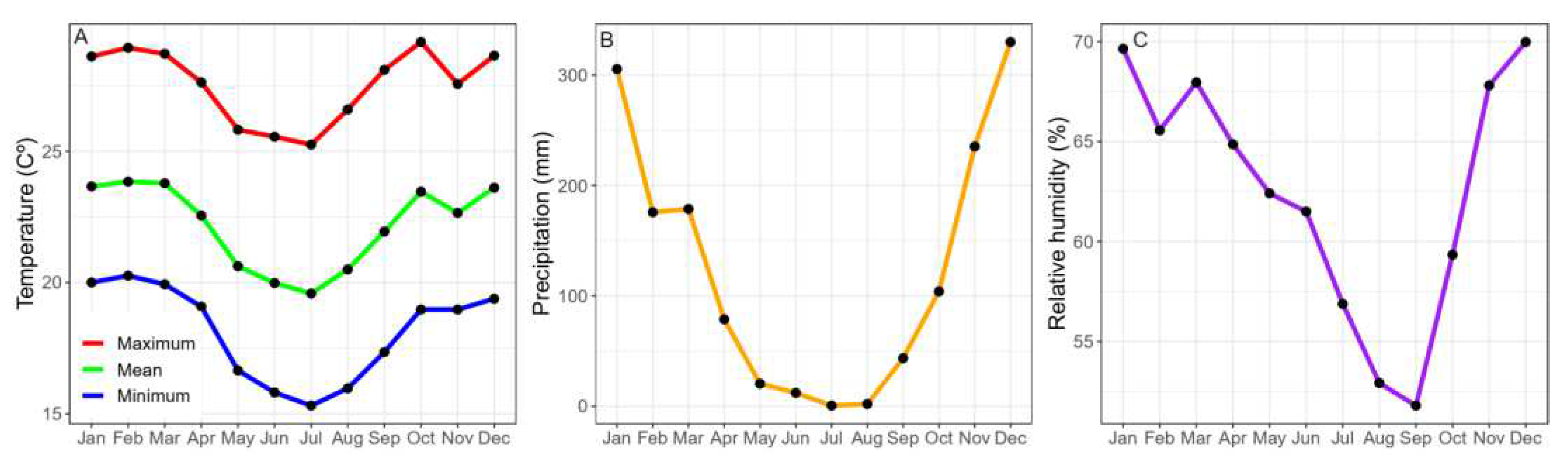
3.2. Monthly Climate Variables (2001–2021)
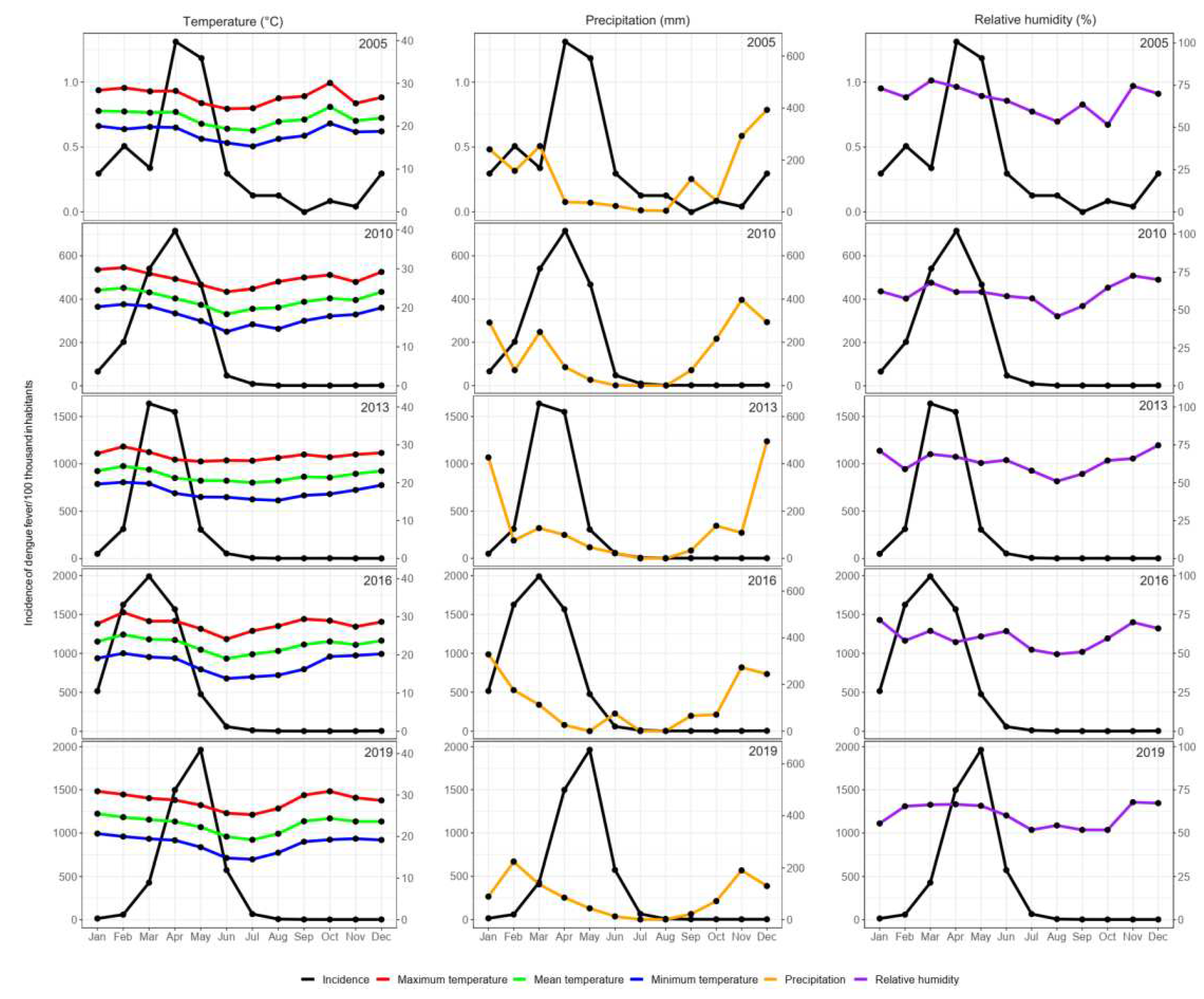
3.3. Dengue Incidence Rates in Epidemic Years in Response to Climatic Variables
3.4. Effect of Climatic Variables on the Incidence of Dengue Fever
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenharo, M. Dengue is breaking records in the Americas—what’s behind the surge? Nature 2023. [Google Scholar] [CrossRef] [PubMed]
- BRASIL. Ministério da Saúde. Série histórica—Casos prováveis de dengue (2000-2023). Avaiable at:https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/dengue/situacao-epidemiologica/serie-historica-casos-provaveis-de-dengue-2000-2023/view.
- 2023 .
- BRASIL. Ministério da Saúde. Série histórica—Casos de óbitos dengue (2000-2023). Avaiable at: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/dengue/situacao-epidemiologica/serie-historica-casos-de-obitos-dengue-2000-2023/view. 2023.
- Araujo, V.E.M.; Bezerra, J.M.T.; Amancio, F.F.; Passos, V.M.A.; Carneiro, M. Increase in the burden of dengue in Brazil and federated units, 2000 and 2015: analysis of the Global Burden of Disease Study 2015. Rev Bras Epidemiol 2017, 20 (Suppl. 1), 205–216. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.S.; Coudeville, L.; Halasa, Y.A.; Zambrano, B.; Dayan, G.H. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg 2011, 84, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, T.; Guzman-Holst, A.; Murray, K.A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat Commun 2020, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Hay, S.I. The Global Expansion of Dengue: How Aedes aegypti Mosquitoes Enabled the First Pandemic Arbovirus. Annu Rev Entomol 2020, 65, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Goncalves Neto, V.S.; Rebelo, J.M. [Epidemiological characteristics of dengue in the Municipality of Sao Luis, Maranhao, Brazil, 1997-2002]. Cad Saude Publica 2004, 20, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Codeco, C.T.; Oliveira, S.S.; Ferreira, D.A.C.; Riback, T.I.S.; Bastos, L.S.; Lana, R.M.; Almeida, I.F.; Godinho, V.B.; Cruz, O.G.; Coelho, F.C. Fast expansion of dengue in Brazil. Lancet Reg Health Am 2022, 12, 100274. [Google Scholar] [CrossRef]
- Pedrosa, M.C.; Borges, M.A.Z.; Eiras, A.E.; Caldas, S.; Cecilio, A.B.; Brito, M.F.; Ribeiro, S.P. Invasion of Tropical Montane Cities by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) Depends on Continuous Warm Winters and Suitable Urban Biotopes. J Med Entomol 2021, 58, 333–342. [Google Scholar] [CrossRef]
- de Sousa, S.C.; Carneiro, M.; Eiras, A.E.; Bezerra, J.M.T.; Barbosa, D.S. Factors associated with the occurrence of dengue epidemics in Brazil: a systematic review. Rev Panam Salud Publica 2021, 45, e84. [Google Scholar] [CrossRef]
- Cunha, M.; Ju, Y.; Morais, M.H.F.; Dronova, I.; Ribeiro, S.P.; Bruhn, F.R.P.; Lima, L.L.; Sales, D.M.; Schultes, O.L.; Rodriguez, D.A.; et al. Disentangling associations between vegetation greenness and dengue in a Latin American city: Findings and challenges. Landsc Urban Plan 2021, 216, None. [Google Scholar] [CrossRef]
- Assis, W.L.; Abreu, M.L. [The urban climate of Belo Horizonte: temporal-spatial analysis of the thermal and water field]. Revista de C. Humanas 2010, 10, 47–63. [Google Scholar]
- Masson-Delmotte, V., P. ; Zhai, A.; Pirani, S.L.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.I.; Gomis, M.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press.; 2021.
- Fouque, F.; Reeder, J.C. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: a look at the evidence. Infect Dis Poverty 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.d.C.M.; Caiaffa, W.T.; Oliveira, C.d.L.; Kroon, E.G.; Pessanha, J.E.M.; Lima, J.A.; Proietti, F.A. Fatores associados à infecção pelo vírus do dengue no Município de Belo Horizonte, Estado de Minas Gerais, Brasil: características individuais e diferenças intra-urbanas. Epidemiologia e serviços de saúde 2008, 17, 217–230. [Google Scholar] [CrossRef]
- PBH. Prefeitura de Belo Horizonte. Dengue. 2023. Availabe online: https//prefeitura.pbh.gov.br/saude/informacoes/vigilancia/vigilancia-epidemiologica/doencas-transmissiveis/dengue (accessed on 21 April 2023).
- Campos, N.B.D.; Morais, M.H.F.; Ceolin, A.P.R.; Cunha, M.; Nicolino, R.R.; Schultes, O.L.; Friche, A.A.L.; Caiaffa, W.T. Twenty-Two years of dengue fever (1996-2017): an epidemiological study in a Brazilian city. Int J Environ Health Res 2021, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.L.; Diaz-Quijano, F.A.; Batista, A.C.; Giatti, L.L. Climatic variables associated with dengue incidence in a city of the Western Brazilian Amazon region. Rev Soc Bras Med Trop 2019, 52, e20180429. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Bambrick, H.; Yakob, L.; Devine, G.; Frentiu, F.D.; Toan, D.T.T.; Thai, P.Q.; Xu, Z.; Hu, W. Heatwaves and dengue outbreaks in Hanoi, Vietnam: New evidence on early warning. PLoS Negl Trop Dis 2020, 14, e0007997. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Panorama Belo Horizonte. Rio de Janeiro: IBGE, 2023. Avaiable at: https://cidades.ibge.gov.br/brasil/mg/belo-horizonte/panorama. 2023.
- PBH. Prefeitura Municipal de Belo Horizonte. Prodabel detalha tamanho e número de bairros das regionais. Belo Horizonte: Prefeitura de Belo Horizonte, 2021. Avaiable at: https://prefeitura.pbh.gov.br/noticias/prodabel-detalha-tamanho-e-numero-de-bairros-das-regionais. 2021.
- Agresti, A. Categorical data analysis; John Wiley & Sons: 2012; Vol. 792.
- Allaire, J. RStudio: integrated development environment for R. Boston, MA 2012, 770, 165–171. [Google Scholar]
- Gabriel, A.F.B.; Abe, K.C.; Guimarães, M.d.P.; Miraglia, S.G.E.K. Health impact assessment of the incidence of dengue associated with precipitation in the city of Ribeirão Preto, São Paulo. Cadernos Saúde Coletiva 2018, 26, 446–452. [Google Scholar] [CrossRef]
- Rose, N.H.; Sylla, M.; Badolo, A.; Lutomiah, J.; Ayala, D.; Aribodor, O.B.; Ibe, N.; Akorli, J.; Otoo, S.; Mutebi, J.P.; et al. Climate and Urbanization Drive Mosquito Preference for Humans. Curr Biol 2020, 30, 3570–3579.e6. [Google Scholar] [CrossRef]
- Powell, J.R.; Tabachnick, W.J. History of domestication and spread of Aedes aegypti--a review. Mem Inst Oswaldo Cruz 2013, 108 (Suppl. 1), 11–17. [Google Scholar] [CrossRef]
- Brown, J.E.; Evans, B.R.; Zheng, W.; Obas, V.; Barrera-Martinez, L.; Egizi, A.; Zhao, H.; Caccone, A.; Powell, J.R. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 2014, 68, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Vasilakis, N. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect Genet Evol 2009, 9, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Saeed, O.; Asif, A. Chapter 2—Dengue virus disease; the origins. Academic Press, Dengue Virus Disease From Origin to Outbreak 2020, 10.1016/B978-0-12-818270-3.00002-3, 9-16. [CrossRef]
- May, R.M.; Anderson, R.M. Epidemiology and genetics in the coevolution of parasites and hosts. Proc R Soc Lond B Biol Sci 1983, 219, 281–313. [Google Scholar] [CrossRef] [PubMed]
- Barh, D.; Tiwari, S.; Rodrigues Gomes, L.G.; Ramalho Pinto, C.H.; Andrade, B.S.; Ahmad, S.; Aljabali, A.A.A.; Alzahrani, K.J.; Banjer, H.J.; Hassan, S.S.; et al. SARS-CoV-2 Variants Show a Gradual Declining Pathogenicity and Pro-Inflammatory Cytokine Stimulation, an Increasing Antigenic and Anti-Inflammatory Cytokine Induction, and Rising Structural Protein Instability: A Minimal Number Genome-Based Approach. Inflammation 2023, 46, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Koelle, K.; Martin, M.A.; Antia, R.; Lopman, B.; Dean, N.E. The changing epidemiology of SARS-CoV-2. Science 2022, 375, 1116–1121. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Ferdous, J.; Saha, O.; Islam, S.; Choudhury, S.D.; Abedin, J.; Hassan, M.M.; Islam, A. Transmission Dynamics and Genomic Epidemiology of Emerging Variants of SARS-CoV-2 in Bangladesh. Trop Med Infect Dis 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.S.A.; Bernardes, A.T.; Barbosa, E.A.G.; Chagas, I.; Dattilo, W.; Reis, A.B.; Ribeiro, S.P. Successive Pandemic Waves with Different Virulent Strains and the Effects of Vaccination for SARS-CoV-2. Vaccines (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Hung, L.S. The SARS epidemic in Hong Kong: what lessons have we learned? J R Soc Med 2003, 96, 374–378. [Google Scholar] [CrossRef]
- Ferreira-de-Lima, V.H.; Lima-Camara, T.N. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: a systematic review. Parasit Vectors 2018, 11, 77. [Google Scholar] [CrossRef]
- Cunha, M.d.C.M. Dengue em Belo Horizonte, 2002 a 2016: Distribuição espaço-temporal e intervenções de requalificação urbana. Universidade Federal de Minas Gerais, 2021.
- Robert, M.A.; Stewart-Ibarra, A.M.; Estallo, E.L. Climate change and viral emergence: evidence from Aedes-borne arboviruses. Curr Opin Virol 2020, 40, 41–47. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Pyke, A.T.; Hall-Mendelin, S.; Day, A.; Mores, C.N.; Christofferson, R.C.; Gubler, D.J.; Bennett, S.N.; van den Hurk, A.F. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One 2013, 8, e68137. [Google Scholar] [CrossRef]
- Watts, D.M.; Burke, D.S.; Harrison, B.A.; Whitmire, R.E.; Nisalak, A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg 1987, 36, 143–152. [Google Scholar] [CrossRef]
- Focks, D.A.; Daniels, E.; Haile, D.G.; Keesling, J.E. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg 1995, 53, 489–506. [Google Scholar] [CrossRef]
- Chan, M.; Johansson, M.A. The incubation periods of Dengue viruses. PLoS One 2012, 7, e50972. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, J.M.; Lazzari, C.R.; Lahondere, C. Effects of the Environmental Temperature on Aedes aegypti and Aedes albopictus Mosquitoes: A Review. Insects 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Alves de Oliveira, B.F.; Bottino, M.J.; Nobre, P.; Nobre, C.A. Deforestation and climate change are projected to increase heat stress risk in the Brazilian Amazon. Communications Earth & Environment 2021, 2, 207. [Google Scholar] [CrossRef]
- Graça, M.; Cruz, S.; Monteiro, A.; Neset, T.-S. Designing urban green spaces for climate adaptation: A critical review of research outputs. Urban Climate 2022, 42, 101126. [Google Scholar] [CrossRef]
- COP28. Conference of the Parties serving as the meeting of the Parties to the Paris Agreement, Fifth session, United Arab Emirates, 30 November to 12 December 2023, Agenda item 4, First global stocktake. Acessed in 14 December 2023 in https://www.cop28.com/en/. 2023.
| Variables | No-Lag Model | 1-Month Lag Model | 2-Month Lag Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% CI | p Value | IRR | 95% CI | p Value | IRR | 95% CI | p Value | ||||
| Maximum temperature | 1.501 | 1.267 | 1.781 | < 0.001 | ||||||||
| Mean temperature |
1.89 | 1.555 | 2.35 | < 0.001 | 1.918 | 1.573 | 2.349 | < 0.001 | ||||
| April | Reference | Reference | Reference | |||||||||
| January | 0.086 | 0.035 | 0.216 | < 0.001 | 0.177 | 0.073 | 0.432 | < 0.001 | 0.068 | 0.028 | 0.166 | < 0.001 |
| February | 0.115 | 0.041 | 0.316 | < 0.001 | 0.429 | 0.179 | 1.021 | 0.055 | 0.401 | 0.168 | 0.953 | 0.036 |
| March | 0.523 | 0.209 | 1.300 | 0.153 | 0.443 | 0.180 | 1.082 | 0.066 | 0.595 | 0.247 | 1.424 | 0.250 |
| May | 0.909 | 0.367 | 2.238 | 0.840 | 0.553 | 0.230 | 1.323 | 0.177 | 0.238 | 0.095 | 0.598 | 0.002 |
| June | 0.353 | 0.135 | 0.914 | 0.035 | 0.639 | 0.237 | 1.715 | 0.393 | 0.082 | 0.034 | 0.200 | < 0.001 |
| July | 0.049 | 0.019 | 0.128 | < 0.001 | 0.279 | 0.085 | 0.909 | 0.0289 | 0.064 | 0.025 | 0.162 | < 0.001 |
| August | 0.006 | 0.002 | 0.015 | < 0.001 | 0.067 | 0.020 | 0.226 | < 0.001 | 0.036 | 0.012 | 0.106 | < 0.001 |
| September | 0.002 | 0.001 | 0.005 | < 0.001 | 0.026 | 0.009 | 0.077 | < 0.001 | 0.033 | 0.011 | 0.103 | < 0.001 |
| October | 0.002 | 0.001 | 0.005 | < 0.001 | 0.009 | 0.003 | 0.022 | < 0.001 | 0.017 | 0.006 | 0.044 | < 0.001 |
| November | 0.005 | 0.002 | 0.012 | < 0.001 | 0.009 | 0.004 | 0.021 | < 0.001 | 0.008 | 0.003 | 0.020 | < 0.001 |
| December | 0.009 | 0.004 | 0.023 | < 0.001 | 0.016 | 0.007 | 0.039 | < 0.001 | 0.009 | 0.004 | 0.022 | < 0.001 |
| AIC | 3325.805 | 3310.732 | 3307.799 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





