Submitted:
18 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Research design
2.3. Blood samples
2.4. Salivary samples
2.5. Measurement of advanced oxidation protein products (AOPP)
2.6. Measurement of products of lipid peroxidation (LPO)
2.7. Nitrite + nitrate (NOx) measurement
2.8. Measurement of glutathione peroxidase (GPx), glutathione reductase (GRd) and catalase (CAT)
2.9. Measurement of α-Klotho
2.10. Salivary cortisol
2.11. Statistical analysis
3. Results
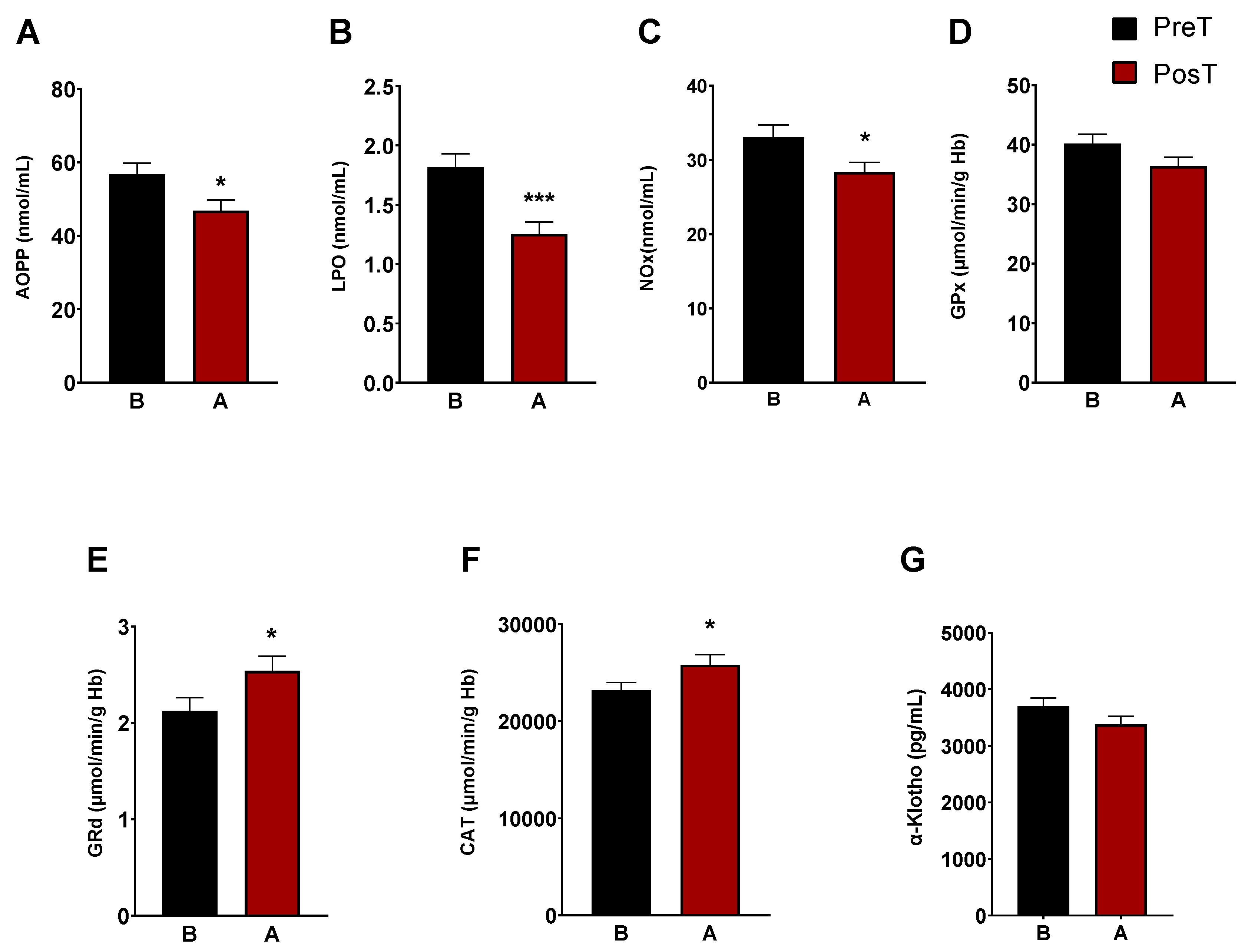
3.1. Oxidative and inflammatory status in the patients before and after treatment
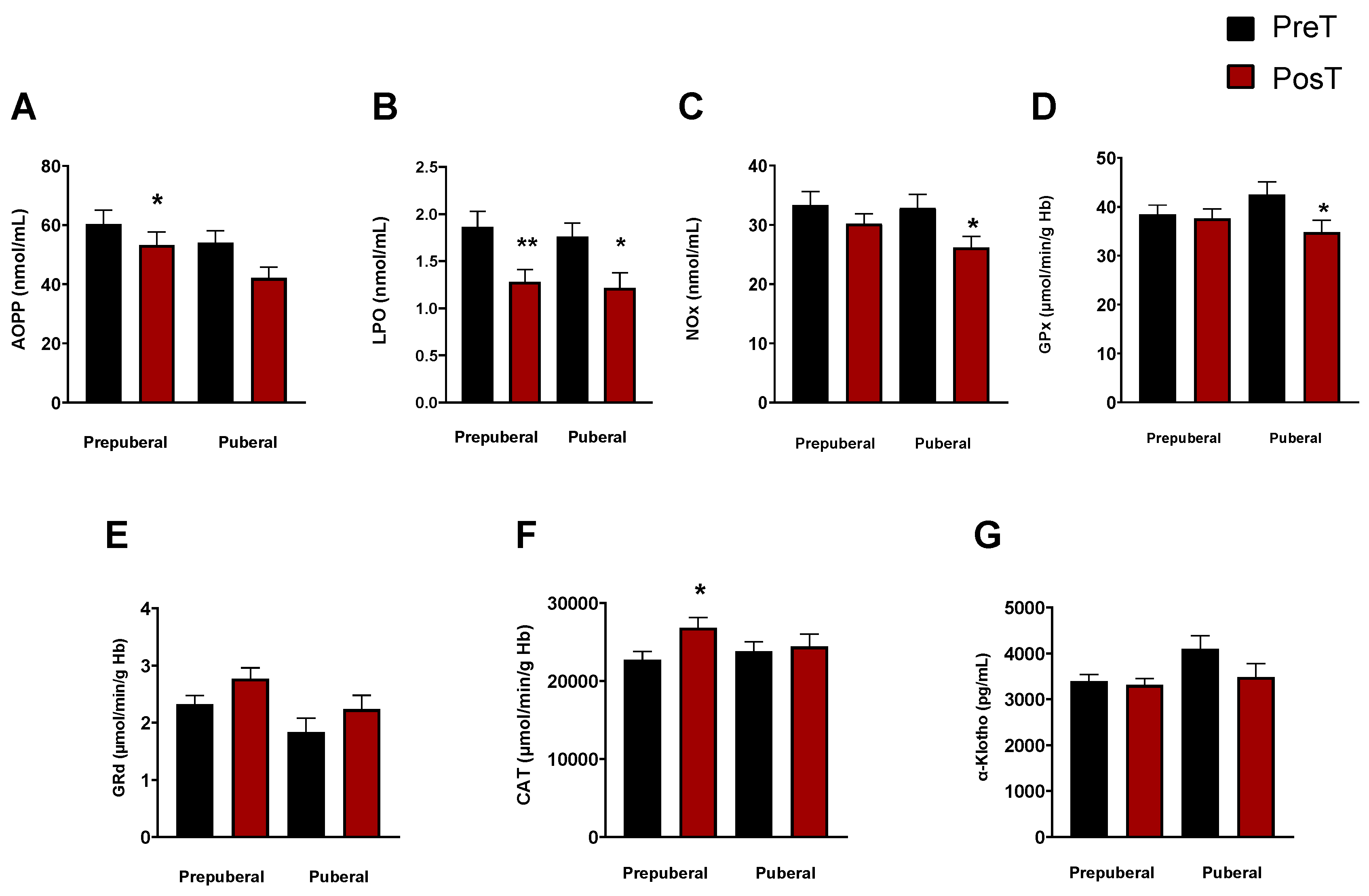
3.2. Assessment of oxidative and inflammatory status as a function of maturational stage: prepuberal vs. puberal children’s groups
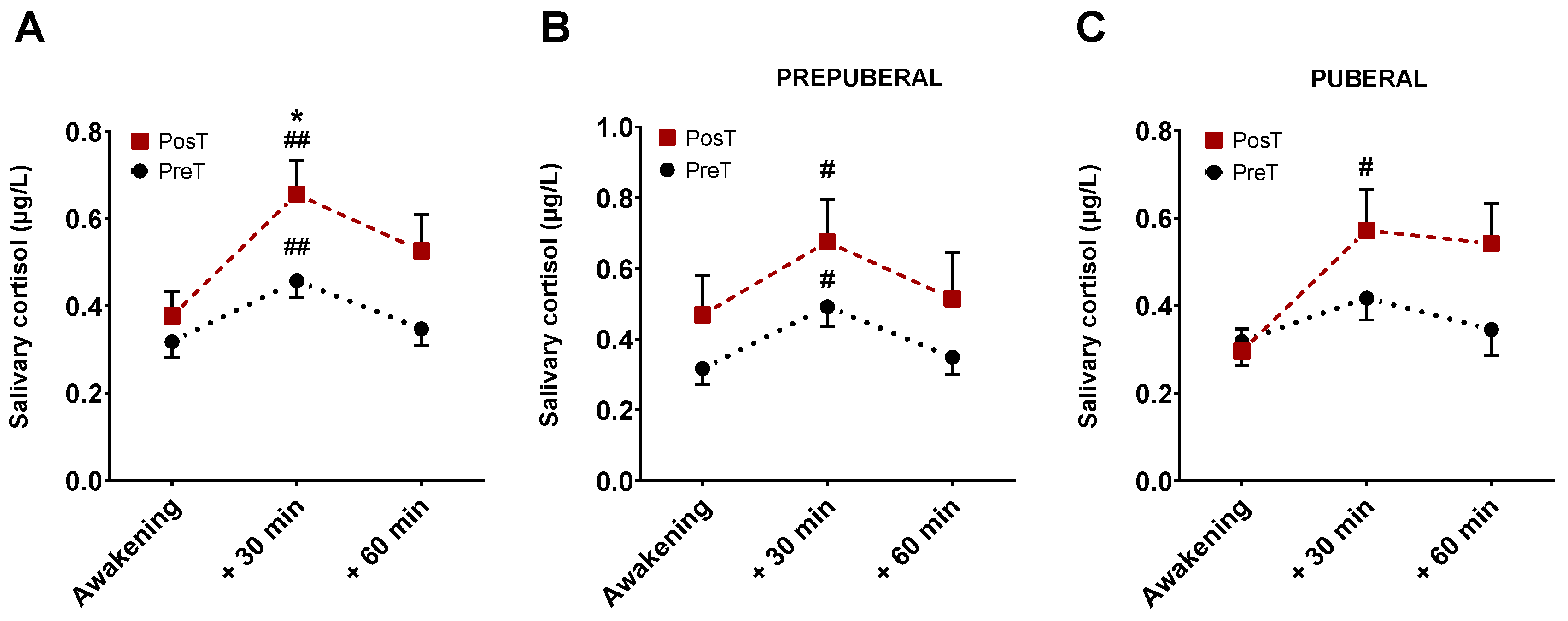
3.3. Salivary cortisol levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-5; 5th ed.; Arlington, VA, USA, 2013.
- Luo, Y.; Weibman, D.; Halperin, J.M.; Li, X. A Review of Heterogeneity in Attention Deficit/Hyperactivity Disorder (ADHD). Front Hum Neurosci 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Collantes, R. González.; Cascajo, A.R.-Sacristán.; García, J.Sánchez. Epidemiología Del TDAH. Revista española de pediatría: clínica e investigación. 2015, 71, 58–61. [Google Scholar]
- Vicario, M.H.; Santos, L.S. Trastorno Por Déficit de Atención e Hiperactividad. Manifestaciones Clínicas y Evolución. Diagnóstico Desde La Evidencia Científica. Pediatría Integral 2014, 18, 609–623. [Google Scholar]
- Catalá-López, F.; Peiró, S.; Ridao, M.; Sanfélix-Gimeno, G.; Gènova-Maleras, R.; Catalá, M.A. Prevalence of Attention Deficit Hyperactivity Disorder among Children and Adolescents in Spain: A Systematic Review and Meta-Analysis of Epidemiological Studies. BMC Psychiatry 2012, 12, 1–13. [Google Scholar] [CrossRef]
- Thapar, A.; Langley, K.; Owen, M.J.; O’Donovan, M.C. Advances in Genetic Findings on Attention Deficit Hyperactivity Disorder. Psychol Med 2007, 37, 1681–1692. [Google Scholar] [CrossRef]
- Anesiadou, S.; Makris, G.; Michou, M.; Bali, P.; Papassotiriou, I.; Apostolakou, F.; Korkoliakou, P.; Papageorgiou, C.; Chrousos, G.; Pervanidou, P. Salivary Cortisol and Alpha-Amylase Daily Profiles and Stress Responses to an Academic Performance Test and a Moral Cognition Task in Children with Neurodevelopmental Disorders. Stress and Health 2021, 37, 45–59. [Google Scholar] [CrossRef]
- Van West, D.; Claes, S.; Deboutte, D. Differences in Hypothalamic–Pituitary–Adrenal Axis Functioning among Children with ADHD Predominantly Inattentive and Combined Types. Eur Child Adolesc Psychiatry 2009, 18, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Jessop, D.S.; Turner-Cobb, J.M. Measurement and Meaning of Salivary Cortisol: A Focus on Health and Disease in Children. Stress 2008, 11, 1–14. [Google Scholar] [CrossRef]
- Ramos-Quiroga, J.A.; Corominas-Roso, M.; Palomar, G.; Ferrer, R.; Valero, S.; Corrales, M.; Richarte, V.; Casas, M. Cortisol Awakening Response in Adults with Attention Deficit Hyperactivity Disorder: Subtype Differences and Association with the Emotional Lability. European Neuropsychopharmacology 2016, 26, 1140–1149. [Google Scholar] [CrossRef]
- Shibuya, I.; Nagamitsu, S.; Okamura, H.; Ozono, S.; Chiba, H.; Ohya, T.; Yamashita, Y.; Matsuishi, T. High Correlation between Salivary Cortisol Awakening Response and the Psychometric Profiles of Healthy Children. Biopsychosoc Med 2014, 8, 1–4. [Google Scholar] [CrossRef]
- Stalder, T.; Kirschbaum, C.; Kudielka, B.M.; Adam, E.K.; Pruessner, J.C.; Wüst, S.; Dockray, S.; Smyth, N.; Evans, P.; Hellhammer, D.H.; et al. Assessment of the Cortisol Awakening Response: Expert Consensus Guidelines. Psychoneuroendocrinology 2016, 63, 414–432. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, M.; Holmberg, K.; Lindblad, F.; Fernell, E.; Ek, U.; Dahllöf, G. Salivary Cortisol Levels and Dental Anxiety in Children with Attention Deficit Hyperactivity Disorder. Eur J Oral Sci 2007, 115, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Mehendale, H.M. Oxidative Stress. Encyclopedia of Toxicology: Third Edition 2014, 735–737. [Google Scholar] [CrossRef]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A.; Campoy, C.; Muñoz-Hoyos, A. Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Arellano, L.; González-García, N.; Salazar-García, M.; Corona, J.C. Antioxidants as a Potential Target against Inflammation and Oxidative Stress in Attention-Deficit/Hyperactivity Disorder. Antioxidants 2020, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Boleti, A.P.; de Oliveira Flores, T.M.; Moreno, S.E.; Dos Anjos, L.; Mortari, M.R.; Migliolo, L. Neuroinflammation: An Overview of Neurodegenerative and Metabolic Diseases and of Biotechnological Studies. Neurochem Int 2020, 136, 104714. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a Risk Factor for Attention Deficit Hyperactivity Disorder. Pharmacol Biochem Behav 2019, 182, 22–34. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+ T Cells in Neurodegenerative Diseases. Front Cell Neurosci 2018, 12, 114. [Google Scholar] [CrossRef]
- Leffa, D.T.; Torres, I.L.S.; Rohde, L.A. A Review on the Role of Inflammation in Attention-Deficit/Hyperactivity Disorder. Neuroimmunomodulation 2019, 25, 328–333. [Google Scholar] [CrossRef]
- Verlaet, A.A.J.; Breynaert, A.; Ceulemans, B.; De Bruyne, T.; Fransen, E.; Pieters, L.; Savelkoul, H.F.J.; Hermans, N. Oxidative Stress and Immune Aberrancies in Attention-Deficit/Hyperactivity Disorder (ADHD): A Case–Control Comparison. Eur Child Adolesc Psychiatry 2019, 28, 719–729. [Google Scholar] [CrossRef]
- Fernández-Mayoralas, D.M.; Fernández-Perrone, A.L.; Muñoz-Jareño, N.; Fernández-Jaén, A. Actualización En El Tratamiento Farmacológico Del Trastorno Por Déficit de Atención/Hiperactividad: Lisdexanfetamina y Guanfacina de Liberación Retardada. Rev Neurol 2017, 64, S1–S8. [Google Scholar] [CrossRef]
- García Campayo, J.; Santed Germán, M.Á.; Cerdán Lanero, C.; Alda Díez, M. Tratamiento Del Trastorno Por Déficit de Atención. Aten Primaria 2007, 39, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch Dis Child 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. [42] Determination of Aldehydic Lipid Peroxidation Products: Malonaldehyde and 4-Hydroxynonenal. In Methods in enzymology; Elsevier, 1990; Vol. 186, pp. 407–421.
- Aebi, H. Catalase in Vitro. Methods Enzymol 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Choudhary, R.; Eesha Saroha, A.; Swarnkar, P.L. Effect of Abscisic Acid and Hydrogen Peroxide on Antioxidant Enzymes in Syzygium Cumini Plant. J Food Sci Technol 2012, 49, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, P.; Malathi, A. Comparative Study of Hemoglobin Estimated by Drabkin’s and Sahli’s Methods. J Postgrad Med 1992, 38, 8. [Google Scholar]
- Fries, E.; Dettenborn, L.; Kirschbaum, C. The Cortisol Awakening Response (CAR): Facts and Future Directions. International journal of Psychophysiology 2009, 72, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two Formulas for Computation of the Area under the Curve Represent Measures of Total Hormone Concentration versus Time-Dependent Change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Spencer, T.J.; Biederman, J.; Mick, E. Attention-Deficit/Hyperactivity Disorder: Diagnosis, Lifespan, Comorbidities, and Neurobiology. J Pediatr Psychol 2007, 32, 631–642. [Google Scholar] [CrossRef]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative Stress in Psychiatric Disorders : Evidence Base and Therapeutic Implications. International Journal of Neuropsychopharmacology 2008, 11, 851–876. [Google Scholar] [CrossRef]
- Jiménez-Fernández, S.; Gurpegui, M.; Garrote-Rojas, D.; Gutiérrez-Rojas, L.; Carretero, M.D.; Correll, C.U. Oxidative Stress Parameters and Antioxidants in Patients with Bipolar Disorder: Results from a Meta-Analysis Comparing Patients, Including Stratification by Polarity and Euthymic Status, with Healthy Controls. Bipolar Disord 2021, 23, 117–129. [Google Scholar] [CrossRef]
- Lopresti, A.L. Oxidative and Nitrosative Stress in ADHD: Possible Causes and the Potential of Antioxidant-Targeted Therapies. ADHD Attention Deficit and Hyperactivity Disorders 2015, 7, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Kul, M.; Unal, F.; Kandemir, H.; Sarkarati, B.; Kilinc, K.; Kandemir, S.B. Evaluation of Oxidative Metabolism in Child and Adolescent Patients with Attention Deficit Hyperactivity Disorder. Psychiatry Investig 2015, 12, 361. [Google Scholar] [CrossRef]
- Selek, S.; Savas, H.A.; Serdar Gergerlioglu, H.; Bulut, M.; Ramazan Yilmaz, H. Oxidative Imbalance in Adult Attention Deficit/Hyperactivity Disorder §. Biol Psychol 2008, 79, 256–259. [Google Scholar] [CrossRef]
- Ceylan, M.; Sener, S.; Cavunt Bayraktar, A.; Kavutcu, M. Oxidative Imbalance in Child and Adolescent Patients with Attention-Deficit/ Hyperactivity Disorder. Prog Neuropsychopharmacol Biol Psychiatry 2010, 34, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Selek, S.; Gergerlioglu, H.S.; Savas, H.A.; Yilmaz, H.R.; Yuce, M.; Ekici, G. Malondialdehyde Levels in Adult Attention-Deficit Hyperactivity Disorder. Journal of Psychiatry and Neuroscience 2007, 32, 435–438. [Google Scholar] [PubMed]
- Joseph, N.; Zhang-James, Y.; Perl, A.; Faraone, S. V. Oxidative Stress and ADHD: A Meta-Analysis. J Atten Disord 2015, 19, 915. [Google Scholar] [CrossRef]
- Guney, E.; Cetin, F.H.; Alisik, M.; Tunca, H.; Torun, Y.T.; Iseri, E.; Taner, Y.I.; Cayci, B.; Erel, O. Attention Deficit Hyperactivity Disorder and Oxidative Stress: A Short Term Follow up Study. Psychiatry Res 2015, 229, 310–317. [Google Scholar] [CrossRef]
- Martins, M.R.; Reinke, A.; Petronilho, F.C.; Gomes, K.M.; Dal-Pizzol, F.; Quevedo, J. Methylphenidate Treatment Induces Oxidative Stress in Young Rat Brain. Brain Res 2006, 1078, 189–197. [Google Scholar] [CrossRef]
- Schmitz, F.; Scherer, E.B.S.; Machado, F.R.; da Cunha, A.A.; Tagliari, B.; Netto, C.A.; Wyse, A.T.S. Methylphenidate Induces Lipid and Protein Damage in Prefrontal Cortex, but Not in Cerebellum, Striatum and Hippocampus of Juvenile Rats. Metab Brain Dis 2012, 27, 605–612. [Google Scholar] [CrossRef]
- Carvalho Muga, M. Methylphenidate-Induced Alterations in Astrocytes: A Comprehensive Characterization, Universidad de Coimbra: Coimbra, 2020.
- Sanches, E.S.; Boia, R.; Leitão, R.A.; Madeira, M.H.; Fontes-Ribeiro, C.A.; Ambrósio, A.F.; Fernandes, R.; Silva, A.P. Attention-Deficit/Hyperactivity Disorder Animal Model Presents Retinal Alterations and Methylphenidate Has a Differential Effect in ADHD versus Control Conditions. Antioxidants 2023, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Tian, Y.; Zhang, H.; Ma, X.; Shang, B.; Zhang, J.; Jiao, Y.; Zhang, Y.; Hu, J.; Wang, Y. Methylphenidate Ameliorates Hypoxia-Induced Mitochondrial Damage in Human Neuroblastoma SH-SY5Y Cells through Inhibition of Oxidative Stress. Life Sci 2018, 197, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.J.; Clement, H.-W.; Gebhardt, S.; Hemmeter, U.M.; Schulz, E.; Krieg, J.-C.; Kircher, T.; Heiser, P. Impact of Psychostimulants and Atomoxetine on the Expression of 8-Hydroxyguanine Glycosylase 1 in Human Cells. J Neural Transm 2010, 117, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Fernandez, S.; Gurpegui, M.; Diaz-Atienza, F.; Pérez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative Stress and Antioxidant Parameters in Patients with Major Depressive Disorder Compared to Healthy Controls before and after Antidepressant Treatment: Results from a Meta-Analysis. J Clin Psychiatry 2015, 76, 13705. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.-C.; Hsieh, C.-L. Antioxidant Effects of Dopamine and Related Compounds. Biosci Biotechnol Biochem 1997, 61, 1646–1649. [Google Scholar] [CrossRef] [PubMed]
- Calvi, J.P.Q.; Cornejo, G.S.E. Metilfenidato: Propiedades, Aplicaciones y Controversias. Revista de Psicología 2022, 12, 189–203. [Google Scholar] [CrossRef]
- Sandoval, V.; Riddle, E.L.; Hanson, G.R.; Fleckenstein, A.E. Methylphenidate Redistributes Vesicular Monoamine Transporter-2: Role of Dopamine Receptors. Journal of Neuroscience 2002, 22, 8705–8710. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo Sanz, G.; Sánchez Herranz, A. Implicación Del Transportador Vesicular de Monoaminas En El Trastorno Por Déficit de Atención/Hiperactividad. Rev. neurol.(Ed. impr.) 2011, 103–108. [CrossRef]
- Hanson, G.R.; Sandoval, V.; Riddle, E.; Fleckenstein, A.E. Psychostimulants and Vesicle Trafficking: A Novel Mechanism and Therapeutic Implications. Ann N Y Acad Sci 2004, 1025, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, A.G.; Schaz, U.; Storch, A.; Liebau, S.; Fegert, J.M.; Boeckers, T.M. Methylphenidate Exerts No Neurotoxic, but Neuroprotective Effects in Vitro. J Neural Transm 2006, 113, 1927–1934. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.S.; Wiersema, J.R.; van der Meere, J.J.; Roeyers, H. Context-Dependent Dynamic Processes in Attention Deficit/Hyperactivity Disorder: Differentiating Common and Unique Effects of State Regulation Deficits and Delay Aversion. Neuropsychol Rev 2010, 20, 86–102. [Google Scholar] [CrossRef]
- Thorn, L.; Hucklebridge, F.; Evans, P.; Clow, A. The Cortisol Awakening Response, Seasonality, Stress and Arousal: A Study of Trait and State Influences. Psychoneuroendocrinology 2009, 34, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Freitag, C.M.; Hänig, S.; Palmason, H.; Meyer, J.; Wüst, S.; Seitz, C. Cortisol Awakening Response in Healthy Children and Children with ADHD: Impact of Comorbid Disorders and Psychosocial Risk Factors. 2009, 34, 1019–1028. [CrossRef]
- Ozgocer, T.; Ucar, C.; Yildiz, S. Daily Cortisol Awakening Response and Menstrual Symptoms in Young Females. Stress and Health 2022, 38, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.C.; Wrath, A.J.; von Dewitz, B.; Marciniuk, K.; Roesler, A.; Napper, S. Attachment Impacts Cortisol Awakening Response in Chronically Depressed Individuals. Psychoneuroendocrinology 2020, 120, 104778. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Lin, X.-X.; Chen, H.; Liu, Y.-Y.; Lin, G.-X.; Wei, L.-X.; Hong, Y.-L. The Change of the Cortisol Levels in Children with ADHD Treated by Methylphenidate or Atomoxetine. J Psychiatr Res 2012, 46, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Huang, Y.-S.; Hsiao, C.-C.; Chen, C.-K. The Trend in Morning Levels of Salivary Cortisol in Children with ADHD during 6 Months of Methylphenidate Treatment. J Atten Disord 2017, 21, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Yang, J.-W.; Ko, Y.-H.; Han, C.; Kim, S.-H.; Lee, M.-S.; Joe, S.-H.; Jung, I.-K. Effects of Methylphenidate and Bupropion on DHEA-S and Cortisol Plasma Levels in Attention-Deficit Hyperactivity Disorder. Child Psychiatry Hum Dev 2008, 39, 201–209. [Google Scholar] [CrossRef]
- Wust, S.; Wolf, J.; Hellhammer, D.H.; Federenko, I.; Schommer, N.; Kirschbaum, C. The Cortisol Awakening Response Normal Values and Confounds. Noise Health 2000, 2, 79. [Google Scholar]
| Parameters | Prepuberal group (n=34) | Puberal group (n=25) |
| Age (years) | 7,74 ± 1,29 | 12,7 ± 1,03 |
| Gender (female/male) | 8 female/26 male | 11 female/14 male |
| ADHD presentation | ADHD combined: 14 | ADHD combined: 7 |
| ADHD inattentive: 14 | ADHD inattentive: 15 | |
| ADHD hyperactive-impulsive: 5 | ADHD hyperactive-impulsive: 2 | |
| Unclassified:1 | Unclassified:1 | |
| Treatment | Methylphenidate | Methylphenidate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





