1. Introduction
Polylactide (PLA) - aliphatic polyester of lactic acid has recently gained great acceptance and is used in many fields of human activity: medicine, pharmaceuticals, packaging, 3D printing [
1,
2,
3,
4,6,7]. However, the use of polylactide is somewhat constrained due to its relatively high cost and hydrophobicity [
2,
3,
4,
5]. To overcome the shortcomings, various PLA-based composite materials have been proposed [7,8,9,10,11,12,13,14]. A study of the properties of these materials showed that they do not have optimal characteristics, including poor adhesion of materials of various natures. To improve the compatibility of PLA with other polymers in composite materials, modification of this polymer by radical graft polymerization seems promising [14,17]. For these purposes, monomers of the acrylic series (acrylic and methacrylic acids) seem preferable; however, polyesters are tend to hydrolytic destruction in the presence of acids; therefore, a less strong methacrylic acid would be a reasonable choice. To generate radicals on the surface of a polylactide film, the method of irradiation with UV light was chosen as it is safe, affordable and allows varying the effect on the polymer object. The properties of the obtained copolymers which are cationites, were determined by the mass of grafted acid and static cation exchange capacity.
The purpose of this work was to investigate the effect of methacrylic acid graft copolymers on the properties of polylactide .
To achieve this goal, the following tasks were solved:
synthesis of copolymers (CP) of PLA and methacrylic acid (MAA);
studying the physicochemical properties of synthesized CP and establishing their structure;
establishing the patterns of graft polymerization;
determination of optimal conditions for the synthesis of CP.
2. Experimental
2.1. Copolymer (CP) synthesis
Samples of PLA films measuring 3*1*0.0012 cm were
preliminarily exposed to UV irradiation for 10, 20, 30, 60 minutes, after which
the resulting samples were placed in a quartz glass test tube. The test tube
was installed at a distance of 15 cm from the irradiation source, parallel to
the axis of incidence of the rays. Next in 10 ml of a 10 - 20% MAA solution
with the addition of 1% Mohr’s salt (Iron (II) ammonium sulfate) was added to
the sample . Next, heating was carried out to 70 o C for 10,
20, 30, 60 minutes. The resulting CP was washed in distilled water and dried to
constant mass [18].
2.2. Determination of the effect of UV irradiation on PLA
Samples of PLA films 3*1*0.0012 cm were placed in a quartz test tube. The test tube was installed at a distance of 15 cm from the irradiation source, parallel to the axis of incidence of the rays. The films were exposed to UV irradiation for 10, 20, 30 min, both from the front side only and from the front and back sides. Next, the resulting samples were dried to constant weight [18].
2.3. Determination of molecular weight of PLA
The molecular weight of PLA before and after irradiation was determined by the viscometric method at a temperature of 30 ºC. The measurements were carried out using an Ostwald capillary viscometer with a capillary diameter of 0.56 mm. Trichloromethane was used as a solvent [
2].
By measuring the flow time of SP solutions of different concentrations, the relative
and specific
viscosity was calculated using the formulas:
where τ is the flow time of the copolymer solution, s ;
τ 0 – solvent flow time, s .
The calculation results were summarized in tables, and the data were presented in the form of a graphical dependence
on c and
c. By extrapolating straight lines to zero concentration, the value of intrinsic viscosity [η] was determined. The viscosity-average molecular weight was calculated using the Mark-Kuhn-Houwink equation:
where K and a are constants for a given polymer-solvent system at a certain temperature;
M – viscosity-average molecular weight, Da [
3].
For this system K =1.31·10
-4 dl /g, α=0.777 [
5].
2.4. Gravimetry and SCEC determination
The resulting CP was weighed and the weight gain ∆
m was determined according to the formula:
where
is the weight gain of the film,
- mass of the base film, g ,
- mass of grafted film, g .
Next, the weighed films were placed in a conical flask, filled with 40 ml of 0.1 N NaOH solution , closed with a ground stopper and stirred periodically. The films were kept in this solution for 30 minutes. Sampling over the film (V = 10 ml) was carried out three times and titrated with a 0.1 N solution of HC l . Phenolphthalein was used as an indicator [
2]. Calculation formulas:
where
and
are the normality of the initial sodium hydroxide solution (0.098) and the normality of the solution after interaction with the cation exchanger, respectively, mEq / cm
3 ,
and
- the volume of hydrochloric acid used for titration of 10 cm
3 of sodium hydroxide, before and after interaction with the cation exchanger, respectively, ml.
where SCEC is the statistical volumetric capacity, mEq / g,
V – volume of sodium hydroxide solution in a flask with film, ml,
– mass of the modified film, g .
2.5. FTIR spectroscopy
FTIR spectra of the resulting films were recorded on an AVATAR 330 spectrometer ( Thermo Nicolet ) in the region of wave numbers 4000-400 cm -1 with an accuracy of 2 cm -1 . The spectra were recorded using the diffuse reflection method using the Smart attachment Diffuse Reflectance . The spectra were analyzed quantitatively using OMNIC software . Peak separation was performed using the NETZSCH – TA 4 ( PeakSep 2) program using a linear baseline and peak shapes described by the Gaussian or Fraser- Suzuki equations [16].
3. Experimental results and discussion
3.1. Determination of the impact of UV irradiation on PLA
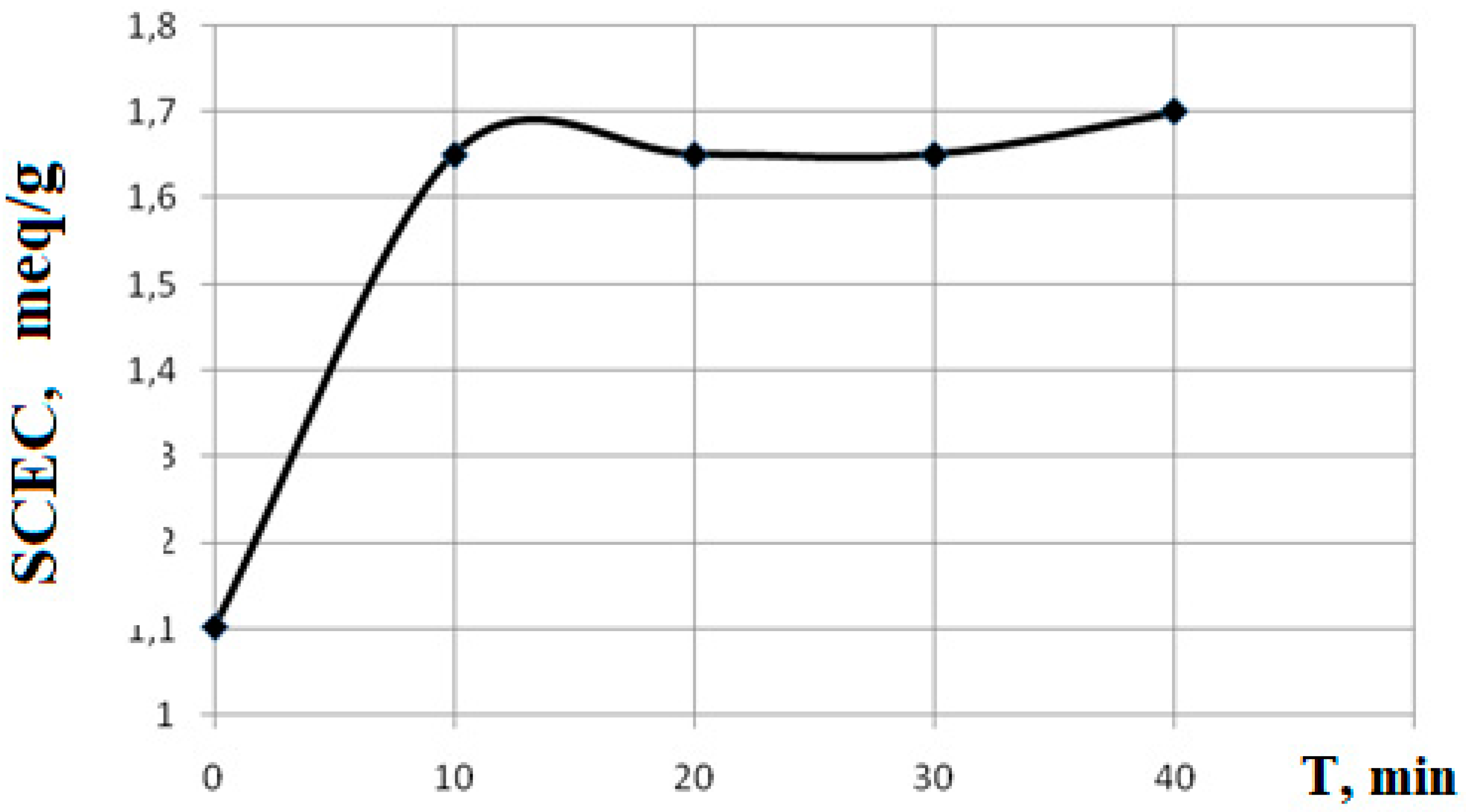
To establish the effect of UV irradiation on the PLA matrix, a series of experiments were carried out on irradiation of films in the absence of other reagents. The action of UV radiation is one of the reasons for the destruction of PLA, which can result in the formation of carboxyl groups. To evaluate the contribution of emerging carboxyl groups to the SCEC value, a number of experiments were carried out with different exposure times to UV irradiation.
Based on experimental data obtained by the acid-base titration method (
Section 2.4), a graph of the dependence of SCEC on irradiation time was constructed.
From the data in
Figure 1 it is clear that the dependence of the SCEC value on the irradiation time is not linear, but has the form of a curve with saturation (reaching a plateau) at 10 minutes. Despite the fact that UV irradiation leads to the destruction of PLA and the formation of carboxyl groups, there are no significant changes in the SCEC value, and there is no change in the mass of the sample.
The viscosity-average molecular weight of polylactide was determined by capillary viscometry (section 2.3). For the unirradiated sample, the molecular weight was 1.4·10 5 Da , for the irradiated sample for 10 minutes - 10.5·10 4 Da , for the irradiated sample for 40 minutes - 8.5·10 4 Da. Thus, when polylactide is exposed to UV radiation, acid groups are formed through destruction, but this process is not highly effective and is accompanied by a decrease in the molecular weight of polylactide, which, with prolonged exposure, can lead to its physical destruction. A solution to the problem of introducing carboxyl groups can be graft polymerization of acrylic or methacrylic acids on a polyester film. The irradiation time should not exceed 10 minutes.
3.2. Synthesis of CP PLA
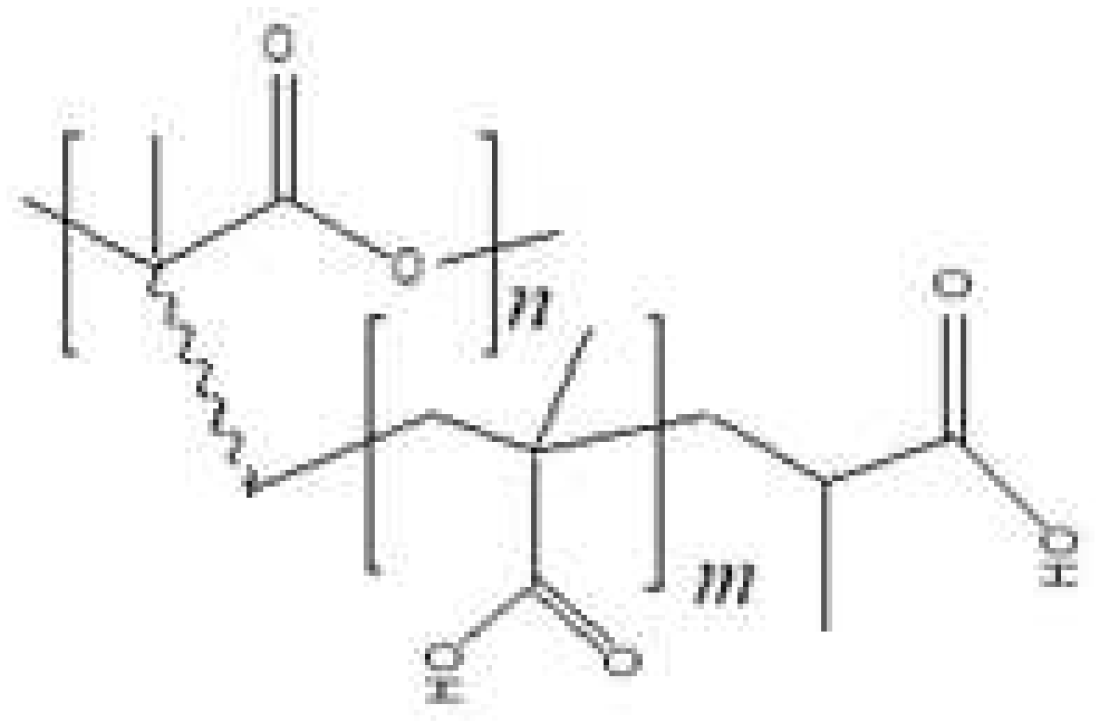
CP PLA and MAA were synthesized using graft polymerization :
Exposure to ultraviolet and other types of radiation on the polylactide matrix in air leads, among other things, to the formation of peroxide groups on the surface of the irradiated material. Under the influence of temperature in the presence of Mohr salt, radicals formed on the surface of the polylactide matrix, by which the grafting polymerization of methacrylic acid took place. As a result, samples of grafted copolymer of polylactide with polymethacrylic acid with varying degrees of grafting were obtained
Figure 2. The samples were characterized by a change in the mass of the dried samples, as well as a difference in properties. During graft polymerization, the samples retained their shape; however, after a significant period of exposure to ultraviolet radiation, the decrease in molecular weight occurred. The change in molecular weight characteristics was accompanied by a deterioration in mechanical strength properties: the films became more rigid and brittle. This fact must be taken into account when choosing the conditions for modifying polylactide products, as well as when determining the conditions of their operation.
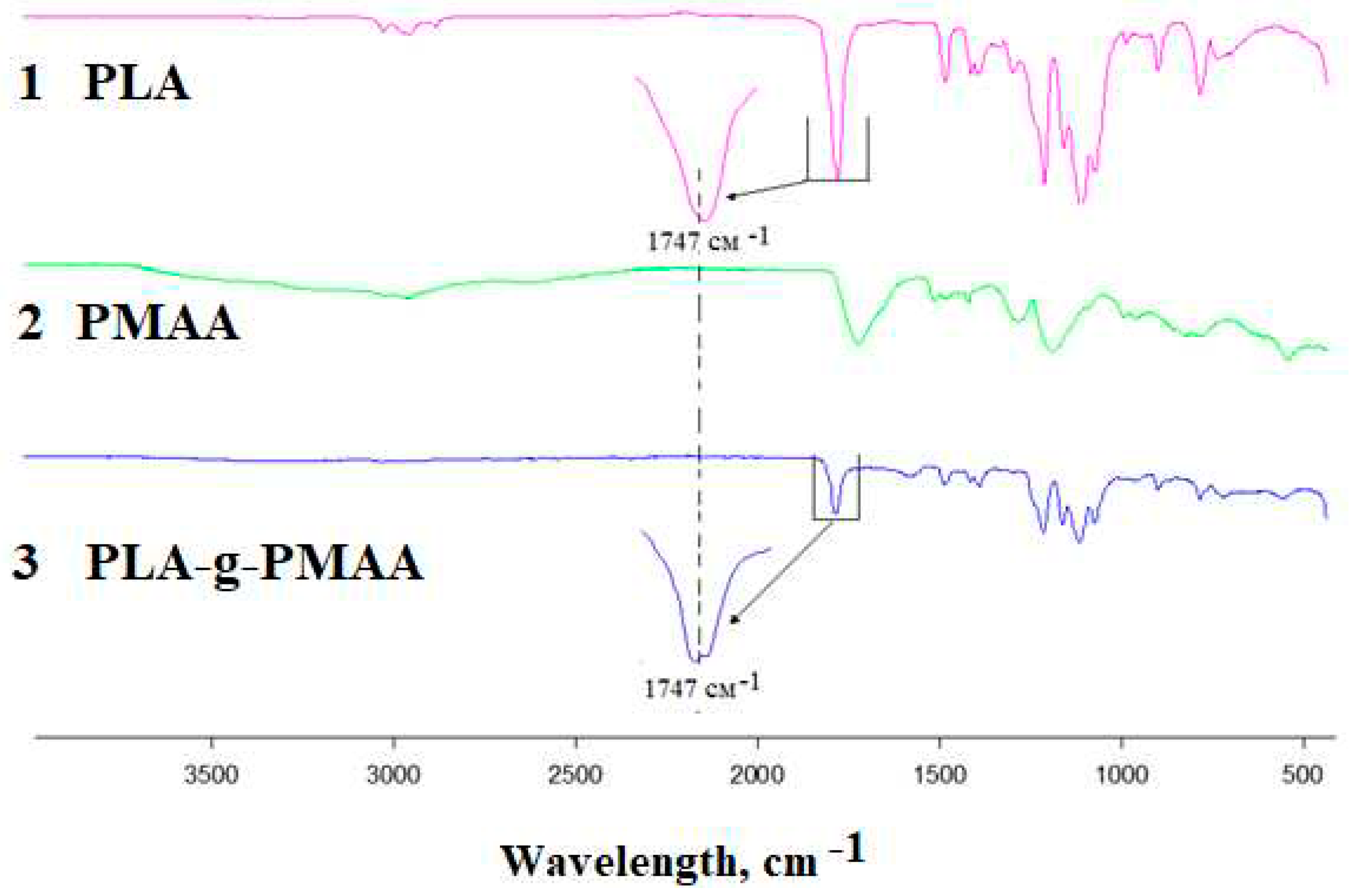
3.3. Study of polymers using FTIR spectroscopy
Study of images of polylactide
Figure 3 polymethacrylic acids and their copolymers using Fourier transform infrared spectroscopy demonstrated the presence of characteristic peaks in the case of polylactide [10,11] at wavelengths 3300-3700, 1700-1760 and 500-1500 cm
-1. as well as the vibrations of the hydroxyl group characteristic of polyacrylic acid - 3550-3500 cm
-1 for free and 3330-2500 cm
-1 for hydrogen-bonded and carboxyl - 1700-1680 cm-1.
The spectra of graft copolymers are generally similar to those for polylactide ; however, instead of a single peak corresponding to the stretching vibrations of the ester group at 1740 cm-1, there is an overlap of two peaks of the ester and carboxylic acid groups , confirming the formation of a graft copolymer.
3.4. Determination of SCEC and weight gain for the resulting CP
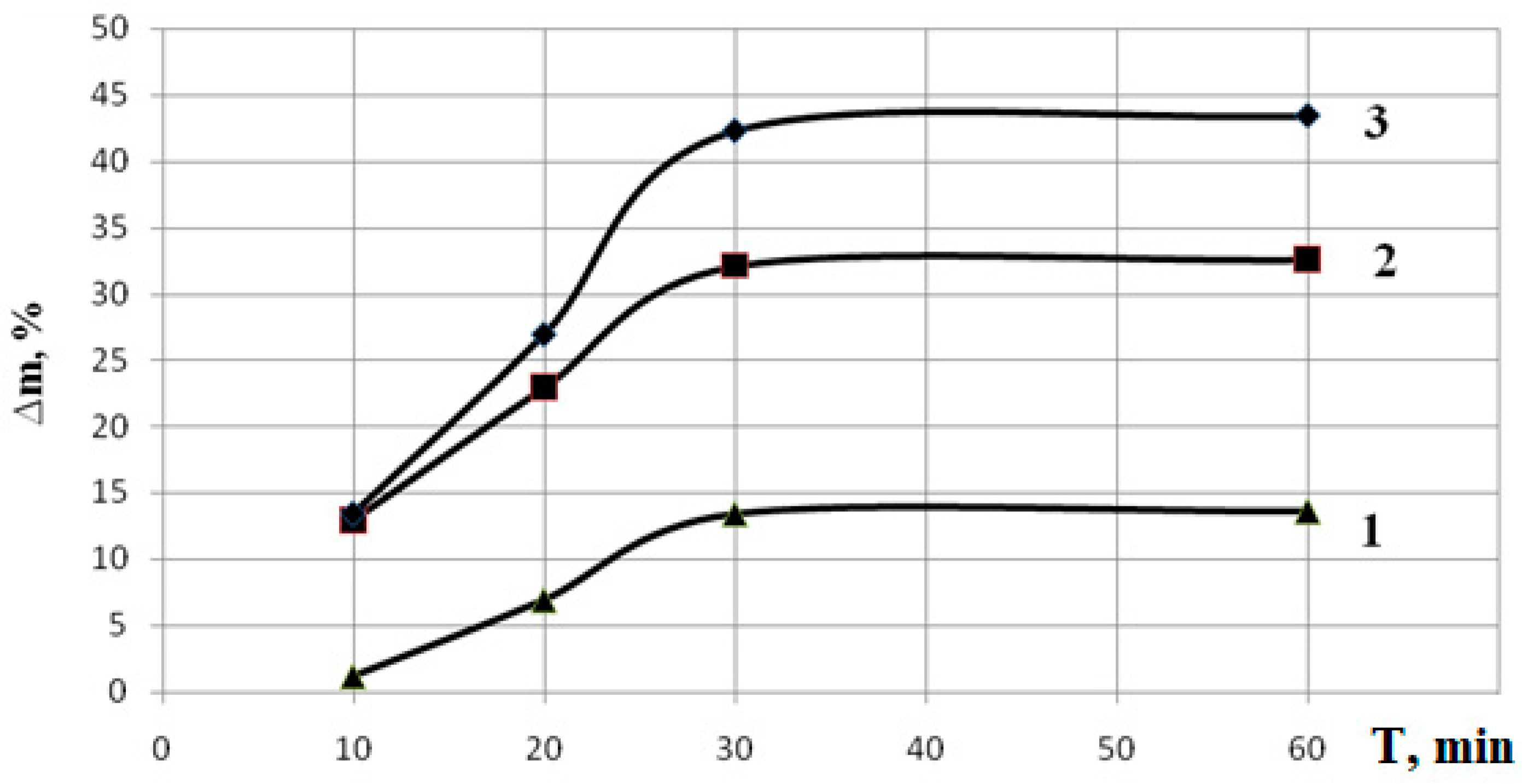
Using the gravimetric method, an increase in the mass of the films was detected and the weight gain was calculated using formula (2.4).
The acid-base titration method (section 2.4) was used to determine the SCEC. Based on the data obtained, graphs of the dependence of SCEC and weight gain ∆ m on the time of exposure to polymerization were plotted .
Figure 4 shows the dependence of the change in mass of CP PLA and MAA on the polymerization time. Treatment of copolymer samples for 30 minutes increases the mass by 10–30%; further increase does not lead to any significant changes in mass.
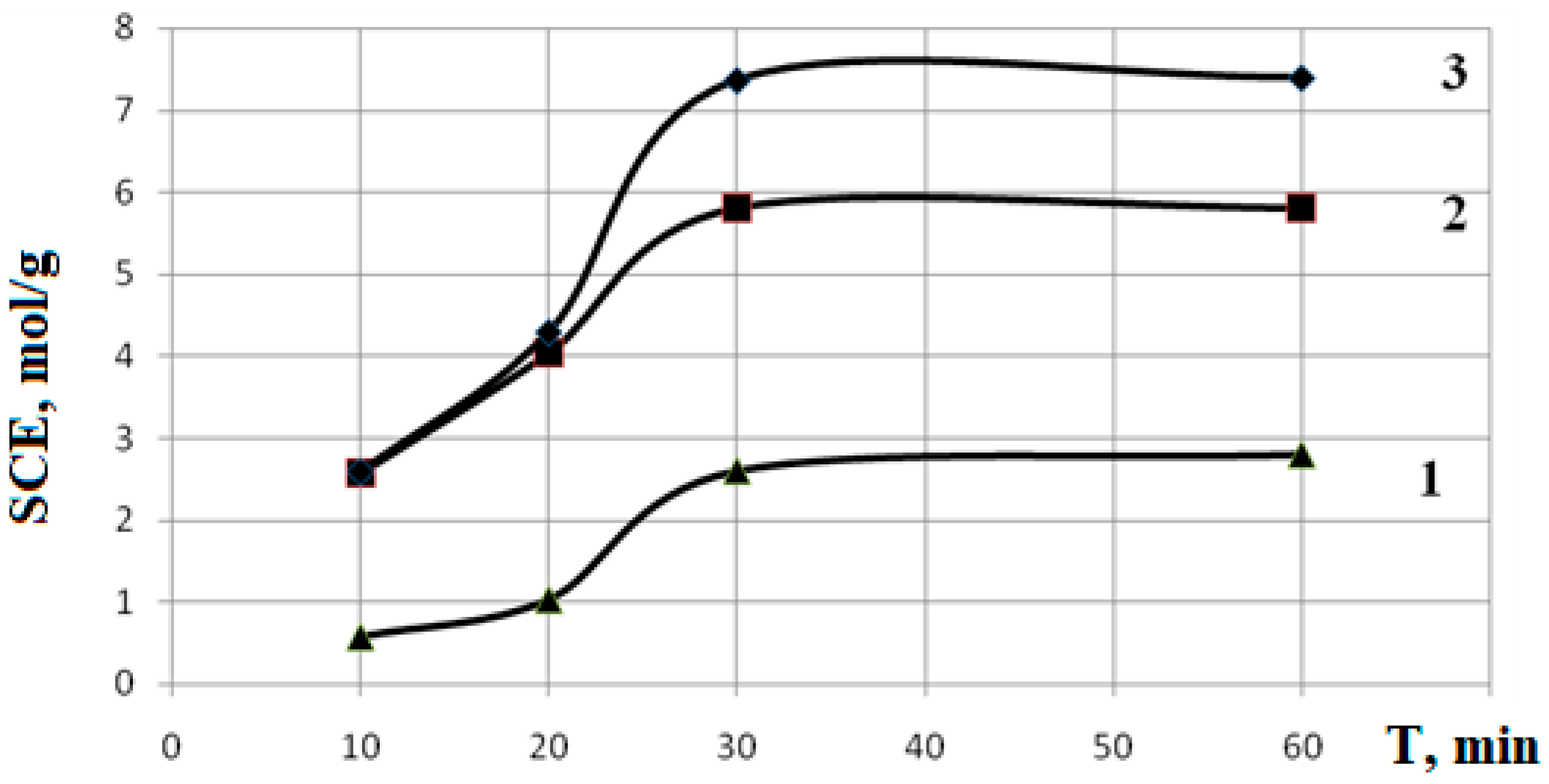
From the data presented in
Figure 5 it is clear that polymerization of samples containing up to 20% MAA for 30 minutes leads to a change in the SCEC value by 2–3 times. Further increase in processing time to 60 minutes does not lead to changes in SCEC.
4. Conclusions
AS result of the work, copolymers of biodegradable polylactide with polymethacrylic acid was synthesized. The influence of copolymerization conditions on the physicochemical properties of the resulting copolymers was determined. The influence of ultraviolet irradiation time and monomer concentration on the properties of the resulting copolymer has been established. Thereby, treatment of the copolymer and methacrylic acid with ultraviolet radiation for up to 30 minutes increases the mass by 10–30%. The change in film mass indicates that increasing the irradiation time increases the amount of reacted (grafted) acid. For copolymers of polylactide and polymethacrylic acid, an increase in the static exchange capacity to 7.5 mol/ g is observed upon irradiation for more than 30 minutes.
References
- Garlotta D. A literature review of poly( lactic acid) / D. Garlotta // Journal of Polymers and the Environment. – 2001. – Vol. 9, No. 2. – P. 63–84. [CrossRef]
- Belov D.A. Biodegradable composite materials for medical purposes based on D , L - polylactide / D.A. Belov, A.N. Bychkova, I.A. Klimovtsova // Materials, technologies, tools. – 2006. – T. 1, No. 1. – P. 71–74. [CrossRef]
- Duda A. Polilaktyd [ poli ( kwas mlekowy )]: synteza , wlasciwosci i zastosowania / A. Duda , S. Penczek // Polimery . – 2003. – T. 48, No. 1. – S. 16–27.
- Poly-Lactic Acid: Production, Applications, Nanocomposites , and Release Studies / M. Jamshidian [et al.] // Comprehensive Reviews in Food Science and Food Safety. – 2010. - Vol. 9. – P. 552-571. [CrossRef]
- Drumright R.E Polylactic Acid Technology / RE Drumright, PR Gruber, DE Henton // Adv. Mater. – 2000. – V. 12, No. 23. – P. 1841-1846. [CrossRef]
- Mehta R. Synthesis of Poly( Lactic Acid): A Review / R. Mehta [et al.] // J. Macromol . Sci. Polymer Rev. – 2005. – V. 45. No. 4. – P. 325-349. [CrossRef]
- Krul L.P. Nanocomposites based on poly-D,L- lactide and multiwall carbon nanotubes / LP Krul , AI Volozhyn , DA Belov , NA Poloiko , AS Artushkevich , SA Zhdanok , AP Solntsev , AV Krauklis , IA Zhukova // Europian Materials Research Society (E- MRS), Spring Meeting. Strasbourg, 2005. France, May 31 - June 3, 2005. Meeting Papers; – Strasbourg, 2005. – H. 2/7.
- Choochottiros S. Synthesis and characterization of polylactide –poly(methyl methacrylate) copolymer by combining of ROP and AGET ATRP / C. Choochottiros , E. Park, I. Chin // Journal of Industrial and Engineering Chemistry. – 2012. – No. 18. – P. 993–1000. [CrossRef]
- Jantas R. Modification of a Polylactide Fiber Surface / R. Jantas [et al.] // Fibers & Textiles in Eastern Europe. – 2010. – Vol. 18. No. 4. – P. 87-91.
- Połowiński S. Modification of PLA Fibers with Bioacceptable Hydrophilic Polymers / S. Połowiński [et al.] // Fibers & Textiles in Eastern Europe. – 2012. – Vol. 20. No. 1. – P. 78-85.
- Janorkar A.V. Modification of Poly(lactic acid) Films: Enhanced Wettability from Surface-Confined Photografting and Increased Degradation Rate Due to an Artifact of the Photografting Process / AV Janorkar , AT Metters , DE Hirt // Macromolecules. – 2004. – No. 37. – P. 9151-9159. [CrossRef]
- Eguiburu J.L Blends of amorphous and crystalline polylactides with poly( methyl methacrylate) and poly(methyl acrylate): a miscibility study / JL Eguiburu [et al.] // Polymer. – 1998. – Vol. 39. No. 26 – P. 6891–6897. [CrossRef]
- Avella M. Radical polymerization of methyl methacrylate in the presence of biodegradable poly( L-lactic acid). Preparation of blends, chemical-physical characterization and morphology / M. Avella [et al.] // Macromol . Chem. Phys. – 2000. - No. 201. – P. 1295–1302. [CrossRef]
- Zhang G. Ultrasonic weld properties of heterogeneous polymers: Polylactide and poly (methyl methacrylate) / G. Zhang [et al.] // Journal of Materials Processing Technology. – 2011. - No. 211. – P. 1358-1363. [CrossRef]
- Dorgan J. R. Fundamental solution and single-chain properties of polylactides / J. R. Dorgan, J. Janzen, D. Knauss // J. polym . Sci. Part B: Polym . Physics – 2005. – Vol. 43, No. 21. – P. 3100-3111. [CrossRef]
- Temperature-Variable FTIR and Solid-State 13 C NMR Investigations on Crystalline Structure and Molecular Dynamics of Polymorphic Poly(L- lactide ) and Poly(L- lactide )/Poly(D- lactide ) Stereocomplex / Pengju Pan [et al.]. Macromolecules 2012. – Vol. 45. – P. 189−197. [CrossRef]
- Improving Polylactide /Starch Biocomposites by Grafting Polylactide with Acrylic Acid –Characterization and Biodegradability Assessment.Chin -San Wu Macromol . Biosci . 2005, 5, 352–361.
- Pikaev A.K. Modern radiation chemistry. Solids and polymers. Applied aspects / A.K. Pikaev, V.I. Spitsin. – M.: Nauka, 1987.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).