1. Introduction
The beginning of the study of paraneoplastic syndromes in humans dates back to the end of the 19
th century [
1,
2,
3], but for domestic animals – only since the beginning of the 21
th century [
4,
5,
6]. During the period of 150 years, various scientists described systemic manifestations of malignant neoplasms and assumed their secondary occurrence against the background of the underlying cancer for humans. Professor Armand Trousseau described paraneoplastic syndrome in the form of migratory thrombophlebitis in humans with cancer of the stomach, uterus or ovary for the first time. The connections between the peripheral nervous system and cancer (of the stomach, pancreas or uterus) was first described by the French scientist, Professor M. Auche [
7]. Paraneoplastic syndromes with damage to the central nervous system were described by the German neurologists [
8,
9]. The term “paraneoplastic syndrome” itself was introduced into clinical practice in order to describe neurological complications in the presence of neoplasia in the patient’s body, but in the absence of direct tumor invasion into the nervous system, as well as during antitumor therapy [
8,
9]. Guichard M. and Vignon G. used the term “paraneoplastic” when describing the differential diagnosis in patients with numerous cranial and radicular neuropathies against the background of metastasis of a uterine tumor, since at autopsy no tumor cells were found in the spinal cord and nerve roots, the authors proposed using the term "paraneoplastic" instead of "neoplastic". At this stage in history, any symptom of unknown etiology accompanying the presence of a tumor in the patient's body was considered paraneoplastic.
The concept of an immune response in neoplasms was proposed by L. Thomas and F. Burnet [
2,
3]. It was based on the assumption of the influence of the body's immune mechanisms on tumor cells and the formation of antitumor antibodies, which in turn lead to damage to healthy tissues of the body [
2,
3].
As an independent “nosological unit”, paraneoplastic syndrome was described by Posner J.B. in 1995 [
10]; the term was used “to define heterogeneous diseases that arise as a result of damage to various parts of the nervous system against the background of a neoplastic process” [
10].
One of the most important stages in the study of carcinogenesis was the proof of the expression of antigens foreign to the body by tumor cells. In the second half of the twentieth century, Soviet immunologist Zil’ber L.A. conducted a number of experiments confirming this hypothesis [
11]. He used the technique of sensitizing experimental animals with tumor tissue in order to obtain a humoral immune response, and then carried out desensitization with normal tissue of the same organ. During repeated administration of the tumor homogenate, the experimental animal responded with anaphylactic shock if specific antigens were present in the tumor. As a result of these studies, it was concluded that there is an antigenic difference between tumor and normal tissue, which subsequently served as the reason for the development of antiserum to detect specific tumor antigens in humans.
Over the years, scientists around the world have described various forms of paraneoplastic syndromes, and created many classifications based on pathogenetic, organ-system principles. At the moment, the recognized and most common classification is based on leading clinical and laboratory signs. In accordance with this classification, paraneoplastic syndromes are divided into endocrinopathies, hematological paraneoplastic syndromes, gastrointestinal, renal, dermatological, neurological, ophthalmological.
According to literary sources [
5,
12,
13,
14,
15,
16,
17,
18], the difficulty of solving this problem is determined by the severity of the disease, the multifactorial nature of its pathogenetic mechanisms and the lack of a scientifically based treatment system that takes into account the clinical forms of ophthalmopathies and the severity of the pathological process in various small domestic animals.
The purpose of this study was to develop a scientifically based algorithm for diagnosing ophthalmopathies against the background of paraneoplastic syndrome in dogs, including a multifactorial system of “hormonal-drug treatment” of paraneoplastic ophthalmopathies.
2. Materials and Methods
The studies were carried out on the basis of the laboratory "Ophthalmology, Oncology and Biochemistry of Animals" (“ROSBIOTECH”, Moscow, Talalikhina st., 33). The study of the influence of exogenous and endogenous factors on the development of a malignant neoplasm is based on the data of our own studies from the total number of animal visits (n=192), individuals with different terms of oncological diseases (n=67) were isolated. The work used a comprehensive methodological approach, including the collection of anamnestic data, clinical examination of the animal, examination of the area of the pathological process, hematological studies, cytomorphological studies, visual diagnostic methods with complete oncological and ophthalmological examinations using the necessary equipment, MRI and CT studies, hematological parameters blood, as well as morphological conclusions, on the basis of which the final diagnosis of the underlying disease was made.
Autopsy and intravital material obtained from animals was subjected to standard histological processing. The eyeballs and organ fragment were immediately placed in labeled sealed containers containing 10% neutral buffered formalin after extraction. All tissues were fixed for at least 7 days with one replacement of the fixing solution. The ratio of the volume of material to formalin was at least 1:10. After fixation, macroscopic examination was performed. Subsequently, after fixation, the pathological material was cut out, during which a macroscopic examination of the eyeballs was performed. At the same time, for each animal, one or two sections of each eyeball (right and left) were obtained, in total 2-4 sections, 4 sections of the organ tissue. After histological examination, the tissues were embedded in paraffin, microtomy was performed to obtain sections with a thickness of 5 μm, and stained with hematoxylin and eosin according to the manufacturer's protocols. The studies were based on case histories of animals with the most common cancers in cats. During the monitoring, the proportion of oncologically ill animals with complaints of visual dysfunction in history from the total number of cancer patients was determined. Anamnestic data were analyzed according to the following criteria: breed, age, sex, living conditions, the presence of bad habits of the owners, the presence of oncological diseases in the hereditary line, data on chronic diseases, long-term use of drugs (immunosuppressors, hormone replacement therapy).
3. Results
Our preliminary clinical and immunological studies allowed us to formulate the basic principles of pathogenetic treatment, which is aimed at suppressing autoimmune reactions. Treatment for cancer included surgical removal of the tumor, chemotherapy and radiotherapy. The choice of treatment protocol depended on the location of the disease, tumor phenotype (if present) and stage of the malignancy (
Table 1). The only chemotherapy was applied using complex protocols in the treatment of dog lymphomas (
Table 1). For mastocytomas and sarcomas in dogs, a multimodal approach was applied using surgery, chemotherapy, and radiotherapy. Treatment of melanoma in dogs was carried out using an integrated approach which combined methods (
Table 1).
Based on a retrospective analysis of the results of treatment of sick animals with various clinical forms of ophthalmopathies, systems for the treatment of eye pathology have been developed, including the use of medications:
- a)
mydriatics,
- b)
anti-inflammatory drugs (corticosteroid and non-steroidal),
- d)
fibrinolytics,
- e)
antihistamines,
- e)
neurotropic drugs,
- g)
vascular strengthening.
It is recommended to change the diet, keeping the animal, exercise, as well as organizing a semi-darkened area for the sick animal.
The intensity of the treatment directly correlated with the severity of the clinical signs of uveitis, taking into account the degree of turbidity of the intraocular fluid, hyperemia of the iris, changes in intraocular pressure, the presence and severity of precipitates, pericorneal injection of the vessels of the eyeball, the presence of posterior synechiae, hypopyon, fibrinous or fibrinous-hemorrhagic exudate, its amount in relation to the volume of the anterior chamber of the eye.
Complex drug treatment for acute and chronic disease was provided according to two schemes:
– Scheme 1 (acute course of paraneoplastic syndrome) - local treatment with steroidal and non-steroidal anti-inflammatory drugs, general anti-inflammatory, desensitizing treatment using anti-inflammatory and antihistamines only for severe ophthalmopathy.
– Scheme 2 (chronic course of paraneoplastic syndrome) - local and general long-term treatment using steroidal and non-steroidal anti-inflammatory drugs, cytostatics, and the use of subconjunctival injections.
During the treatment, hematological blood test parameters, the immune status of sick animals, as well as changes in the condition of the eye were monitored: manifestations of clinical signs of anterior and posterior uveitis, fundus images were recorded, intraocular pressure was measured, and changes in the severity and nature of exudate were monitored in its initial presence.
The main criteria for prescribing treatment regimens were the clinical characteristics and severity of endogenous anterior and posterior uveitis; subsequent study of hematological values made it possible to adjust the treatment taking into account these indicators. The goal of using mydriatic cycloplegics is to achieve moderate mydriasis. For pupil dilation and cycloplegia, M-anticholinergics were used in the form of a 0.1% solution of atropine sulfate, Midriacil (Tropicamide) in local instillations. Relaxation of the ciliary muscle (cycloplegia) and the iris sphincter reduces ocular pain. Mydriasis reduces the risk of posterior synechiae formation as a result of a reduction in the contact area between the iris and the lens, but may contribute to an increase in intraocular pressure.
The use of glucocorticosteroids is due to their anti-inflammatory and immunosuppressive effects; they inhibit immunological reactions by suppressing proliferative processes, reducing increased vascular permeability. In case of damage to the corneal epithelium, local instillations and subconjunctival injections were not prescribed. The frequency of topical use of anti-inflammatory drugs depended on the degree of the inflammatory process, with the maximum frequency of use being 4–6 times a day in combination with other drugs. When using glucocorticosteroids, and in case of paraneoplastic ophthalmopathies it is quite long-term, it is necessary to remember the possibility of side effects associated with their use - the development of cataracts and glaucoma. In severe anterior uveitis, characterized by a pronounced inflammatory process, as well as in posterior uveitis, glucocorticosteroids were prescribed systemically in immunosuppressive doses (Prednisolone 1-2 mg/kg, 2 times per day).
Nonsteroidal anti-inflammatory drugs were prescribed by local instillation (1–4 times per day), depending on the severity of clinical signs of uveitis. The hormonal-drug treatment modes are presented in the
Table 2.
From the data in the
Table 2 it follows that for the local treatment of paraneoplastic ophthalmopathies the following modes were used:
1) Atropine sulfate 0.1%, Mydriacyl (Tropicamide), 1–2 times a day, the frequency and duration of use of the drugs depended on the condition of the iris, and their use also requires regular monitoring of intraocular pressure, after achieving moderate mydriasis, the frequency of use decreased,
2) Dexamethasone eye drops 0.1% solution, from 1-2 to 4-6 times a day,
3) Indomethacin (Indocollir), Nepafenac (Nevanac), eye drops 1–2 to 3–4 times a day,
4) subconjunctival injections were performed once a day once or daily for 3–5 days; components of the mixture for subconjunctival injections: Novocaine 0.5% - 0.5-1.0 ml, Dexamethasone 4 mg/ml - 0.2-0.4 ml (possibly adding, Atropine sulfate 0.1% - 0.05 ml). The solution was injected subconjunctivally into both the lower and upper eyelid vault.
4. Conclusions
In 2022–2023, several groups of experimental animals (dogs) were formed; measurements of more than 10 parameters were carried out, including physiological, hematological and morphological indicators of more than 50 samples (samples), i.e., about 500 measurements were taken in total.
A clinical and ophthalmic criteria are given (
Table 1 and
Table 2,
Appendix A) for the development of a method for the treatment of primary and secondary ophthalmopathies in dogs. The obtained results can be recommended for use in the diagnosis and treatment of paraneoplastic ophthalmopathies in dogs.
Funding
The work was carried out within the framework of the topic: “Etiopathogenesis and development of methods for diagnosing, preventing and treating immune-mediated paraneoplastic ophthalmopathies in animals” (project code FSMF-2022-0003 of the Ministry of Higher Education and Science of the Russian Federation) of the Research Laboratory of Ophthalmology, Oncology and Biochemistry of Animals, Federal State Budgetary Educational Institution of Higher Education “Russian Biotechnological University (ROSBIOTECH)”. (Moscow, Russia).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, internationally recognized guidelines (concerning experiments with animals), and approved by the Institutional Review Board of the Institute of Veterinary Medicine, Veterinary Sanitary Expertise and Agrosafety of the Federal State Budgetary Educational Institution of Higher Education “Russian Biotechnological University (ROSBIOTECH)” (protocol code 11_2022, Moscow, 125080, Russia and date of approval: 7 November 2022).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A. Research Results of the Magnetic Resonance Imaging (MRI)
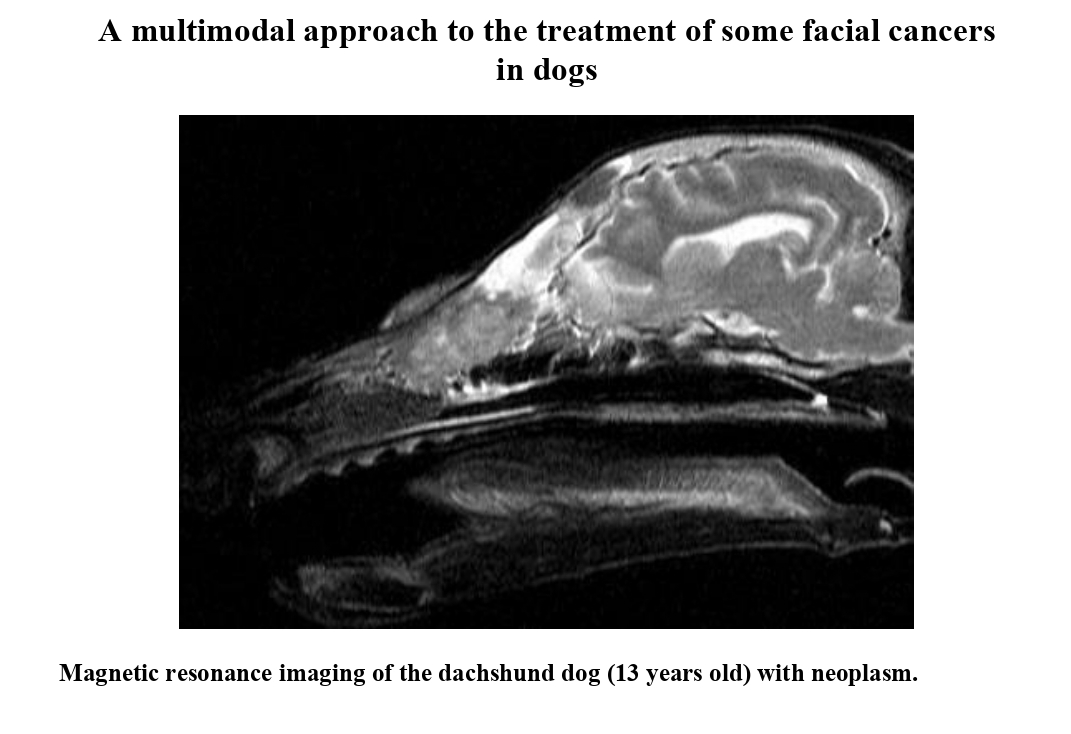
Figure A1.
Dachshund dog, 13 years old, 9 kg. T2-VI mode sagittal plane. Heterogeneous hyperintense signal from the nasal cavity, areas of the ethmoid bone and frontal sinus – neoplasm.
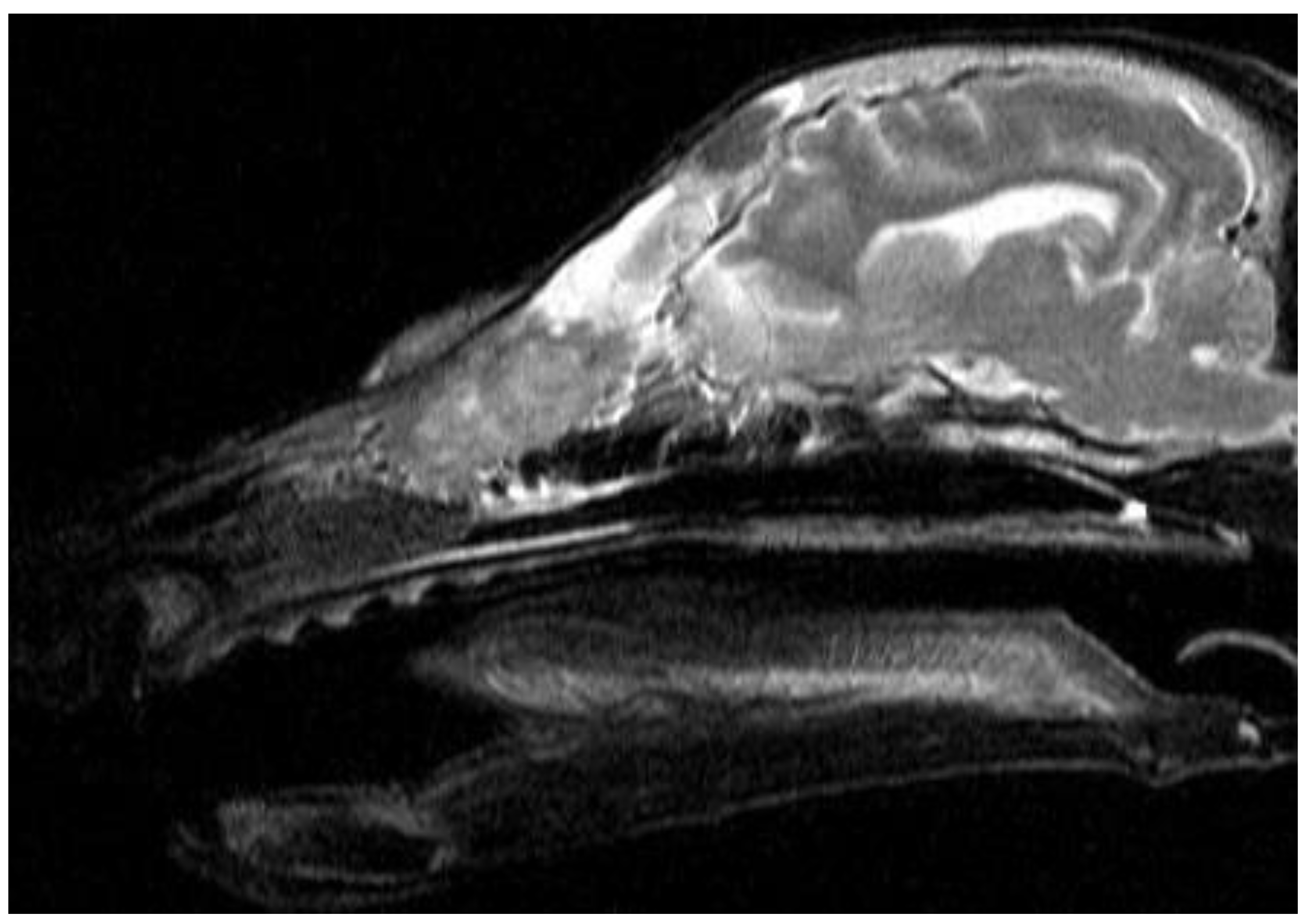
Figure A1.
Dachshund dog, 13 years old, 9 kg. T2-VI mode sagittal plane. Heterogeneous hyperintense signal from the nasal cavity, areas of the ethmoid bone and frontal sinus – neoplasm.
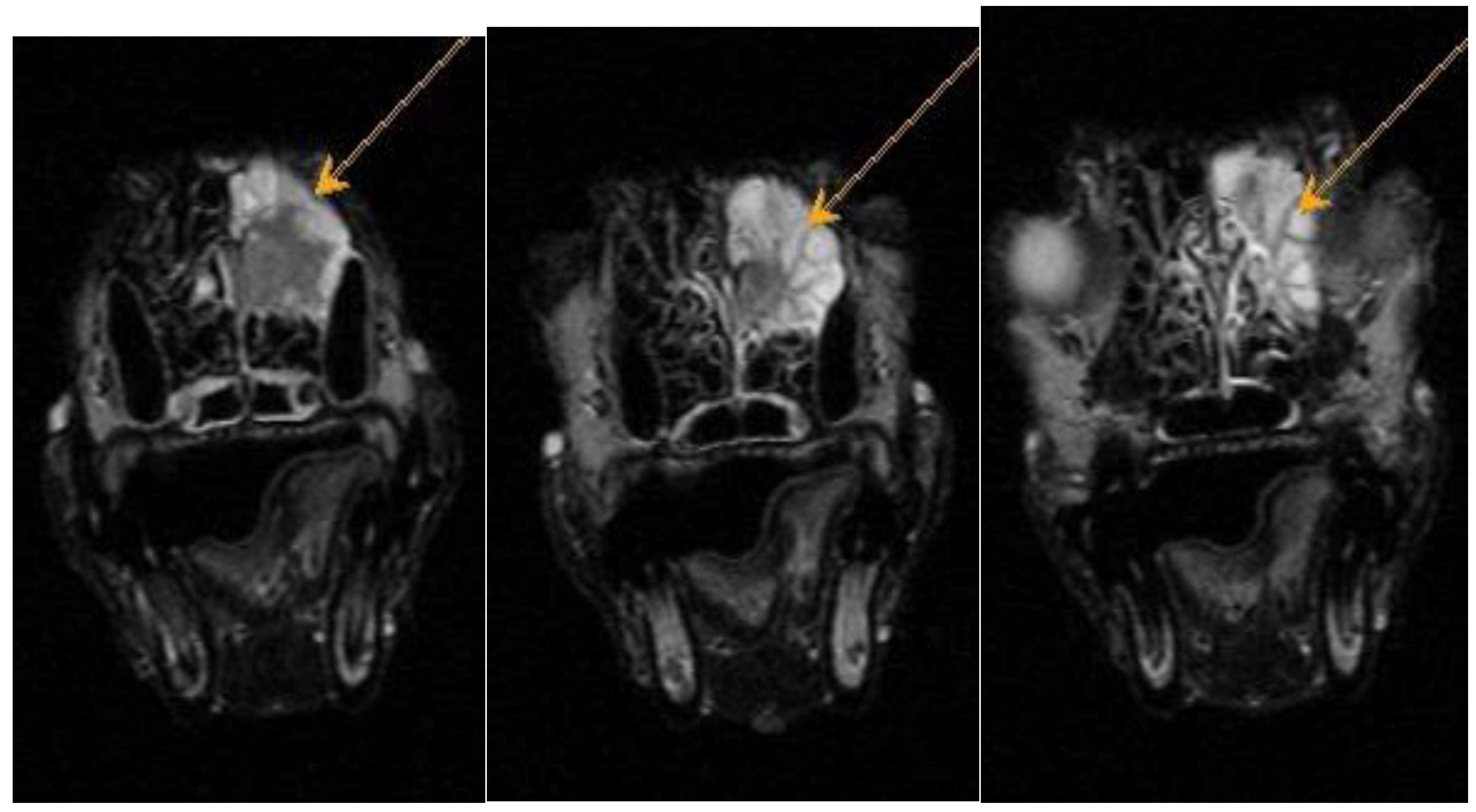
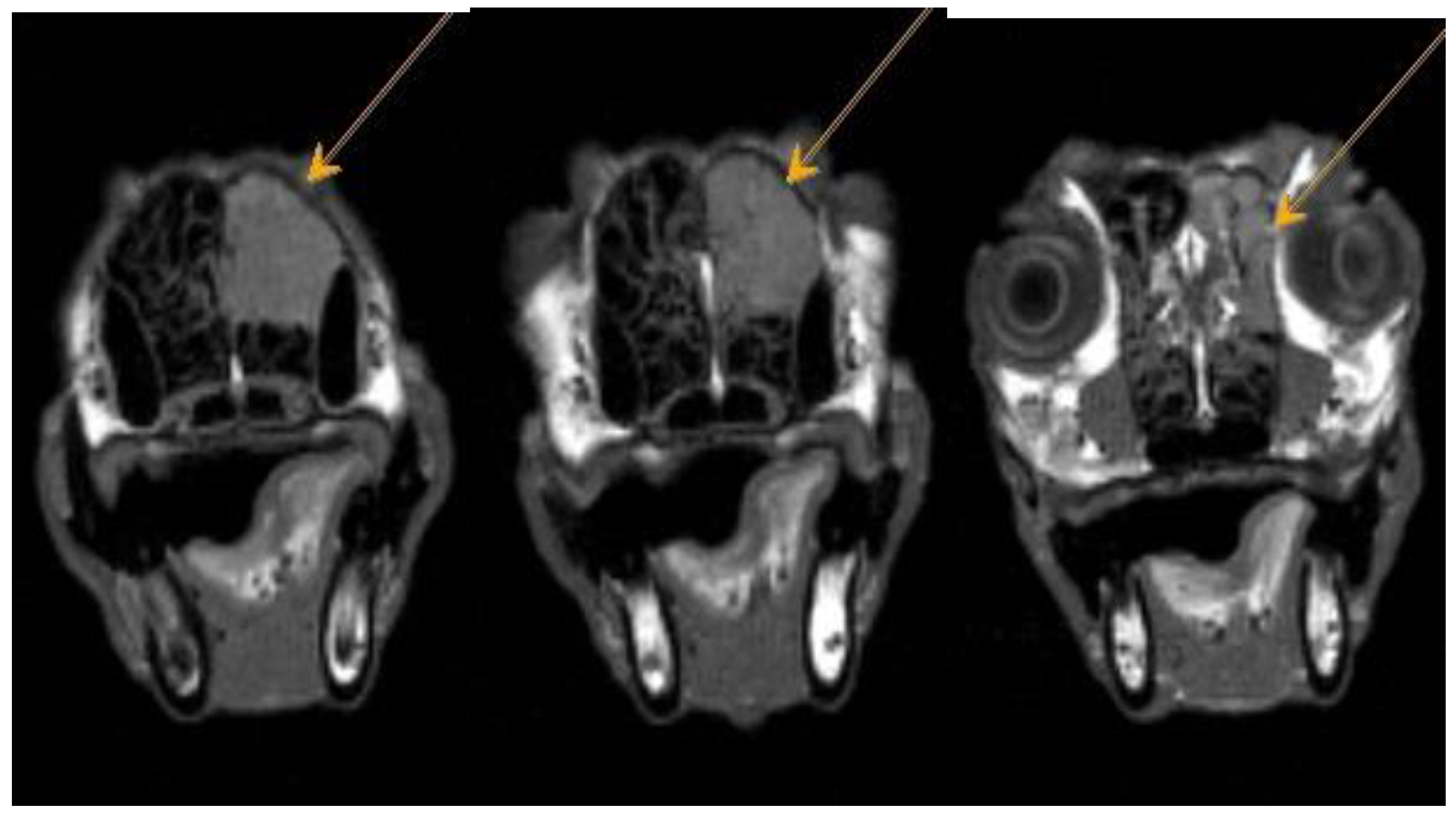
Figure A2.
Dachshund dog, 13 years old, 9 kg. T2-VI mode axial plane. Heterogeneous hyperintense signal from the area of the ethmoid bone – neoplasm.
Figure A2.
Dachshund dog, 13 years old, 9 kg. T2-VI mode axial plane. Heterogeneous hyperintense signal from the area of the ethmoid bone – neoplasm.
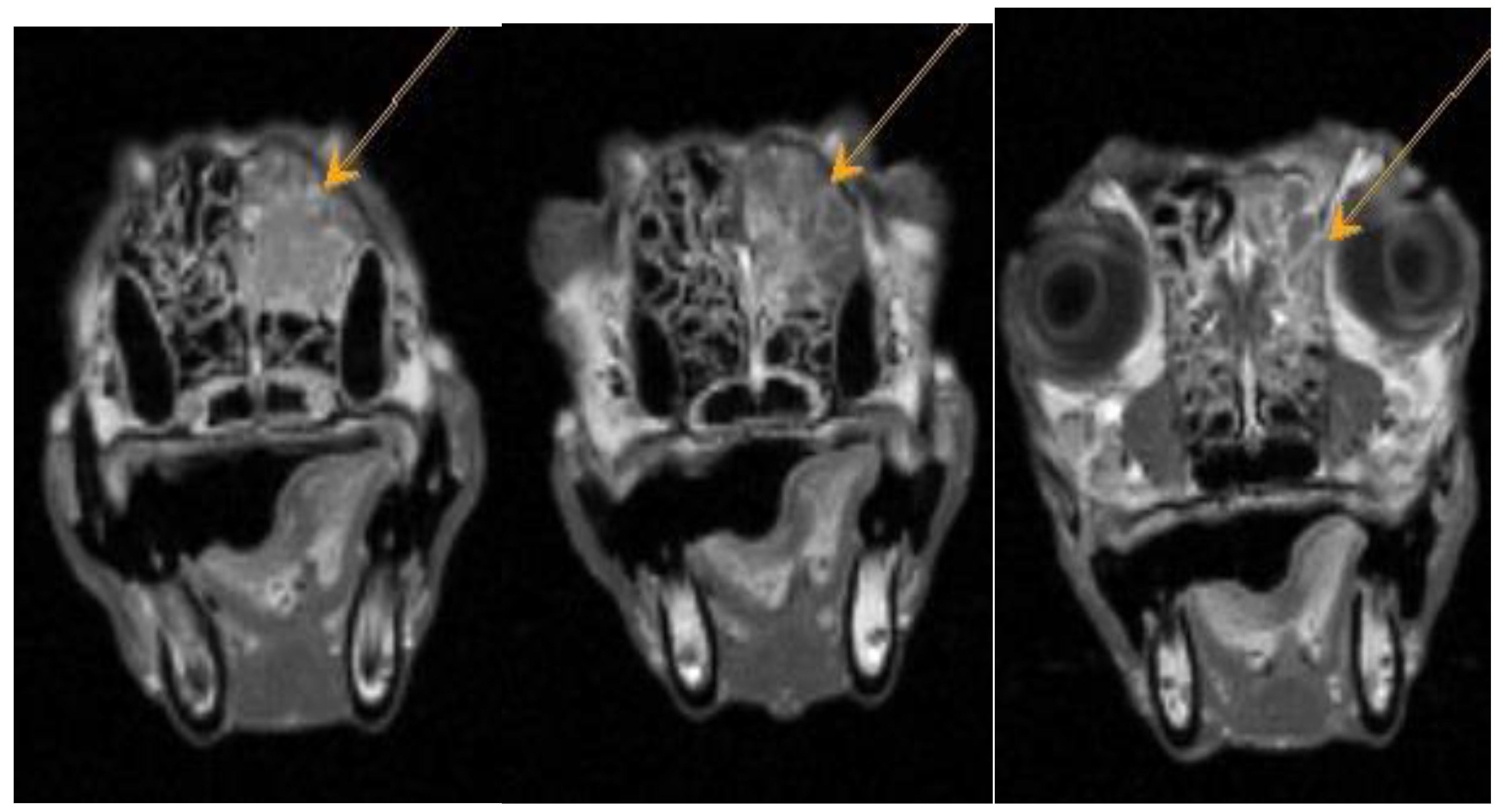
Figure A3.
Dachshund dog, 13 years old, 9 kg. FLAIR mode axial plane. Heterogeneous hyperintense signal from the area of the ethmoid bone – neoplasm.
Figure A3.
Dachshund dog, 13 years old, 9 kg. FLAIR mode axial plane. Heterogeneous hyperintense signal from the area of the ethmoid bone – neoplasm.
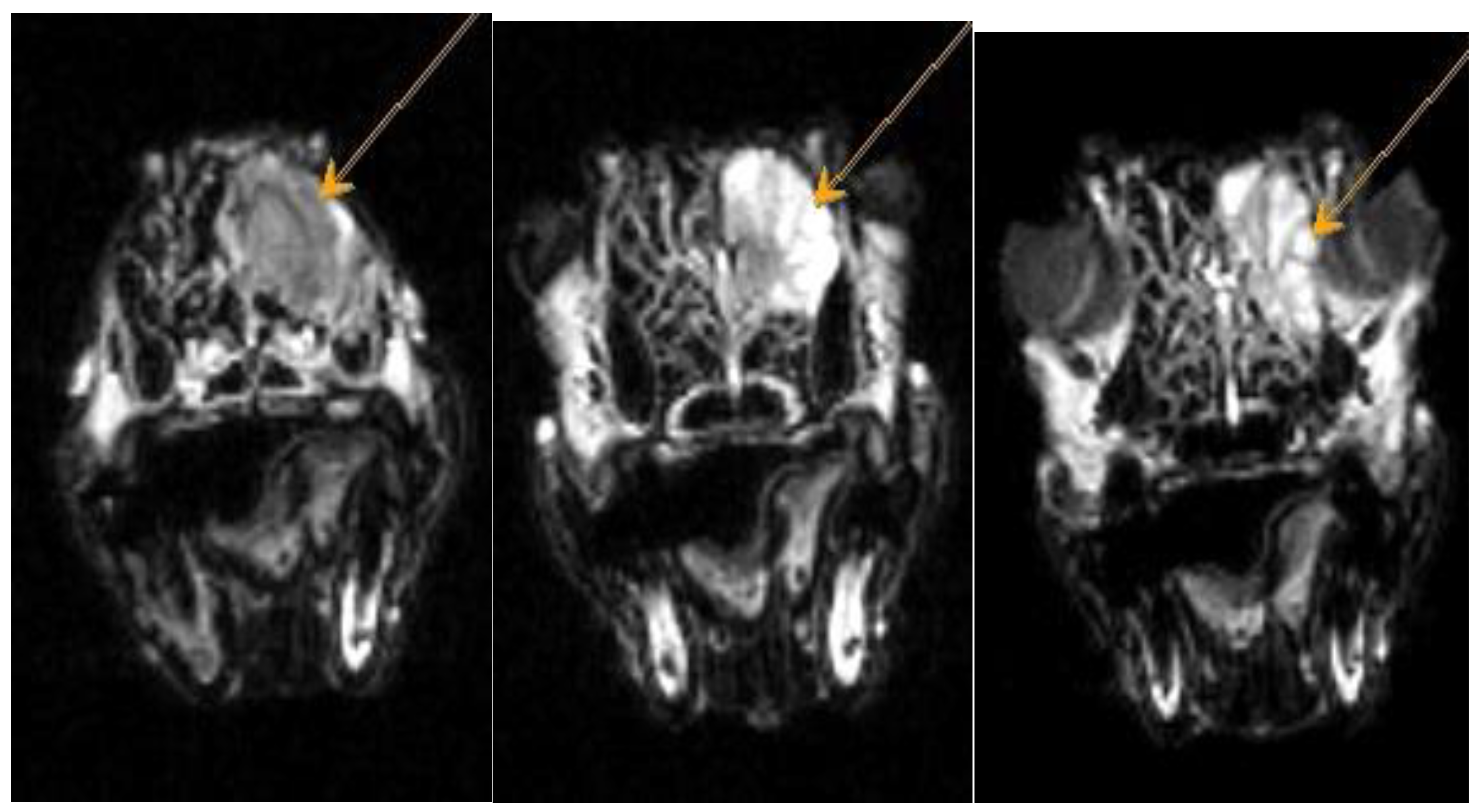
Figure A4.
Dachshund dog, 13 years old, 9 kg. T1-VI mode axial plane. Heterogeneous weakly hyperintense signal from the nasal cavity - neoplasm.
Figure A4.
Dachshund dog, 13 years old, 9 kg. T1-VI mode axial plane. Heterogeneous weakly hyperintense signal from the nasal cavity - neoplasm.
Figure A5.
Dachshund dog, 13 years old, 9 kg. T1-VI mode axial plane, after administration of a contrast agent (CA). Diffuse focal accumulation of CA in the nasal cavity is a neoplasm.
Figure A5.
Dachshund dog, 13 years old, 9 kg. T1-VI mode axial plane, after administration of a contrast agent (CA). Diffuse focal accumulation of CA in the nasal cavity is a neoplasm.
References
- Walusinski, O. Armand Trousseau (1801–1867), a neurologist before neurology. Revue Neurologique. 2020. 176(7–8): 531-542. [CrossRef]
- Shnayder N. A., Dykhno Yu. A. Short hystory of the study of paraneopastic neurological syndrome. Rossiiskii onkologicheskii zhurnal (Russian Journal of Oncology). 2016. 21(1–2): 105–109. (In Russ.). [CrossRef]
- Schneider N.A., Kantimirova E.A. Paraneoplastic polyneuropathy: definition, etiopathogenesis, diagnosis. Siberian Medical Review. 2010; 61 (1): 12–6.
- Sotnikova L.F. New in Pet Ophthalmology. Veterinary medicine. 2006. No. 4. P. 23-27.
- Kopenkin E.P., Sotnikova L.F. Eye diseases of small domestic animals. Moscow: Partnership of scientific publications KMK "Author's Academy", 2008. 186 p.
- Zernii E.Y., Gancharova O.S., Baksheeva V.E., Golovastova M.O., Savchenko M.S., Serebryakova M.V., Zamyatnin A.A., Philippov P.P., Senin I.I., Ishutina I.E., Kabanova E.I., Sotnikova L.F. Mechanisms of perioperative corneal abrasions: alterations in the tear film proteome. Biochemistry (Moscow) Supplement. Series B: Biomedical Chemistry. 2017. 11(2): 186-193. [CrossRef]
- Auche M. Des nevrites peripheriques chez les cancereux. Rev. Med. 1890. 10: 785–807.
- Guichard M.M.A., Vignon G. La polyradiculonéurite cancéreuse métastatique. J. Méd. Lyon. 1949. 30: 197–207.
- Guichard M.M.A., Cabanne F., Tommasi M., Fayolle J. Polyneuropathies in cancer patients and paraneoplastic polyneuropathies. Lyon Med. 1956. 41: 309–29.
- Posner J.B. Paraneoplastic Syndromes. In Neurologic Complications of Cancer. Contemporary Neurology Series 45. ed. Davis F.A., Company Philadelphia, 1995. p. 353.
- Zil’ber L.A., Abelev G.I. Virusology and Immunology of Cancer. [Virusologiya i immunologiya raka]. Moscow: MedGis ; 1962. 458 p. (in Russian).
- Cohen D.A., Bhatti M.T., Pulido J.S., Lennon V.A., Dubey D., Flanagan E.P., Pittock S.J., Klein C.J., Chen J.J. Collapsin Response-Mediator Protein 5-Associated Retinitis, Vitritis, and Optic Disc Edema. Ophthalmology. 2020. 127(2): 221–229. [CrossRef]
- Gordon L., Dinkin M. Paraneoplastic Syndromes in Neuro-ophthalmology. Continuum (Minneap Minn). 2019. 25(5): 1401–1421. [CrossRef]
- Graus F., Dalmau J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019. 16(9): 535–548. [CrossRef]
- Henry K., Paraneoplastic syndromes: Definitions, classification, pathophysiology and principles of treatment. Semin Diagn Pathol. 2019. 36(4): 204–210. [CrossRef]
- Becquart O., Lacotte J., Malissart P., Nadal J., Lesage C., Guillot B., Du Thanh A.J. Myasthenia Gravis Induced by Immune Checkpoint Inhibitors. Immunother. 2019. 42(8): 309–312. [CrossRef]
- Annus Á., Bencsik K., Obál I., Kincses Z.T., Tiszlavicz L., Höftberger R., Vécsei L. Paraneoplastic Neuromyelitis Optica Spectrum Disorder: A case report and review of the literature. J. Clin. Neurosci. 2018. 48: 7–10. [CrossRef]
- Beauchemin P., Iorio R., Traboulsee A.L., Field T., Tinker A.V., Carruthers R.L. Paraneoplastic Neuromyelitis Optica Spectrum Disorder: A single center cohort description with two cases of histological validation. Mult. Scler. Relat. Disord. 2018. 20: 37–42. [CrossRef]
Table 1.
Cancer treatment protocols: lymphomas and mastocytomas in dogs. .
Table 1.
Cancer treatment protocols: lymphomas and mastocytomas in dogs. .
| Treatment method |
Drug, dosage, method of administration |
| Lymphomas |
| Chemotherapy |
1-st week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 2 mg/kg, orally |
2-nd week. Cyclophosphamide, 250 mg/m2, subcutaneous.
Prednisolone, 2 mg/kg, orally |
3-rd week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 1 mg/kg, orally |
4-th and 5-th week. Doxorubicin, 1 mg/kg intravenously for animals up to 15 kg; for animals whose weight is more than 15 kg - 30 mg/m2.
Prednisolone, 1 mg/kg, orally |
6-th week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 2 mg/kg, orally |
7-th week. Cyclophosphamide, 250 mg/m2, subcutaneous.
Prednisolone, 2 mg/kg, orally [a] |
8-th week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 1 mg/kg, orally |
9-th and 10-th week. Doxorubicin, 1 mg/kg intravenously for animals up to 15 kg; for animals whose weight is more than 15 kg - 30 mg/m2.
Prednisolone, 1 mg/kg, orally [b] |
11-th week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 2 mg/kg, orally |
12-th week. Cyclophosphamide, 250 mg/m2, subcutaneous.
Prednisolone, 2 mg/kg, orally [a] |
13-th week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 1 mg/kg, orally |
14-th and 15-th week. Doxorubicin, 1 mg/kg intravenously for animals up to 15 kg; for animals whose weight is more than 15 kg - 30 mg/m2.
Prednisolone, 1 mg/kg, orally |
16-th week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 2 mg/kg, orally |
17-th week. Cyclophosphamide, 250 mg/m2, subcutaneous.
Prednisolone, 2 mg/kg, orally [a] |
18-th week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 1 mg/kg, orally |
19-th and 20-th week. Doxorubicin, 1 mg/kg intravenously for animals up to 15 kg; for animals whose weight is more than 15 kg - 30 mg/m2.
Prednisolone, 1 mg/kg, orally |
| |
21-th week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 2 mg/kg, orally |
| |
22-th week. Cyclophosphamide, 250 mg/m2, subcutaneous.
Prednisolone, 2 mg/kg, orally [a] |
| |
23-th week. Vincristine, 0.5 mg/m2, intravenously.
Prednisolone, 1 mg/kg, orally |
| |
24-th week. Doxorubicin, 1 mg/kg intravenously for animals up to 15 kg; for animals whose weight is more than 15 kg - 30 mg/m2.
Prednisolone, 1 mg/kg, orally [c] |
[a] if the animal is diagnosed with renal or central nervous system lymphoma, on these days Cyclophosphamide should be replaced with Cytarabine (600 mg/m2, subcutaneously, every 12 hours for 2 days);
[b] if the animal achieves complete remission at week 9, treatment should be continued until week 11;
[c] if the patient achieves complete remission at week 25, treatment is discontinued and the animal is monitored monthly to ensure timely detection of relapse. |
| Mastocytomas, approach I |
| Chemotherapy |
1. Imatinib in “mono mode”. Imatinib, 10 mg/kg per day, orally.
2. If there was no effect from Imatinib, the protocol was changed to Vinblastine in “mono mode”. Vinblastine, at a dose of 2 mg/m2 (intravenously, weekly).
3. Vincristine in “mono mode”, at a dose of 0.5 mg/m2 (weekly) or Lomustine orally in mono mode, at a dose of 60–90 mg/m2 (weeks 1, 22 and 42) |
| Radiobeam |
single focal dose (ROD) 5 Gy,
total focal dose (SOD) 45–50 Gy |
| Mastocytomas, approach II |
| Surgical |
1. Poorly differentiated - resection of the neoplasm involving healthy tissue of at least 3 cm plus fascia.
2. Moderately differentiated - resection of the tumor with the inclusion of healthy tissue of at least 2 cm plus fascia.
3. Highly differentiated - resection of the neoplasm involving healthy tissue of at least 1 cm plus fascia. |
| Melanoma |
| Chemotherapy |
Carboplatin in “mono mode” at a dosage of 300 mg/m2 with an interval of 21 days, intravenously.
|
|
Radiobeam
|
Before and after surgery with morphological confirmation of invasion into surrounding tissues :
single focal dose (ROD) 5 Gy,
total focal dose (SOD) 45–50 Gy. |
Table 2.
Scheme of hormonal-drug treatment in dogs.
Table 2.
Scheme of hormonal-drug treatment in dogs.
| Local treatment |
General treatment |
| drugs |
duration |
drugs |
duration |
Corticosteroids
Dexamethasone, 0.1% |
up to 2 months |
Anti-inflammatory
Prednisolone, 1–2 mg/kg |
14–30 days |
Nonsteroidal anti-inflammatory drugs:
Indomethacin,
Nepafenac |
up to 2 months |
Antihistamines:
Clemasteel
Suprastin |
30–40 days, alternating each 10 days |
Mydriatic cycloplegics:
Atropine sulfate,
0.1%, Midriacil |
until the posterior synechiae rupture; 2–3 months for severe miosis |
Vascular strengthening agents:
Calcium chloride,
Calcium gluconate |
10–20 days
1.5–2 months, alternating |
Subconjunctival mixture:
0.5% Novocaine,
0.5-1.0 ml
4 mg/ml Dexamethasone,
0.2-0.4 ml |
1–5 days |
Compliance with dietary feeding |
up to 1 months |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).