Submitted:
15 December 2023
Posted:
18 December 2023
You are already at the latest version
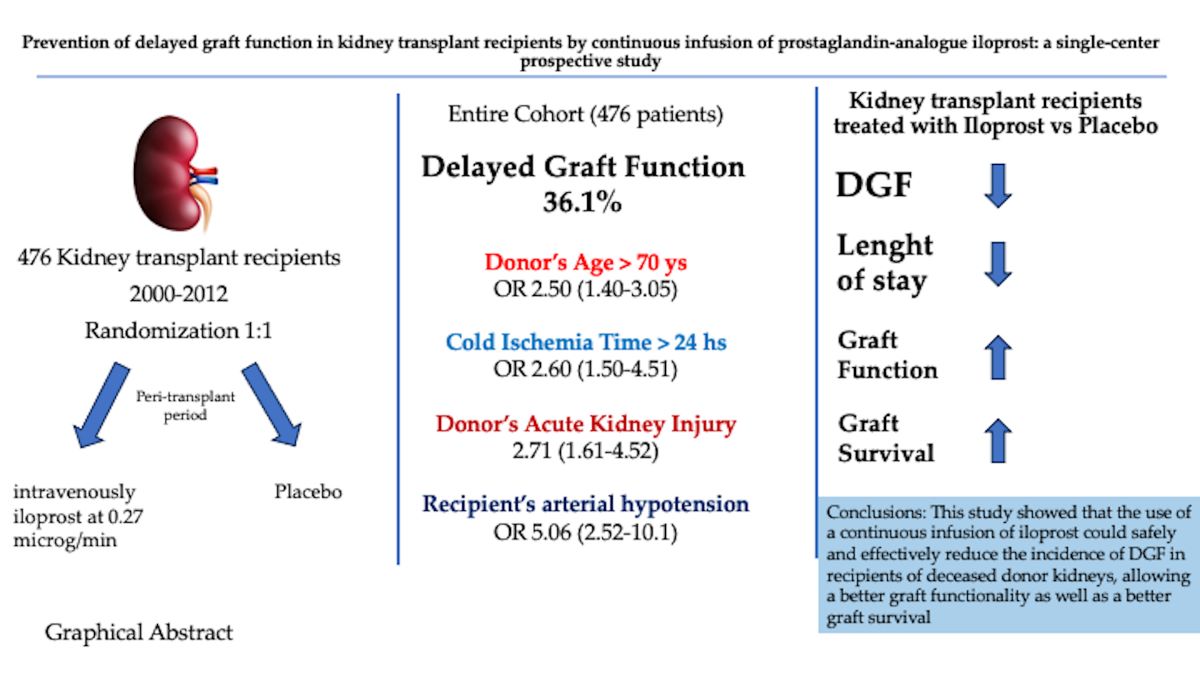
Abstract
Keywords:
1. Introduction
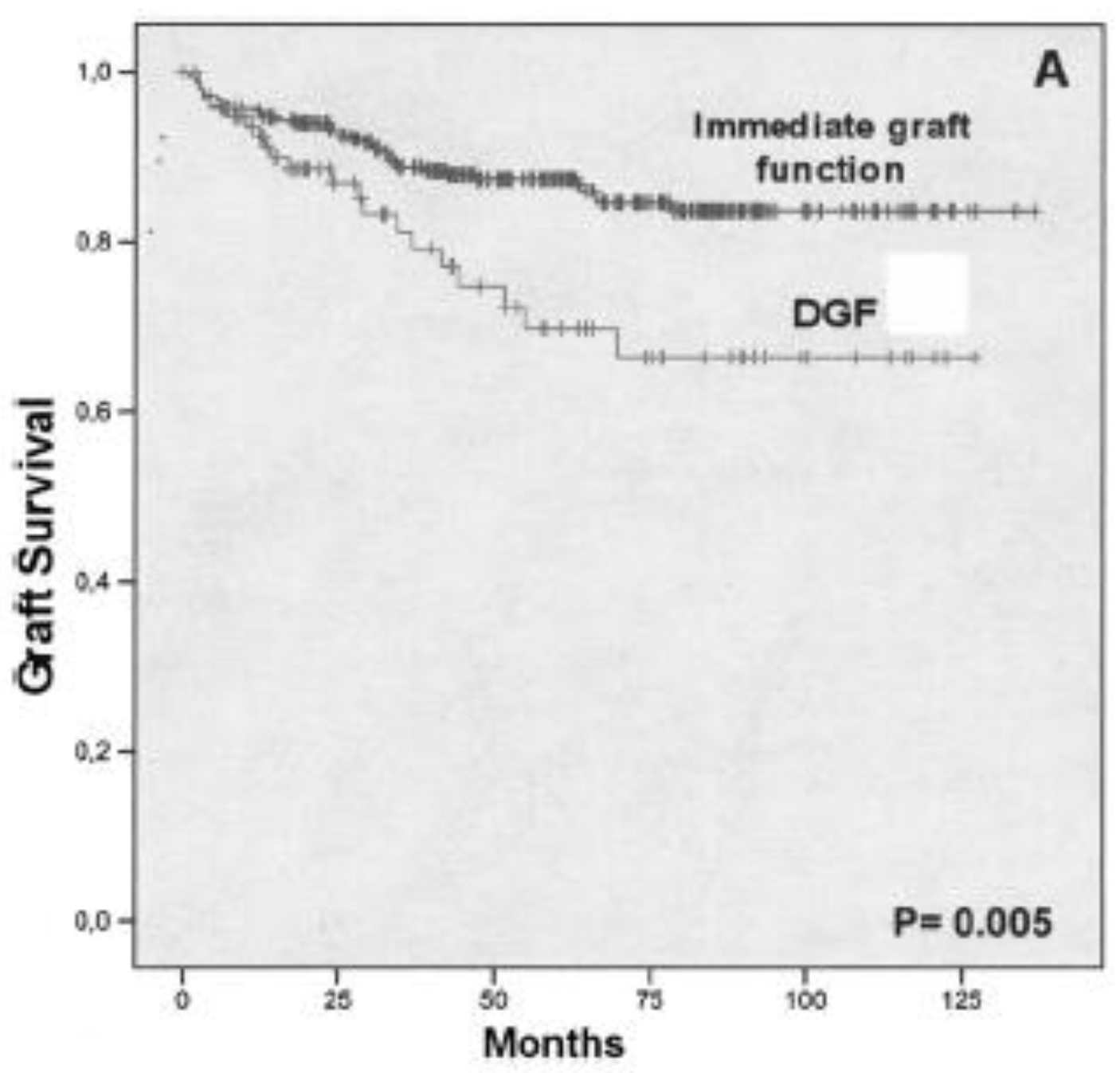
2. Results
3. Discussion
4. Materials and Methods
4.1. Study population
4.2. Statistical analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004; 4(3),378-83. [CrossRef]
- Stratta, R.J.; Rohr, M.S.; Sundberg, A.K.; Armstrong, G.; Hairston, G.; Hartmenn, E.; Farney, A.C.; Roskopf, J.; Iskandar, S.S.; Adams, P.L. Increased kidney transplantation utilizing expanded criteria deceased organ donors with results comparable to standard criteria donor transplant. Ann Surg, 2004; 239, 688-695. [CrossRef]
- Remuzzi, G.; Cravedi, P.; Perna, A.; Dimitrov, B.; Turturro, M.; Locatelli, G.; Rigotti, P.; Baldan, N.; Betaini, M.; Valente, U.; Scalamogna, M.; Ruggenenti P. Long-term outcome of renal transplantation from older donors. N Engl J Med 2006; 354, 343-52. [CrossRef]
- Veroux, P.; Veroux, M.; Puliatti, C.; Valastro, M.; Di Mare, M.; Gagliano, M.; Macarone, M.; Cappello, D.; Spataro, M.; Giuffrida G. Kidney transplantation from cadaveric donors unsuitable for other centers and older than 60 years of age. Transplant Proc. 2005, 37:2451-2453. [CrossRef]
- Mesnard, B.; Territo, A.; Campi, R.; Hevia, V.; Andras, I.; Piana, A.; Pecoraro, A.; Boissier, R.; Prudhomme, T.; EAU-Young Academic Urologist (YAU) group of Kidney Transplantation. Kidney transplantation from elderly donors (> 70 years): a systematic review. World J Urol. 2023, 41(3),695-707. [CrossRef]
- Corona, D.; Ekser, B.; Gioco, R.; Caruso, M.; Schipa, C.; Veroux, P.; Giaquinta, A.; Granata, A.; Veroux M. Heme-Oxygenase and Kidney Transplantation: A Potential for Target Therapy? Biomolecules. 2020, 10(6):840. [CrossRef]
- Cooper, M.; Wiseman, A.C.; Doshi, M.D.; Hall, I.E.; Parsons, R.F.; Pastan, S.; Reddy, K.S.; Schold, J.D.; Mohan, S.; Hippen, B.E. Understanding Delayed Graft Function to Improve Organ Utilization and Patient Outcomes: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Am J KidneyDis. 2023 S0272-6386(23)00860-0. [CrossRef]
- Schrezenmeier, E.; Müller, M.; Friedersdorff, F.; Khadzhynov, D.; Halleck, F.; Staeck, O.; Dürr, M.; Zhang, K.; Eckardt, K.U.; Budde, K.; Lehner, L.J. Evaluation of severity of delayed graft function in kidney transplant recipients. Nephrol Dial Transplant. 2022 , 37(5):973-981. [CrossRef]
- Lapointe, I.; Lachance, J.G.; Noël, R.; Côté, I.; Caumartin, Y.; Agharazii, M.; Houde, I.; Rousseau-Gagnon, M.; Kim, S.J.; De Serres, S.A. Impact of donor age on long-term outcomes after delayed graft function: 10-year follow-up. Transpl Int. 2013, 26(2):162-9. [CrossRef]
- Li, M.T.; Ramakrishnan, A.; Yu, M.; Daniel, E.; Sandra, V.; Sanichar, N.; King, K.L.; Stevens, J.S.; Husain, S.A.; Mohan, S. Effects of Delayed Graft Function on Transplant Outcomes: A Meta-analysis. Transplant Direct. 2023, 9(2):e1433. [CrossRef]
- Swanson, K.J.; Bhattarai, M.; Parajuli, S. Delayed graft function: current status and future directions. Curr Opin Organ Transplant. 2023,28(1):1-7. [CrossRef]
- Feldman, H.I.; Gayner, R.; Berlin, J.A.; Roth, D.A.; Silibovsky, R.; Kushner, S.; Brayman, K.L.; Burns, J.E.; Kobrin, S.M.; Friedman, A.L.; Grossman, R.A.. Delayed graft function reduces renal allograft survival independent of acute rejection. Nephrol Dial Transplant 1996; 11: 1306-1313. [CrossRef]
- Heylen, L.; Pirenne, J.; Naesens, M.; Sprangers, B.; Jochmans, I. "Time is tissue"-A minireview on the importance of donor nephrectomy, donor hepatectomy, and implantation times in kidney and liver transplantation. Am J Transplant. 2021, 21(8):2653-2661. [CrossRef]
- Quiroga, I.; Mcshane, P.; Koo, D.D.; Gray, D.; Friend, P.J.; Fuggle, S.; Darby, C. Major effects of delayed graft function and cold ischemia time on renal allograft survival. Nephrol Dial Transplant 2006; 21: 1689-96.
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004; 364:1814-27. [CrossRef]
- Ponticelli, C.; Reggiani, F.; Moroni, G. Delayed Graft Function in Kidney Transplant: Risk Factors, Consequences and Prevention Strategies. J Pers Med. 2022, 12(10):1557. [CrossRef]
- Salguero, J.; Chamorro, L.; Gómez-Gómez, E.; de Benito, P.; Robles, J.E.; Campos, J.P. Kidney Survival Impact of Delayed Graft Function Depends on Kidney Donor Risk Index: A Single-Center Cohort Study. J Clin Med. 2023 12(19):6397. [CrossRef]
- Dahmane, D.; Audard, V.; Hiesse, C.; Pessione, F.; Bentaarit, B.; Barrou, B.; Rondeau, E.; Cohen, S.; Lang, P.; Grimbert, P. Retrospective follow-up of transplantation of kidney from marginal donors. Kidney Int 2006; 69: 546-532. [CrossRef]
- Carter, J.T.; Chan, S.; Roberts, J.P.; Feng, S. Expanded criteria donor kidney allocation: marked decrease in cold ischemia and delayed graft function at a single center. Am J Transplant 2005; 5, 2745-53. [CrossRef]
- Vacher-Coponat, H.; Purgus, R.; Indreis, M.; Moal, V.; Luciani, H.; Lechevallier, E.; Delaporte, V.; Luccioni, A.; Julian, H.; Reviron, D.; Dussol, B.; Berland, Y. Cold ischemia time in renal transplantation is reduced by a timesheet in a French transplant center. Transplantation 2007, 83, 561-55. [CrossRef]
- Hollenbeck, M.; Dinter, K.; Torsello, G.; Kock, M.; Willers, R.; Sandmann, W.; Grabensee, B. Prostaglandin E1 reduces the risk of delayed graft function after cadaveric renal transplantation. Nephrol Dial Transplant 1999, 14(suppl.4), 32-33. [CrossRef]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.L.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; Held, P.J. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002 74(9), 1281-1286. [CrossRef]
- Veroux, M.; Zerbo, D.; Basile, G.; Gozzo, C.; Sinagra, N.; Giaquinta, A.; Sanfiorenzo, A.; Veroux, P. Simultaneous Native Nephrectomy and Kidney Transplantation in Patients With Autosomal Dominant Polycystic Kidney Disease. PLoS One. 2016 11(6):e0155481. [CrossRef]
- Cavaleri, M.; Veroux, M.; Palermo, F.; Vasile, F.; Mineri, M.; Palumbo, J.; Salemi, L.; Astuto, M.; Murabito, P. Perioperative Goal-Directed Therapy during Kidney Transplantation: An Impact Evaluation on the Major Postoperative Complications. J Clin Med. 2019, 8(1), 80. [CrossRef]
- Veroux, P.; Veroux, M.; Macarone, M.; Bonanno, M.G.; Tumminelli, M.G. Efficacy of a novel method of intravenous infusion of the prostaglandin analogue iloprost for the treatment of lower-limb critical ischemia:an open-label, nonrandomized study in two cohorts. Curr Ther Res 2004, 65, 255-265. [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4),c179-84. [CrossRef]
- Nita, G.E.; Gopal, J.P.; Khambalia, H.A.; Moinuddin, Z.; van Dellen, D. Kidney Transplantation From Donors With Acute Kidney Injury: Are the Concerns Justified? A Systematic Review and Meta-Analysis. Transpl Int. 2023, 36:11232. [CrossRef]
- Haas, M.;Loupy, A.; Lefaucheur C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Bouatou, Y.; Becker, J.U.; Cornell, L.D.; Duong van Huyen, J.P.; Gibson, I.W.; Kraus, E.S.; Mannon, R.B.; Naesens, M.; Nickeleit, V.; Nickerson, P.; Segev, D.L.; Singh, H.K.; Stegall, M.; Randhawa, P.; Racusen, L.; Solez, K.; Mengel, M. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative end points for next-generation clinical trials. Am J Transplant 2018,18, 293–307. [CrossRef]
- Fernández, A.R.; Sánchez-Tarjuelo, R.; Cravedi, P.; Ochando, J.; López-Hoyos, M. Review: Ischemia Reperfusion Injury-A Translational Perspective in Organ Transplantation. Int J Mol Sci. 2020 21(22),8549. [CrossRef]
- Browne, B.J.; Holt, C.O.; Emovon, O.E. Delayed graft function may not adversely affect short-term renal allograft outcome. Clin Transplant 2003; 17 suppl 9: 35-8. [CrossRef]
- Hauet, T.; Goujon, J.M.; Vandewalle, A.; Baumert, H.; Lacoste, L.; Tillement, J.P.; Eugene, M.; Carretier, M. Trimetazidine reduces renal dysfunction by limiting the cold ischemia/reperfusion injury in autotransplanted pig kidneys. J Am Soc Nephrol. 2000, 11(1), 138-148. [CrossRef]
- Emrecan, B.; Tulukoglu, E.; Bozok, S.; Kestelli, M.; Onem, G.; Küpelioglu, A.; Yagdi, S.; Gürbüz, A. Effects of iloprost and pentoxifylline in renal ischemia reperfusion in rabbit model. Eur J Med Res 2006, 11, 295-299.
- Aytacoglu, B.N.; Sucu, N.; Tamer, L.; Polat, A.; Gul, A.; Degirmenci, U.; Mavioglu, I.; Dikmengil, M. Iloprost for the attenuation of ischaemia/reperfusion injury in a distant organ. Cell Biochem Funct 2006, 24, 341-346. [CrossRef]
- Neumayer, H.H.; Schreiber, M.; Wagner, K. Prevention of delayed graft function in cadaveric kidney transplants by the calcium antagonist diltiazem and the prostacyclin-analogue iloprost. Outcome of a prospective randomized clinical trial. Prog Clin Biol Res 1989, 301, 289-295.
- Kassis, H.M.; Minsinger, K.D.; McCullough, P.A.; Block, C.A.; Sidhu, M.S.; Brown, J.R. A Review of the Use of Iloprost, A Synthetic Prostacyclin, in the Prevention of Radiocontrast Nephropathy in Patients Undergoing Coronary Angiography and Intervention. Clin Cardiol. 2015, 38(8), 492-8. [CrossRef]
- Hansen, J.M.; Christensen, N.J.; Fogh-Andersen, N.; Strandgaard, S. Effects of the prostacyclin analogue iloprost on cyclosporine-induced renal hypoperfusion in stable renal transplant recipients. Nephrol Dial Transplant 1996, 11, 340-346. [CrossRef]
- Grosso, G.; Corona, D.; Mistretta, A.; Zerbo, D.; Sinagra, N.; Giaquinta, A.; Cimino, S.; Ekser, B.; Giuffrida, G.; Leonardi, A.; Gula, R.; Veroux, P.; Veroux, M. Delayed graft function and long-term outcome in kidney transplantation. Transplant Proc. 2012, 44(7), 1879-83. [CrossRef]
| DGF (n=172) |
No DGF (n=304) |
P value | |
|---|---|---|---|
| Mean Donor’s age (yr) | 57.2±15.8 | 47.1±18.8 | <.001 |
| Donor’s age | |||
| <55 years | 76 (44.1) | 191 (62.8) | .032 |
| 66-70 years | 49 (28.4) | 77 (25.3) | .543 |
| > 70 years | 47 (27.3) | 36 (11.8) | <.001 |
| Donors with AKI | 41 (23.8) | 38 (12.5) | .001 |
| Female Donor | 75 (43.6) | 155 (50.9) | .121 |
| Use of vasopressors | 165(95.9) | 285 (93.7) | .744 |
| Stay in ICU | 5.4±4.0 | 4.9±3.9 | .251 |
| Mean Cold Ischemia time (min) | 1070±450 | 882±331 | <.001 |
| Cold ischemia time | |||
| < 24 hours | 132 (76.7) | 270 (88.9) | .635 |
| > 24 hours | 40 (23.3) | 34 (11.1) | < .001 |
| Recipient’s age | 52.9±11.4 | 46.0±11.9 | < .001 |
| Male recipients (%) | 110(63.9) | 193(63.4) | .884 |
| Recipient’s Hypotension | 30 (17.4) | 12 (3.9) | <.001 |
| Waiting list (months) | 24.4±21.2 | 16.5±14.4 | .001 |
| Time on dialysis (months) | 65.0±50.8 | 39.9±32.7 | < .001 |
| Characteristics | OR | 95% CI | P value |
|---|---|---|---|
| Donor age | |||
| Donor age < 70 years | reference | ||
| Donors age > 70 years | 2.50 | 1.40-3.05 | <.001 |
| Cold ischemia time | |||
| < 24 h | reference | ||
| >24 h | 2.60 | 1.50-4.51 | < .001 |
| Donors with AKI | 2.71 | 1.61-4.52 | .021 |
| Time on dialysis | |||
| < 12 months | reference | ||
| 12-24 months | 1.01 | 0.66-1.56 | .908 |
| >24 months | 2.87 | 1.91-4.33 | < .001 |
| Recipient age > 60 ys | 3.39 | 2.14-5.38 | < .001 |
| Recipient’s hypotension | 5.06 | 2.52 – 10.1 | < .001 |
| Group and characteristics | Treatment group (N=238) | Control Group (N=238) | P value |
|---|---|---|---|
| Donor | |||
| Age (yr) | 50.9 ± 20.4 | 50.7± 19.8 | .845 |
| Male Sex (%) | 91 (38.2) | 69 (28.9) | .032 |
| Terminal Serum Creatinine (mg/dl) | 1.13 ± 0.3 | 1.11 ± 0.3 | .532 |
| Use of vasoactive amines (%) | 200 (84%) | 203 (85.2) | .624 |
| Diabetes (%) | 18 (7.5) | 13 (5.4) | .223 |
| Arterial Hypertension > 10 ys (%) | 95 (39.9) | 92 (38.6) | .498 |
| Cold Ischemia Time (hr) | 17.3±7.4 | 13.8±6.2 | <.001 |
| Cerebral haemorrhage/ischemia brain death (%) | 141 (59.2) | 158 (66.3) | .147 |
| Non traumatic brain death (%) | 93 (39) | 78 (32.7) | .122 |
| Other cause of brain death | 4 (1.6) | 2 (0.8) | .554 |
| Use of vasoamine drugs | 225 (94.5) | 228 (95.7) | .922 |
| Stay in ICU | 4.9±3.8 | 5.3±4.2 | .279 |
| Recipient | |||
| Age (yr) | 49±11.1 | 47.9±12 | .324 |
| Male sex (%) | 159 (66.8) | 142 (59.6) | .424 |
| Time on Dialysis (mo) | 50±23.4 | 47.1±26.2 | .113 |
| Time on waiting list (mo) | 23.9±33 | 15±16 | < .001 |
| Peritoneal dialysis (%) | 5 (2.1) | 9 (3.7) | .433 |
| Dual transplant (%) | 17 (7.1) | 11 (4.6) | .115 |
| HCV seropositivity | 36 (15.1) | 11(4.6) | <.05 |
| Delayed graft function (%) | 51 (21.4) | 121 (50.9) | < .001 |
| Discontinuation of dialysis (dy) | 10.5±8.3 | 13.4±6.7 | .016 |
| Primary Non Function (%) | 6 (2.5%) | 6(2.5%) | 1 |
| Acute rejection | 16 (6.7) | 25 (10.5) | .141 |
| Immunosuppression | |||
| Induction (basiliximab) | 68 (28.5) | 55 (23.1) | .753 |
| Induction (thymoglobuline) | 22 (9.2) | 24 (10) | .883 |
| Tacrolimus | 150 (63) | 164 (68.9) | .214 |
| MMF | 205 (86.1) | 185 (77.7) | .301 |
| Sirolimus | 36 (15.1) | 26 (10.9) | .112 |
| Cyclosporine | 29 (12.1) | 55 (23.1) | .108 |
| Everolimus | 14 (5.8) | 19 (7.9) | .323 |
| Hospital stay | 10.5±4.4 | 13.3±6.4 | <.05 |
| 30-day acute rejection | 22 (9.2) | 25 (10.5) | .212 |
| Postoperative Death (30-day) | 3 (1.2) | 4 (0.8) | .823 |
| 1-y Serum Creatinine (mg/dL) | 1.41±0.61 | 1.60±0.65 | .008 |
| 5-y Serum Creatinine (mg/dL) | 1.50±0.62 | 1.66±0.81 | .045 |
| 10-y Serum Creatinine (mg/dL) | 1.54±0.76 | 1.64±0.55 | .525 |
| 1-y Graft Survival | 93.3% | 92% | < .05 |
| 5-y Graft Survival | 83% | 83.7% | < .05 |
| 10-y Graft Survival | 75% | 74% | .183 |
| 1-y Patient Survival | 96.7% | 92.1% | .185 |
| 5-y Patient Survival | 96.3% | 93.7% | .211 |
| 10-y patient Survival | 80% | 77% | .172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





