1. Introduction
Platelets in recent years have been in short supply and high demand. Approximately one in five cardiac surgery patients require platelets. In our safety-net hospital, however, the incidence is over 40%. Renal dysfunction, long cardiopulmonary bypass (CPB) times, and absence of tranexamic acid (TXA) are well-described major risk factors for requiring platelet transfusions. Patient blood management protocols (PBM) should be individualized according to each hospital’s demonstrated needs. Thromboelastograms (TEG) are one method to prevent unnecessary platelet transfusions, but their efficacy in cardiac surgery is not as well studied as in other settings [
1].
Our institution is a safety-net hospital, constrained by finances and staffing, preventing us from purchasing a point-of-care TEG. However, we have a TEG that requires hours for results and about $700 vs. $50 for a platelet count. Staffing limitations prevented prolonged intensive care unit (ICU) stay, necessitating that the surgical department after summer of 2022 offered CPB only if expected duration was shorter and kidney function better, along with requiring TXA be administered uniformly.
2. Methods
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Louisiana State University Health Sciences Center Shreveport (IRB, 1501 Kings Highway, Shreveport, Louisiana, USA 71103, Fax (318) 813-1360, Telephone (318) 813-1350, FWA 00000653, 00000178 IRB) on May 9, 2023. Our retrospective observational study protocol was designed to examine electronic medical records (IRB approved protocol number = STUDY00002388) by first assigning a new study subject number into the data set, masking any personal information, thereby introducing negligible risk of loss of privacy. Furthermore, because retrospective chart review cannot be practicably carried out without the waiver, as it is not practical to contact this many patients, IRB waived any requirement for each individual to sign informed consent. The authorization of research use and disclosure of protected health information by signed informed consent was also waived based upon an IRB general statement regarding “secondary research on data or specimens (no consent required) exemption.” The IRB Chairman signed that, “Based on the information above, the IRB finds that this waiver request meets all the legal requirements for a Waiver of Authorization under HIPAA (45 CFR Part 160 and 164) and 45 CFR 46.” Therefore, our study was in compliance with the terms of the Health Insurance Portability and Accountability Act of 1996 of the United States. All the data used for this research are available, upon reasonable request, from the Louisiana State University Health Sciences Center Shreveport, USA.
We compared all 32 consecutive patients undergoing CPB from January through March in 2022 to the 29 patients from January through March in 2023. Exclusion criteria included protected classes of pediatrics, pregnancy, prisoners, and anyone cognitively impaired.
Our primary outcome variable was how early was the timing of each platelet transfusion, in terms of hours after separation from CPB through 7 days postoperatively. Secondary outcome variables included: creatinine at postoperative day two, along with total number of platelet units, fresh frozen plasma (FFP), cryoprecipitate (CRYO), and packed red blood cells (PRBC) transfused within 7 days. Confounders included preoperative creatinine, duration of CPB, TXA dose, age, weight, height, anticoagulants, gender, and platelet and hemoglobin values immediately preceding platelet transfusion.
Significant differences in means and standard deviations were compared using two tailed unpaired t-tests with SAS 9.3 software, after demonstrating parametric distribution. Chi Square analysis was performed for categorical variables.
CPB did not include retrograde arterial prime (which boosts hemoglobin), return of autologous blood siphoned off prior to exposure to tubing of CPB, nor Hemobag collection-system of pump salvage. Cellsaver (inferior to Hemobag in terms of preserving platelets) was used to salvage the blood after CPB in pump tubing. Dosing for TXA by protocol was 1gram followed by 1mg/kg/hour.
3. Results
After excluding 4 prisoners in 2022, 61 patients were evaluated: 28 in 2022 and 29 in 2023 (
Table 1). Coronary artery bypass grafting was performed during CPB at an equally prevalent rate (65%) among both groups.
Preoperatively creatinine tended to be higher in 2022 at 1.5 vs 1.1, reaching significance postoperatively at 1.9 vs 1.1 (p=0.02,
Table 2). Bypass times decreased from 174 to 124 minutes (p=0.06). TXA use increased from 18 of 28 patients to 26 of 29 patients.
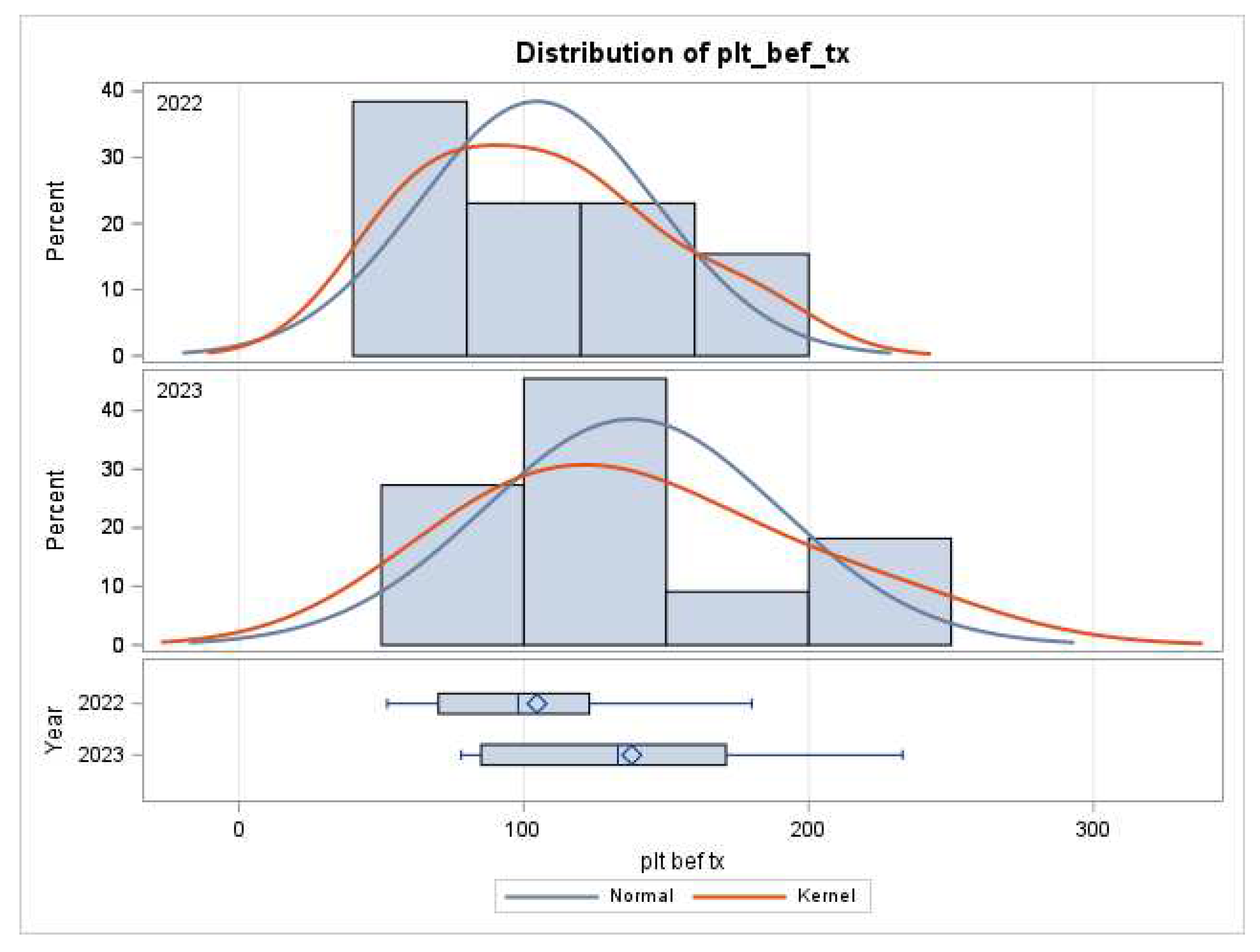
Platelet counts increased on bypass from 142 to 173 (p=0.06). Platelet counts were drawn an hour earlier before termination of CPB (1.6 vs 2.6, p=0.03). Essentially the same number of platelet counts (5.6 in 2022 vs 4.9 in 2023, p=0.11) were drawn within 48 hours in both groups. Platelet counts rose from 105 to 138 before transfusing the first unit of platelets (p=0.10,
Figure 1) and 93 to 133 before the second unit in 2023 (p=0.15).
TEG data was available in only 2 patients, only in the intensive care unit and only in 2022. Platelet and hemoglobin nadirs were higher and occurred at day 3 (8g/dL vs 8.8g/dL, p=0.04; 98 vs 138, p=0.005). Hemoglobin before the second platelet transfusion was significantly lower in 2022 (7.7g/dL vs 9.5g/dL, p=0.03).
Transfusions for red cells tended to drop from 1.97 to 1.21 units, within 7 days. This trend toward fewer average PRBC transfusions per patient began within the first 48 hours, dropping from 2.0 to 1.2 (p=0.28). Cryoprecipitate tended to rise from 0.36 units per patient to 0.69 in 2023 (p=0.23). FFP tended to decline from 0.93 to 0.83 units per patient (p=0.84).
The average number of units of platelets transfused per patient non-significantly rose from 0.75 to 0.93 (p=0.59). If exposed to platelets, the average number of units tended to rise from 2.1 to 2.5 (p=0.49). Exposure differences are equivocal, with 11 of 29 patients in 2023 receiving platelets versus 13 of 28 in 2022 (
Table 3, p=0.5).
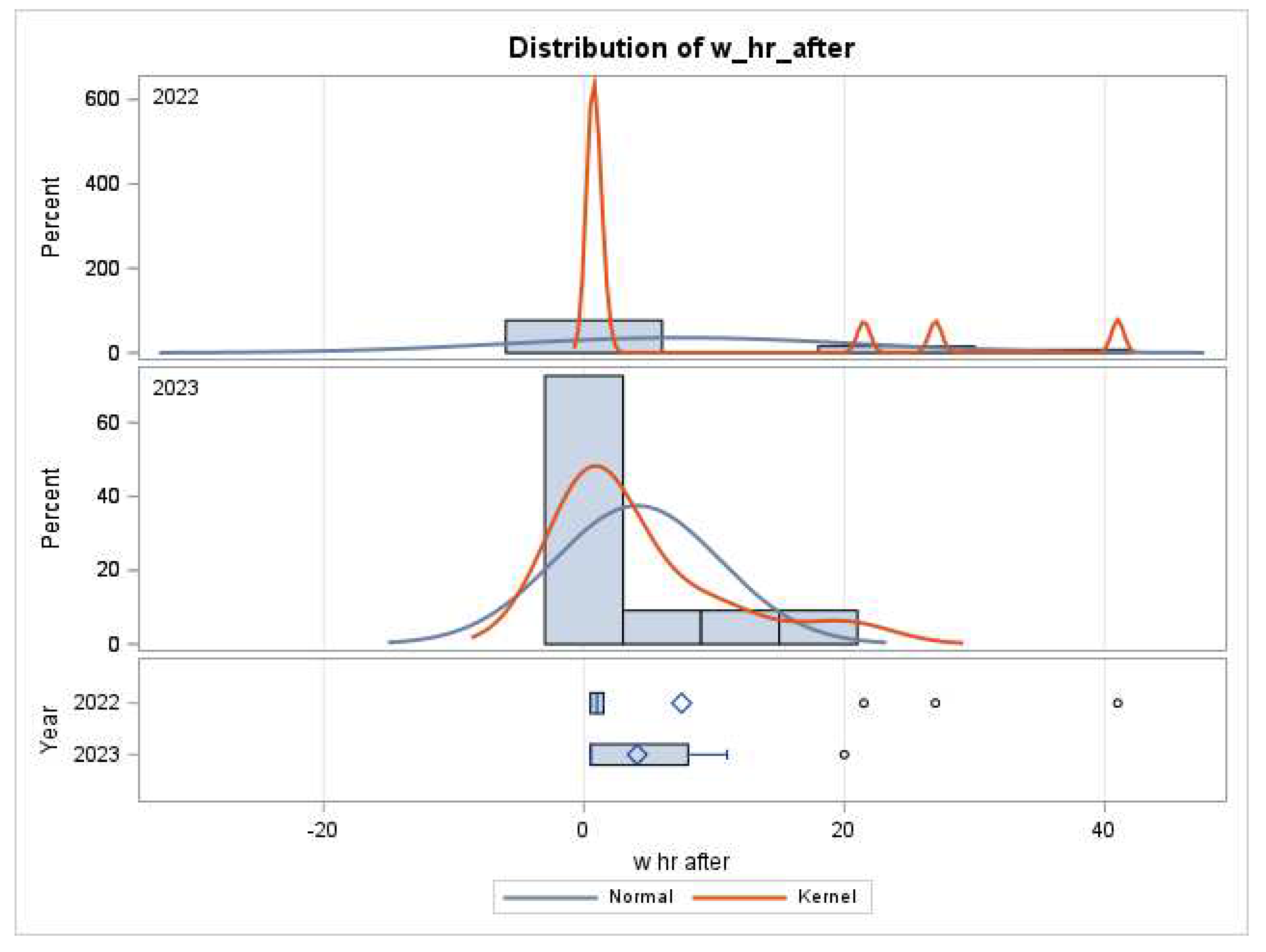
In 2022, the first unit of platelets tended to be administered later (7.5 vs 4.1 hours, p =0.4,
Figure 2). In 2022, 8 of the 13 patients receiving platelets received a second unit, while in 2023, 5 of the 11 had a second unit. The second unit of platelets was given after platelet counts were available from a blood draw on average 1.6 hours after termination of CPB in 2023 vs 8.8 hours in 2022 (p=0.26). The timing of platelet transfusion was sooner (
Figure 2 and
Figure 3). Four patients received a 3
rd bag in 2023 compared to 2 patients in 2022 at a significantly higher platelet count in 2023 (101 vs 46, p=0.04). Three patients received a 4
th bag in 2023 compared to 1 patient in 2022.
4. Discussion
This is the first study we are aware of that examines the
timing of platelet transfusions after separation from bypass, relative to platelet and hemoglobin levels. Our protocol remained measurement of one platelet count during bypass, but prothrombin only hours after arrival in intensive care. Our study measured confounders of age, weight, height, anemia, anticoagulants, duration of CPB, and creatinine based upon previous research [
2,
3]. Despite a conscious shift toward preoperative selection of patients with better renal function, requiring shorter surgeries, and universal TXA, more platelets tended to be transfused earlier. Also contrary to what we expected, platelet count and hemoglobin were higher immediately prior to platelet transfusions.
Lower length of stay and components transfused are sometimes associated with the introduction of TEG. For example, in a recent study, platelet administration fell from 27% to 16%, p =0.001. These investigators obtained point-of-care (POC-TEG) results within a couple of minutes. However, average number of platelet units transfused per patient did not decline [
4]. In their data, mortality was only 2.5% and re-exploration rate was only 2%. In a safety-net hospital such as ours, patients often have more platelet dysfunction arising from more comorbidities, and thus may be expected to require more platelet transfusions. A lower number needed to treat (NNT) would result from power analysis, making a safety-net hospital fertile ground for future research about POC-TEG.
Blood transfusion contributes to mortality. After hours of severe hemorrhage, necessitating massive blood transfusions, re-exploration for tamponade may be required. Acute kidney injury may develop after these hemodynamic stressors. Deppe et al. found TEG was not associated with decrease in duration of intensive care unit or hospital length of stay, stroke, or mortality [
5]. However, they found that TEG was associated with a decrease in acute kidney injury, declining from 7.8% to only 6% incidence. In Deppe’s data, re-exploration rates were 4.2%, regardless of being before or after adoption of TEG, emphasizing that TEG data has shown variable results. Some authors state it is useful for reducing platelet transfusions [
4,
5]. Deppe’s group showed NNT with TEG is 9 to reduce one unit of transfusion of any product, and NNT is 6.6 for FFP [
5]. In his study, platelet use was reduced by 21%. This only represented a drop in incidence from 20.5% to 19.5% after adoption of TEG, p=0.03 [
5].
In contrast, another study found the mean number of platelets transfused remained the same at 1.28 before instituting frequent TEG compared to 1.26 after [
2]. Their study also found that FFP transfusions dropped by about 3-fold, and packed red blood cells per patient dropped from 2.99 to 2.38, achieving a 40% decrease in mean units of any product transfused. Astonishingly, the opposite effect was noted in Canada at 12 hospitals studied in a randomized controlled trial (RCT) comprising 7402 patients. Fewer PRBC were transfused (RR 0.9, CI 0.85-0.98, p=0.02) but platelet transfusions were reduced the most (RR 0.77, CI 0.68-0.87). Stated another way, the Canadian study showed a 24% reduction in average number of platelets transfused per person and NNT 16.7 [
6]. Those authors summarized that an integrated transfusion algorithm reduced red blood cell transfusions, platelet transfusions, and major bleeding following cardiac surgery, without reducing fresh frozen plasma transfusion. Other research describes the TEG as
not helpful in discriminating coagulopathy in the presence of antiplatelet medications, during obstetrical, spine, aortic or cardiac surgery, and trauma. In cardiac surgery, many patients received 30mcg/kg recombinant Factor VIIa and other treatments in the same TEG arms, confounding the results [
7].
During very long courses of CPB, such as ECMO, rather than a TEG, a daily PT, PTT, fibrinogen, and CBC are recommended [
8]. During shorter CPB durations, a landmark trial compared TXA at 2mg/kg/hr vs 16mg/kg/hr [
9]. They demonstrated allogeneic red blood cell transfusion occurred in 333 of 1525 patients (21.8%) in the high-dose group and 391 of 1506 patients (26.0%) in the low-dose group (RR 0.84; P = 0.004). Outcomes of postoperative seizure, thrombotic events, kidney dysfunction, or death occurred in 17.6% of patients in the high-dose group and 16.8% of patients in the low-dose group, P = 0.003. Fourteen of the 15 prespecified secondary end points were not significantly different between groups, including seizure, which occurred in 1% in the high-dose group and 0.4% in the low-dose group (95% CI, -0.0% to 1.2%; P = 0.05). No decrease in platelet transfusions was demonstrated. A limitation of our investigation is that we administered only 1gram followed by 1mg/kg/hr in both groups, while double or triple that dose might be more effective.
The national shortage of platelets is severe [
10]. Our patients received an abundance of platelets in 2023, perhaps indicating a need for monitoring with POC-TEG, or they began with more unfavorable unmeasured characteristics. The American College of Surgeons (ACS) published trauma practice guidelines stating that clinical judgment remains the leading method, alongside administration of 6 PRBC, and at least 3 FFP, one Cryoprecipitate 10 or 20 pack, and only thereafter one platelet apheresis [
11]. The ACS recommends repeating platelet, fibrinogen, and hemoglobin counts every hour during massive transfusion, or until stable, and adding point-of-care TEG where available [
5]. Two weaknesses of our study are that our TEG requires two hours and delivery to the operating room of a unit of platelets varies widely depending upon severity of daily shortages. Platelets not immediately used require placement on an agitator machine (cost
$2500) maintained in an operating room. Possibly our patients received more platelets because once delivered they must be immediately used.
Other researchers examined over 12,000 cardiac surgeries and found no association between platelet transfusion and mortality (OR 1.28, 99% CI: 0.49-3.35) [
12,
13]. When matching 195 patients who received cryoprecipitate at a median time of 1.7 hours after arrival in the intensive care unit with 743 controls, there was no increased mortality (OR 1.1; 99%. CI 0.43-2.84) or kidney injury (OR 1.03). Decreased infections appeared in those who received platelets, which may be secondary to earlier administration synergistic with other blood components [
6,
12]. European practice guidelines list TEG as Class IIa, as helpful to “consider.” However, their larger hospitals are equipped with POC-TEG [
14]. Thromboembolic events were decreased with TEG, but length of stay and intensive care unit stay did not change [
5].
5. Limitations
The greatest weakness in the present investigation is a relatively small sample size, and a lack of TEG, fibrinogen or prothrombin levels. Saillant comments that while TEG is important, “we routinely overlook hemostatic adjuncts that may reduce blood loss, such as RiaSTAP,” a fibrinogen concentrate [
10]. Saillant also recommends drawing more frequent platelet counts (giving platelets if bleeding and under 100), but first correcting fibrinogen levels (giving cryoprecipitate if under 200) and prothrombin times or INR (giving FFP if greater than 1.5). The greatest strength is that, to our knowledge, this is the first study that describes
timing between separation from bypass and each platelet transfusion.
6. Conclusions
Patient blood management (PBM) flowcharts individualized to a cardiac surgery center need to include a specific TXA dose. In one study of 27,729 patients, only medium or high dose TXA resulted in less patients exposed to platelet transfusion, although not lower average units per patient [
16]. A meta-analysis showed a bolus technique is equally effective in reducing the average transfused red cells by one unit as is a bolus followed by a continuous infusion [
17]. A large randomized controlled trial showed that high doses such as 80mg/kg versus moderate doses of 50mg/kg are associated with average transfused units dropping from 4.1 to 2.5, p=0.02 [
18]. These investigators defined low dose as 1g bolus and 400mg/hr plus 0.5g in the CPB prime and high dose as 30mg/kg followed by 15mg/kg/hour and 2mg/kg in the prime [
19].
Second, PBM flowcharts need to include trigger values of hemoglobin, fibrinogen, and prothrombin time, or TEG before transfusion of each type of component. Our population unfortunately has high incidence of malnutrition, leading to microvascular bleeding and more platelet transfusions [
20,
21]. However, if hemoglobin is too high, viscosity is a risk factor for coronary thrombosis [
22]. Fibrinogen has been shown to be the most important component to thicken blood and slow flow [
23].
To further discriminate the effect of fibrinogen upon transfusion, investigators studied TEG during bypass before and after transfusion of platelet versus cryoprecipitate, containing fibrinogen [
24]. They found platelets improved alpha and maximal amplitude (MA), markers of fibrinogen and platelet function, respectively. Cryoprecipitate after CPB also improved MA. In cyanotic congenital heart disease, TEG showed hypofibrinogenemia but only the Sonoclot unmasked dysfunctional platelets [
25]. Interpretation of one institution’s TEG should be within the context of their comprehensive PBM [
26].
Similar in size to our analysis, a study of 28 infants without TEG retrospectively, followed by 40 with TEG prospectively demonstrated fewer platelet transfusions (36+/-12 vs. 49+/-27ml/kg, p=0.028) [
27]. Reviews have recently elucidated the difference of TEG in determining if coagulopathy stems from platelet dysfunction or hypofibrinogenemia [
28,
29]. A relative risk of 0.77 for platelet transfusion was found during a systematic database review of various TEG technologies [
30]. When massive trauma did not permit time for examination of lab values, the 12 PRBC:6 FFP:1 platelet ratio was not inferior to 6:6:1 ratio for mortality at 30 days; nevertheless, such anemia may result in more platelet transfusions [
31]. Instead of waiting for hours for laboratory values, we suggest creating a local PBM algorithm beginning with higher TXA doses and ending CPB with stat fibrinogen and prothrombin time (or, better yet, POC-TEG) triggering transfusion of appropriate types of blood components.
Funding
This research received no external funding.
Acknowledgments
The authors want to acknowledge Runhua Shi, MD, PhD Statistician, and Professor of Internal Medicine, Louisiana State University Health Sciences Center at Shreveport for his contributions in generating statistical methods, tables, and figures in this manuscript. Moreover, they are grateful to the Paolo Procacci Foundation for the support in the publishing process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chegini A. Evaluating the importance of patient blood management during COVID-19 pandemic. Anesth Pain Med. 11(6):e112910. 2022. [CrossRef]
- Fleming K, Redfern RE, March RL, et al. TEG-directed transfusion in complex cardiac surgery: Impact on blood product usage. J Extra Corpor Technol. 49(4):283-290. 2017. [CrossRef]
- Zaffar N, Joseph A, Mazer CD, et al. The rationale for platelet transfusion during cardiopulmonary bypass: an observational study. Can J Anaesth. 60(4):345-354. 2013. [CrossRef]
- Hasan O, Tung RC, Freeman H, et al. Thromboelastography after cardiopulmonary bypass: Does it save blood products? Kans J Med. 15:27-30. 2022.
- Deppe AC, Weber C, Zimmermann J, et al. Point-of-care thromboelastography/thromboelastometry-based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. J Surg Res. 203(2):424-433. 2016. [CrossRef]
- Karkouti K, Callum J, Wijeysundera DN, et al. Point-of-Care hemostatic testing in cardiac surgery: A stepped-wedge clustered randomized controlled trial. Circulation. 134(16):1152-1162. 2016.
- Erdoes G, Faraoni D, Koster A, et al. Perioperative considerations in management of the severely bleeding coagulopathic patient. Anesthesiology. 138(5):535-560. 2023. [CrossRef]
- Skidmore KL, Rajabi A, Nguyen, et al. Veno-venous extracorporeal membrane oxygenation: Anesthetic considerations in clinical practice. Anesthesiol Pain Med. In press. 2023. [CrossRef]
- Shi J, Zhou C, Pan W, et al. Effect of high- vs low-dose tranexamic acid infusion on need for red blood cell transfusion and adverse events in patients undergoing cardiac surgery: The OPTIMAL randomized clinical trial. JAMA. 328(4):336-347. 2022.
- Saillant NN, Kornblith LZ, Moore H, et al. The national blood shortage-An impetus for change. Ann Surg. 275(4)641-643. 2022. [CrossRef]
- TQIP: Massive transfusion in trauma guidelines – Google Scholar. Accessed September 27, 2023. https://scholar.google.com/scholar_lookup?title=ACS+TQIP+Massive+Transfusion+in+Trauma+Guidelines&publication_year=2015&.
- Fletcher CM, Hinton JV, Xing Z, et al. Platelet transfusion after cardiac surgery. J Cardiothorac Vasc Anesth. 37(4):528-538. 2023. [CrossRef]
- Hinton JV, Xing Z, Fletcher CM, et al. Cryoprecipitate transfusion after cardiac surgery. Heart Lung Circ. 32(3):414-423. 2023. [CrossRef]
- Boer C, Meesters MI, et al. 2017. Task force on patient blood management for adult cardiac surgery of the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Cardiothoracic Anesthesiology (EACTA). Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth. 32(1):88-120. 2018.
- Research for FDA. Published online March 7, 2022. Accessed September 27, 2023. https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/riastap.
- Lin PS, Yao YT, Tian LJ, et al. Cardiovascular Anesthesia (EICA) Group. The efficacy and safety of intravenous administration of tranexamic acid in patients undergoing cardiac surgery: Evidence from a single cardiovascular center. Medicine (Baltimore). 19;102(20):e33819. 2023. [CrossRef]
- Guo J, Gao X, Ma Y, et al. Different dose regimens and administration methods of tranexamic acid in cardiac surgery: a meta-analysis of randomized trials. BMC Anesthesiol. 19:129. 2019.
- Hodgson S, Larvin JT, Dearman C. What dose of tranexamic acid is most effective and safe for adult patients undergoing cardiac surgery? Interact Cardiovasc Thorac Surg. Sep;21(3):384-8. 2015.
- Couture P, Lebon JS, Laliberte E, et al. Low-dose versus high-dose tranexamic acid reduces the risk of nonischemic seizures after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. Oct;31(5):1611-1617. 2017. [CrossRef]
- Kuhn V, Diederich L, Keller TCS 4th, et al. Red blood cell function and dysfunction: Redox regulation, nitric oxide metabolism, anemia. Antioxid Redox Signal. May 1;26(13):718-742. 2017.
- Jeong SK, Cho YI, Duey M, et al. Cardiovascular risks of anemia correction with erythrocyte stimulating agents: should blood viscosity be monitored for risk assessment? Cardiovasc Drugs Ther. Apr;24(2):151-60. 2010.
- Beris AN, Horner JS, Jariwala S, et al. Recent advances in blood rheology: a review. Soft Matter. Dec 8;17(47):10591-10613. 2021. [CrossRef]
- Varchanis S, Dimakopoulos Y, Wagner C, et al. How viscoelastic is human blood plasma? Soft Matter. May 30;14(21):4238-4251. 2018.
- Tirotta CF, Lagueruela RG, Salyakina D, et al. Interval changes in ROTEM values during cardiopulmonary bypass in pediatric cardiac surgery patients. J Cardiothorac Surg. Jul 22;14(1):139. 2019. [CrossRef]
- Bhardwaj V, Malhotra P, Hasija S, et al. Coagulopathies in cyanotic cardiac patients: An analysis with three point of care testing devices. Ann Card Anaesth. Apr-Jun:20(2)212-218. 2017.
- Gorlinger K, Dirkmann D, Hanke AA. Potential values of transfusion protocols in cardiac surgery. Curr Opin Anaesthesiol. Apr;26(2):230-43. 2013. [CrossRef]
- Naguib AN, Carrillo SA, Corridore M, et al. A ROTEM-guided algorithm aimed to reduce blood product utilization during neonatal and infant cardiac surgery. J Extra Corpor Technol. Jun;55(2):60-69. 2023. [CrossRef]
- Agarwal S, Abdelmotieleb M. Viscoelastic testing in cardiac surgery. Oct;60 Suppl 6:S52-S60. 2020. [CrossRef]
- Demailly Z, Wurtz V, Barbay V, et al. Point-of-care viscoelastic hemostatic assays in cardiac surgery patients: Comparison of thromboelastography 6S, Thromboelastometry Sigma, and Quantra. J Cardiothorac Vasc Anesth. Jun;37(6):948-955. 2023. [CrossRef]
- Whiting P, Al M, Westwood M, et al. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of hemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess. Jul;19(58):1-228. 2015.
- Holcomb JB, Tilley BC, Baraniuk S, et al. “Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA Feb 3;313(5):471-82. 2015.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).