Submitted:
13 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methodology
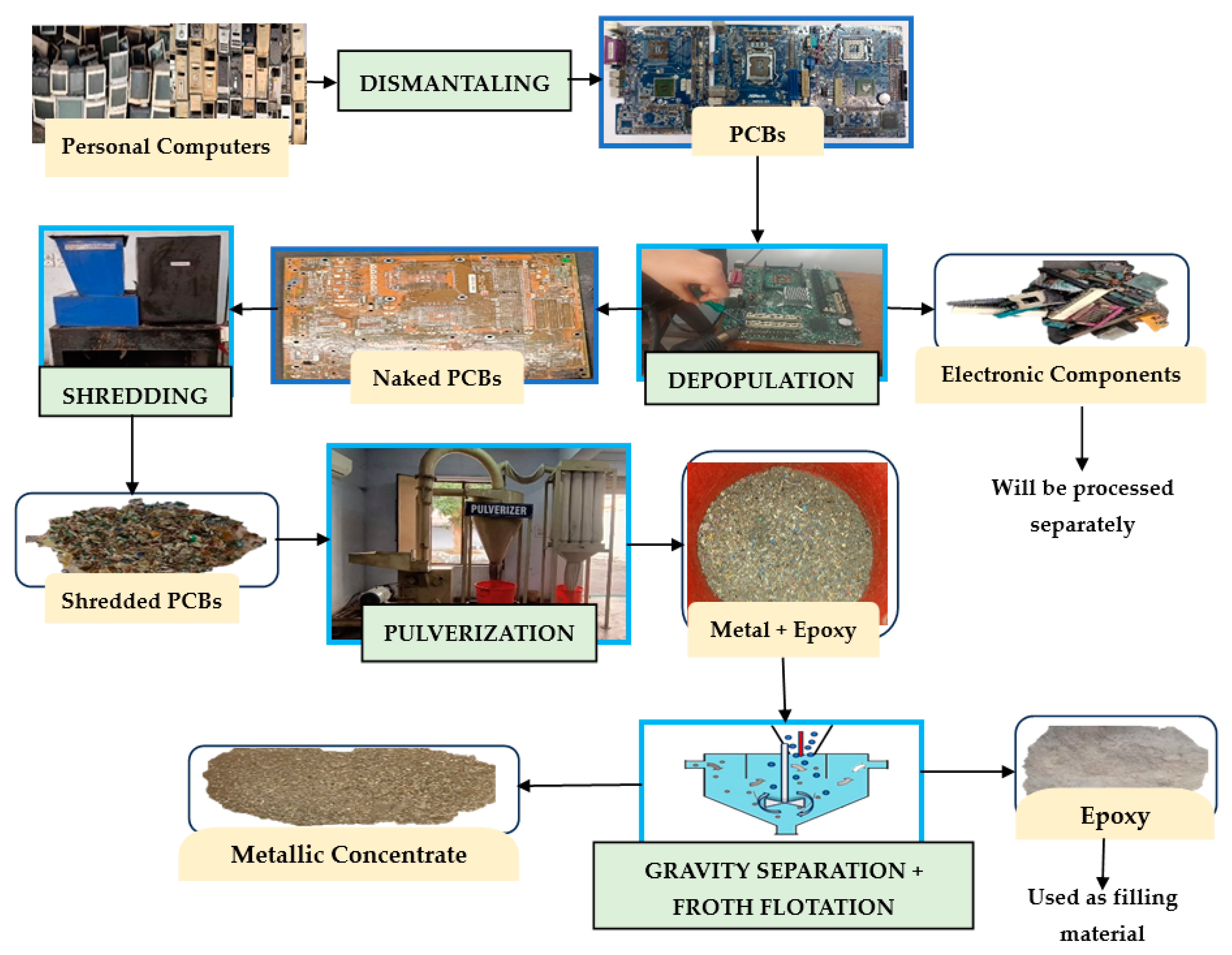
2.1. Raw Material and Chemical reagents
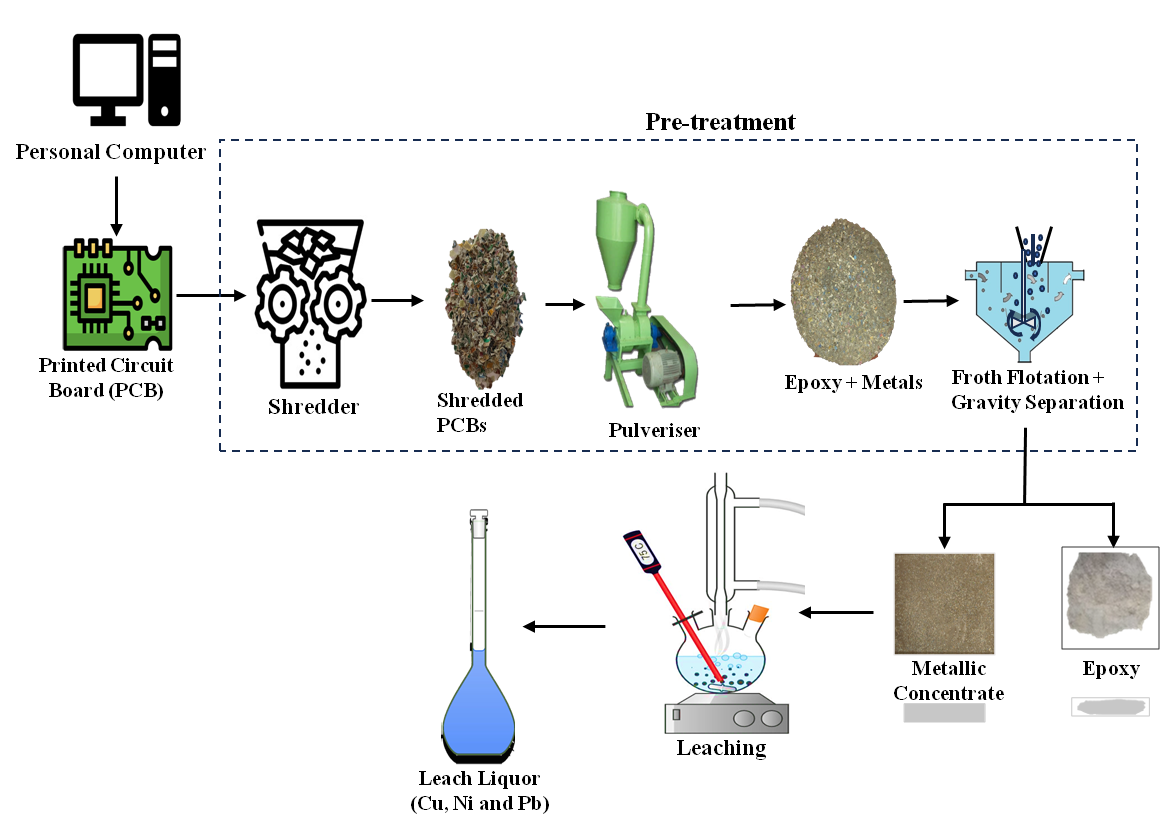
2.2. Pre-treatment Processes
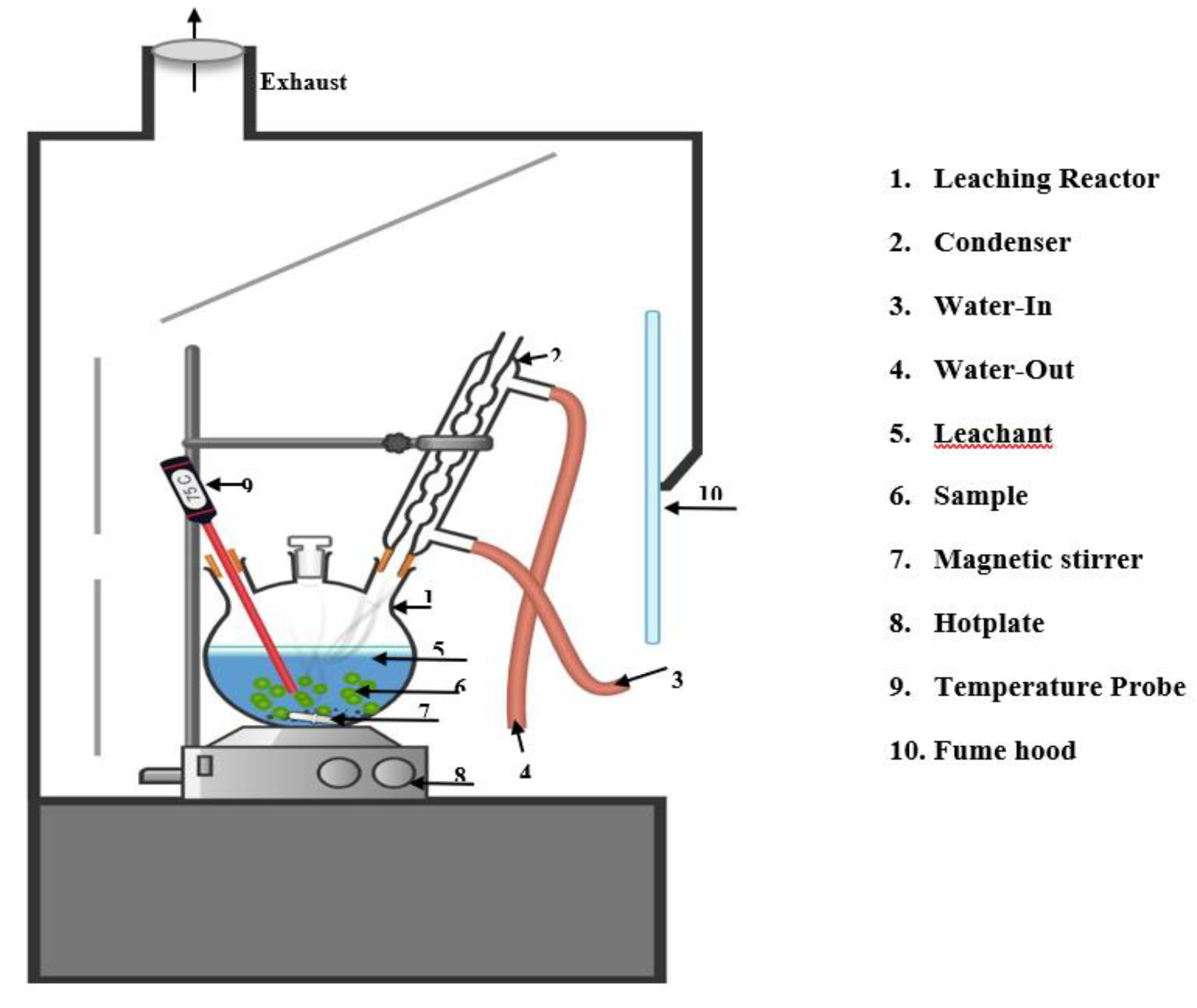
2.3. Methodology
2.4. Analytical procedure
3. Results and Discussion
3.1. Leaching Studies
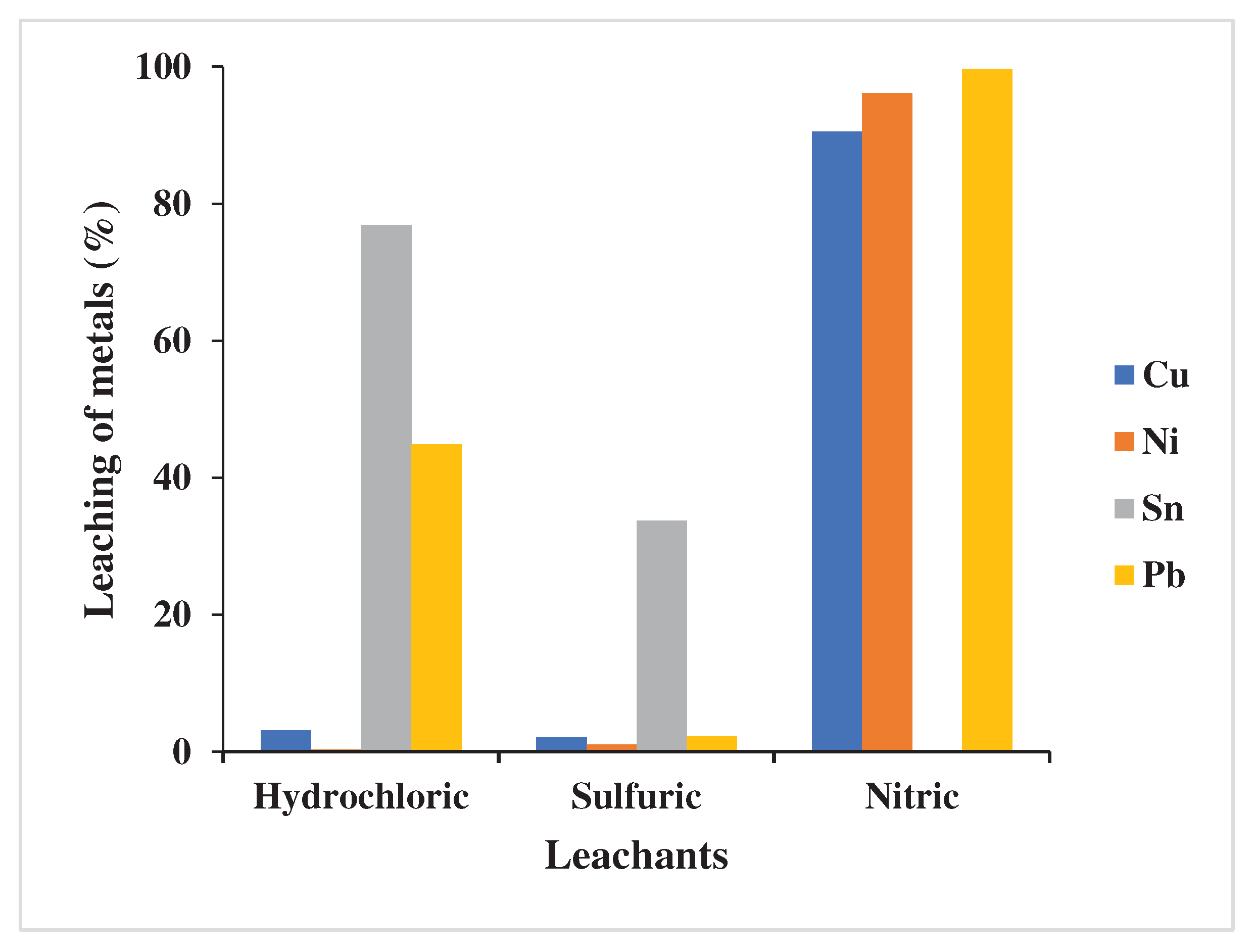
3.1.1. Selection of leachant
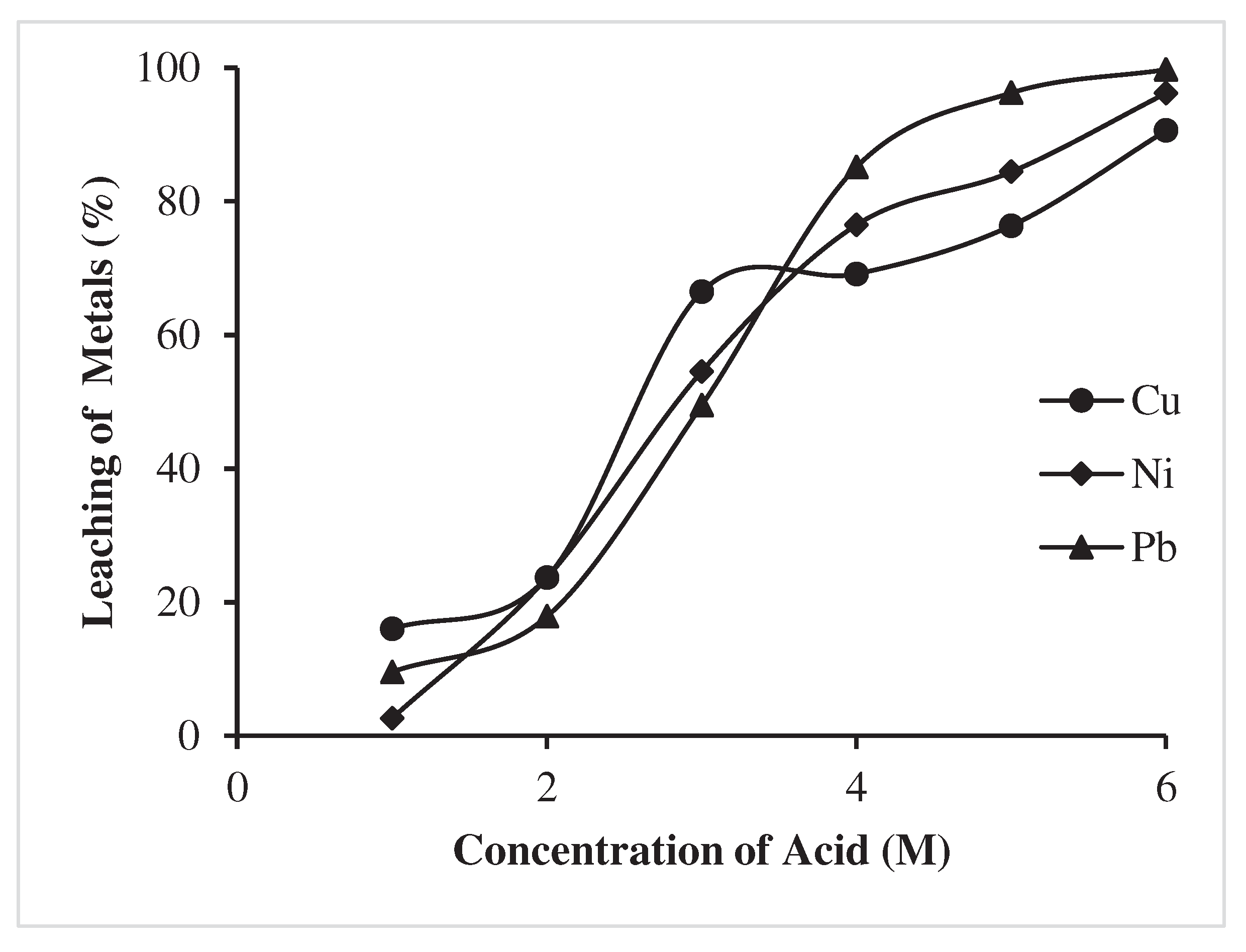
3.1.2. Effect of leachant concentration
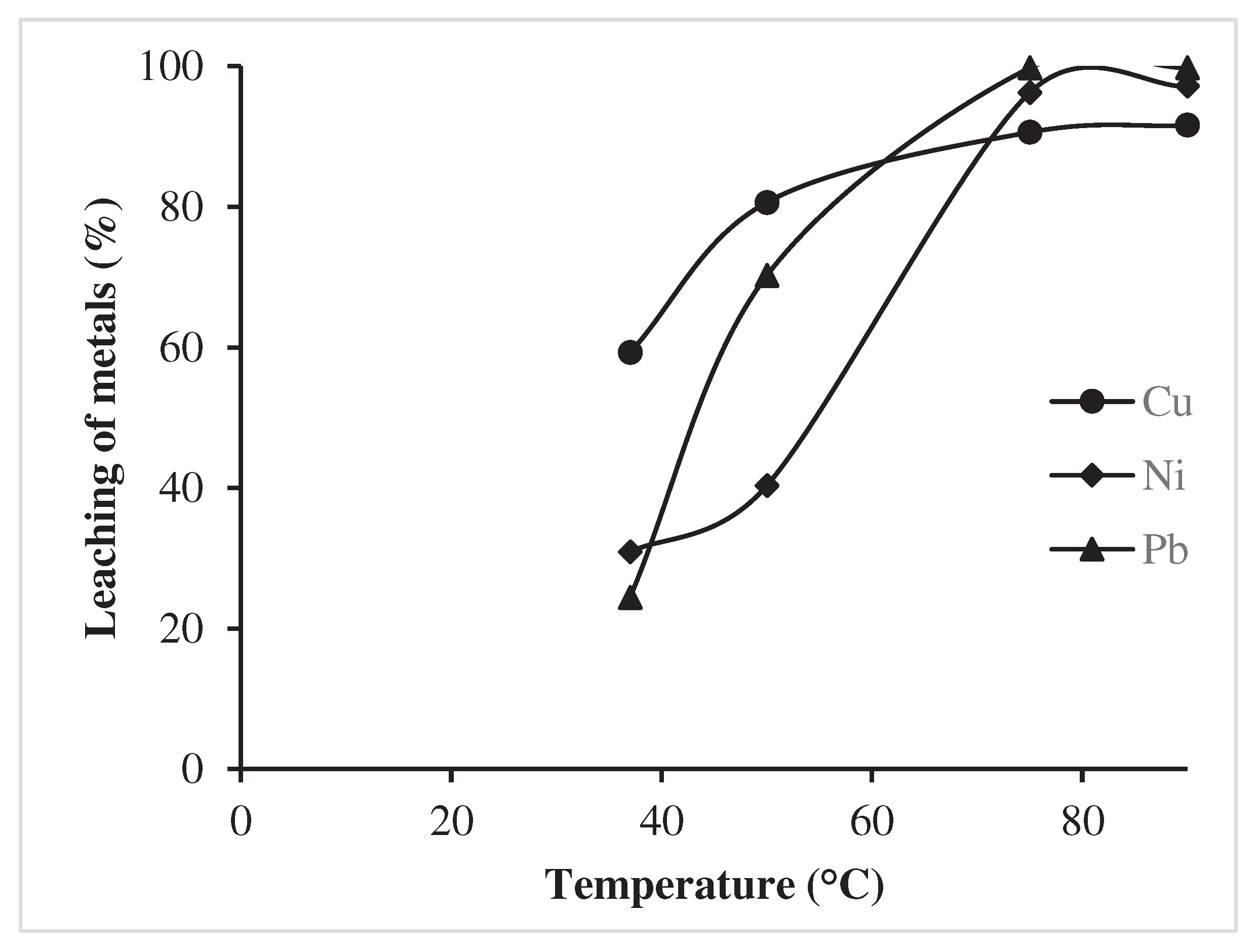
3.1.3. Effect of temperature
3.1.4. Effect of time
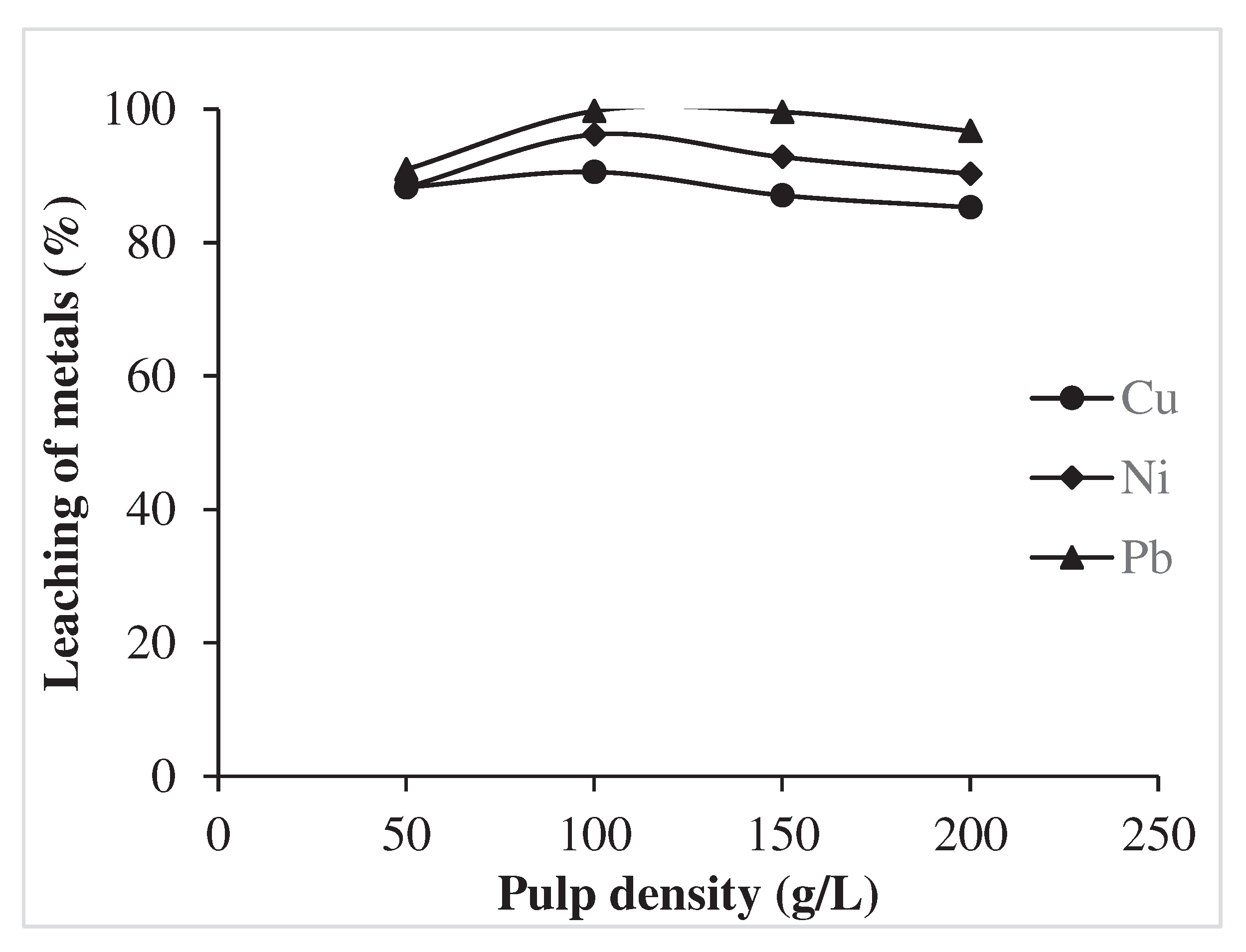
3.1.5. Effect of pulp density
3.2. Characterization studies
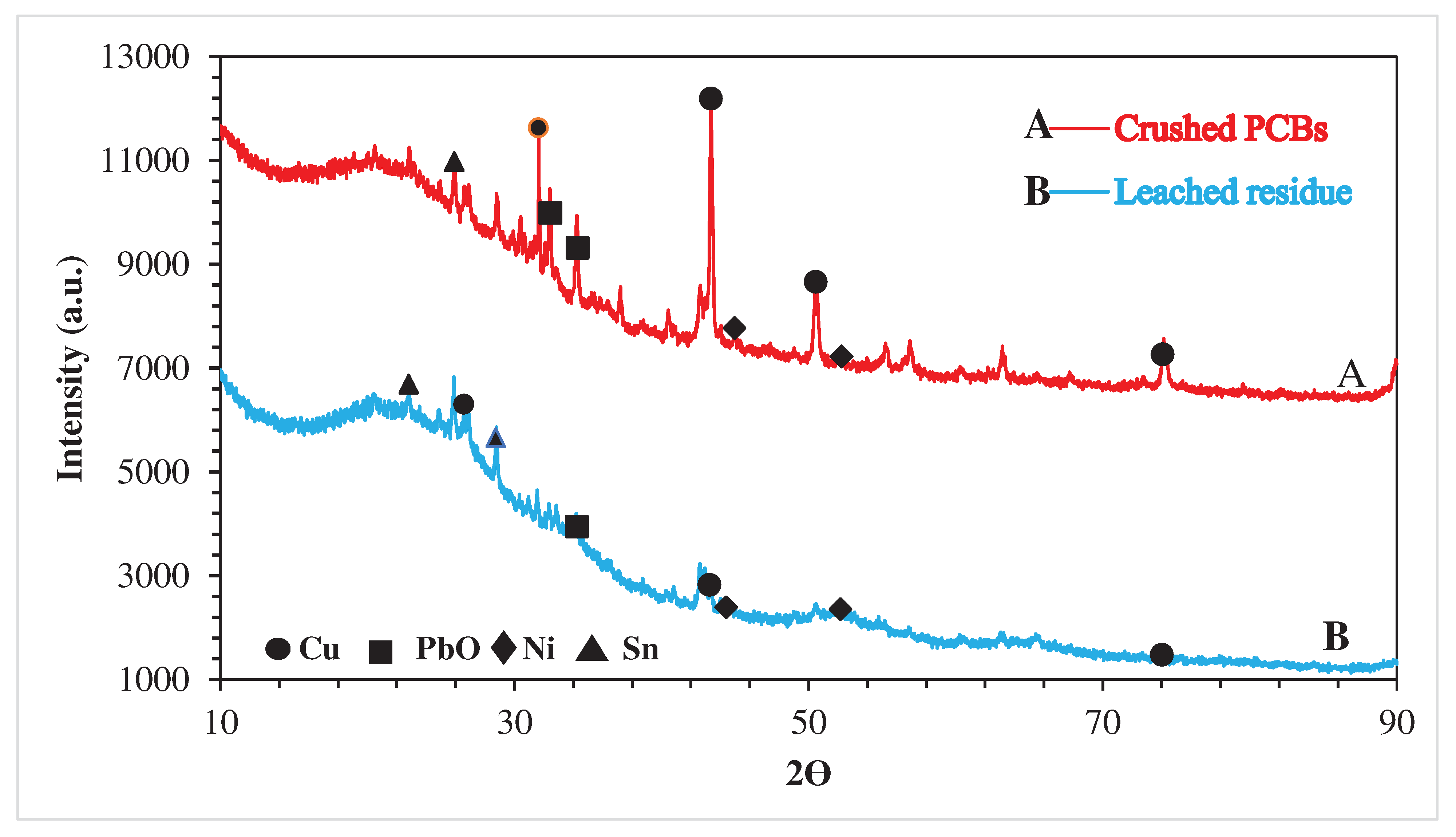
3.2.1. X-ray Powder Diffraction (XRD)
3.2.2. Scanning Electron Microscopy (SEM-EDS)
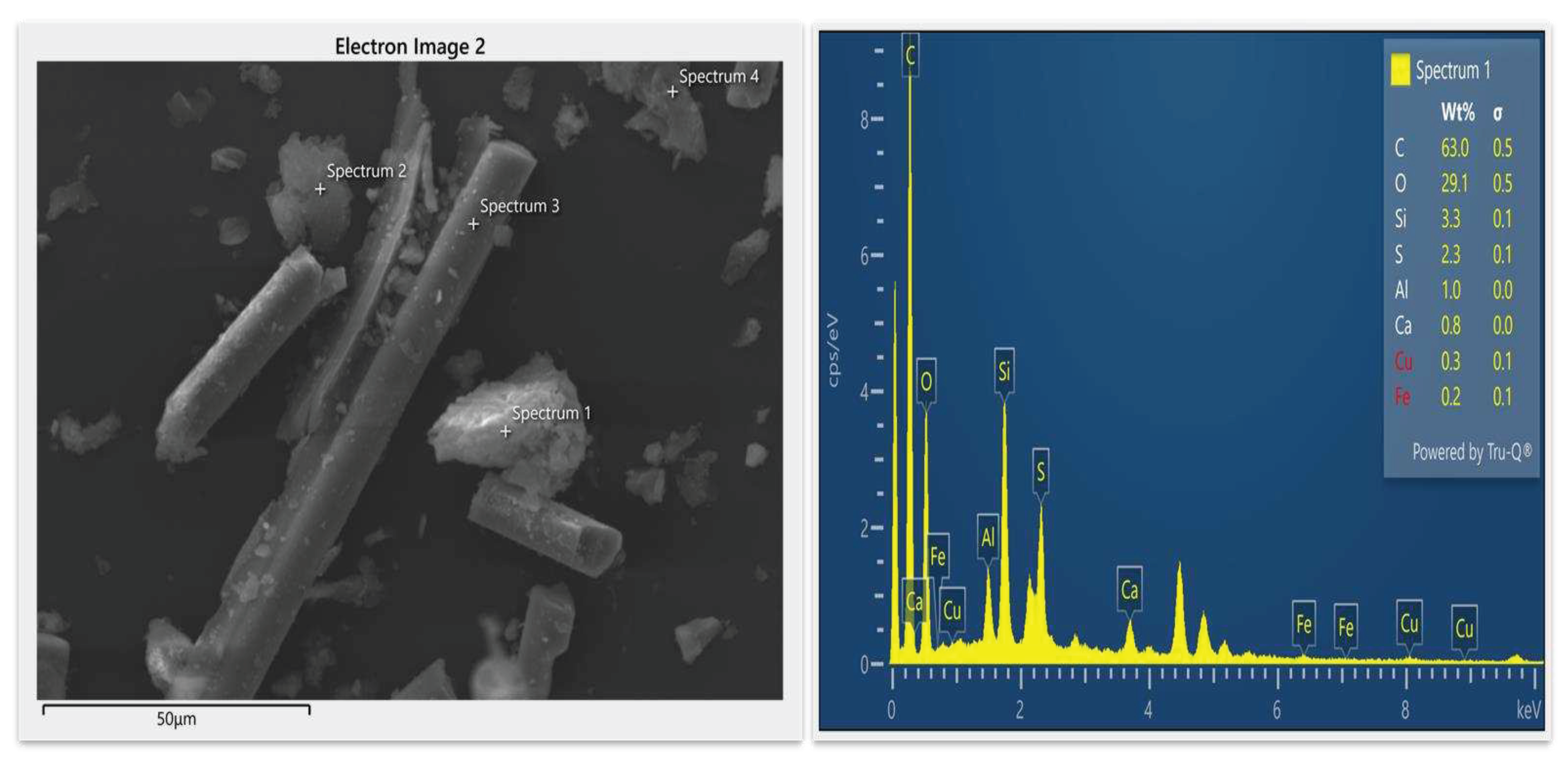
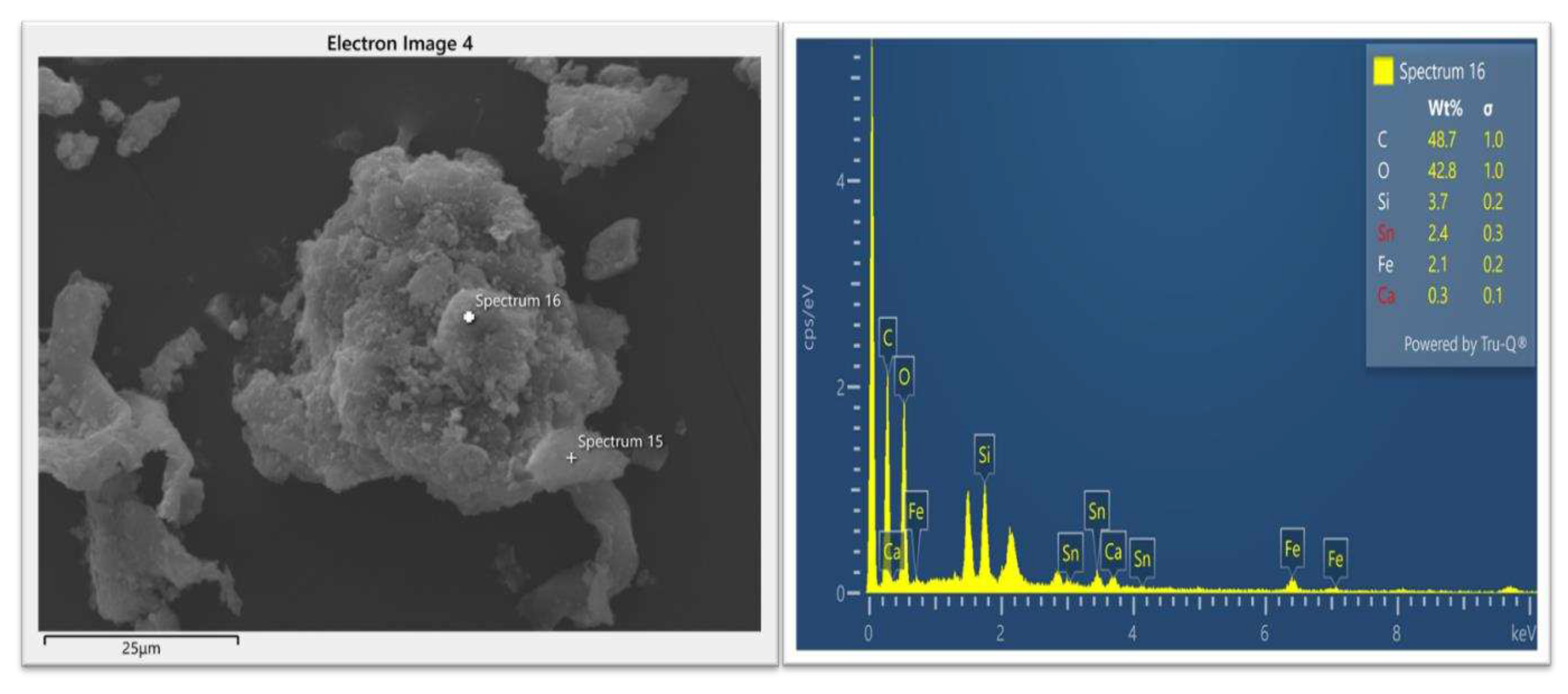
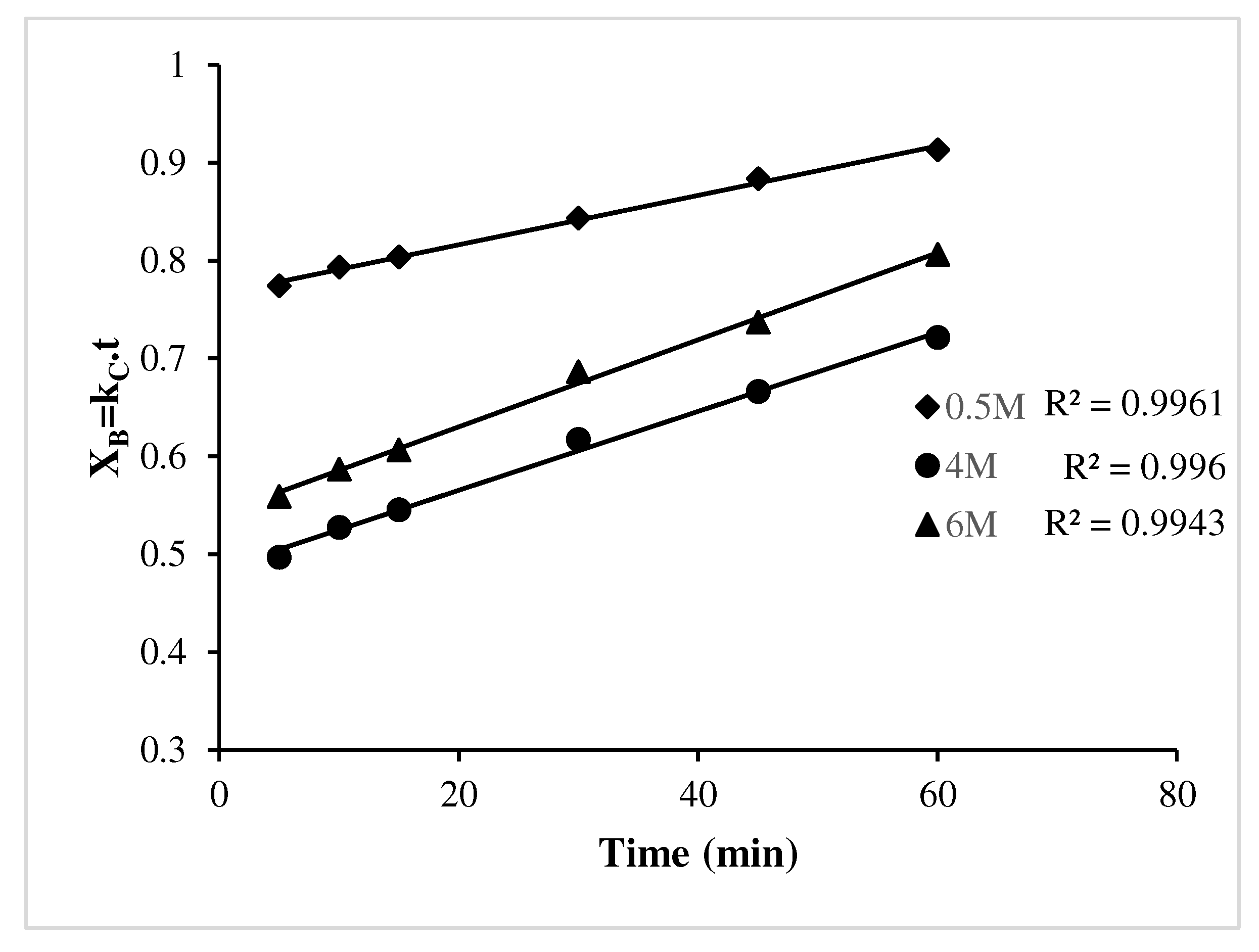
3.3. Scientific validation of leaching
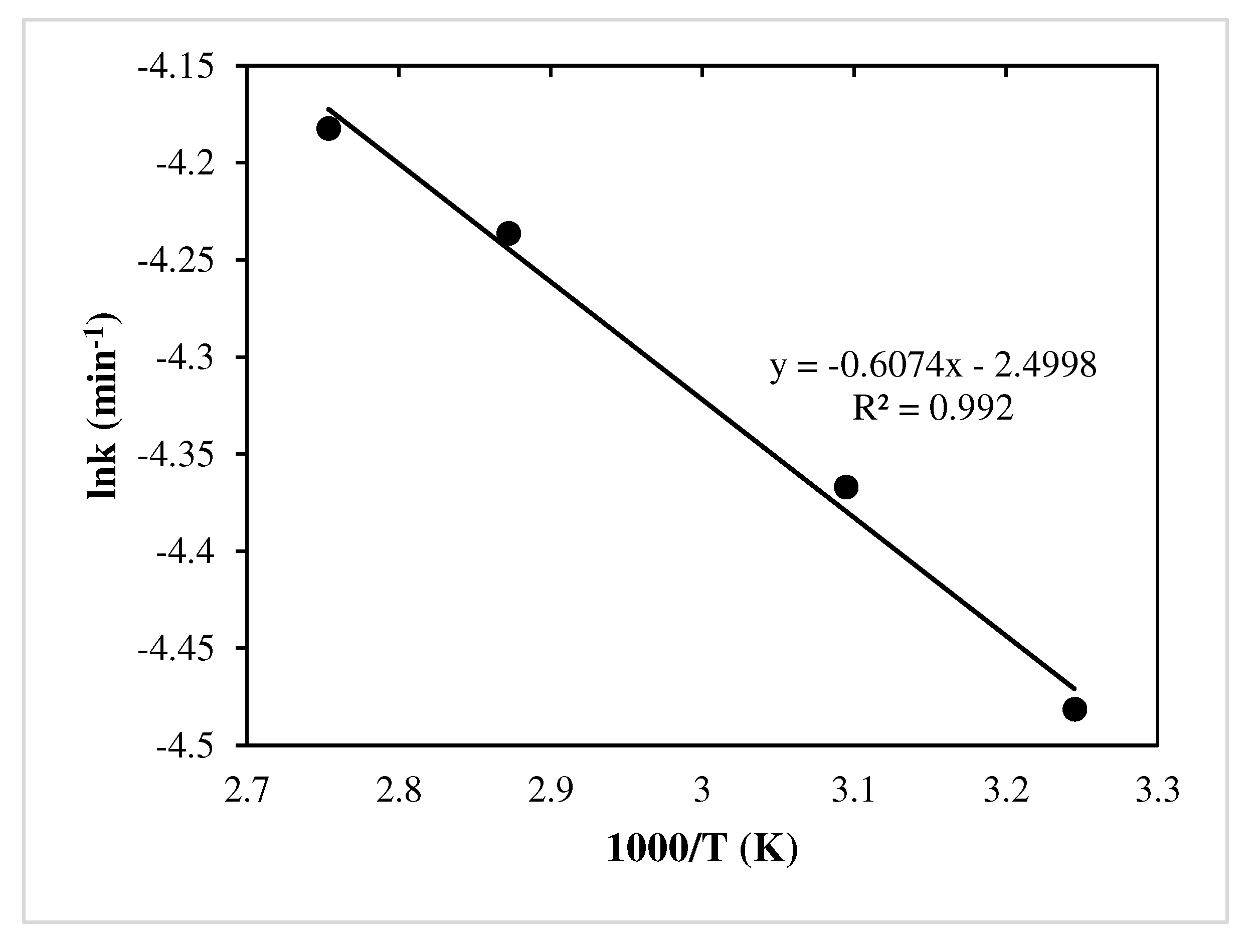
Arrhenius Equation:
4. Conclusion
- The metals liberation was found to be ~ 99.90%. However, epoxy resins or plastic were removed 99.99%.
- The optimum condition for recovery or dissolution of 90.58% Cu, 96.19% Ni and 99.72% Pb were found at 6M Nitric acid, temperature 75°C, pulp density 100 g/L and time 60 min.
- Film diffusion control dense constant size small particles-all geometries (XB = kc.t) was found to be fitted well with the leaching kinetics for Cu.
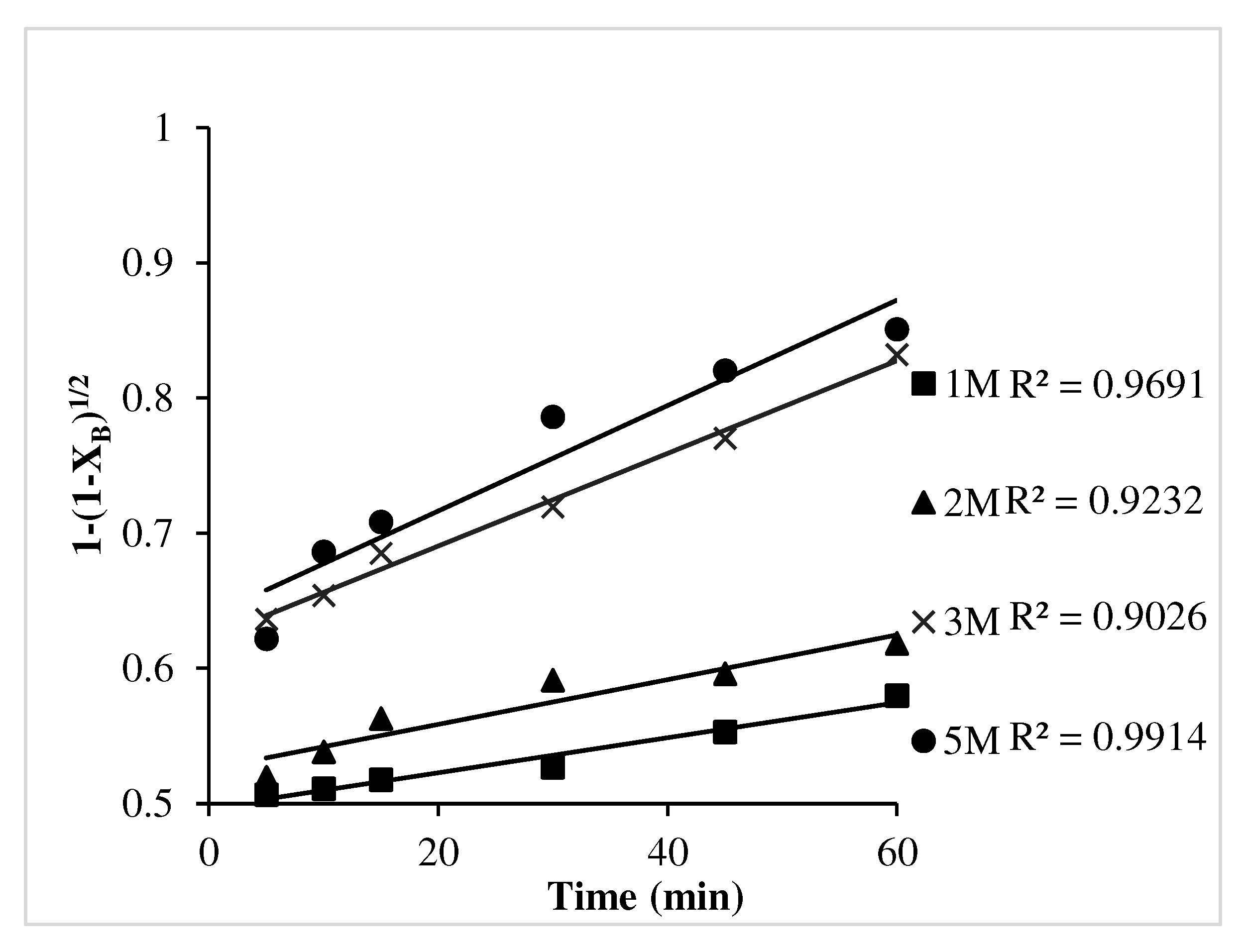
- Chemical reaction control dense constant size model (1-(1-XB)1/2) was found to be fitted well with the leaching kinetics for Ni.
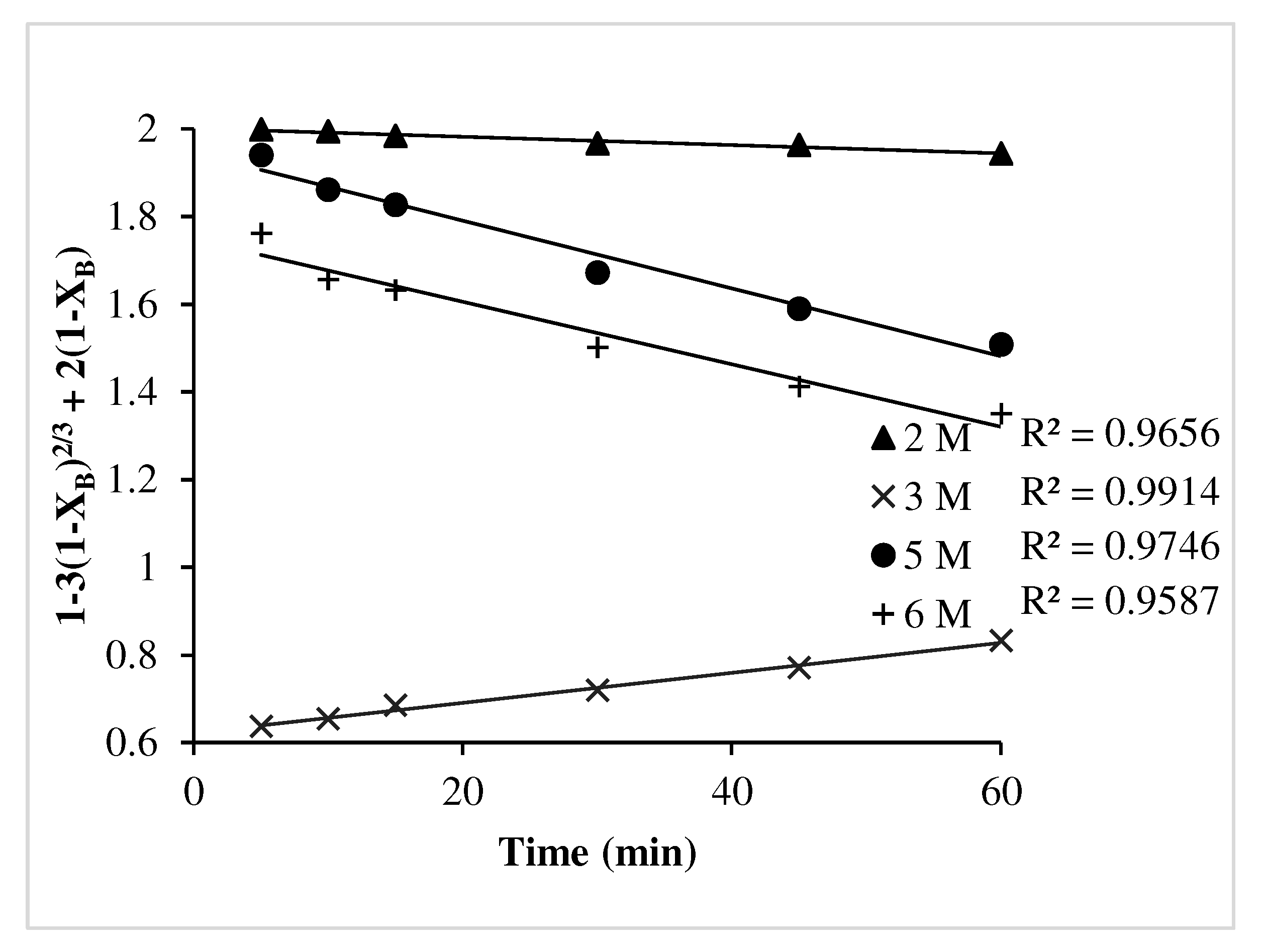
- Ash diffusion control dense constant size model (1-3(1-XB)2/3 + 2(1-XB)) was found to be fitted well with the leaching kinetics for Pb.
- The activation energy is found 19.42 kJ/ mol for Cu.
- Nitric leaching in close-loop system can result in high metal recovery rates or a larger percentage of the target metal could be leached without emitting obnoxious gases. Since nitric acid is a strong oxidizing agent that can facilitate metal dissolution faster. The efficiency is crucial for economic and environmental reasons, as it reduces waste and increases the overall yield of valuable metals.
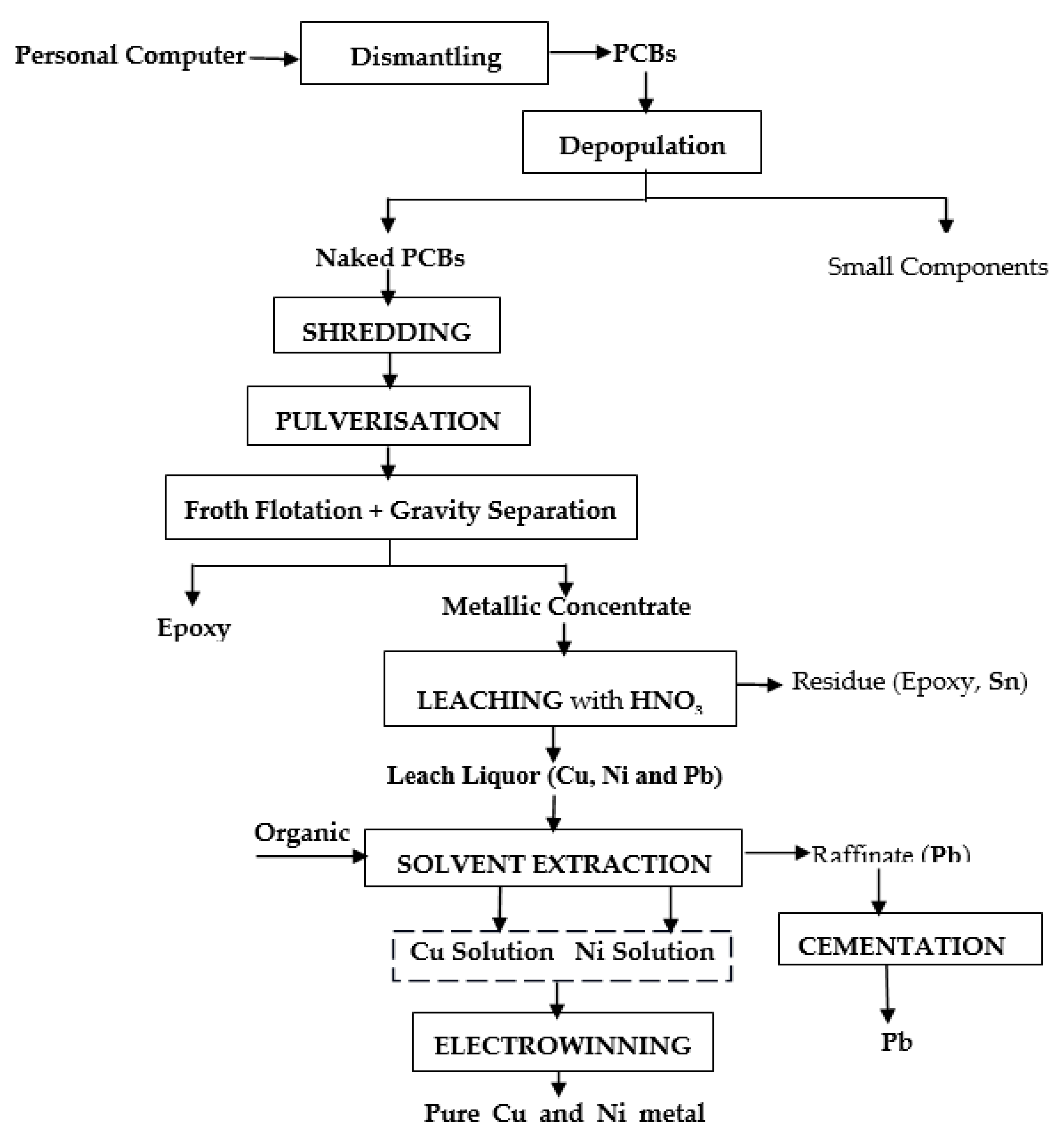
- The generated leach liquor could further be processed using solvent extraction, cementation and electrowinning process to get purified metal product. The complete process flow-sheet is shown in figure 14.
- The process is viable and eco-friendly at laboratory scale and has potential for commercial exploitation. The leached residues and effluents produced during the experiment will be properly treated and can be reused or using standard procedure before their final disposal to the environment.
- Whereas the complete recycling will not only reduce the loss of valuable metals but will also aid in the establishment of an organized sector for e-waste recycling, taking into account environmental regulations, and raising public awareness about the loss of valuables caused by the dumping of such wastes into the environment.
Acknowledgments
Disclosure statement
References
- Gande, V. V.; Vats, S.; Bhatt, N.; & Pushpavanam, S. Sequential recovery of metals from waste printed circuit boards using a zero-discharge hydrometallurgical process. Cleaner Engineering and Technology, 2021, 4, 100143. [CrossRef]
- Ahirwar, R.; Tripathi, A. K. E-waste management: A review of recycling process, environmental and occupational health hazards, and potential solutions. Environmental Nanotechnology, Monitoring & Management, 2021, 15, 100409. [CrossRef]
- Hao, J.; Wang, Y.; Wu, Y.; Guo, F. Metal recovery from waste printed circuit boards: A review for current status and perspectives. Resources, Conservation and Recycling, 2020, 157, 104787. [CrossRef]
- Chancerel, P., & Rotter, S. Recycling-oriented characterization of small waste electrical and electronic equipment. Waste Management, 2009, 29(8), 2336–2352. [CrossRef]
- Zhang, X.; Tang, J.; Gao, P.; Ding, Y. A review on hydrometallurgical recovery of precious and base metals from waste printed circuit boards. Journal of Hazardous Materials, 2020 ,400, 123051.
- Rocchetti, L.; Amato, A.; Beolchini, F. Printed circuit board recycling: A patent review. Journal of Cleaner Production, 2018, 178, 814–832. [CrossRef]
- Choubey, P. K.; Panda, R.; Jha, M. K.; Lee, J. C.; Pathak, D. D. Recovery of copper and recycling of acid from the leach liquor of discarded Printed Circuit Boards (PCBs), Separation and Purification Technology, 2015, 156, 269-275.
- Jha, M. K., Choubey, P. K., Jha, A. K., Kumari, A., Lee, J., Kumar, V., & Jeong, J. Leaching studies for tin recovery from waste e-scrap. Waste Management, 2012, 32(10), 1919–1925. [CrossRef]
- Cui, H; Anderson, C. G. Literature Review of Hydrometallurgical Recycling of Printed Circuit Boards (PCBs). J Adv Chem Eng, 2016, 6, 142. [CrossRef]
- Barragan, J. A.; Ponce de León, C.; Alemán Castro, J. R.; Peregrina-Lucano, A.; Gómez-Zamudio, F.; & Larios-Durán, E. R. Copper and Antimony Recovery from Electronic Waste by Hydrometallurgical and Electrochemical Techniques. ACS Omega, 2020, 5(21), 12355–12363. [CrossRef]
- Elbashier, E.; Mussa, A.; Hafiz, M.; & Hawari, A. H. Recovery of rare earth elements from waste streams using membrane processes: An overview. Hydrometallurgy, 2021, 204, 105706. [CrossRef]
- Nekouei, R. K.; Pahlevani, F; Mayyas, M; Maroufi, S; Sahajwalla, V. Direct transformation of waste printed circuit boards into high surface area t-SnO2 for photocatalytic dye degradation, Journal of Environmental Chemical Engineering, 2019, 7( 3),103133, . [CrossRef]
- Chen, Y.; Liang, S.; Xiao, K.; Hu, J.; Hou, H.; Liu, B.; Yang, J. A cost-effective strategy for metal recovery from waste printed circuit boards via crushing pretreatment combined with pyrolysis: Effects of particle size and pyrolysis temperature. Journal of Cleaner Production, 2021, 280, 124505. [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Panda, S. Intensified acidophilic bioleaching of multi-metals from waste printed circuit boards (WPCBs) of spent mobile phones. Journal of Chemical Technology & Biotechnology, 2020. [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E. Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Minerals Engineering, 2012, 25(1), 28–37. [CrossRef]
- Ippolito, N.M.; Passadoro, M.; Ferella, F.; Pellei, G.; Vegliò, F. Recovery of Metals from Printed Circuit Boards by Gold-REC 1 Hydrometallurgical Process. Sustainability, 2023, 15, 7348. [CrossRef]
- Panda, R.; Jha, M.K.; Dinkar, O.S.; Pathak, D.D. Reclamation of Precious Metals from Small Electronic Components of Computer Hard Disks. Rare Metal Technology 2020. The Minerals, Metals & Materials Series. Springer, Cham. [CrossRef]
- Gámez, S.; Garcés, K.; de la Torre, E.; Guevara, A. Precious metals recovery from waste printed circuit boards using thiosulfate leaching and ion exchange resin. Hydrometallurgy. 2019. [CrossRef]
- Gulliani, S.; Volpe, M.; Messineo, A.; Volpe, R. Recovery of metals and valuable chemicals from waste electric and electronic materials: a critical review of existing technologies. Royal Society of Chemistry RSC Sustainability, 2023, 1, 1085–1108. [CrossRef]
- Rao, M. D.; Singh, K. K.; Morrison, C. A.; Love, J. B. Optimization of process parameters for the selective leaching of copper, nickel and isolation of gold from obsolete mobile phone PCBs, Cleaner Engineering and Technology, 2021, 4, 100180. [CrossRef]
- Kumar, M.;Lee, J. C.;Kim, M.S.; Jeong, J.;Yoo, K. Leaching of metals from waste printed circuit boards (WPCBs) using sulfuric and nitric acids. Environmental engineering and management journal, 2014, 13. 2601-2607. [CrossRef]
- Birloaga, I. De Michelis, F. Ferella, M. Buzatu, F. Veglio, Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery, Waste Manag., 2013, 33 (4), 935–941, . [CrossRef]
- Y. Yazici and H. A. Deveci, Extraction of metals from waste printed circuit boards (WPCBs) in H2SO4 CuSO4 NaCl solutions, Hydrometallurgy, 2013, 139, 30–38. [CrossRef]
- Behnamfard, M. Mehdi and F. Veglio, Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation, Waste Manage., 2013, 33(11), 2354–2363. [CrossRef]
- D. Bas, H. Deveci and E. Y. Yazici, Treatment of manufacturing scrap TV boards by nitric acid leaching, Sep. Purif. Technol., 2014, 130(2), 151–159. [CrossRef]
- Y. J. Park and D. J. Fray, Recovery of high purity precious metals from printed circuit boards, J. Hazard. Mater., 2009, 164(2–3), 1152–1158, . [CrossRef]
- Ashiq, J. Kulkarni, M. Vithanage, Hydrometallurgical Recovery of Metals from E-waste, Electron. Waste Manage. Treat. Tech, 2019, l., 225–246. [CrossRef]
- Akcil, C. Erust, C.S. Gahan, M. Ozgun, M. Sahin, A. Tuncuk, Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants–A review, Waste Manag., 2015, 45, 258–271, . [CrossRef]
- Birloaga, V. Coman, B. Kopacek, F. Veglio, An advanced study on the hydrometallurgical processing of waste computer printed circuit boards to extract their valuable content of metals, Waste Manage., 2014, 34 (12), 2581– 2586, . [CrossRef]
- J. Li and J. D. Miller, Reaction kinetics of gold dissolution in acid thiourea solution using ferric sulfate as oxidant, Hydrometallurgy, 2007, 89(3), 279–288. https://www.sciencedirect.com/science/article/pii/S0304386X07001697.
- M. Gurung, B. Adhikari, H. Kawakita, K. Ohto, K. Inoue and S. Alam, Selective Recovery of Precious Metals from Acidic Leach Liquor of Circuit Boards of Spent Mobile Phones Using Chemically Modified Persimmon Tannin Gel, Ind. Eng. Chem. Res., 2012, 51, 11901–11913, . [CrossRef]
- Tesfaye, F., Lindberg D. and Hamuyuni, J., Valuable Metals and Energy Recovery from Electronic Waste Streams, Energy Technology, 2017, 103-116. [CrossRef]
- G. Liu, De’an Pan, Y. Wu, H. Yuan, L.u. Yu, W. Wang, An integrated and sustainable hydrometallurgical process for enrichment of precious metals and selective separation of copper, zinc, and lead from a roasted sand, Waste Manage., 2021, 132, 133–141, . [CrossRef]
- Gulliani, Sahil and Volpe, Maurizio and Messineo, Antonio and Volpe, Roberto, Recovery of metals and valuable chemicals from waste electric and electronic materials: a critical review of existing technologies, RSC Sustain.,2023,1,5,1085-1108", RSC. [CrossRef]
- F.-R. Xiu, H. Weng, Y. Qi, G. Yu, Z. Zhang, F.-S. Zhang, M. Chen, A novel recovery method of copper from waste printed circuit boards by supercritical methanol process: Preparation of ultrafine copper materials, Waste Manage., 2017, 60, 643–651, . [CrossRef]
- J. Li, T. Xu, J. Liu, J. Wen and S. Gong, A review of current progress of supercritical fluid technologies for e-waste treatment, Environ. Sci. Pollut. Res., 2021, 28(33), 44622– 44637. [CrossRef]
- Wang and F.-S. Zhang, Degradation of brominated flame retardant in computer housing plastic by supercritical fluids, J. Hazard. Mater., 2012, 205–206, 156–163. [CrossRef]
- Z.Y. Zhang, F.S. Zhang (Eds.). A green process for copper recovery from waste printed circuit boards. Adv. Mater. Res. Trans Tech Publ., 2014, . [CrossRef]
- P. Jadhao, G. Chauhan, K. Pant, K. Nigam, Greener approach for the extraction of copper metal from electronic waste, Waste Manage., 2016, 57, 102–112, . [CrossRef]
- G. Chauhan, K. K. Pant and K. D. P. Nigam, Environmental Science Processes & Impacts Chelation technology: a promising green approach for resource management and waste minimization, Environ. Sci. Technol., 2015, 17, 12–40, . [CrossRef]
- Altansukh, K. Haga, N. Ariunbolor, K. Shigeru and A. Shibayama, Leaching and Adsorption of Gold from Waste Printed Circuit Boards Using Iodine-Iodide Solution and Activated Carbon, Eng. J., 2016, 20, 29–40, . [CrossRef]
- Chaurasia, K. Singh, T. Mankhand, Extraction of tin and copper by acid leaching of PCBs, Int. J. Metall. Eng., 2013, 2, 243–248.
- E. Hsu, K. Barmak, A.C. West, A.-H.-A. Park, Advancements in the treatment and processing of electronic waste with sustainability: a review of metal extraction and recovery technologies, Green Chem., 2019, 21 (5), 919–936, . [CrossRef]
- Kumar, V.; Lee, J.; Jeong, J.; Jha, M. K.; Kim, B.; Singh, R. Recycling of printed circuit boards (PCBs) to generate enriched rare metal concentrate. Journal of Industrial and Engineering Chemistry, 2015, 21, 805–813. [CrossRef]
- Park, Y.; Eom, Y.; Yoo, K.; Jha, M.K. Leaching of copper from waste-printed circuit boards (PCBs) in sulfate medium using cupric ion and oxygen. Metals, 2021, 11, 1369. [CrossRef]
- Jha, M. K.; Kumari, A.; Choubey, P. K.; Lee, J.; Kumar, V.; fJeong, J. Leaching of lead from solder material of waste printed circuit boards (PCBs). Hydrometallurgy, 2012, 28–34. [CrossRef]
- Ronald William Missen, Charles A. Mims, Bradley A. Saville - MISSEN, R. W.: Introduction to Chemical Reaction Engineering and Kinetics, 2002.

| Leaching Details |
Targeted Metals | Remarks | References |
|---|---|---|---|
| Sulfuric acid Leaching With oxidizing agent H2O2 |
Cu |
Pros: Higher leaching efficiency; Reduced Corrosivity; Enables selective leaching Cons:
|
22-24 |
| Aqua regia | Au, Ag |
Pros: Specificity for metals leaching Cons:
|
21, 25-26 |
| Cyanide leaching | Au, Ag, Pd and Pt |
Pros: Highly efficient to recover gold Cons: Toxicity; Regulatory Challenges; Limited Selectivity |
27-28 |
| Thiourea Leaching CS(NH2)2 |
Au, Ag |
Pros: High selectivity of gold and silver Cons:
|
22, 29-30 |
| Thiosulphate Leaching |
Au, Ag |
Pros: Less toxicity; Lower environmental impact and non-corrosivity Cons:
|
22, 28-29,31-32 |
| Halide Leaching |
Au |
Pros: Higher recovery of base metal as well as precious metal Cons: Emission of toxic gases like chlorine gas |
33-34 |
| Supercritical methanol (SCM) process |
Cu |
Pros: Ease of implementation; no additional reducing agents required Cons:
|
35-37 |
| Cuprous Chloride synthesis |
Cu |
Pros: Non-corrosive acids applicability during the process Cons: Limited to copper recovery. |
23, 38 |
| Chelation technology |
Cu |
Pros: Effective in extracting toxic metals Cons: Expensive and lack of selectivity |
39-40 |
| Iodine Leaching |
Precious metals |
Pros: Iodine/iodide is a superior substitute for chlorine/chloride due to its rapid kinetics, non-toxicity, and high selectivity towards precious metals. Cons: Iodine elevated cost and high consumption rate prevent the industrialization. |
41 |
| Nitric acid leaching |
Cu, Pb, Sn |
Pros: Dissolution percent is higher; Versatile in extracting more metals. Cons:
|
25, 42-43 |
|
HNO3 Leaching (Our work) |
Cu, Pb, Ni | Fast Kinetic reaction due to powerful oxidizing agent; facilitating the dissolution of various metal; Maximum recovery and less time consumption;Closed-loop systems curbs NOx formation and helps to reuse chemicals by reducing the overall environmental impact | Present Research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





