Submitted:
11 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
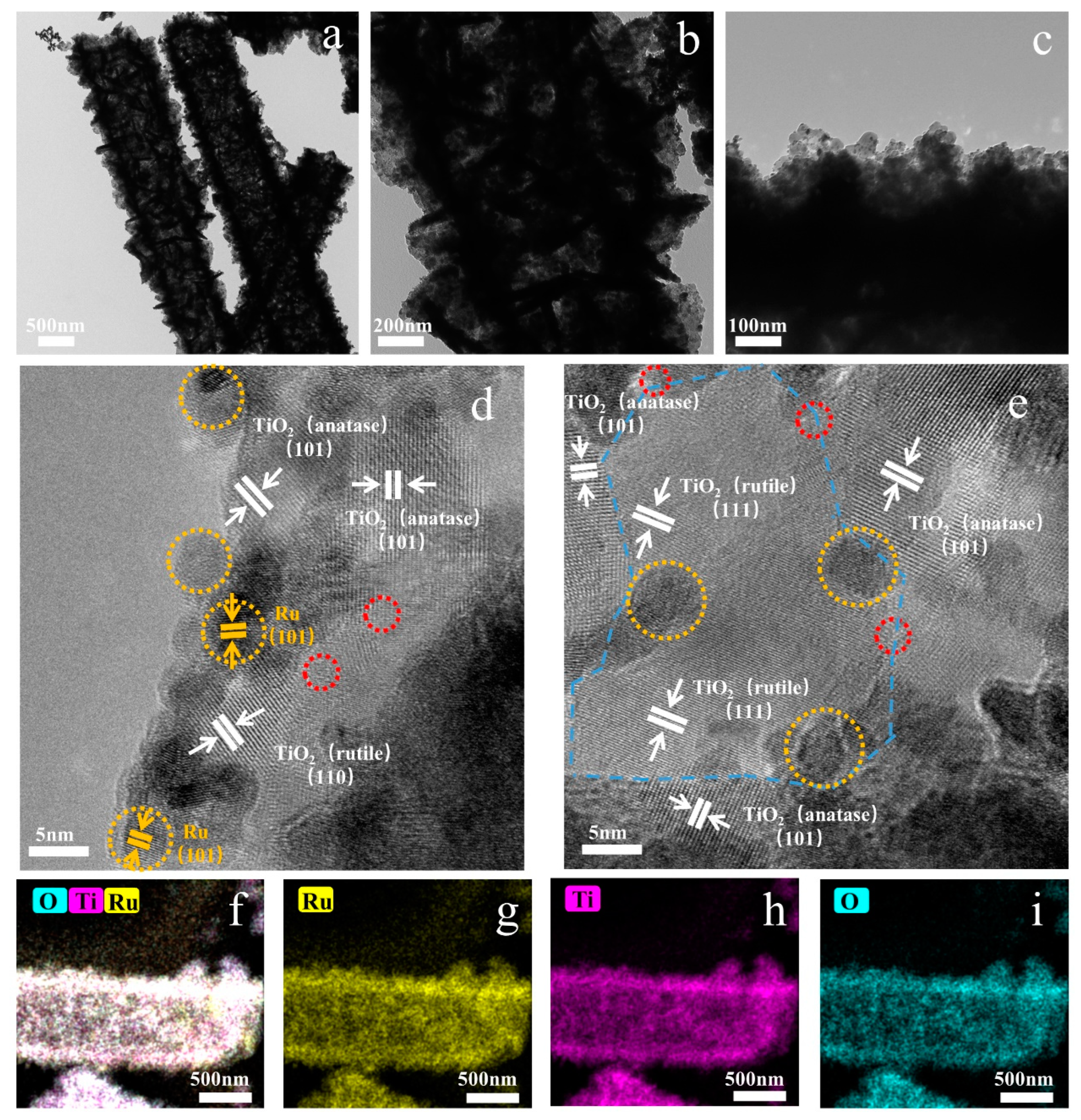
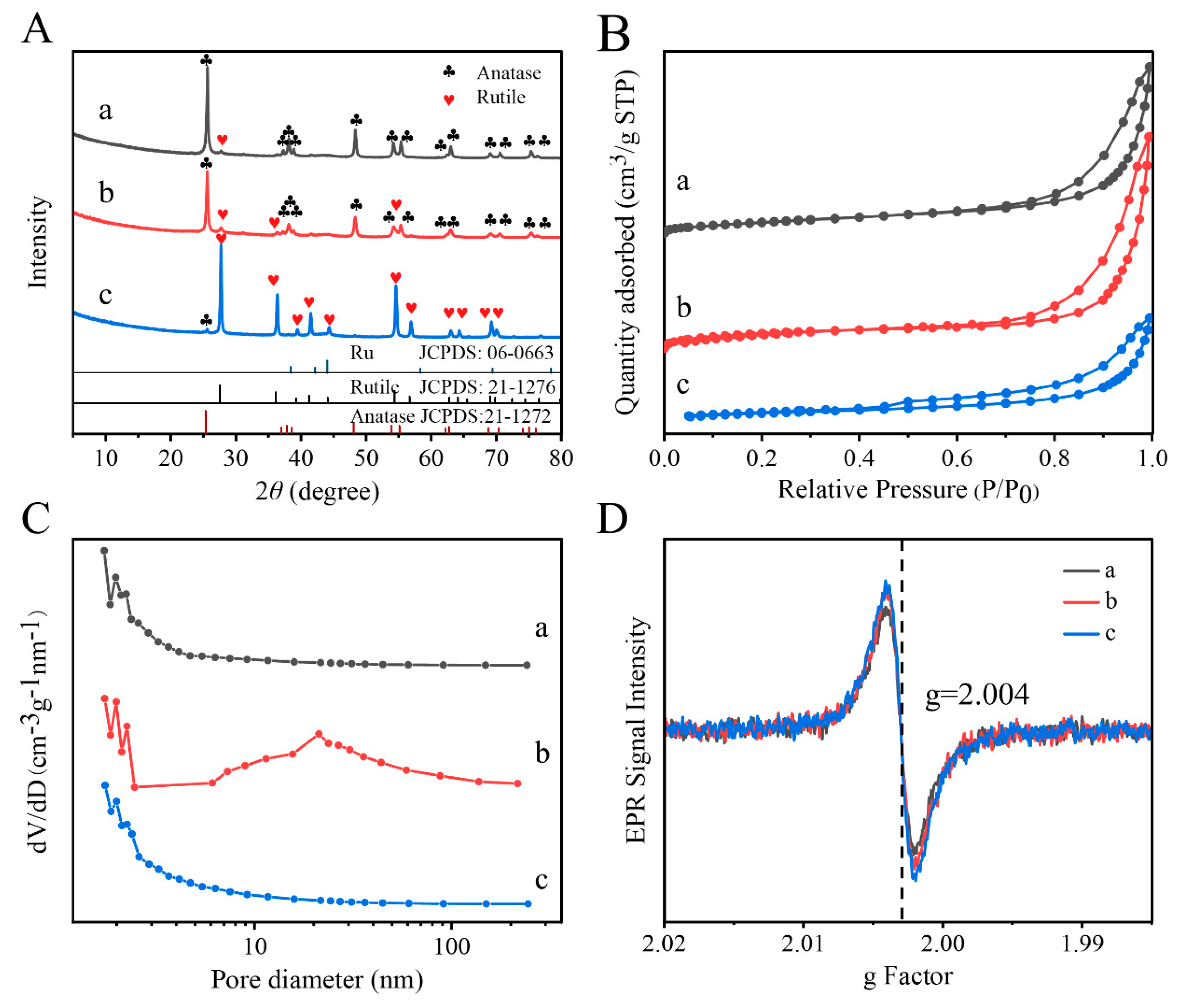
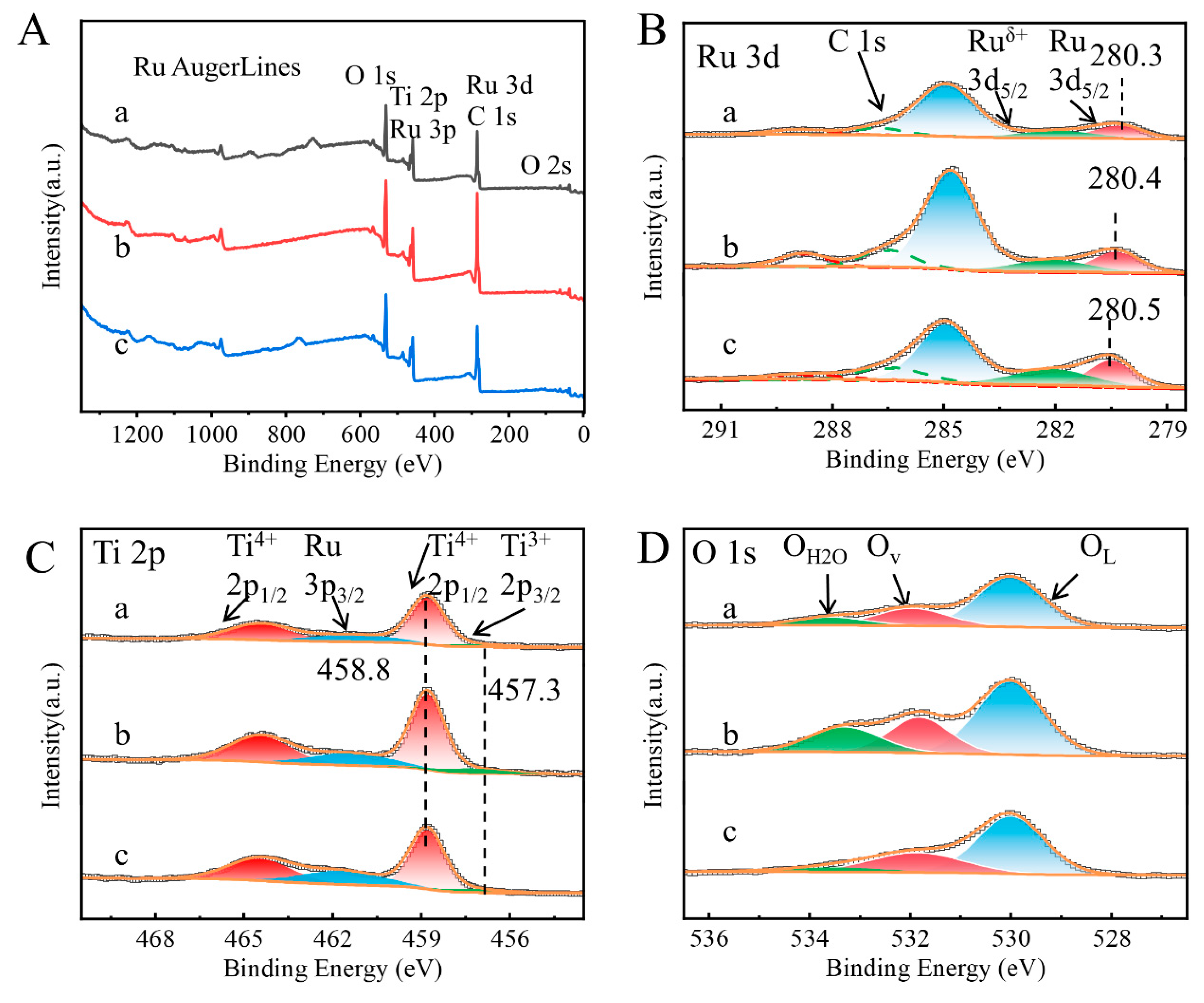
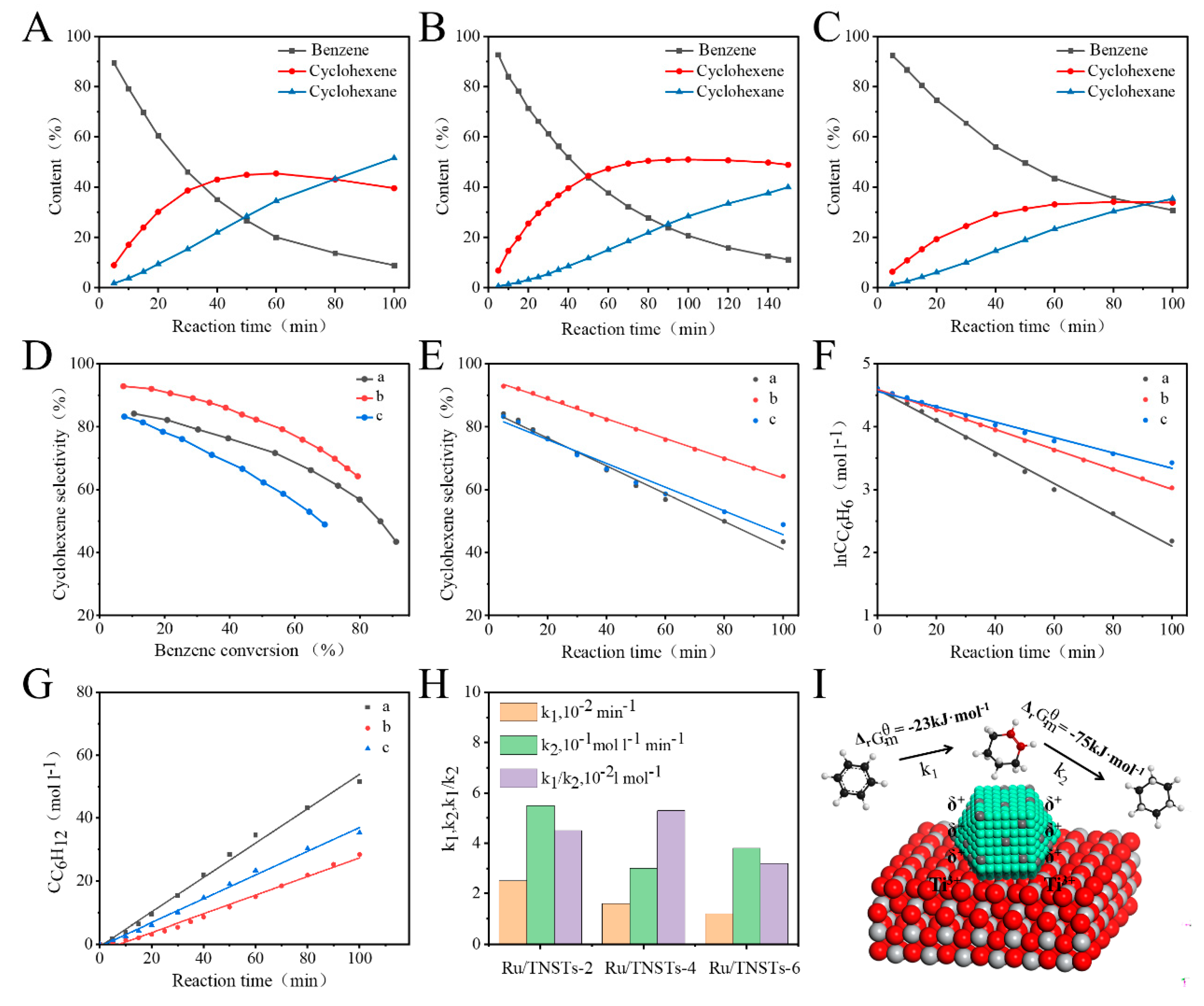
2. Results and Discussion
3. Experimental section
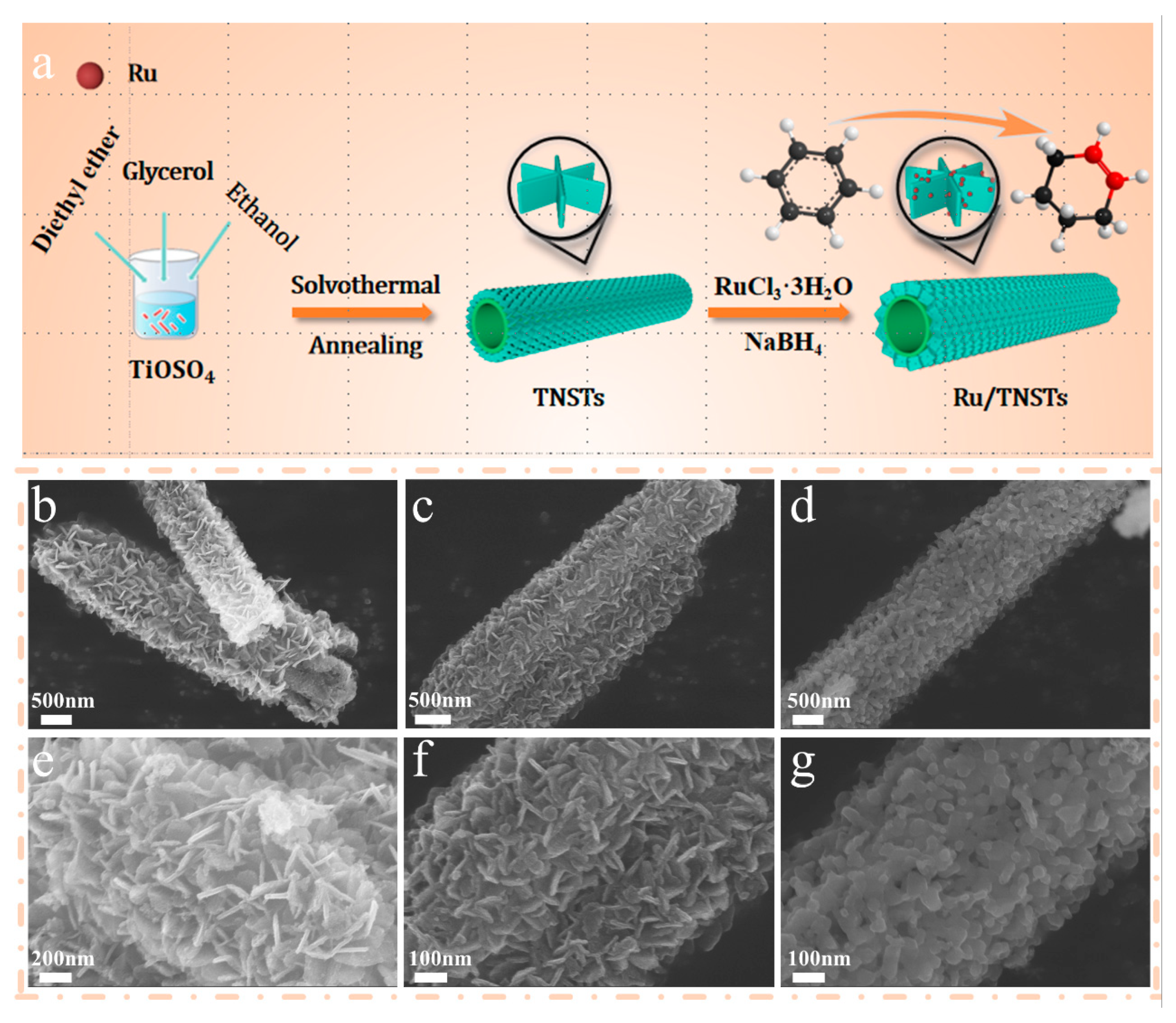
3.1. Synthesis of TiO2 nanosheets assembled nanotubes (TNSTs)
3.2. Preparation of TNSTs supported Ru catalysts
3.3. Catalytic testing
3.4. Instrumentation and characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, J.-C.; Doka Dari, M.; Tang, X.-Q.; Yuan, P.-Q. Diffusion of benzene through water film confined in silica mesopores: Effect of competitive adsorption of solvent. Chemical Engineering Science 2020, 224. [Google Scholar] [CrossRef]
- Spod, H.; Lucas, M.; Claus, P. Selective Hydrogenation of Benzene to Cyclohexene over 2Ru/La2O3-ZnO Catalyst without Additional Modifiers. ChemCatChem 2016, 8, 2659–2666. [Google Scholar] [CrossRef]
- Melgo, M.S.; Lindner, A.; Schuchardt, U. Wacker oxidation of cyclohexene in the presence of Pd(NO3)2/CuSO4/H3PMo12O40. Applied Catalysis A: General 2004, 273, 217–221. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, H.; Peng, Z.; Gao, J.; Li, B.; Liu, Z.; Liu, S. Selective Hydrogenation of Benzene: Progress of Understanding for the Ru-Based Catalytic System Design. Industrial & Engineering Chemistry Research 2019, 58, 13794–13803. [Google Scholar] [CrossRef]
- Foppa, L.; Dupont, J. Benzene partial hydrogenation: advances and perspectives. Chem Soc Rev 2015, 44, 1886–1897. [Google Scholar] [CrossRef]
- He, H.; Meyer, R.J.; Rioux, R.M.; Janik, M.J. Catalyst Design for Selective Hydrogenation of Benzene to Cyclohexene through Density Functional Theory and Microkinetic Modeling. ACS Catalysis 2021, 11, 11831–11842. [Google Scholar] [CrossRef]
- Nagahara, H.; Ono, M.; Konishi, M.; Fukuoka, Y. Partial hydrogenation of benzene to cyclohexene. Applied Surface Science 1997, 121-122, 448–451. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, G. Promoting the performances of Ru on hierarchical TiO2 nanospheres exposed {0 0 1} facets in benzene semi-hydrogenation by manipulating the metal-support interfaces. Journal of Catalysis 2020, 382, 97–108. [Google Scholar] [CrossRef]
- Sun, H.; Fan, Y.; Sun, X.; Chen, Z.; Li, H.; Peng, Z.; Liu, Z. Effect of ZnSO4, MnSO4 and FeSO4 on the Partial Hydrogenation of Benzene over Nano Ru-Based Catalysts. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Lin, H.; Wang, Z.; Li, Z.; Li, B.; Liu, Z.; Liu, S. Surface engineering on a nanocatalyst: basic zinc salt nanoclusters improve catalytic performances of Ru nanoparticles. Journal of Materials Chemistry A 2016, 4, 17694–17703. [Google Scholar] [CrossRef]
- Zhong, Z.; Luo, B.; Lin, C.; Yin, T.; Tian, Z.; Wang, C.; Chen, Y.; Wu, Y.; Shu, R. Ultrafast microfluidic preparation of highly dispersed Ru/TiO2 catalyst for the hydrodeoxygenation of lignin-derived phenolic compounds. Fuel 2023, 340. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, L.; Yun, R.; Pu, M.; Zhang, B.; Xiang, X. Increasing the Activity and Selectivity of TiO2-Supported Au Catalysts for Renewable Hydrogen Generation from Ethanol Photoreforming by Engineering Ti3+ Defects. ACS Sustainable Chemistry & Engineering 2019, 7, 13856–13864. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Yan, Z.; Xu, C.; Zhang, W.; Ban, H.; Li, C. Activation reconstructing CuZnO/SiO2 catalyst for CO2 hydrogenation. Journal of Catalysis 2022, 412, 10–20. [Google Scholar] [CrossRef]
- Wang, S.; Feng, K.; Zhang, D.; Yang, D.; Xiao, M.; Zhang, C.; He, L.; Yan, B.; Ozin, G.A.; Sun, W. Stable Cu Catalysts Supported by Two-dimensional SiO(2) with Strong Metal-Support Interaction. Adv Sci (Weinh) 2022, 9, e2104972. [Google Scholar] [CrossRef]
- Diao, Y.; Wang, H.; Chen, B.; Zhang, X.; Shi, C. Modulating morphology and textural properties of Al2O3 for supported Ni catalysts toward plasma-assisted dry reforming of methane. Applied Catalysis B: Environmental 2023, 330. [Google Scholar] [CrossRef]
- Muravev, V.; Simons, J.F.M.; Parastaev, A.; Verheijen, M.A.; Struijs, J.J.C.; Kosinov, N.; Hensen, E.J.M. Operando Spectroscopy Unveils the Catalytic Role of Different Palladium Oxidation States in CO Oxidation on Pd/CeO(2) Catalysts. Angew Chem Int Ed Engl 2022, 61, e202200434. [Google Scholar] [CrossRef] [PubMed]
- Tsiotsias, A.I.; Hafeez, S.; Charisiou, N.D.; Al-Salem, S.M.; Manos, G.; Constantinou, A.; AlKhoori, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; et al. Selective catalytic deoxygenation of palm oil to produce green diesel over Ni catalysts supported on ZrO2 and CeO2–ZrO2: Experimental and process simulation modelling studies. Renewable Energy 2023, 206, 582–596. [Google Scholar] [CrossRef]
- Hu, X.; Fan, Q.; Tan, M.; Luo, Y.; Wu, X.; Manuputty, M.Y.; Ding, J.; Choksi, T.S.; Kraft, M.; Xu, R.; et al. Investigating the impact of dynamic structural changes of Au/rutile catalysts on the catalytic activity of CO oxidation. Carbon Energy 2023. [Google Scholar] [CrossRef]
- Wang, K.; He, S.; Lin, Y.; Chen, X.; Dai, W.; Fu, X. Photo-enhanced thermal catalytic CO2 methanation activity and stability over oxygen-deficient Ru/TiO2 with exposed TiO2 {001} facets: Adjusting photogenerated electron behaviors by metal-support interactions. Chinese Journal of Catalysis 2022, 43, 391–402. [Google Scholar] [CrossRef]
- Wang, F.; Kishimoto, H.; Develos-Bagarinao, K.; Yamaji, K.; Horita, T.; Yokokawa, H. Encroachment of titanium oxide on Ni surface for Ni/TiO2 under reducing atmosphere. Solid State Ionics 2016, 288, 130–134. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Z.; Fan, G.; Yang, L.; Li, F. Regulating Surface-Interface Structures of Zn-Incorporated LiAl-LDH Supported Ru Catalysts for Efficient Benzene Hydrogenation to Produce Cyclohexene. ChemCatChem 2022, 14. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhou, C.; Jin, F.; Chen, C.; Jiang, L. Ru/TiO2 catalyst for selective hydrogenation of benzene: Effect of surface hydroxyl groups and spillover hydrogen. Applied Surface Science 2020, 525. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, L.; He, D. Ru nanoparticles on TiO2 with various anatase-to-rutile ratios tuned by selective chemical dissolution: Effect of support polymorph composition on selective benzene hydrogenation. Applied Catalysis A: General 2019, 575, 65–73. [Google Scholar] [CrossRef]
- Zhou, G.; Dou, R.; Bi, H.; Xie, S.; Pei, Y.; Fan, K.; Qiao, M.; Sun, B.; Zong, B. Ru nanoparticles on rutile/anatase junction of P25 TiO2 : Controlled deposition and synergy in partial hydrogenation of benzene to cyclohexene. Journal of Catalysis 2015, 332, 119–126. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, G. Distinguishing the roles of TiO2 {1 0 1}, {0 0 1}, and {0 1 0} facets in benzene semi-hydrogenation over Ru/TiO2 catalysts. Applied Surface Science 2021, 535. [Google Scholar] [CrossRef]
- Shi, R.; Wang, X.; Zhou, G. Electronic metal − support interaction directed electron-deficient nanoparticulate Ru on Ti3C2 MXene-derived TiO2 nanoflowers for robust benzene semi-hydrogenation. Applied Surface Science 2023, 624. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, F.; Shi, R. Nanoparticulate Ru on morphology-manipulated and Ti3+ defect-riched TiO2 nanosheets for benzene semi-hydrogenation. Journal of Catalysis 2021, 398, 148–160. [Google Scholar] [CrossRef]
- Zhu, F.; Wen, J.; Guo, H.; An, J.; Wang, G.; Ren, G.; Ma, X. Low-temperature catalytic performance improvement of Ru/TiO2{001} for o-dichlorobenzene oxidation. Chemical Engineering Journal 2023, 473. [Google Scholar] [CrossRef]
- Lin, W.; Chen, Y.; Zhang, Y.; Zhang, Y.; Wang, J.; Wang, L.; Xu, C.C.; Nie, R. Surface Synergetic Effects of Ni–ReOx for Promoting the Mild Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol. ACS Catalysis 2023, 13, 11256–11267. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Zhou, L.; Liu, Y.; Yang, Y.; Zhang, L.; Shang, Z.; Li, H.; Xiao, T.; Zhang, C.; et al. Efficient hydrodeoxygenation of guaiacol to phenol over Ru/Ti–SiO2 catalysts: the significance of defect-rich TiOx species. Green Chemistry 2022, 24, 5822–5834. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, H.; Zhu, X.; Xing, Z.; Liu, Q.; Liu, T.; Gao, S.; Lu, S.; Chen, G.; Asiri, A.M.; et al. Identifying the Origin of Ti(3+) Activity toward Enhanced Electrocatalytic N(2) Reduction over TiO(2) Nanoparticles Modulated by Mixed-Valent Copper. Adv Mater 2020, 32, e2000299. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, S.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. Novel Highly Active Anatase/Rutile TiO2 Photocatalyst with Hydrogenated Heterophase Interface Structures for Photoelectrochemical Water Splitting into Hydrogen. ACS Sustainable Chemistry & Engineering 2018, 6, 10823–10832. [Google Scholar] [CrossRef]
- Lan, K.; Wang, R.; Wei, Q.; Wang, Y.; Hong, A.; Feng, P.; Zhao, D. Stable Ti3+ Defects in Oriented Mesoporous Titania Frameworks for Efficient Photocatalysis. Angewandte Chemie International Edition 2020, 59, 17676–17683. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Lyu, S.; Tian, Y.; Zhao, D.; Ye, J.; She, M.; Song, S.; Ding, T.; Li, X. Identification of active sites for preferential oxidation of CO over Ru/TiO2 catalysts via tuning metal–support interaction. Chemical Engineering Journal 2023, 475. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, Q.; Wang, Q.; Wang, M.; Zuo, J.; Chen, H.; Kuang, Q.; Xie, Z. Effect of Rutile Content on the Catalytic Performance of Ru/TiO2 Catalyst for Low-Temperature CO2 Methanation. ACS Sustainable Chemistry & Engineering 2021, 9, 14288–14296. [Google Scholar] [CrossRef]
- Qiu, J.-Y.; Feng, H.-Z.; Chen, Z.-H.; Ruan, S.-H.; Chen, Y.-P.; Xu, T.-T.; Su, J.-Y.; Ha, E.-N.; Wang, L.-Y. Selective introduction of surface defects in anatase TiO2 nanosheets for highly efficient photocatalytic hydrogen generation. Rare Metals 2022, 41, 2074–2083. [Google Scholar] [CrossRef]
- Chen, L.-N.; Wang, S.-H.; Zhang, P.-Y.; Chen, Z.-X.; Lin, X.; Yang, H.-J.; Sheng, T.; Lin, W.-F.; Tian, N.; Sun, S.-G.; et al. Ru nanoparticles supported on partially reduced TiO2 as highly efficient catalyst for hydrogen evolution. Nano Energy 2021, 88. [Google Scholar] [CrossRef]
- Tang, M.; Tong, Q.; Li, Y.; Jiang, R.; Shi, L.; Shen, F.; Wei, Y.; Liu, Z.; Liu, S.; Zhang, J.; et al. Effective and selective electrocatalytic nitrate reduction to ammonia on urchin-like and defect-enriched titanium oxide microparticles. Chinese Chemical Letters 2023, 34. [Google Scholar] [CrossRef]
- Zhou, G.; Dong, Y.; He, D. Bimetallic Ru–M/TiO2 (M = Fe, Ni, Cu, Co) nanocomposite catalysts facribated by galvanic replacement: Structural elucidation and catalytic behavior in benzene selective hydrogenation. Applied Surface Science 2018, 456, 1004–1013. [Google Scholar] [CrossRef]
- Hao, F.; Zheng, J.; Ouyang, D.; Xiong, W.; Liu, P.; Luo, H. Selective hydrogenation of benzene over Ru supported on surface modified TiO2. Korean Journal of Chemical Engineering 2021, 38, 736–746. [Google Scholar] [CrossRef]
- Liu, S.C.; Guo, Y.Q.; Yang, X.L.; Ji, Y.L.; Luo, G. Kinetic Equations for Liquid-Phase Selective Hydrogenation of Benzene to Cyclohexene. Chinese Journal of Catalysis 2003, 24, 42–46. [Google Scholar]
- Fan, C.; Zhu, Y.-A.; Zhou, X.-G.; Liu, Z.-P. Catalytic hydrogenation of benzene to cyclohexene on Ru(0001) from density functional theory investigations☆. Catalysis Today 2011, 160, 234–241. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Zhang, K.; Wang, J.; Sun, B.; Li, H.; Qiao, P.; Wang, L.; Zhou, W. Ti3+ Self-Doped Black TiO2 Nanotubes with Mesoporous Nanosheet Architecture as Efficient Solar-Driven Hydrogen Evolution Photocatalysts. ACS Sustainable Chemistry & Engineering 2017, 5, 6894–6901. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, T.; Jiang, T.; Wang, W.; Liu, H.; Fan, H.; Zhang, Z.; Han, B. Ru–Zn supported on hydroxyapatite as an effective catalyst for partial hydrogenation of benzene. Green Chem. 2013, 15, 152–159. [Google Scholar] [CrossRef]
- Fan, G.-Y.; Jiang, W.-D.; Wang, J.-B.; Li, R.-X.; Chen, H.; Li, X.-J. Selective hydrogenation of benzene to cyclohexene over RuCoB/γ-Al2O3 without additive. Catalysis Communications 2008, 10, 98–102. [Google Scholar] [CrossRef]
- Yu, X.-L.; Li, Y.; Xin, S.-M.; Yuan, P.-Q.; Yuan, W.-K. Partial Hydrogenation of Benzene to Cyclohexene on Ru@XO2 (X = Ti, Zr, or Si). Industrial & Engineering Chemistry Research 2018, 57, 1961–1967. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



