Submitted:
09 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Sample Collection
2.3. Light Transmission Aggregometry
2.4. Platelet Function Analyzer
2.5. Nitrite-Nitrate (NOx)
2.6. RT-qPCR Analyses
2.7. Statistical Analysis
3. Results
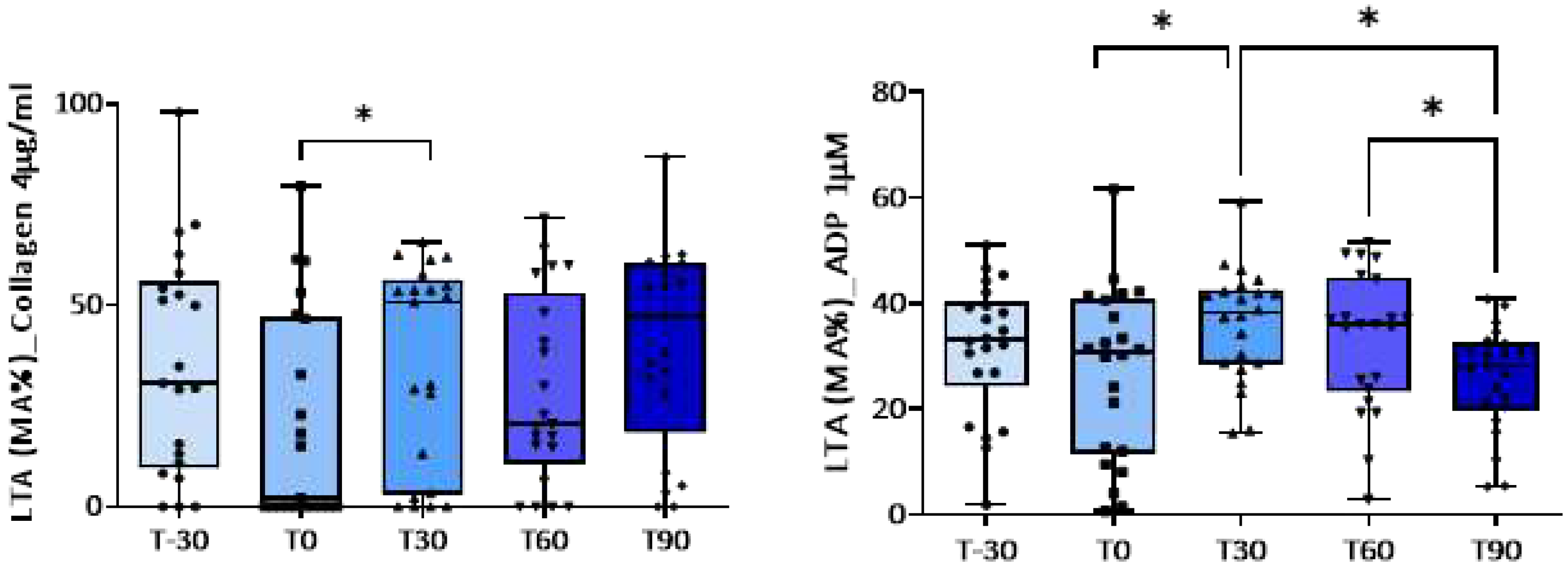
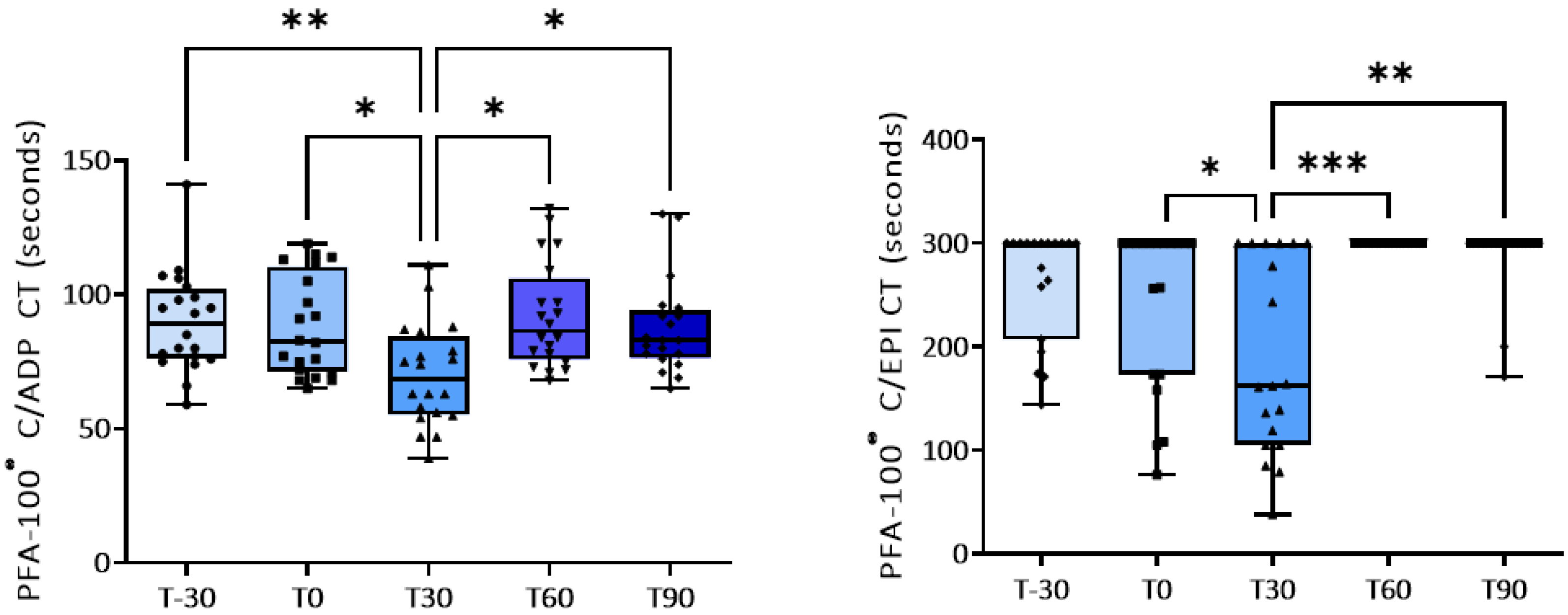
3.1. Effect of Training on Platelet Activation
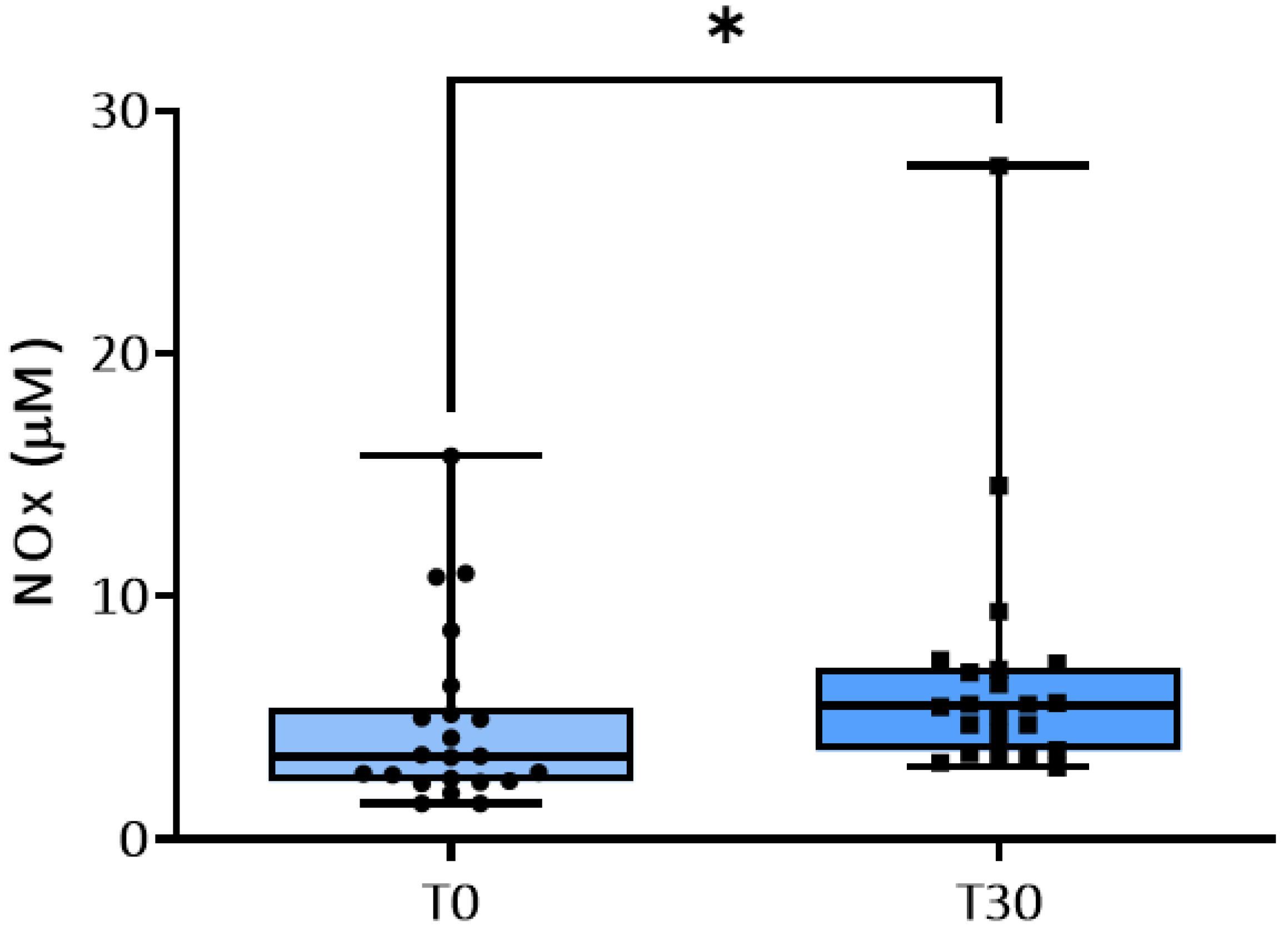
3.2. NO Metabolism
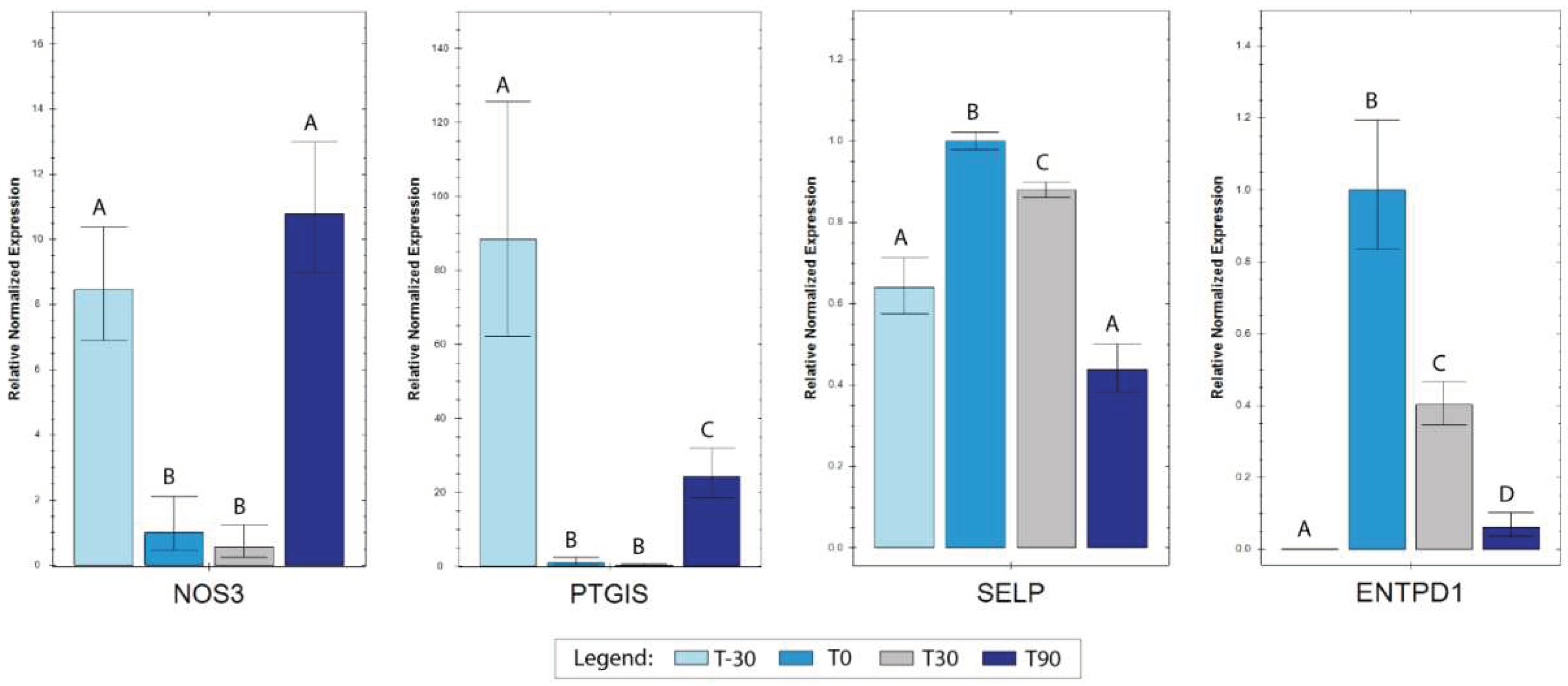
3.3. RT-qPCR Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Assenza, A.; Tosto, F.; Casella, S.; Fazio, F.; Giannetto, C.; Piccione, G. Changes in Blood Coagulation Induced by Exercise Training in Young Athletic Horses. Research in Veterinary Science 2013, 95, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.S.; Ali, N.; El-Sayed Ali, Z. Aggregation and Activation of Blood Platelets in Exercise and Training. Sports Med 2005, 35, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Arfuso, F.; Fazio, F.; Giudice, E.; Pietro, S.D.; Bruschetta, D.; Piccione, G. Different Training Schedules Influence Platelet Aggregation in Show Jumping Horses. Polish Journal of Veterinary Sciences 2017, 20, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kingston, J.K.; Sampson, S.N.; Beard, L.A.; Meyers, K.M.; Sellon, D.C.; Bayly, W.M. The Effect of Supramaximal Exercise on Equine Platelet Function. Equine Vet J Suppl 1999, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Bazzano, M.; Giannetto, C.; Marafioti, S.; Fazio, F. Training-Induced Changes in Clotting Parameters of Athletic Horses. J Vet Sci 2014, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Grasso, F.; Fazio, F.; Giudice, E. The Effect of Physical Exercise on the Daily Rhythm of Platelet Aggregation and Body Temperature in Horses. The Veterinary Journal 2008, 176, 216–220. [Google Scholar] [CrossRef]
- Piccione, G.; Fazio, F.; Giudice, E.; Grasso, F.; Caola, G. Exercise-Induced Changes in the Clotting Times and Fibrinolytic Activity during Official 1600 and 2 000 Meters Trot Races in the Standardbred Horses. Acta Vet. Brno 2005, 74, 509–514. [Google Scholar] [CrossRef]
- McKeever, K.H.; Hinchcliff, K.W.; Kociba, G.J.; Reed, S.M.; Muir, W.W. Changes in Coagulation and Fibrinolysis in Horses during Exercise. Am. J. Vet. Res. 1990, 51, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Monreal, L.; Angles, A.M.; Monreal, M.; Espada, Y.; Monasterio, J. Changes in Haemostasis in Endurance Horses: Detection by Highly Sensitive ELISA-Tests. Equine Veterinary Journal 1995, 27, 120–123. [Google Scholar] [CrossRef]
- Piccione, G.; Assenza, A.; Casella, S.; Giannetto, C.; Tosto, F.; Caola, G. Modifications of Platelet Aggregation during Treadmill Section and Obstacle Course in Athletic Horse. Acta vet. (Beogr.) 2010, 60, 165–172. [Google Scholar] [CrossRef]
- Karampour, S.; Gaeini, A.A. Response of Coagulation and Anti-Coagulant Factors of Elite Athletes Following Acute Resistance and High-Intensity Interval Training. J Sports Med Phys Fitness 2018, 58, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Marafioti, S.; Giannetto, C.; Panzera, M.; Fazio, F. Effect of Dietary Supplementation with Omega 3 on Clotting Time, Fibrinogen Concentration and Platelet Aggregation in the Athletic Horse. Livestock Science 2014, 161, 109–113. [Google Scholar] [CrossRef]
- Miglio, A.; Falcinelli, E.; Mezzasoma, A.M.; Cappelli, K.; Mecocci, S.; Gresele, P.; Antognoni, M.T. Effect of First Long-Term Training on Whole Blood Count and Blood Clotting Parameters in Thoroughbreds. Animals (Basel) 2021, 11, 447. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlström, M.; Weitzberg, E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell Metabolism 2018, 28, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Piknova, B.; Nghiem, K.; Lozier, J.N.; Schechter, A.N. Inhibitory Effect of Nitrite on Coagulation Processes Demonstrated by Thrombelastography. Nitric Oxide 2014, 40, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, N.; Gatica, S.; Olivares, P.; Cabello-Verrugio, C.; Simon, F. Endothelial Cells Exhibit Two Waves of P-Selectin Surface Aggregation Under Endotoxic and Oxidative Conditions. Protein J 2019, 38, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Chi, L.; Zhao, R.; Tourdot, B.E.; Yalavarthi, S.; Jacobs, B.N.; Banka, A.; Liao, H.; Koonse, S.; Anyanwu, A.C.; et al. Ectonucleotidase Tri(Di)Phosphohydrolase-1 (ENTPD-1) Disrupts Inflammasome/Interleukin 1β-Driven Venous Thrombosis. J Clin Invest 2019, 129, 2872–2877. [Google Scholar] [CrossRef] [PubMed]
- Boudreaux, M.K.; Koehler, J.; Habecker, P.L.; Piero, F.D. Evaluation of the Genes Encoding CD39/NTPDase-1 and CD39L1/NTPDase-2 in Horses with and without Abnormal Hemorrhage and in Horses with Pathologic Evidence of Exercise-Induced Pulmonary Hemorrhage. Veterinary Clinical Pathology 2015, 44, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, C.; Yabuki, T.; Inoue, H.; Tone, Y.; Hara, S.; Hatae, T.; Nagata, M.; Takahashi, E.I.; Tanabe, T. Human Gene Encoding Prostacyclin Synthase (PTGIS): Genomic Organization, Chromosomal Localization, and Promoter Activity. Genomics 1996, 36, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.T.-V.; Elbatreek, M.H.; Fuchß, T.; Grädler, U.; Schmidt, H.H.H.W.; Shah, A.M.; Wallace, A.; Knowles, R. Nitric Oxide Synthase Inhibitors into the Clinic at Last. Handb Exp Pharmacol 2020. [CrossRef]
- Vedmedovska, N.; Bokucava, D.; Kivite-Urtane, A.; Rovite, V.; Zake-Nikitina, L.; Klovins, J.; Fodina, V.; Donders, G.G.G. The Correlation Between Abnormal Uterine Artery Flow in the First Trimester and Genetic Thrombophilic Alteration: A Prospective Case-Controlled Pilot Study. Diagnostics (Basel) 2020, 10. [Google Scholar] [CrossRef]
- Miglio, A.; Cappelli, K.; Capomaccio, S.; Mecocci, S.; Silvestrelli, M.; Antognoni, M.T. Metabolic and Biomolecular Changes Induced by Incremental Long-Term Training in Young Thoroughbred Racehorses during First Workout Season. Animals (Basel) 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Segura, D.; Monreal, L.; Espada, Y.; Pastor, J.; Mayós, I.; Homedes, J. Assessment of a Platelet Function Analyser in Horses: Reference Range and Influence of a Platelet Aggregation Inhibitor. The Veterinary Journal 2005, 170, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, E.; Francisci, D.; Schiaroli, E.; Minuz, P.; Orsini, S.; Malincarne, L.; Sebastiano, M.; Mezzasoma, A.M.; Pasticci, M.B.; Guglielmini, G.; et al. Effect of Aspirin Treatment on Abacavir-Associated Platelet Hyperreactivity in HIV-Infected Patients. International Journal of Cardiology 2018, 263, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, E.; Guglielmini, G.; Torti, M.; Gresele, P. Intraplatelet Signaling Mechanisms of the Priming Effect of Matrix Metalloproteinase-2 on Platelet Aggregation. J Thromb Haemost 2005, 3, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.K.; Heilmann, E.J.; Sio, R.; Garcia, C.; Davidson, R.M.; Ostgaard, R.A. Description of an in Vitro Platelet Function Analyzer--PFA-100. Semin Thromb Hemost 1995, 21 Suppl 2, 106–112. [Google Scholar] [CrossRef]
- Gresele, P.; Bury, L.; Mezzasoma, A.M.; Falcinelli, E. Platelet Function Assays in Diagnosis: An Update. Expert Rev Hematol 2019, 12, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Segura, D.; Monreal, L.; Espada, Y.; Pastor, J.; Mayós, I.; Homedes, J. Assessment of a Platelet Function Analyser in Horses: Reference Range and Influence of a Platelet Aggregation Inhibitor. Vet. J. 2005, 170, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Momi, S.; Caracchini, R.; Falcinelli, E.; Evangelista, S.; Gresele, P. Stimulation of Platelet Nitric Oxide Production by Nebivolol Prevents Thrombosis. Arterioscler Thromb Vasc Biol 2014, 34, 820–829. [Google Scholar] [CrossRef]
- Marañón, G.; Muñoz-Escassi, B.; Manley, W.; García, C.; Cayado, P.; de la Muela, M.S.; Olábarri, B.; León, R.; Vara, E. The Effect of Methyl Sulphonyl Methane Supplementation on Biomarkers of Oxidative Stress in Sport Horses Following Jumping Exercise. Acta Veterinaria Scandinavica 2008, 50, 45. [Google Scholar] [CrossRef]
- Cappelli, K.; Amadori, M.; Mecocci, S.; Miglio, A.; Antognoni, M.T.; Razzuoli, E. Immune Response in Young Thoroughbred Racehorses under Training. Animals 2020, 10, 1809. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, K.; Felicetti, M.; Capomaccio, S.; Nocelli, C.; Silvestrelli, M.; Verini-Supplizi, A. Effect of Training Status on Immune Defence Related Gene Expression in Thoroughbred: Are Genes Ready for the Sprint? The Veterinary Journal 2013, 195, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Meazza, C.; Salvadori, M.; Paltrinieri, S. Thromboelastometric Profiles of Horses Affected by Exercise-Induced Pulmonary Hemorrhages. Veterinary Medicine International 2010, 2010, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kingston, J.K.; Bayly, W.M.; Meyers, K.M.; Sellon, D.C.; Wardrop, K.J. Evaluation of Binding of Fibrinogen and Annexin V to Equine Platelets in Response to Supramaximal Treadmill Exercise. Equine Vet J Suppl 2002, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Kornreich, B.; Enyeart, M.; Jesty, S.A.; Nydam, D.V.; Divers, T. The Effects of Pentoxifylline on Equine Platelet Aggregation. J Vet Intern Med 2010, 24, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Lanier, C.J.; Taintor, J.S.; Christopherson, P.W.; Spangler, E.A. Effect of Lactic Acid Addition to Equine Whole Blood on Platelet Aggregation Measured by Impedance Aggregometry. Veterinary Clinical Pathology 2022, 51, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Giannetto, C.; Fazio, F.; Assenza, A.; Piccione, G. Nictemeral Profile of Platelet Aggregation and Clotting Parameters in Horses during Training. Bulletin of the Veterinary Institute in Puławy 2009, 53, 801–806. [Google Scholar]
- Norris, J.W.; Watson, J.L.; Tablin, F.; Kozikowski, T.A.; Knych, H.K. Pharmacokinetics and Competitive Pharmacodynamics of ADP-Induced Platelet Activation after Oral Administration of Clopidogrel to Horses. Am J Vet Res 2019, 80, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.B.; Divers, T.J.; Watts, A.E.; Ness, S.L.; Frye, A.H.; Stokol, T.; Fubini, S.L. Effects of Clopidogrel on the Platelet Activation Response in Horses. American Journal of Veterinary Research 2013, 74, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Roscher, K.A.; Failing, K.; Moritz, A. Inhibition of Platelet Function with Clopidogrel, as Measured with a Novel Whole Blood Impedance Aggregometer in Horses. Vet J 2015, 203, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Heber, S.; Volf, I. Effects of Physical (In)Activity on Platelet Function. Biomed Res Int 2015, 2015, 165078. [Google Scholar] [CrossRef] [PubMed]
- Di Francescomarino, S.; Sciartilli, A.; Di Valerio, V.; Di Baldassarre, A.; Gallina, S. The Effect of Physical Exercise on Endothelial Function. Sports Med 2009, 39, 797–812. [Google Scholar] [CrossRef]
- Green, D.J.; Maiorana, A.; O’Driscoll, G.; Taylor, R. Effect of Exercise Training on Endothelium-Derived Nitric Oxide Function in Humans. The Journal of Physiology 2004, 561, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.; Dwyer, K.; Enjyoji, K.; Robson, S.C. Ecto-Nucleotidases of the CD39/NTPDase Family Modulate Platelet Activation and Thrombus Formation: Potential as Therapeutic Targets. Blood Cells Mol Dis 2006, 36, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindemann, U.; Moggio, A.; Dutsch, A.; Kessler, T.; Sager, H.B. The Impact of Exercise on Immunity, Metabolism, and Atherosclerosis. International Journal of Molecular Sciences 2023, 24, 3394. [Google Scholar] [CrossRef]
- Rivero, J.-L.L.; Ruz, A.; Martí-Korff, S.; Estepa, J.-C.; Aguilera-Tejero, E.; Werkman, J.; Sobotta, M.; Lindner, A. Effects of Intensity and Duration of Exercise on Muscular Responses to Training of Thoroughbred Racehorses. Journal of Applied Physiology 2007, 102, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
| March (T-30) | April (T0) | May (T30) | June (T60) | July (T90) |
|---|---|---|---|---|
| Untrained horses | Start of training | Incremental training | Incremental training | Incremental training |
| 15 min Walk 10 min Trot Rest 10 min Trot Walk |
15 min Walk 10 min Trot 6 min Canter every Tuesday: 1 min Gallop |
15 min Walk 10 min Trot 6 min Canter every Tuesday: 2 min Gallop |
15 min Walk 10 min Trot 6 min Canter every Tuesday: 3 min Gallop |
15 min Walk 10 min Trot 6 min Canter every Tuesday: 4 min Gallop |
| Gene | Primer Forward | Primer Reverse | Amp. Length |
Accession |
| SELP | GCTGACAATCCAGGAAGCCC | CGCTTTGAGCAGTCAAGGGA | 146 | NM_001081792 |
| ENTPD1 | TTGAGCCACCAAGACCAGAAG | ATTCTGGGTCAACCCCACAG | 125 | XM_001500628 |
| PTGIS | TTCCTGAGTCCGCAGAAGGA | TCGCTTCCCGTCCTTGTAAA | 117 | XM_001501166 |
| NOS3 | TTCGGGAGAGTGAGCTGGTA | CAATCCCGCGCATCAAAGAC | 109 | XM_001504650 |
| B2M | TCCTGCTCGGGCTACTCTC | TGCTGGGTGACGTGAGTAAA | 83 | NM_001082502 |
| SDHA | GCGCGCTTCAGACGATTTAT | CCAGTGCTCCTCAAATGGCT | 146 | XM_014734954 |
| Maximal amplitude (%) | T-30 | T0 | T30 | T60 | T90 |
|---|---|---|---|---|---|
| Collagen (4g/ml) | 35.4±6.0 | 21.0±5.7 | 35.0±5.0 p=0.05 vs. T0 |
28.8±5.1 | 40.4±5.4 |
| ADP (1M) | 31.5±2.7 | 26.9±3.4 | 35.9±2.2 p=0.008 vs. T0; p=0.013 vs. T90 |
33.1±2.8 p=0.029 vs. T90 |
25.7±2.1 |
| ADP (5M) | 58.4±3.7 | 57.8±1.9 | 61.0±3.0 | 60.8±4.6 | 60.5±3.6 |
| ADP (10M) | 63.5±2.3 | 63.3±1.6 | 64.4±2.2 | 59.3±2.2 | 62.3±2.1 |
| A23187 (5M) | Not done | 24.2±5.1 | 30.9±5.2 | 41.8±5.3 p=0.03 vs. T0; p=0.04 vs. T90 |
22.0±4.9 |
| PFA-100® (sec) | T-30 | T0 | T30 | T60 | T90 |
|---|---|---|---|---|---|
| C-ADP | 89.8±4.1 | 88.2±4.1 | 70.0±4.2 p=0.03 vs. T0; p=0.0021 vs. T-30 |
92.0±4.4 p=0.011 vs. T30 |
87.6±3.9 p=0.019 vs. T30 |
| C-EPI | 262.0±12.5 | 242.4±18.0 | 190.0±21.0 p=0.045 vs. T0 |
300±0.0 p=0.0006 vs. T30 |
287.0±8.3 p=0.0021 vs. T30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





