1. Introduction
OSCC is the most common type of cancer in the head and neck area. Every year, approximately 300.000 new cases are diagnosed worldwide [
1]. When diagnosed at advanced stages, it is related to a higher morbidity and a very poor prognosis. Despite, a
djuvant radiation therapy or combined chemoradiotherapy are frequently used for treatment, nowadays treatment is generally based on surgical resection of primary tumor, which may be followed by radio, chemo and immunotherapy. The selection of adjuvant treatments relies in factors as tumor location, histological grading, histopathological features (such as
extracapsular extension in N+ disease and/or presence of positive surgical margins), radiographic findings and the TNM system grading [
2].
In recent years, immunotherapy has been added to the therapeutic arsenal against many different tumors, supported by the results of different clinical trials [
3,
4,
5,
6,
7,
8]. This immunotherapy is based on the different survival strategies of tumor cells and their relationship with the microenvironment. One of these potential tumor escape routes from the action of the immune system is the expression by tumor cells of the PD1 ligand protein (PD-L1) which, when interacting with the PD1 protein of cytotoxic CD8 lymphocytes, induces anergy in these and prevents them from attacking the tumor cells. Blocking the binding of PD-L1 to PD-1 by an immune checkpoint inhibitor (anti-PD-L1 or anti-PD-1) allows T cells to destroy tumor cells [
9,
10]. PD-L1 expression has been studied by immunohistochemistry in many tumors, including head and neck squamous cell carcinomas; however, its expression levels are characterized by high inter- and intratumoral heterogeneity due to factors that are not completely known. Even in squamous cell carcinomas of the head and neck, variable expression is observed depending on the location of the primary tumor [
11]. The immune checkpoint PD1 and its ligand PD-L1 are related to the prognosis in many solid tumors, whether its role in oral squamous cell carcinomas (OSCC) remains unclear: most published series and systematic reviews include heterogeneity of the cohorts (geographical region, tumor site, sex distribution, habits such as alcohol and tobacco),
immunohistochemistry (IHC) tools, even treatment protocols, this leading to controversial and non-conclusive results without significant clinical relevance [
12].
The aim of this study is to assess the immunohistochemical expression of PD-L1 in squamous cell carcinomas of the oral cavity with a reliable and accurate method and its possible association with different histopathological, clinical and prognostic variables in a cohort of patients with OSCC from a single institution with a peculiar distribution including a majority of females and just 50% of regular smokers, and determine an accurate and reliable method for measuring PD-L1 in OSCC.
2. Materials and Methods
Retrospective observational study of 65 OSCC from 65 patients was performed. All patients were collected form Hospital Universitario 12 de Octubre, Madrid between 01/2020 and 05/2022. Tumors were stained for PD-L1 using CPS, considering PD-L1 as positive when CPS ≥ 1. ESTROBE guidelines were followed to write this article. Ethical approval was granted by the CEIM/CEI of Hospital Universitario 12 de Octubre.
Clinicopathologic features, including age at diagnosis, sex, smoking history (never smoker, current/former smoker), tumor sites (Tongue, cervical metastasis from a previous resected OSCC, gum, buccal mucosa, floor of the mouth, Retromolar trigone), pathologic TNM stage (the American Joint Committee on Cancer 8th edition of the Oral Cancers staging system) and comorbidity that can alter the immune system (Diabetes mellitus (DM), chronic corticosteroids, transplant recipients and chronic obstructive pulmonary diasease (COPD)) were analyzed in this study. Histologic differentiation was designated as either well, moderately or poorly differentiated, extranodal extension (ENE) of metastatic lymph nodes was classified a positive or negative.
The patients were followed up until death or 1st of November 2022. Overall survival (OS) was defined as the interval from the day of surgery to the day of death for any cause, Disease-free survival (DFS) from the day of surgery to the day of disease progression on CT scan / other image test or death by any cause, and Disease-Specific Survival (DSS) from the day of surgery to the day of death from OSCC.
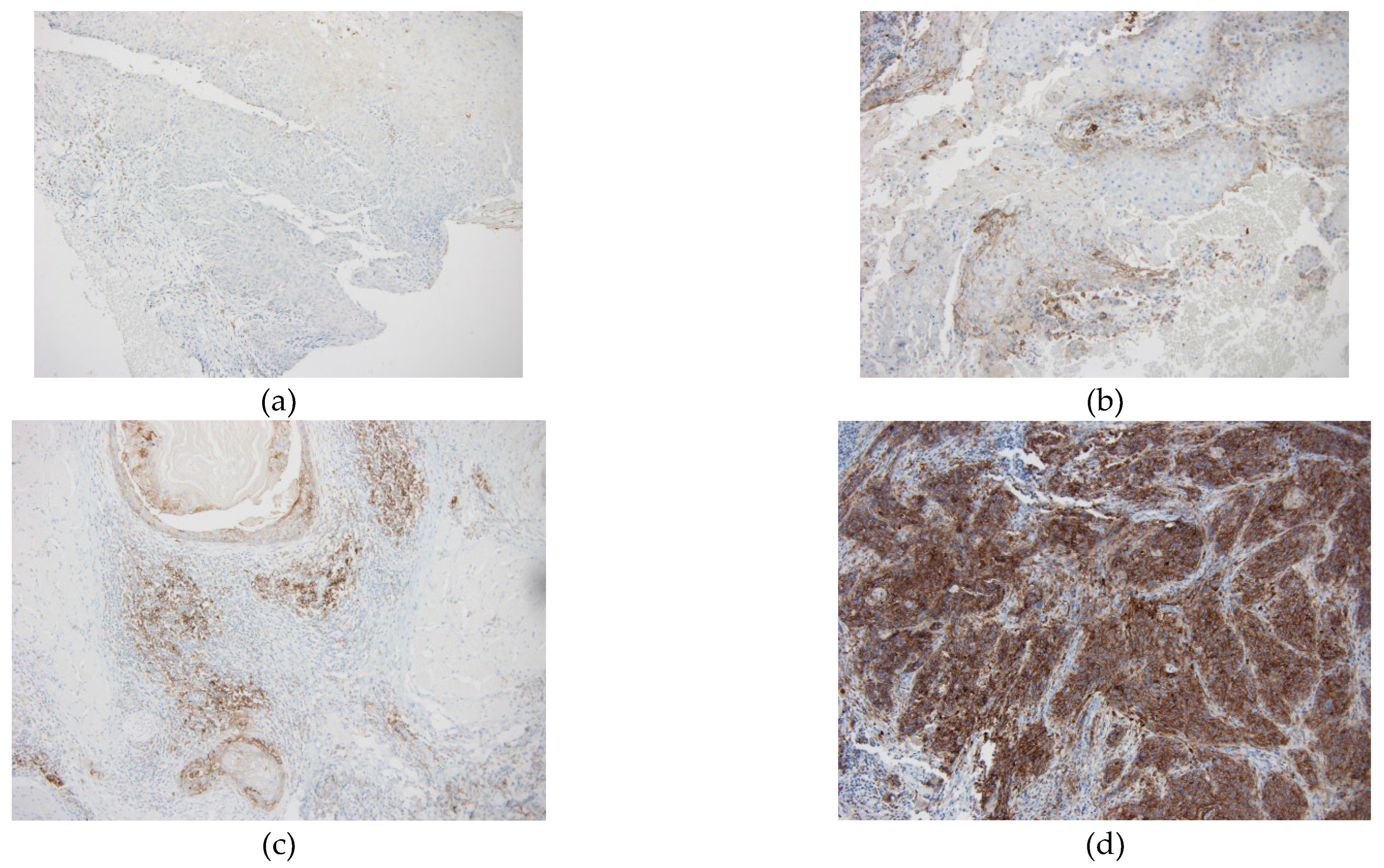
The immunohistochemical study was performed in the Pathology department of our hospital using sections of 4-micron thickness of tissue embedded in paraffin and fixed in formalin. We used a laboratory test with Dako 22C3 anti-PD-L1 primary antibody (Agilent Dako, Ca, USA, catalog number K8005) on a Ventana Benchmark ULTRA platform. Antigen retrieval was performed using Cell Conditioning 1antigen retrieval solution (Ventana Medical Systems) for 64 min at 95ºC. The specimens were incubated with primary anti-PD-L1 monoclonal antibody at a concentration of 1:25 for 64 min at 37ºC, and with the Optic View DAB IHQ Detection Kit. Internal control with normal tonsil was performed to assess the validity of PD-L1 immunoreaction. Cases were considered adequate for assessment if at least 100 tumor cells were observed. We considered positive partial or complete membrane staining in infiltrative tumor cells and membrane and /or cytoplasmic staining of immune cells (lymphocytes and macrophages) close to the tumor. CPS (combined positive score) was used to calculate the expression level of PD-L1. CPS less than 1 was considered negative. Our trained pathologist assessed the PD-L1 expression with CPS evaluation (number of positive cells, immune plus tumor, divided by the number of viable tumor cells, multiplied by 100) in the stained slides of HNSCC in a blinded fashion without knowledge of clinical data.
CPS classification as high (20), low (1-20) or negative (<1) was based on the KEYNOTE-048 study [
3]. In this study, pembrolizumab with chemotherapy improved OS vs cetuximab with chemotherapy in the patients with CPS of 20 or more and CPS of 1 or more. In hence, clinically relevant cut-offs of ≥1 and ≥ 20 for CPS were used. Due to such clinical relevance, we used the same cutoff points (
Figure 1).
IBM SPSS 24.0 (IBM Corp.) was used for statistical analysis. The chi-squared test was used for clinicopathological variables with categorical data unless any category had a value <5, in which case Fisher’s exact test was used. Continuous data were analysed using either Mann–Whitney’s U or Kruskal–Wallis’s H tests. Survival out- comes were calculated using Kaplan–Meier curves and the log-rank test. The reliability for the CPS score on biopsy and surgical specimen was determined by calculating the correlation coefficient (IC). We defined IC as poor (95% CI <0.5), moderate 0.5≥95% CI < 0.75), good (0.75 ≥ 95% CI < 0.9), or excellent (95% CI ≥ 0.9).
3. Results
3.1. CPS measured in biopsy and surgical piece
65 tumors from 65 patients were analyzed. PD-L1 was measured in two occasions, first in the biopsy of the tumor and later in the piece of surgical resection. CPS was scored in biopsy and sample of the surgical piece in 23 cases. PD-L1 measurement in the biopsy was available in 52 tumors, of which 6 (11’53%) tumors were PD-L1-, while 46 (88’46%) tumors were PD-L1 +, with a mean CPS of 34.83. PD-L1 measurement in the surgical piece was available in 38 tumors, of which 7 (18’42%) were PD-L1 negative and 31 tumors (81’58%) were PD-L1 positive, mean CPS of 48.23. The correlation coefficient between the PD-L1 expression in biopsy and surgical piece was 0.83 (p 0.0001). 60 tumors (89’55 %) were PD-L1 positive in the biopsy or in the piece of surgical resection, at least on one occasion. 37 (55%) tumors had a high CPS (>20). In 5 tumors the CPS was higher in the biopsy than in the surgical piece, while 6 tumors had a higher CPS in the surgical piece. Simplifying these measurements for statistical analysis, we consider a tumor as PD-L1 positive when a positive value has been obtained in one of the two PD-L1 measurements. In the case of being positive in both measurements, we consider the highest value for statistical analysis.
3.2. Clinicopathological correlation
A comparison was made between PD-L1 status (as a categorized variable) and clinicopathological variables (
Table 1). Mean age of the patients was 67.92 (Ds 14.24). Using 60 years as the cutoff point, no statistically significant differences were observed in PD-L1 status and age (CPS ≥1 vs <1 p 0.89; CPS ≥20 vs <20 p 0.47). In our sample there were 29 (44.61%) men and 36 women (55.38%). No significant difference was seen between the sex of the patient for the expression of PD-L1 (p 0.92). 32 (49.23%) patients were nonsmokers, 33 (50.76%) patients were smokers or exsmokers. No significant differences were seen between smoking status for the expression of PD-L1 (p 0.65). There was no significant correlation between being or not a smoker and sex (p 0.47), so the sex of the patient did not act as a confounding factor between smoking and PD-L1 expression in our sample.
18 (27.69%) patients were immunosuppressed (DM, chronic corticosteroids, transplant recipients and COPD), 47 (72.30%) not being immunosuppressed. No statistically significant differences were seen between the expression of PD-L1 and immune status of patients (p 0.96) neither significant difference was seen between the immune status and the degree of high, low or negative expression of PD-L1 (p 0.43). Most common location of OSCCs was the tongue, 22 (33.84%) patients. No significant differences were seen between the tumor location and the expression of PD-L1 (p 0.074), nor between the location, smoking status and the PD-L1 expression (p 0.3). 23 (35.38%) tumors were T4, the most common tumor size. 86.95% of T4 tumors expressed PD-L1 in the surgical piece (tumor resection), compared with 54.54% in T1 tumors. Although, all T4 tumors express PD-L1 at least once (there were 3 T4 tumors PD-L1 positive in the biopsy and PD-L1 negative in the surgical piece). Significant differences were seen between status expression of PD-L1 and the tumor size when CPS cutoff value was set at ≥1 and ≥20 (p < 0.001, p 0.009 respectively). This significant difference is explained because most of the PD-L1 negative tumors are T1. 32 (50.79%) of tumors showed lymph node involvement (pN+). 100% of the tumors N+ were PD-L1+ at least in one measurement (biopsy or surgical piece). There were 3 N+ tumors PD-L1 positive in the biopsy and PD-L1 negative in the surgical piece. Prevalence of pN+ was 59.38% for PD-L1 high expression versus 40.63% for low expression. Cervical node extension significantly correlated with the PD-L1 expression (CPS ≥ 1) (p 0.004). Similar to the observed with the tumor size, this significant difference is explained because most of the PD-L1 negative tumors are N0. No significant differences are seen when we compared the cervical node extension (N0 vs N+) between the high (CPS ≥ 20) vs low expression (CPS < 20) of PD-L1 (p 0.7). 43 (66.15%) tumors were advanced stages (III and IV), of which 36 (83.72%) were stage IV, all of them were PD-L1 +. 97.67% of the advanced tumors expressed PD-L1 + while 71.42% of the early tumors expressed PD-L1. Significant difference was seen between tumor staged and PD-L1 expression (p <0.001) when CPS cutoff value was set at 1. Neither the grade of tumor differentiation nor extranodal extension showed no significant association to PD-L1 expression (p 0.12; p 0.13 respectively).
The study included 59 (90.76%) primary tumors and 6 (9.23%) recurrences. 52 (80%) of the primary tumors were PD-L1 positive, of which 30 (57.7%) with a high expression (CPS > 20), and 22 (42.30%) with a low expression (CPS <20). 100% of the recurrences, the 6 tumors, expressed PD-L1, 3 of them with a high expression and the other 3 with a low expression. No significant differences were seen between primary and recurrence tumors for the expression of PD-L1 (p 0.38).
3.3. Survival
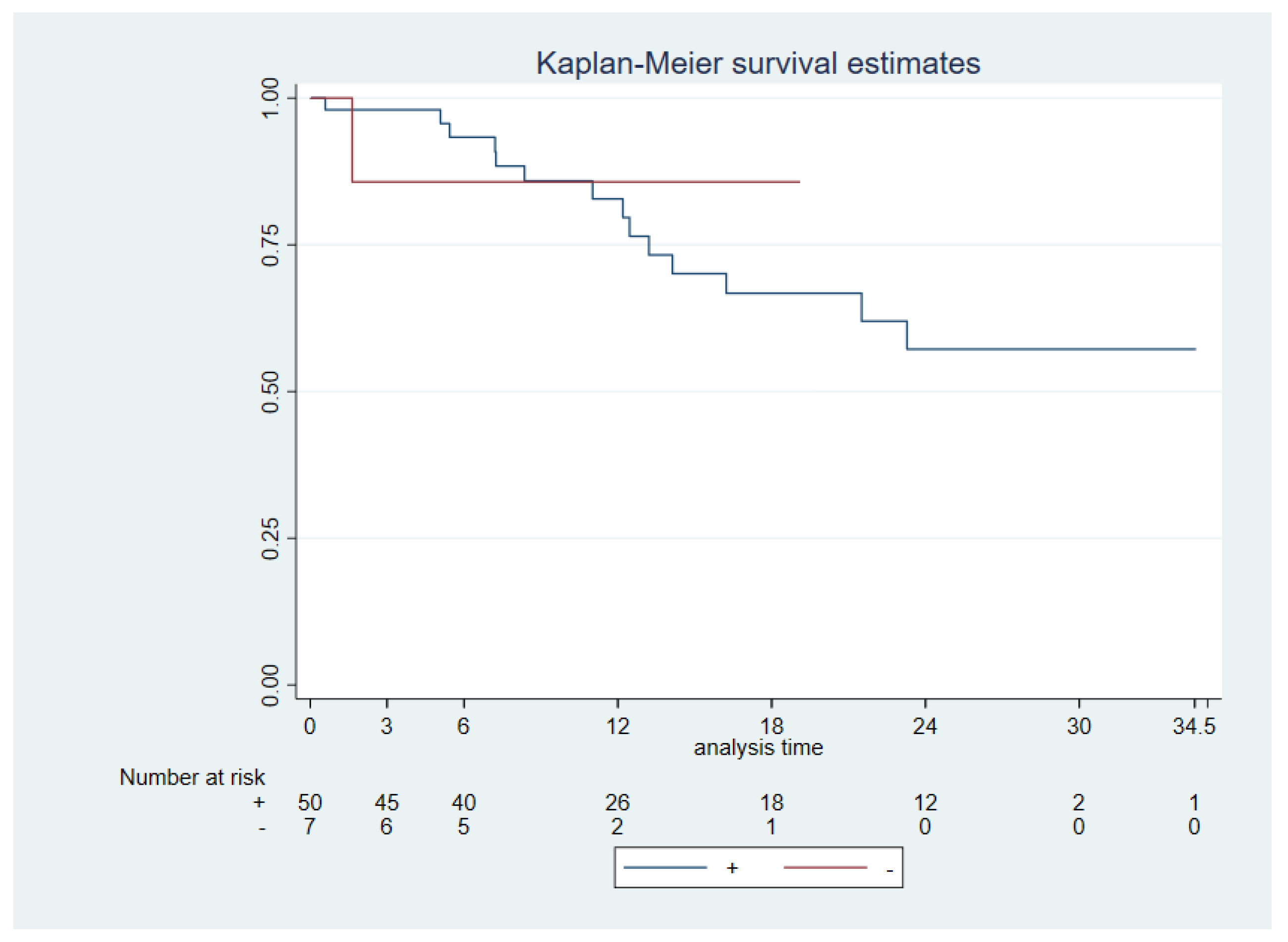
57 patients underwent surgery as first treatment, 3 patients were initially treated with chemoradiotherapy, 4 patients just radiotherapy and 1 best supportive care. We decided to restrict the survival analysis, using the data of the patients at the time of the first tumor surgically treated, which is why only 57 patients were included in the survival analysis.
Patients’ follow-up ranged from 2 to 34.5 months with 11 cancer-specific deaths. The median follow-up of the patients was 13.68 months [6.36,14.17]. Median OS was reached 11.9 months and interquartile range [6.36,19.11]. A significantly worse OS was found for N+ status (N0 vs N+, p 0.0006), advanced stages (I & II vs III & IV, p 0.0067), tumor size (p 0.003) and extra nodal extension (ENE - vs ENE +, p 0.0033). No significant difference was seen between PD-L1 +/- (CPS ≥1 vs CPS <1) neither PD-L1 high (CPS ≥20) vs low (CPS <20) and OS (p 0.97 and 0.64, respectively) (
Figure 2).
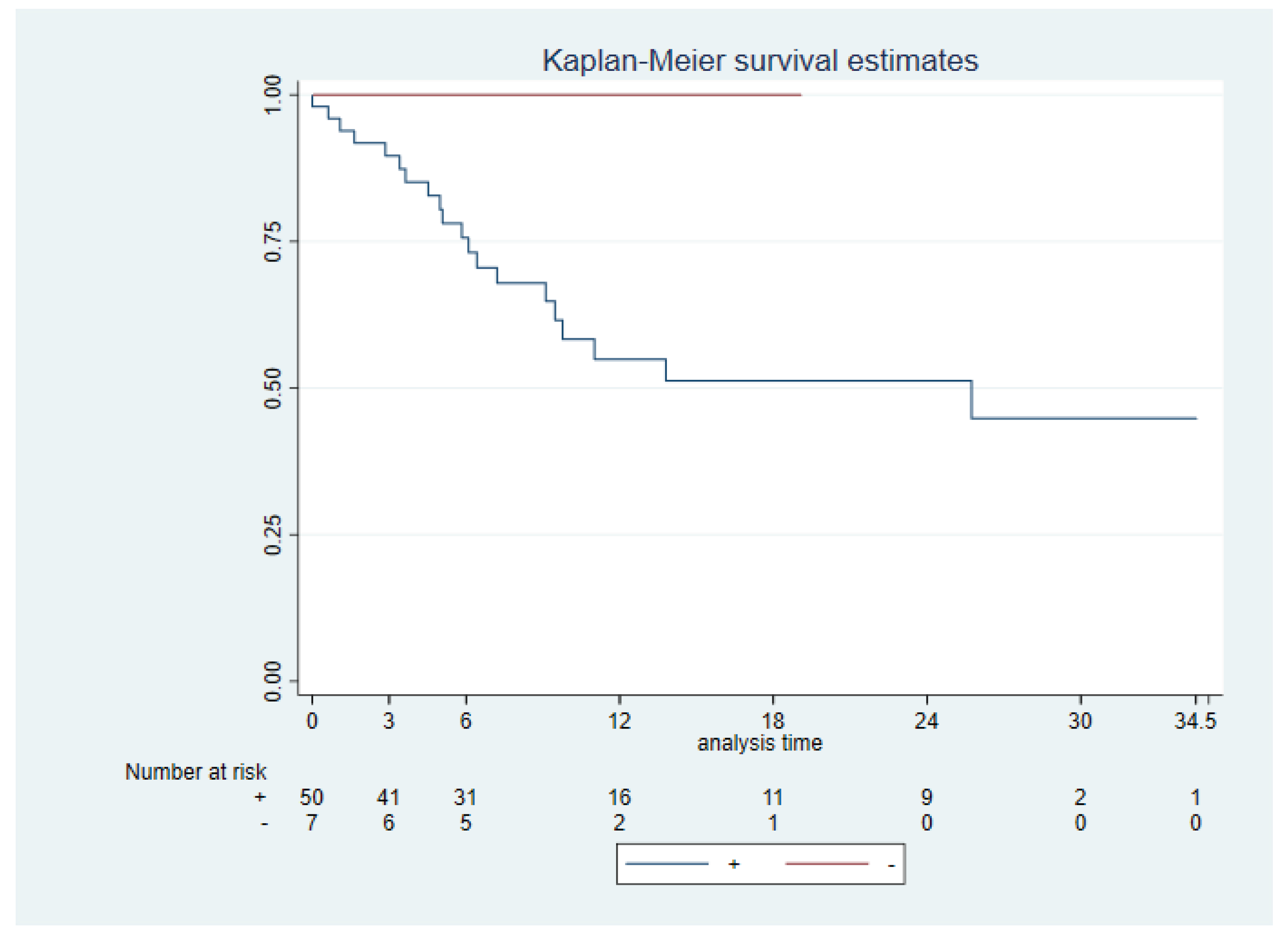
Disease Free Survival (DFS) was measured from the months of surgery to the day of disease progression on CT scan / other image test or death by any cause. ENE was close to achieving a significant difference in DFS (p 0.061). No significant difference was seen between PD-L1 +/- (CPS ≥1 vs CPS <1) neither PD-L1 high (CPS ≥20) vs low CPS <20) and DSF (p 0.11 and p 0.88, respectively) (
Figure 3). We had 33,89% of tumor recurrences (20 tumors). 12 of these tumors were PD-L1 high and 7 of them were PD-L1 low. Just one tumor was PD-L1 negative in the biopsy made of the recurrence, previously this tumor was PD-L1 low (CPS 10).
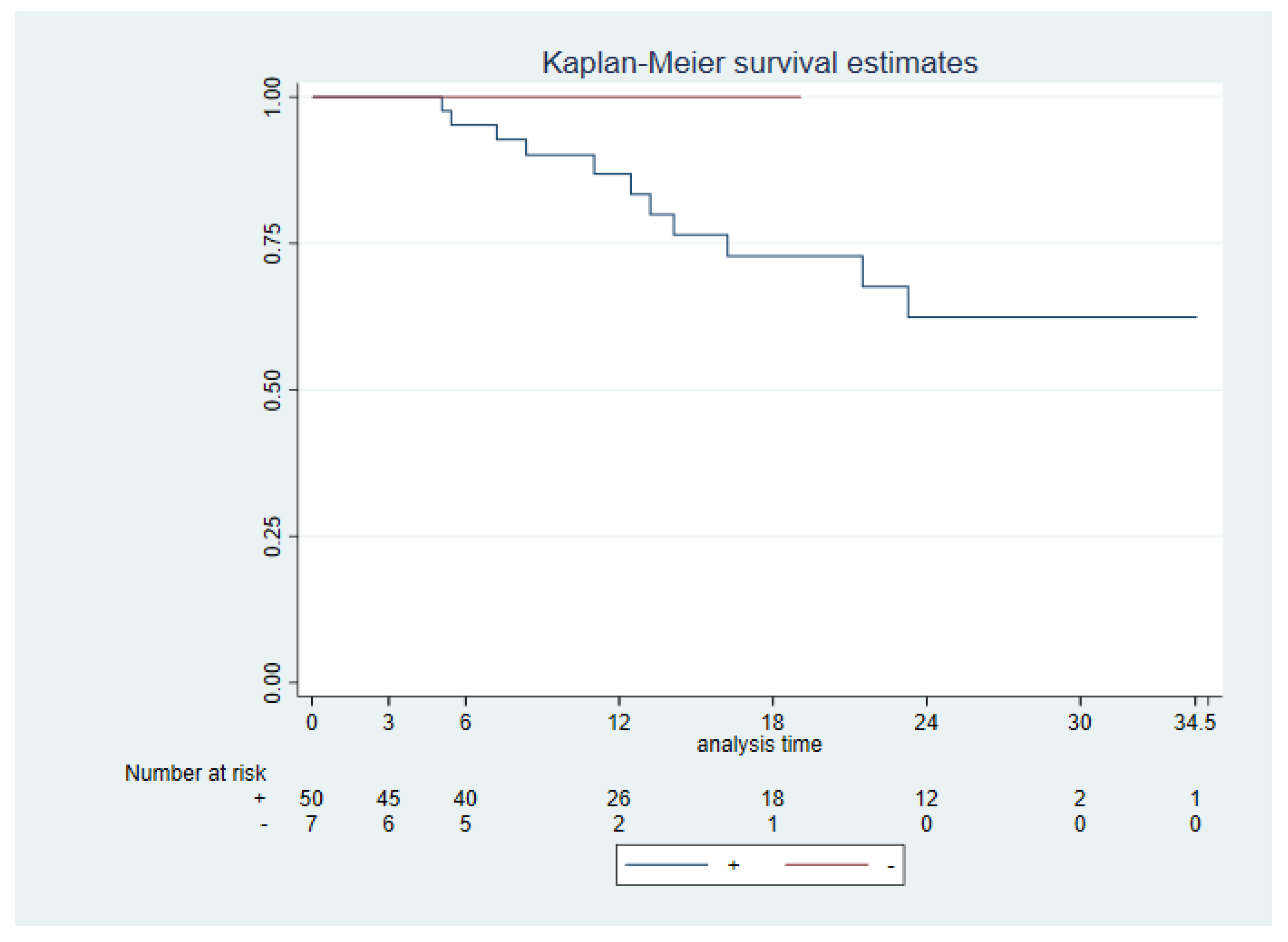
All patients who died due to the OSCC were PD-L1 positive, despite this no significant difference was found between PD-L1 +/- (+CPS ≥1 vs CPS <1) neither PD-L1 high (CPS ≥20) vs low CPS <20) and DSS (p 0.43, p 0.99, respectively) (
Figure 4). A significantly worse DSS was seen for ENE + (ENE + vs ENE -, p 0.003), cervical node extension (N+ vs N-, p 0.0005), advanced stages (I & II vs III & IV, p 0.005) and tumor size (p 0.0014).
4. Discussion
Measurement of PD-L1 was made using the scoring system Combined Positive Score (CPS), which measures the expression of PD-L1 both in tumor cells and in immune cells. The presence of immune cells in the tumor will have an impact on an adequate clinical response to immunotherapy [
13]. When CPS is above 1% is used to predict response to anti-PD-L1 therapies when its level is above 1% [
3,
14]. Another way to measure PD-L1 is the TPS, but this score does not take into account the presence of immune cells in the tumor, so it should be complemented with the IC (tumor-infiltrating immune cells) to estimate the response of immune checkpoint inhibitors (ICIs) [
15].
In our study 58 (89.23%) out of the 65 tumors were PD-L1 positive, 36
(55.38%) tumors had a high CPS (>20). Very similar expression frequencies of PD-L1 in head and neck epidermoid tumors were found in other studies in the literature, Dilinaer Wusiman et al [
16] and the KETNOTE-048 study [
3] reported 89.9% and 85% with a CPS ≥ 1, 43.7% and 43% when CPS> 20, respectively. Both studies used 22C3 assay for immunohistochemical study, as we did. In contrast to this, a prevalence of 48% (CPS>1) was found in the cohort of Miranda et al [
17]. Other studies used TPS for PD-L1 measurement, such as Levounel et al. [
18], reporting a 58% prevalence of PD-L1+ OSCC in their cohort, cut off TPS >5%.
In our cohort, 5 tumors the CPS was higher in the biopsy than in the surgical piece, while 6 tumors had a higher CPS in the surgical piece. There were 3 N+ and T4 tumors PD-L1 positive in the biopsy and PD-L1 negative in the surgical piece. Gaetano Paolino et al [
19] reported PD-L1 expression usually higher in lymph nodes metastasis than in surgical specimens and lower expression was found in biopsies. Jacob H. Rasmussen [
20] considers that the most important factor in the variations of PD-L1 measurement in HNSCC is the heterogenicity of the tumor itself.
In spite of this, we had a good correlation coefficient between the CPS scores of the biopsy and the surgical specimen (0.83 p 0.0001). This added to the internal tests with amygdala, which provide internal validity to the results, make us consider our measurements and method as valid and reliable. Simona Crosta et al [
21] compared 5 PD-L1 measurement protocols with PD-L1 detection with microarrays and found inter-observer reliability was moderate, with an ICC of 0.774 (95% CI (0.651; 0.871), so considering this, specific training is needed for PD-L1 measurement as it influences patients’ therapeutic decisions. Similar results were described in the study by Andrea Ambrosini-Spaltro et al. who observed a concordance between tumor resection of head and neck epidermoid tumors and tumor biopsy was 86.7% (k = 0.688) [
11].
Positive expression of PD-L1 (CPS ≥ 1) was significantly more frequent in advanced T stages. In our cohort, most of the PD-L1 negative tumors were T1 (71’42%). These results are in the line of other studies reported in the literature. One explanation for this observed phenomenon could be that when the tumor begins to express PD-L1 it develops a faster growth due to the inhibition of the immune system. However, other studies have related PD-L1 expression (CPS ≥ 1) with smaller tumor size [
16,
22,
23]. It is been suggested than this may be due to complex molecular mechanisms which may stop tumor mitosis [
24]. Further study of the relationship between PD-L1 and tumor size is needed.
No significant correlation was found between PD-L1 (CPS ≥ 1) sex, smoking status nor tumor location. Some studies [
18,
25] have suggested that PD-L1 could be involved in the oncogenesis of tongue tumors and PD-L1 may be overexpressed in early stages of tongue cancer. Levounel et al. [
18] found a significantly higher TPS in tongue tumors, and suggested that PD-L1 could be involved in the oncogenesis of tongue tumors in non-smoking female patients. A recent pilot study [
26] have shown a significant increase in PD-L1 expression in progressing dysplasia to OSCC in comparison with non-progressive dysplasia, so PD-L1 could be related with tumor development and oncogenesis. In this line, one metanalysis [
27], showed PD-L1 expression is significantly higher in non-smoking patients. One of the explanations found in the literature for the lower expression of PD-L1 in smoking patients is the lower signaling of INF gamma, which is a potent inducer of PD-L1 [
28]. Something to take into consideration is that in our study we included in the same group patients who were smokers and ex-smokers, without having access to a record of the pack-year index, which could mean that this measurement was artifactual in our study. One solution to objectify the effect of smoking would be to measure its mutational signature, as performed by Mandal et al., 2016 [
29], who thus associated smoking with lower T-cell infiltrate and IFN-γ signaling.
When using cutoff point at 60 years old no statistically significant differences were observed in PD-L1 status (CPS ≥1 vs <1 p 0.611; CPS ≥20 vs <20 p 0.17). In this line, Levounel et al. [
27] describe no significant association between PD-L1 status and age (>56, >60, >65). Age plays an important role on PD-L1 regulation and individual response to immunotherapy, being its response reduce in elderly patients. Meta-analysis published by Yi Ming et al. suggested that patients older that 75 might not get OS benefit from antiPD-L1 treatment in solid tumors [
30].
No differences in PD-L1 expression were observed in OSCC in immunosuppressed or non-immunosuppressed patients. Dilinaer Wusiman et al. [
16] found a significantly lower incidence of PD-L1 HNSCC tumors in diabetic type II patients.
PD-L1 was not associated with a worse prognosis in terms of overall and disease-specific survival in our study. The number of survival-related events in our study was not statistically powered to draw any conclusions about how PD-L1 affects survival. In addition, our study could have biases due to being conducted retrospectively at a single institution, so even if we had found a correlation between PD-L1 and survival we would have to take these results with caution. Reviewing the literature, Lin YM et al. [
31] described high PD-L1 expression as an independent risk factor in males and smokers and PD-L1 could be an independent prognostic marker for OSCC patients who are male or who have a smoking habbit. In the same line, two meta-analysis suggest that PD-L1 may have a poor impact in patients survival in HNSCC. In OSCC, Levounel et al. [
27] found that overexpression of PD-L1 had a worse survival measured as DSS and DFS. The other meta-analysis, done by Wu L et al [
32], suggest that PD-L1 may play an important role as an effective marker of poor prognosis in salivary gland carcinomas. On the contrary, X. Sun et al [
24], found better prognosis in gastrointestinal stromal tumors that expressed PD-L1 and had tumor infiltrating CD8+ cells. Wei-Fa Yang et al [
33] reviewed 23 studies of PD-L1’s prognosis in HNSCC and found no significant difference between PD-L1-positive and -negative HNSCC patients in OS and DSS. Despite the meta-analyses, the lack of consensus on how we measure PD-L1 makes us unsure of its implication in the survival of head and neck tumors, so, once the method is standardized and further survival study of PD-L1 in head and neck tumors are published, another meta-analysis could be done. Further studies with a greater number of OSCC are needed to elucidate the influence of PD-L1 in the prognosis of OSCC.
Another implication of PD-L1 in the prognosis of oral cavity tumors would be neoadjuvant treatment. Ju WT et al. [
34] conducted a pilot neoadjuvant study
in oral cavity tumors with camrelizumab and apatinib in 20 patients with locally advanced tumors. Tumors with CPS greater than 10 achieved a greater pathological response and neoadjuvant treatment was safe for subsequent surgery. More studies will be necessary to determine neoadjuvant therapy with antiPD-L1 drugs, however, the results seem promising.
5. Conclusions
This study proposes a measurement of PD-L1 with a valid (with internal amygdala controls) and reliable method (CI 0.83 p 0.0001) and relates PD-L1 expression to the main clinicopathological variables and prognostic values. We obtained an OSCC PD-L1+ expression of 89.23% (CPS>1), similar to that found in other articles. PD-L1+ tumors were associated with larger tumor size, lymph node metastasis and advanced stages. We found no correlation of PD-L1 with other clinical variables. PD-L1 + tumors were not associated with worse prognosis in our sample.
Author Contributions
“Conceptualization, G.S.A. and V.J.G.; methodology, J.A.J.; software, I.P.H.; validation, G.S.A. and I.Z.R.; formal analysis, C. R. F.; investigation, I.P.H and F.L.J.; resources, F.L.J.; data curation, I.P.H, F.L.J. P.C.P.; writing—original draft preparation, F.L.J.; writing—review and editing, F.L.J.; visualization, I.Z.R.; supervision, G.S.A.; project administration, G.S.A.; funding acquisition, G.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of “Comité de Ética de Investigación del Hospital Universitario 12 de Octubre” (CPMP/ICH/135/95 date 14/03/2023).”
Informed Consent Statement
The study was conducted on left-over specimens from surgical samples. Cases were completely anonymized and used in blind.
Acknowledgments
The authors would like to thank Prof. Gregorio Sánchez Aniceto for his patience.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ettinger KS, Ganry L, Fernandes RP. Oral Cavity Cancer. Oral Maxillofac Surg Clin North Am. 2019, 31, 13–29. [Google Scholar] [CrossRef]
- Burtness B, Harrington KJ, Greil R, Soulières D, C. D. KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928, Epub 2019 Nov 1. Erratum in: Lancet 2020, 395, 272; Erratum in: Lancet 2020, 395, 564; Erratum in: Lancet 2021, 397, 2252. [Google Scholar] [CrossRef]
- Kiyota N, Hasegawa Y, Takahashi S, C. D. A randomized, open-label, phase III clinical trial of nivolumab vs. therapy of investigator’s choice in recurrent squamous cell carcinoma of the head and neck: a subanalysis of Asian patients versus the global population in CheckMate 141. Oral Oncol 2017, 73, 138–146. [Google Scholar] [CrossRef]
- Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, Iwata H, Masuda N, Otero MT, Gokmen E, Loi S, Guo Z, Zhao J, Aktan G, Karantza V, Schmid P; KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K; KEYNOTE-590 Investigators. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, Fradet Y, Oudard S, Vulsteke C, Morales Barrera R, Fléchon A, Gunduz S, Loriot Y, Rodriguez-Vida A, Mamtani R, Yu EY, Nam K, Imai K, Homet Moreno B, Alva A; KEYNOTE-361 Investigators. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, Esteban E, Isla D, Martinez-Marti A, Faehling M, Tsuboi M, Lee JS, Nakagawa K, Yang J, Samkari A, Keller SM, Mauer M, Jha N, Stahel R, Besse B, Peters S; EORTC-1416-LCG/ETOP 8-15 – PEARLS/KEYNOTE-091 Investigators. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Wu T, Tang C, Tao R, Yong X, Jiang Q, Feng C. PD-L1-Mediated Immunosuppression in Oral Squamous Cell Carcinoma: Relationship With Macrophage Infiltration and Epithelial to Mesenchymal Transition Markers. Front Immunol. 2021, 12, 693881. [Google Scholar] [CrossRef]
- Qiao X-w, Jiang J, Pang X, Huang M-c, Tang Y-j, Liang X and Tang Y. The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer. Front. Immunol. 2020, 11, 1721. [Google Scholar] [CrossRef]
- Andrea Ambrosini-Spaltro et al. PD-L1 expression in head and neck carcinoma by combined positive score: a comparison among preoperative biopsy, tumor resection, and lymph node metastasis. Virchows Archiv 2022, 481, 93–99. [CrossRef]
- Crosta,S. ;Boldorini,R.; Bono, F.; Brambilla, V.; Dainese, E.; Fusco, N.; Gianatti, A.; L’Imperio, V.; Morbini, P.; Pagni, F. PD-L1 Testing and Squamous Cell Carcinoma of the Head and Neck: A Multicenter Study on the Diagnostic Reproducibility of Different Protocols. Cancers 2021, 13, 292. [Google Scholar] [CrossRef]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014, 515, 568–71. [Google Scholar] [CrossRef]
- Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, Le QT, Lee NY, Leidner R, Lewis RL, Licitra L, Mehanna H, Mell LK, Raben A, Sikora AG, Uppaluri R, Whitworth F, Zandberg DP, Ferris RL. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019, 7, 184. [Google Scholar] [CrossRef]
- Paver EC, Cooper WA, Colebatch AJ, Ferguson PM, Hill SK, Lum T, Shin JS, O’Toole S, Anderson L, Scolyer RA, Gupta R. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology. 2021, 53, 141–156. [Google Scholar]
- Wusiman D, Guo L, Huang Z, Li Z, Liu S, Ying J, Li W, An C. The clinicopathological significance of PD-L1 expression assessed by the combined positive score (CPS) in head and neck squamous cell carcinoma. Pathol Res Pract. 2022, 236, 153934. [Google Scholar] [CrossRef]
- Miranda-Galvis M, Rumayor Piña A, Sales de Sá R, Almeida Leite A, Agustin Vargas P, Calsavara VF, Lópes Pinto CA, Teng Y, Kowalski LP. PD-L1 expression patterns in oral cancer as an integrated approach for further prognostic classification. Oral Dis. 2021, 27, 1699–1710. [Google Scholar] [CrossRef]
- Lenouvel D, González-Moles MÁ, Ruiz-Ávila I, Chamorro-Santos C, González-Ruiz L, González-Ruiz I, Ramos-García P. Clinicopathological and prognostic significance of PD-L1 in oral cancer: A preliminary retrospective immunohistochemistry study. Oral Dis. 2021, 27, 173–182. [Google Scholar] [CrossRef]
- Paolino G, Pantanowitz L, Barresi V, Pagni F, Munari E, Moretta L, Brunelli M, Bariani E, Vigliar E, Pisapia P, Malapelle U, Troncone G, Girolami I, Eccher A. PD-L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathol Res Pract. 2021, 226, 153605. [Google Scholar] [CrossRef]
- Rasmussen JH, Olin AB, Lelkaitis G, Hansen AE, Andersen FL, Johannesen HH, Kjaer A, Fischer BM, Specht L, Bentzen SM, von Buchwald C, Wessel I, Vogelius IR. Intratumor heterogeneity is biomarker specific and challenges the association with heterogeneity in multimodal functional imaging in head and neck squamous cell carcinoma. Eur J Radiol. 2021, 139, 109668. [Google Scholar] [CrossRef]
- Crosta S, Boldorini R, Bono F, Brambilla V, Dainese E, Fusco N, Gianatti A, L’Imperio V, Morbini P, Pagni F. PD-L1 Testing and Squamous Cell Carcinoma of the Head and Neck: A Multicenter Study on the Diagnostic Reproducibility of Different Protocols. Cancers (Basel). 2021, 13, 292. [Google Scholar] [CrossRef]
- Sanchez-Canteli M, Granda-Díaz R, Del Rio-Ibisate N, Allonca E, López-Alvarez F, Agorreta J, Garmendia I, Montuenga LM, García-Pedrero JM, Rodrigo JP. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol Immunother. 2020, 69, 2089–2100. [Google Scholar] [CrossRef]
- Chen J, Gu P, Wu H. Uncovering PD-L1 and CD8+ TILS Expression and Clinical Implication in Cervical Squamous Cell Carcinoma. Biomed Res Int. 2020, 2020, 8164365. [Google Scholar]
- Sun X, Shu P, Fang Y, Yuan W, Zhang Q, Sun J, Fu M, Xue A, Gao X, Shen K, Hou Y, Sun Y, Qin J, Qin X. Clinical and Prognostic Significance of Tumor-Infiltrating CD8+ T Cells and PD-L1 Expression in Primary Gastrointestinal Stromal Tumors. Front Oncol. 2021, 11, 789915. [Google Scholar] [CrossRef]
- Yoshida S, Nagatsuka H, Nakano K, Kogashiwa Y, Ebihara Y, Yano M, Yasuda M. Significance of PD-L1 Expression in Tongue Cancer Development. Int J Med Sci. 2018, 15, 1723–1730. [Google Scholar] [CrossRef]
- Dave K, Ali A, Magalhaes M. Increased expression of PD-1 and PD-L1 in oral lesions progressing to oral squamous cell carcinoma: a pilot study. Sci Rep. 2020, 10, 9705. [Google Scholar] [CrossRef]
- Lenouvel D, González-Moles MÁ, Ruiz-Ávila I, Gonzalez-Ruiz L, Gonzalez-Ruiz I, Ramos-García P. Prognostic and clinicopathological significance of PD-L1 overexpression in oral squamous cell carcinoma: A systematic review and comprehensive meta-analysis. Oral Oncol. 2020, 106, 104722. [Google Scholar] [CrossRef]
- de la Iglesia JV, Slebos RJC, Martin-Gomez L, Wang X, Teer JK, Tan AC, Gerke TA, Aden-Buie G, van Veen T, Masannat J, Chaudhary R, Song F, Fournier M, Siegel EM, Schabath MB, Wadsworth JT, Caudell J, Harrison L, Wenig BM, Conejo-Garcia J, Hernandez-Prera JC, Chung CH. Effects of Tobacco Smoking on the Tumor Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2020, 26, 1474–1485. [Google Scholar] [CrossRef]
- Mandal R, Şenbabaoğlu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, Lee KW, Ganly I, Hakimi AA, Chan TA, Morris LG. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016, 1, e89829. [Google Scholar]
- Weng, Yi Ming; Peng, Min; Hu, Meng Xue; Yao, Yi; Song, Qi Bin. Clinical and molecular characteristics associated with the efficacy of PD-1/PD-L1 inhibitors for solid tumors: a meta-analysis. OncoTargets and Therapy 2018, 11, 7529–7542. [Google Scholar] [CrossRef]
- Lin YM, Sung WW, Hsieh MJ, Tsai SC, Lai HW, Yang SM, Shen KH, Chen MK, Lee H, Yeh KT, Chen CJ. High PD-L1 Expression Correlates with Metastasis and Poor Prognosis in Oral Squamous Cell Carcinoma. PLoS One. 2015, 10, e0142656. [Google Scholar]
- Wu L, Jiang C, Zhu Z, Sun Y, Zhang T. Prognostic role of PD-L1 expression in patients with salivary gland carcinoma: A systematic review and meta-analysis. PLoS One. 2022, 17, e0272080. [Google Scholar]
- Yang WF, Wong MCM, Thomson PJ, Li KY, Su YX. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2018, 86, 81–90. [Google Scholar] [CrossRef]
- Ju WT, Xia RH, Zhu DW, Dou SJ, Zhu GP, Dong MJ, Wang LZ, Sun Q, Zhao TC, Zhou ZH, Liang SY, Huang YY, Tang Y, Wu SC, Xia J, Chen SQ, Bai YZ, Li J, Zhu Q, Zhong LP. A pilot study of neoadjuvant combination of anti-PD-1 camrelizumab and VEGFR2 inhibitor apatinib for locally advanced resectable oral squamous cell carcinoma. Nat Commun. 2022, 13, 5378. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).