1. Introduction
Agriculture remains an important sector for most countries, representing the main source of food for the world's population. However, it faces an important challenge: producing more and better while increasing sustainability with a reasonable use of natural resources [
1].
The jalapeño pepper is a variety of chili with high production in Mexico due to the infinite number of uses that its fruit has and its intermediate pungency [
2,
3,
4,
5,
6,
7,
8]. The annual area worked is approximately 30 thousand hectares, distributed in practically all states. However, its production, like that of other agricultural products, has been complicated due to global warming; Consequently, it is necessary to look for alternatives to increase production[
9] and favor the conservation of water from the soil, such as soilless systems [
10]. Soilless cultivation is recognized worldwide for its ability to support efficient and intensive plant production, it is a flexible method that allows the grower to have full control over the growing environment, including the active root zone[
11]. Compared to soil-based cultivation, soilless production can be more profitable, producing higher yields and faster harvests in smaller areas [
12]. In soilless cultivation, the nutrient solution that drains out of the root zone can be easily collected and recycled, greatly increasing water use efficiency and minimizing environmental impacts from fertilizer waste [
13].
Aeroponics is a soilless cultivation technique [
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25], it is considered a potential method for cultivation of herbal plants due to the high growth rate and improvement in quantity and quality of production [
26,
27]. In aeroponic crops, to achieve maximum efficiency, it is important to analyze the environments with which the plants interact. Although, interactions between plants and aerial environments have been frequently studied, studies in the root are few due to the complexity of their environment [
28]. The root requires an optimal temperature range to have an adequate growth and production rate. In general, the optimal root temperature tends to be lower than the optimal leaf area temperature. Crop roots have different optimal root temperatures depending on the species [
29,
30].
In addition to the use of efficient cultivation techniques, in agriculture there is a great need for the development of technological tools that support resource management and the adequate detection of pests and diseases. Globally, around 40% of crops are discarded due to various diseases and pests.
Technologies such as expert systems, artificial intelligence and computer vision have made it possible to address different problems that traditional agriculture presents, improving resource management. Image analysis techniques [
31] are actively introduced in agriculture to quickly recognize the reactions and damage to crops due to environmental changes, they are a tool that allows for accurate estimate of various variables that reflect the growth of crops and physiological characteristics. This technology has attracted significant attention for crop monitoring and yield prediction, as it allows, in a non-invasive way, to characterize crop conditions [
32,
33]. The images that are mainly used are hyperspectral [
34,
35,
36,
37], multispectral [
38,
39,
40] and those obtained from the spectra: far infrared (IR), Visible (VIS) [
41], short wave and near infrared (SWIR/ NIR).
The global water problem, the high consumption of fresh water in irrigating crops and climate change requires an efficient use of water resources. Irrigation management is crucial to optimize crop yield and quality, while conserving limited water resources and protecting environmental sustainability [
42]. There are studies that suggest that digital processing of RGB and thermal images can be fundamental in the non-invasive monitoring of water stress in plants. Timely detection of water stress in crops can help manage irrigation accurately and minimize yield loss [
43].
The objectives of this research are to: 1) design and evaluate the operation of the jalapeño pepper crop in a greenhouse aeroponic growth chamber, 2) define a vision system for capturing multispectral images, 3) validate the efficiency of the aeroponic system through development of algorithms for image analysis that allow determining the growth pattern and the main characteristics of the phenological stages of jalapeño pepper plants in aeroponics, 4) describe the growth of the root system in a non-invasive way.
2. Results and Discussion
2.1. Vegetative Growth and Fruiting of Aeroponic Jalapeño Pepper Cultivation
The development of aeroponic crops of jalapeño pepper in a greenhouse has the advantage of having greater control over the crop and, in turn, healthier fruits. Fertigation of jalapeño pepper plants with the nutrients that the plant needs at the time it is required by them suggests good growth for the plant, the root and the fruits. When transplanting the seedlings to the aeroponic system, supports were placed that guide the plant in its growth (See
Figure 1).
During the 60 days that the jalapeno pepper cultivation lasted in the aeroponic system, the growth of the fruits was monitored and it was calculated that 98% of the fruits developed healthily without imperfections. The jalapeño pepper fruits reached an average length of up to 11.5 cm, see
Figure 2.
Table 1 shows the yield and the number of corresponding fruits for each of the four crops. The ANOVA and Tukey statistical tests were applied to check whether the null hypothesis of the means of the four crops were equally met. The results indicated that there was no significant difference between the means of crops 2, 3, and 4. While crop 1 presented a significant difference in both yield and the number of fruits.
2.2. Leaf and Root Morphometric Characterization
The average morphometric characteristics evaluated from images captured in the VIS for the leaf system and in the NIR for the root system of the four crops are represented in
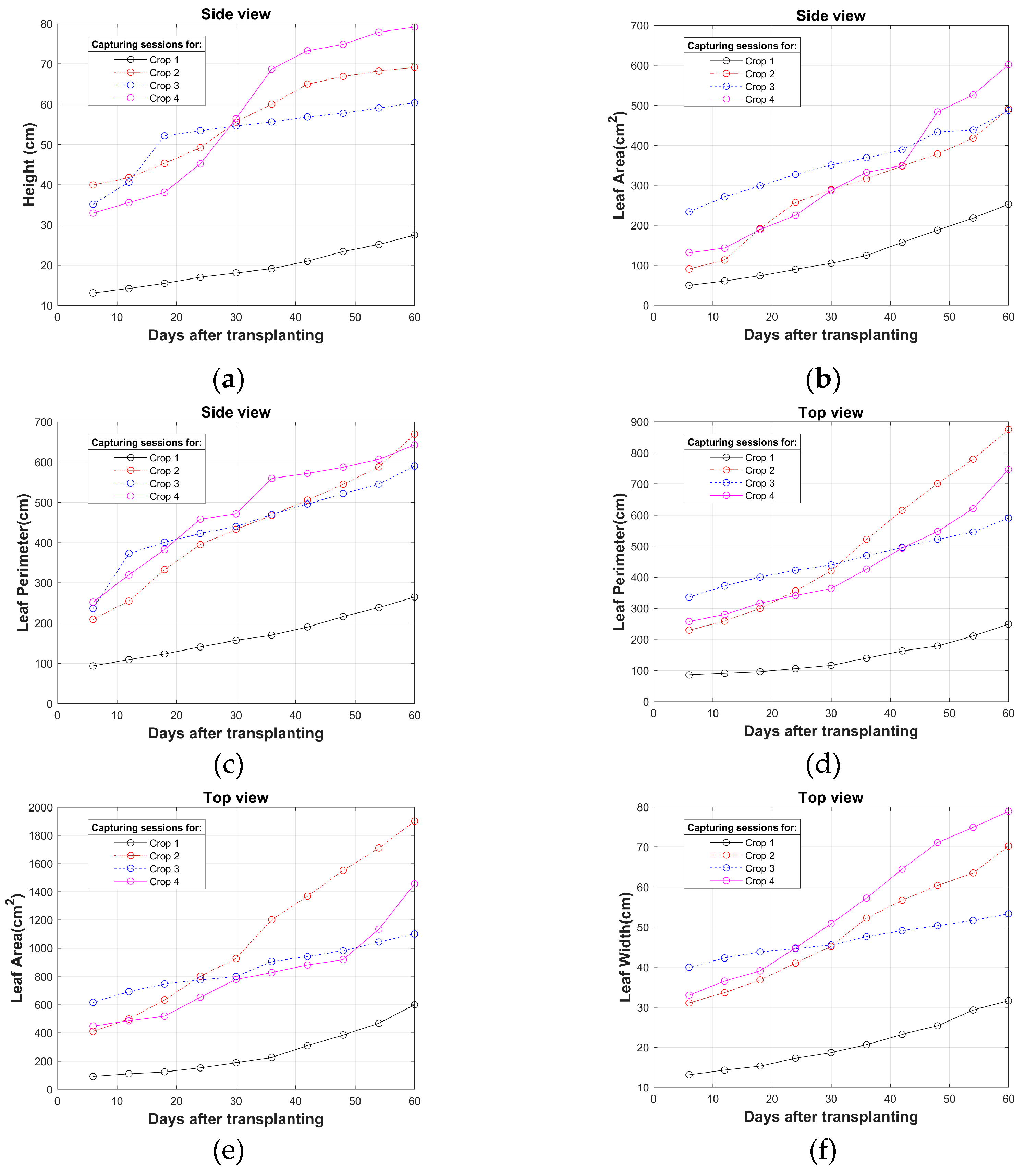
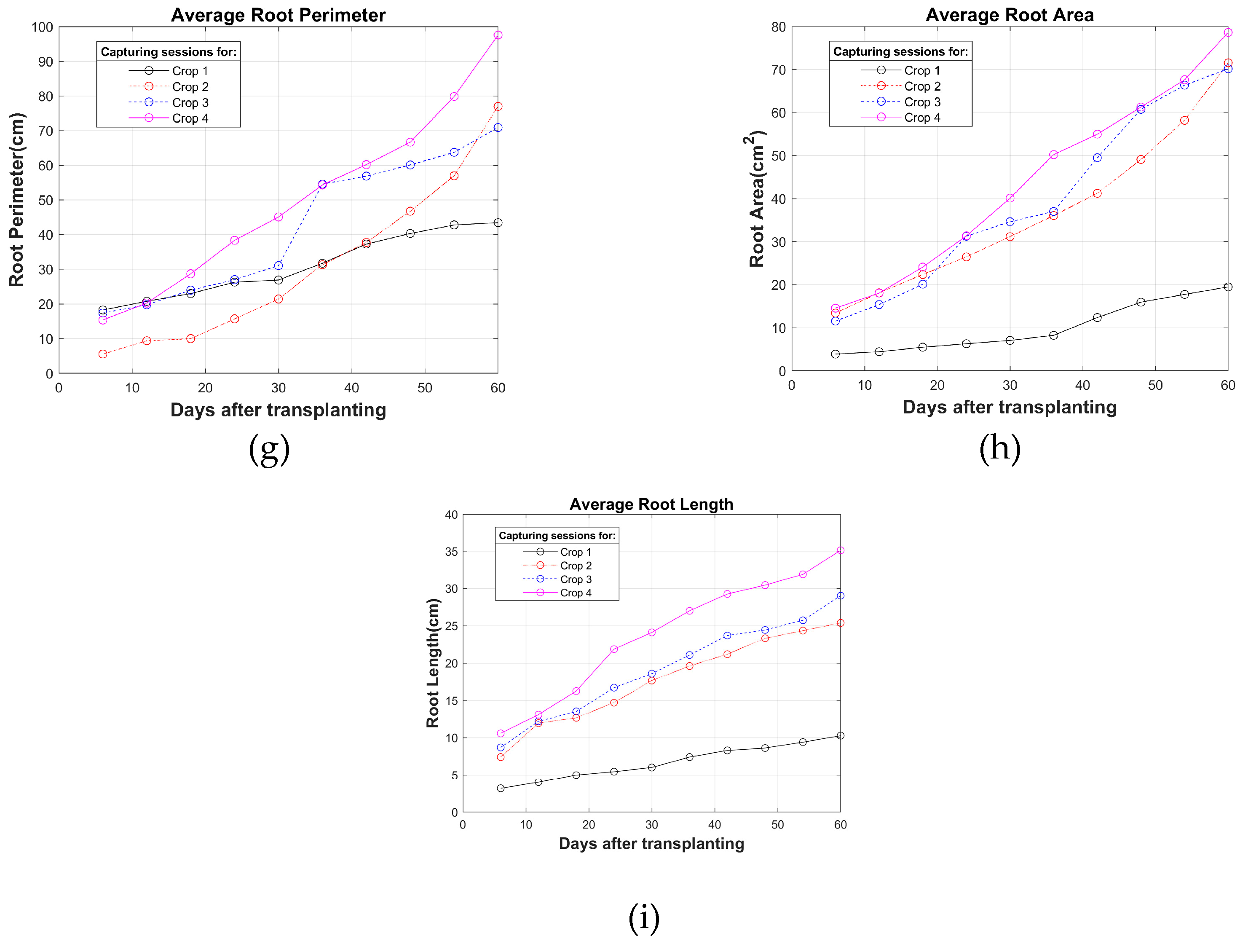
Figure 3. As can be observed, the growth curve of crop 1 shows decreases in its characteristics due to the cultivation of adjustments in fertigation times and in the moments in which nutrient solution for plant growth and fruiting is applied. In crops 2 to 4 the fertigation characteristics are similar. In the graphs, each circle refers to the average of the characteristic calculated in each capture session.
2.3. Average Temperature Obtained during a Period of Water Stress
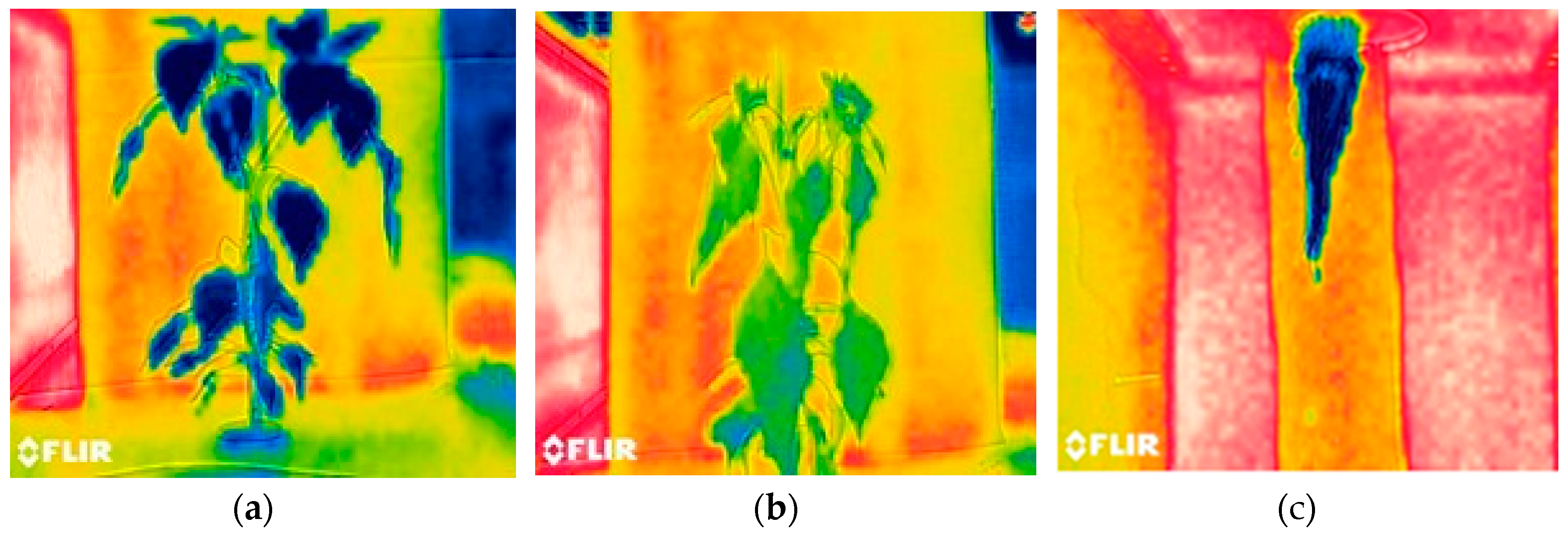
The thermal analysis was carried out under two conditions: when the plant was in optimal conditions (without stress) and when the plant suffered water stress in high temperature conditions. In
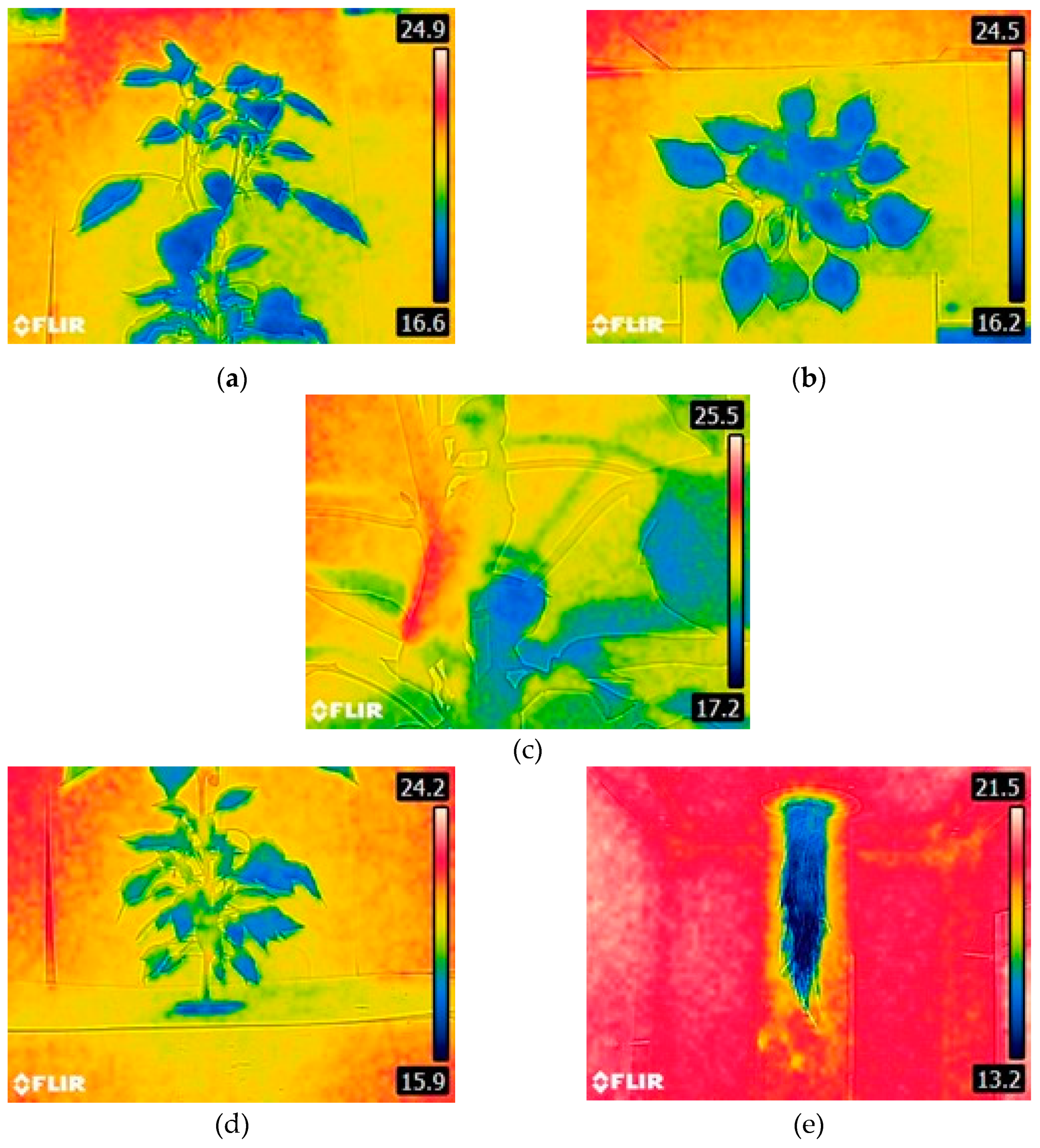
Figure 4, you can see three samples of thermal images in different conditions, (a) the thermal image of the leaf system in optimal state where you can see the uniform color of the plant with a tendency to low temperatures, (b) the thermal image refers to a plant that has suffered from water stress and in addition the greenhouse was at maximum temperature, the plant presented loss of turgor as a mechanism of adaptation to high temperatures and the lack of fertigation, it can be seen that the inclination of the leaves is totally affected, (c) Thermal image of the root system of a plant that suffered stress, the root temperature was not so altered due to the internal temperature and relative humidity conditions of the aeroponic system.
The results of the analysis of the thermal images of the plants with water stress are shown in
Table 2 and
Table 3 where the variables are: ambient temperature (Ta), internal temperature of the chamber (Tc), leaf temperature (Th), root temperature (Tr). In
Table 3 it is possible to see that if a plant is being water stressed, but the ambient temperature is optimal for the crop, the temperature of both the leaf and the root of the plant continue to be highly correlated with the ambient temperature.
In
Table 3, the condition of plants that suffer from water stress and that were also exposed to high ambient temperatures were evaluated.
When a plant suffers water stress and the ambient temperature of the greenhouse is high (exceptional case), its temperature is 3.7 to 5 °C above a plant in optimal condition and when it suffers water stress at optimal growth temperatures the plant is up to 1.3 °C above the temperature of a plant in optimal condition.
The repeatability and efficiency of the aeroponic cultivation technique for jalapeño peppers has been verified through the statistical analysis of the morphometric and thermal parameters of the root, leaf and fruit of 4 crops. According to Ampim et al.
[9] who conclude that the need to increase food production by reducing the impact on the environment, the proposed technique contributes to satisfy this need since jalapeño pepper crops have been obtained with a considerable amount of average fruits per plant in the first cutting (
Table 1) and with reduced water consumption (21.06 liters per crop per day). Furthermore, our proposal complements the characterizations reported by Ramírez-Meras et al.
[4] and Santillano-Cazarez et al.
[6] enabling broader studies in order to evaluate the quality and quantity of harvest under various stress conditions in plants or those that present some type of disease. Finally, V. Singh et al.
[31] and J. Gómez-Camperos et al.
[32] correspondingly in their publications conclude that vision systems represent a reliable alternative for the timely detection of pests and diseases in plants. However, the proposed aeroponic technique also makes it possible to detect stress, pests and diseases in the root before their effects manifest themselves in the leaves or stem.
3. Materials and Methods
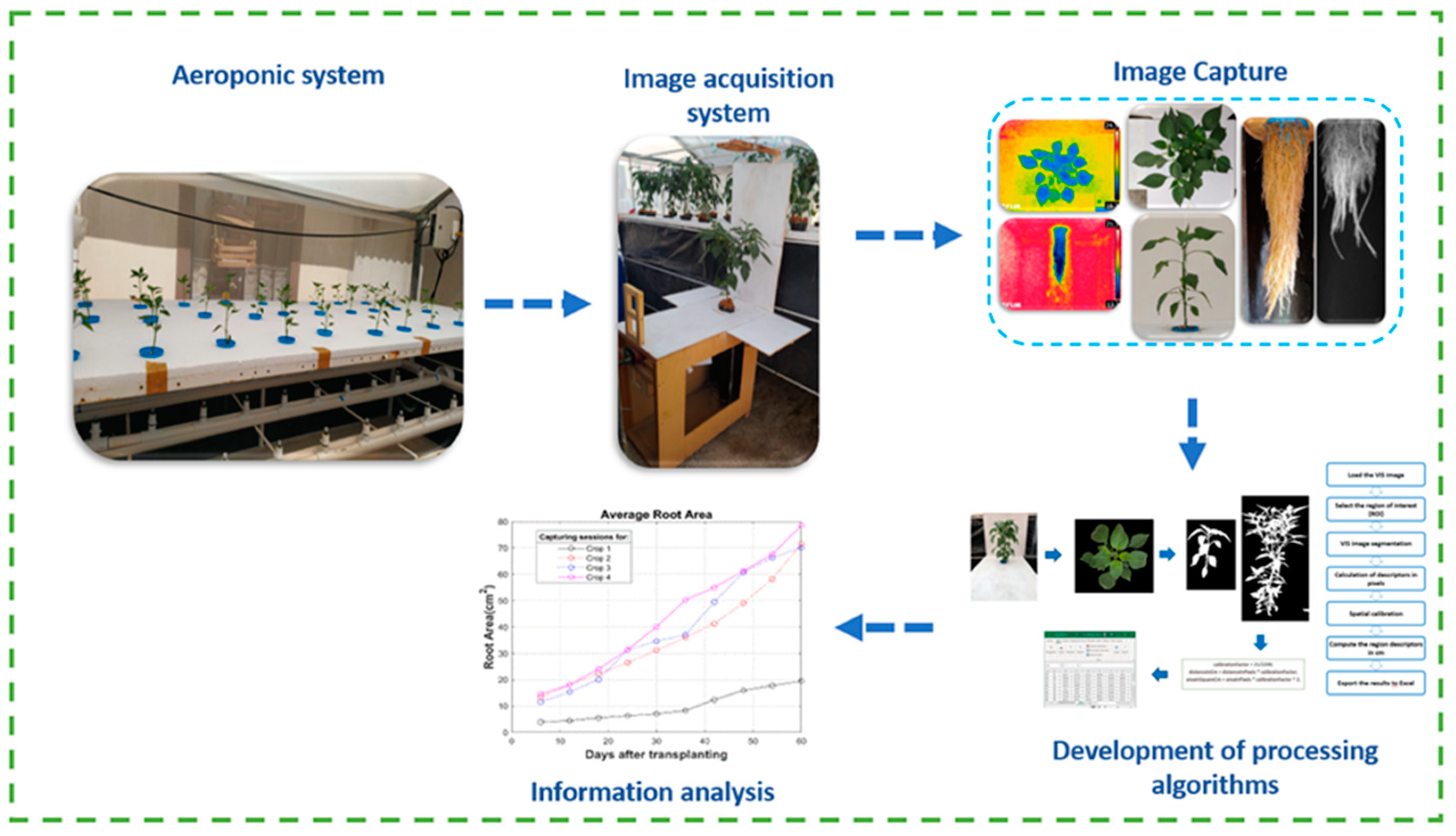
The validation process of aeroponic cultivation of jalapeño pepper through the analysis of the thermal and morphometric properties of the plants and fruits is presented in
Figure 5. Each of the validation stages is described below:
3.1. Aeroponic Cultivation of Jalapeño Peppers
Four jalapeño pepper cultures were cultivated in an aeroponic growth chamber installed within a 16 m2 greenhouse, located in Celaya, Guanajuato, Mexico. The greenhouse is located in a semi-dry/semi-warm region with an average temperature of 28.19 °C, 63.98 % average relative humidity value, average solar radiation of 149.26 W/m2 and a photoperiod of 13 hours. The duration of each crop is 120 days from the day of germination to the day of harvest, of which the first 60 days are for seedling growth and the following 60 days are for crop development within the aeroponic growth chamber.
On day 60 after germination, the seedlings are transplanted to the aeroponic growth chamber. The first three crops were cultivated during 2021. Crop 1 was transplanted on February 10 and harvested on April 12. The seedlings of the second crop were transplanted to the growth chamber on April 14 and harvested on June 14. The third crop was transplanted on October 8 and harvested on December 12. The fourth crop was grown from February 4 to May 6, 2022.
3.2. Aeroponic System
An aeroponic growth chamber was designed and built for the cultivation of 30 jalapeño pepper plants (Capsicum annuum L.) (see
Figure 6), for the design of the chamber the growth characteristics of the plant were considered (height, width and root length), the structure of the chamber is rectangular in shape, its dimensions are 3.3 m long by 1.2 m wide by 1.4 m high. The system has an inspection window to observe the development of the root system in a non-invasive way, an automatic timing system, a fertigation system through nebulization that includes a nutrient solution recirculation section and a nutrient solution tank of 120 l that was separated from the chamber to facilitate the preparation and measurement of the variables involved (pH and electrical conductivity (EC)). Inside the chamber, ideal conditions must be provided for the root to develop adequate functions.
At the top of the chamber structure, three removable lines were installed that serve as support for the vertical staking of the jalapeño pepper plants.
The aeroponic jalapeño pepper crops were developed in four phenological stages: seedling development, vegetative growth, flowering and fruiting. The first stage consists of seedling growth, the seeds are germinated in agricultural foam and covered with inert substrate composed of a mixture of 50% vermiculite and 50% perlite. The seedlings grow for 59 days with a floating root irrigation system. Fertigation begins when the first leaves of the plant appear and is cultivated with the vegetative growth nutrient solution [
13] prepared at 25% of its concentration for 20 days and the following 29 days with 100% concentration, during this stage the aim is for the plant to form its root and leaf system in order to be transplanted to the aeroponic growth chamber.
The vegetative growth stage begins on day 60 after germination with the transplant of the pepper seedling to the growth chamber. From this stage onwards, flowering/fruiting nutrient solution with 100% concentration is used.
The flowering and fruiting of the plants begins approximately on day 78 after germination and ends on the day of harvest. During this period the plants are fed with 100% flowering/fruiting nutrient solution.
In the nutrition of jalapeño pepper plants, two different formulas of commercial nutrient solution are used, the first Steiner type is applied for the vegetative growth stage [
44] and the second is for the reproductive stage (flowering/fruiting) [
45]. In the vegetative growth stage, the electrical conductivity of the nutrient solution is EC = 3586 µS / cm and the total dissolved solids in the water is TDS = 1715 ppm, the values of the reproductive stage are: EC = 3412 µS / cm and TDS = 1706 ppm.
In this article, the results of four crops are reported. The first had the purpose of defining the appropriate fertigation times for the growth of plants in the aeroponic system. The following three crops were used to characterize the phenolic stages and contrast the quality and quantity of production.
According to what was observed in the different tests with fertigation times, it was established that the favorable frequencies for the cultivation of jalapeño pepper in the aeroponic system should be divided into two moments: from the day of transplant to the first day of flowering. The times are 24 minutes of shutdown for 18 seconds of fertigation, one day after the first flower appears the shutdown time changes to 18 minutes of shutdown and 18 seconds of fertigation, this suggests that in the second stage the crop requires 25% more of nutrient solution.
The climatic conditions inside the greenhouse affect the absorption and distribution of nutrients in the plant, this was the reason why the environmental variables of temperature, relative humidity and solar radiation inside the greenhouse were recorded daily throughout the vegetative period of 60 days for each crop. The average solar radiation inside the greenhouse was obtained in a photoperiod of 13 hours, the maximum and minimum temperatures recorded throughout the day and the average relative humidity were reported.
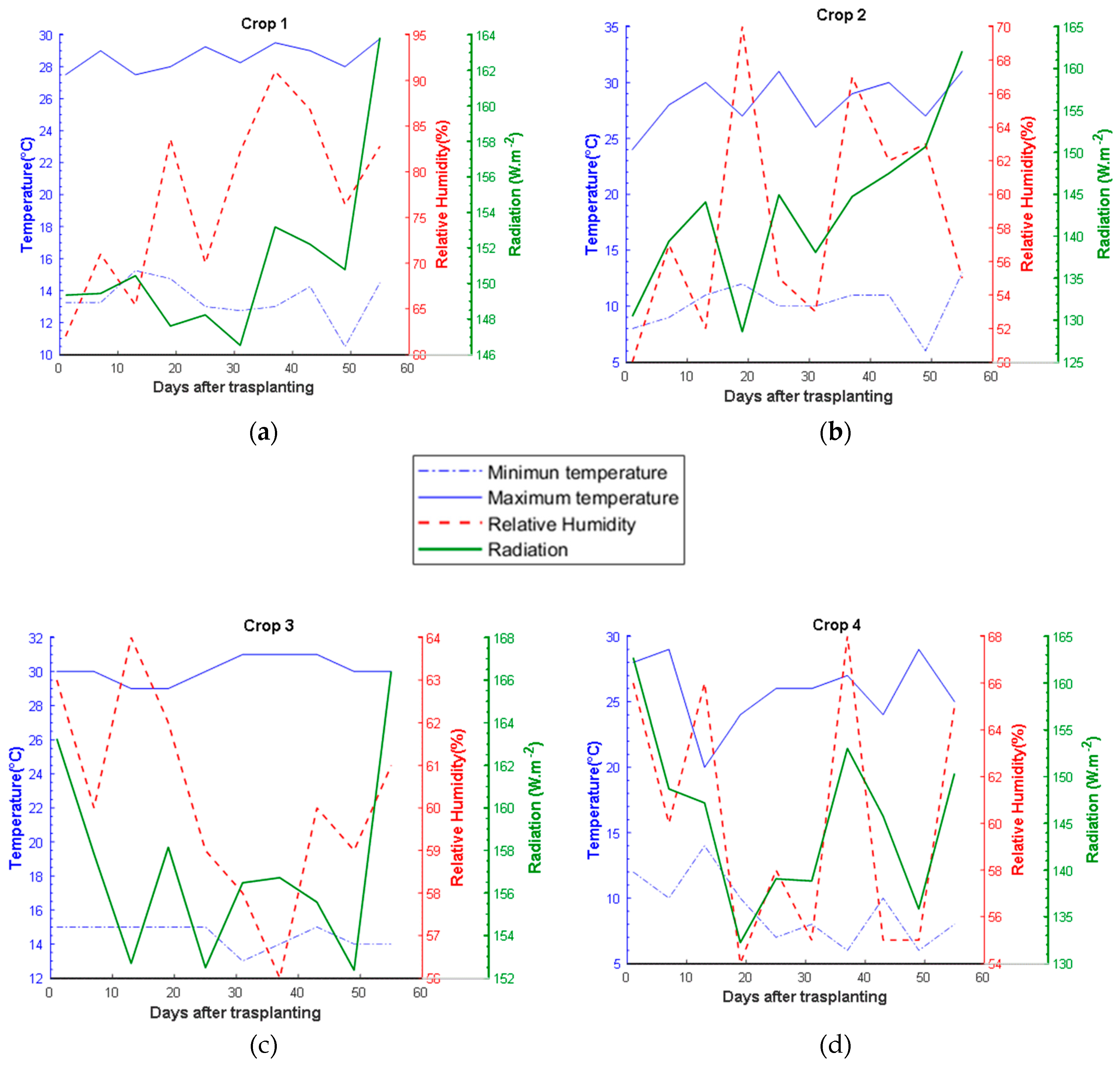
Figure 7 shows the behavior of these variables in the four crops of jalapeno pepper.
The average ambient temperature, relative humidity and solar radiation inside the greenhouse during the growth period were 28.19 ± 1.78 °C, 63.98 ± 5.80 % and 149.26 ± 6.33 W/m2 (mean ± standard deviation), respectively. The behavior of ambient temperature presented statistically different temperature averages for the four crops.
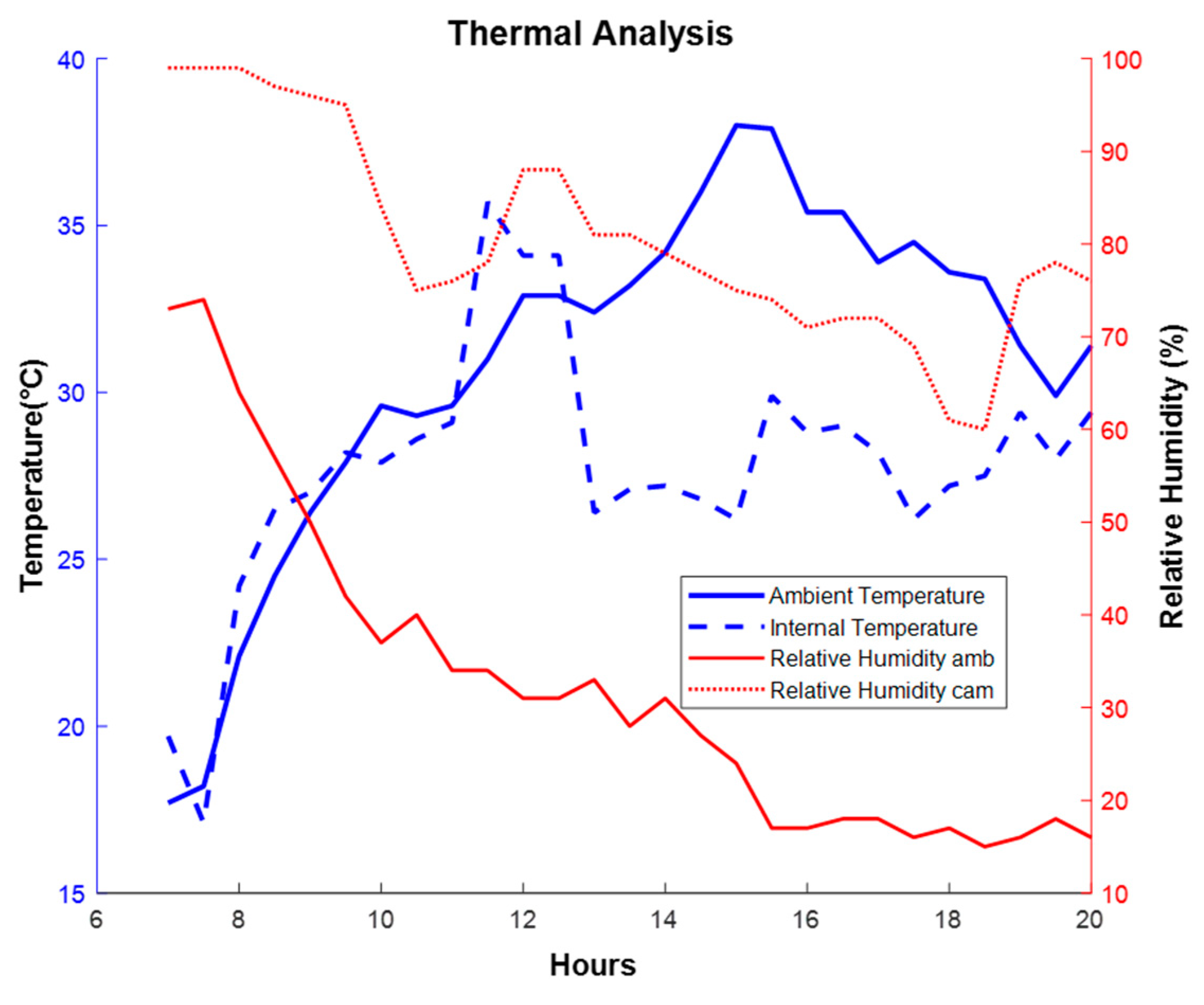
The temperature and relative humidity of the greenhouse environment and the internal part of the aeroponic growth chamber were monitored. The variables were recorded during a 13-hour photoperiod starting at 7:00 a.m. and ending at 8:00 p.m., see
Figure 8.
The dashed-lines refer to the variables measured in the internal part of the aeroponic growth chamber, the blue lines indicate the temperature variables and the red relative humidity. It is possible to see that approximately after 15 hours the ambient temperature reaches its maximum value and the relative humidity of the ambient reaches its minimum value, while in the internal part of the chamber the maximum temperature is reached approximately after11 hours and the relative humidity values remain above 60%. At the time of day when the environmental conditions are not favorable for the plant, the internal temperature of the chamber to which the root system is exposed remains 15 °C lower and the relative humidity is approximately 75%, as a consequence of the fertigation process which guarantees adequate conditions for the root system [
46].
3.3. Image Acquisition System
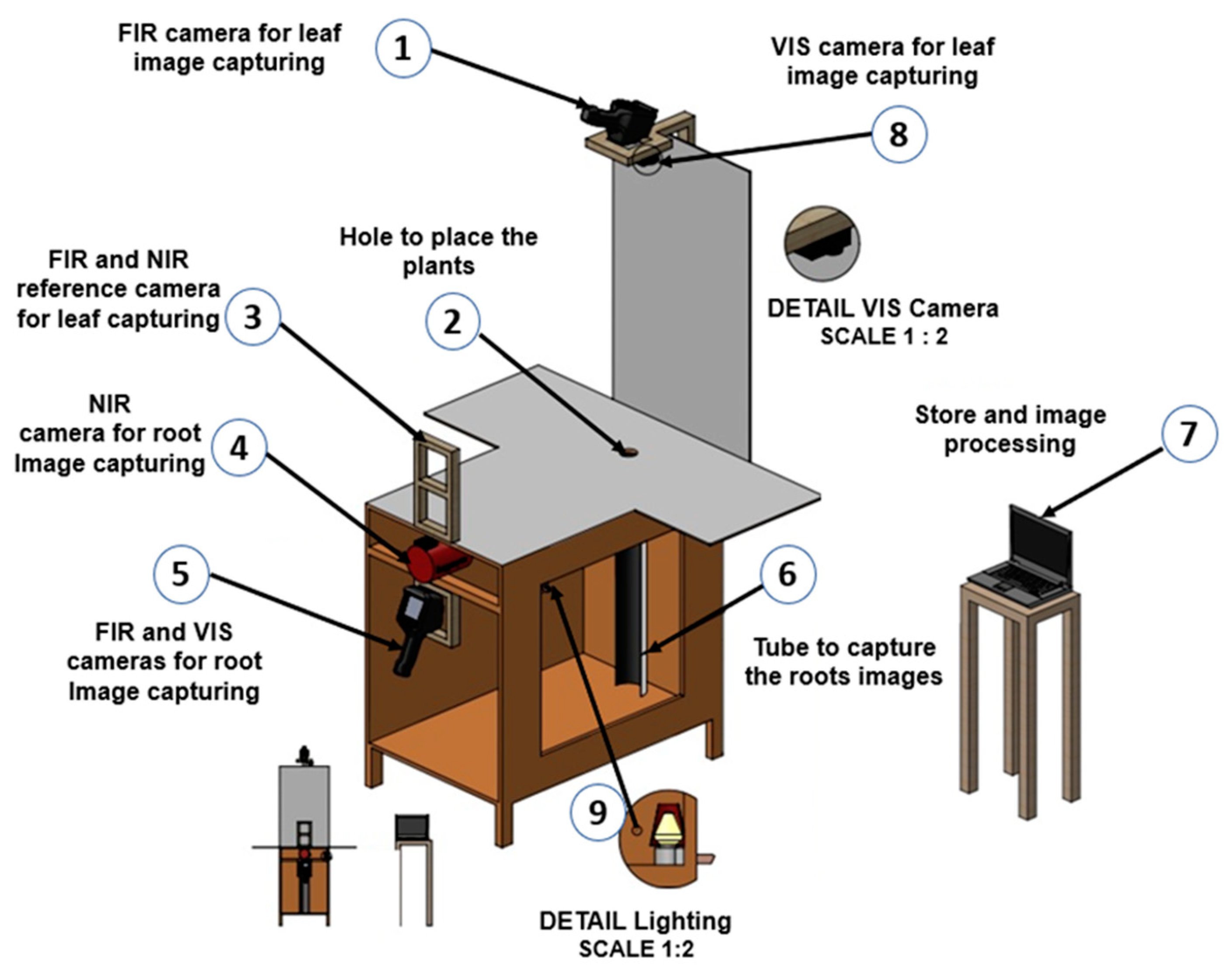
Figure 9 shows the multispectral image acquisition system used in monitoring the jalapeño pepper plants during the four crops. The system allows obtaining images of the top and side views of the plant to acquire morphometric and thermal characteristics of both the leaf system and the root system. Three specialized cameras were attached for the spectra: Visible (VIS), near infrared (NIR) and far infrared (IR). VIS spectrum images were captured with a GoPro Hero4 camera with a spectral range of 400–700 nm. The NIR spectrum images were obtained with an Allied Vision G-132 Cool Big-eye NIR camera with: Sony ICX285 EXview HAD CCD sensor, 1280 x 1024-pixel resolution, extended sensitivity ranging from 350 nm to 1000 nm. The near-infrared images are captured with the camera connected to a computer and to improve the lighting conditions, a 7.5 W white light lamp was indirectly installed. The IR spectrum images were acquired with a FLIR E4 infrared camera with wavelength range: 7500-13000 nm, operating temperature range of -20 to 250 °C with an accuracy of ± 2% for ambient temperature of 10 to 35 °C, IR resolution of 80 x 60 pixels, field of view of 45 x 34, minimum focal length of 0.5 m and thermal sensitivity of 0.15 °C.
Additionally, in the design of the prototype supports were installed that serve as a reference point for the placement of the three cameras. The support located for the captures from the top view was placed taking as a reference the maximum height that the plant can have, which is 90 cm.
3.4. Image Capture
A total of 120 jalapeño pepper plants were transplanted, 30 for each of the four crops, having a growth period of 60 days in a growth chamber. Monitoring of growth parameters for each set of 30 plants was carried out through ten image capture sessions. The first session began a week after transplanting the seedlings to the aeroponic system. This waiting time allowed the seedlings to adapt to the system. Each imaging session was performed every five days at 7:00 am, ensuring constant lighting conditions. Additionally, thermal images of plants suffering from water stress under high temperature and optimal temperature conditions were acquired.
As part of non-invasive monitoring, samples were taken in situ within the greenhouse. An average of 345 images total were obtained, the number of images depends on the growth of the plant and the number of images required to capture the total area of the plant. Approximately 210 images of the leaf system and 135 images of the root system were acquired corresponding to the thirty jalapeño pepper plants and the three spectra, see
Table 4.
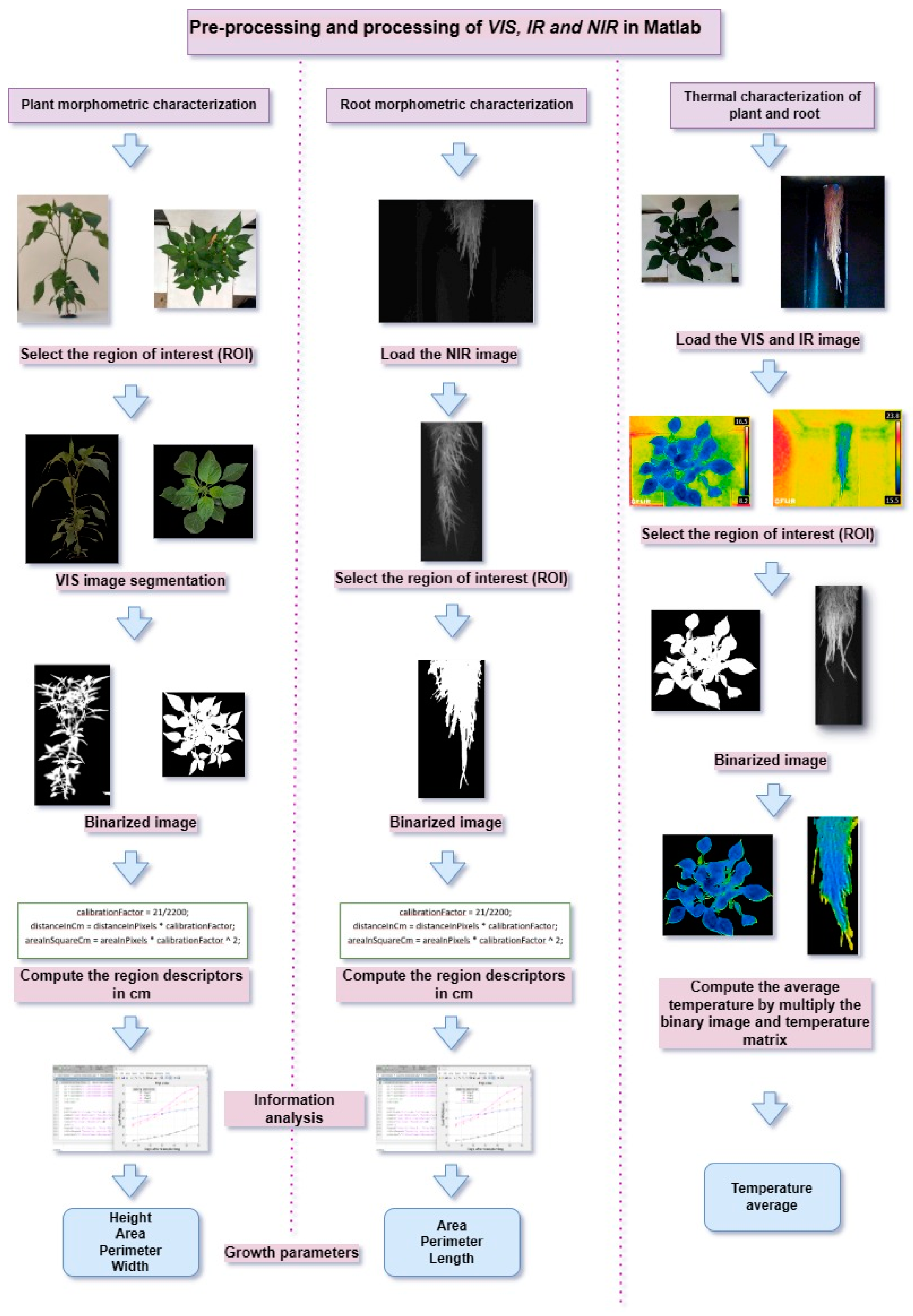
3.5. Image Processing
Multispectral images were analyzed using processing algorithms developed with Matlab R2017b (The MathWorks Inc., Natick, MA, USA). Algorithms were designed for the analysis of visible and infrared images, see
Figure 10.
Images were captured from the top view and the side view. The purpose of capturing the plant from the top view is to evaluate the leaf area and perimeter; these parameters indicate the growth of the plant in relation to the number of leaves and their size. The image captured from the side view is to obtain the height of the plant. The thermal images were obtained from the lateral view for the root and the top and lateral view for the leaf. With these samples, the average temperature for the different parts of the plant was calculated.
3.5.1. Thermal Measurement of Leaf, Root and Fruit Using IR Images
The thermal analysis was carried out under two circumstances: when the plant was in optimal climatic conditions and when the plant suffered water stress and was exposed to high temperatures. Characteristic temperature parameters acquired with a thermal imaging camera were extracted. The camera was configured with an emissivity factor (ε) of 0.96 [
46] and the images were taken from a distance of less than 1 m, capturing the lateral and top view for the leaves, lateral for the root and the fruit. Two images were captured for each part of the plant, an IR image and a VIS image. The image in the Visible spectrum was used to remove the background from the IR image.
An algorithm was designed to evaluate the average temperature of the plants using the following procedure: 1) Load the VIS and IR images, 2) select the region of interest (ROI), 3) binarize the VIS image, 4) obtain the temperature matrix, 5) evaluate the average temperature by multiplying the binary image by the temperature matrix and analyze the results.
Figure 11 shows some of the infrared images captured.
3.5.2. Measurement of Morphometric Parameters Using Images Captured in the Visible Spectrum
The morphometric plant descriptors were calculated from images acquired in the Visible spectrum (
Figure 12), height, area and perimeter were evaluated using a side view image, width and area are evaluated using a side view image. superior. First, the image was obtained with the region of interest, this image was processed with the Matlab Color Thresholder APP, subsequently, the RGB image was analyzed to obtain a mask image which was used to segment the region of interest.
3.5.3. Measurement of Morphometric Parameters Using Images from the NIR Spectrum
The morphometric parameters of the root system were evaluated with the acquired near-infrared images, the processing steps include: selection of the ROI region of interest, image enhancement (contrast), binarization by thresholding, calculation of length, perimeter and area in pixels, spatial calibration to calculate the parameters in cm (Calibration Factor = Object length in cm / Object pixels number), and analysis of results.
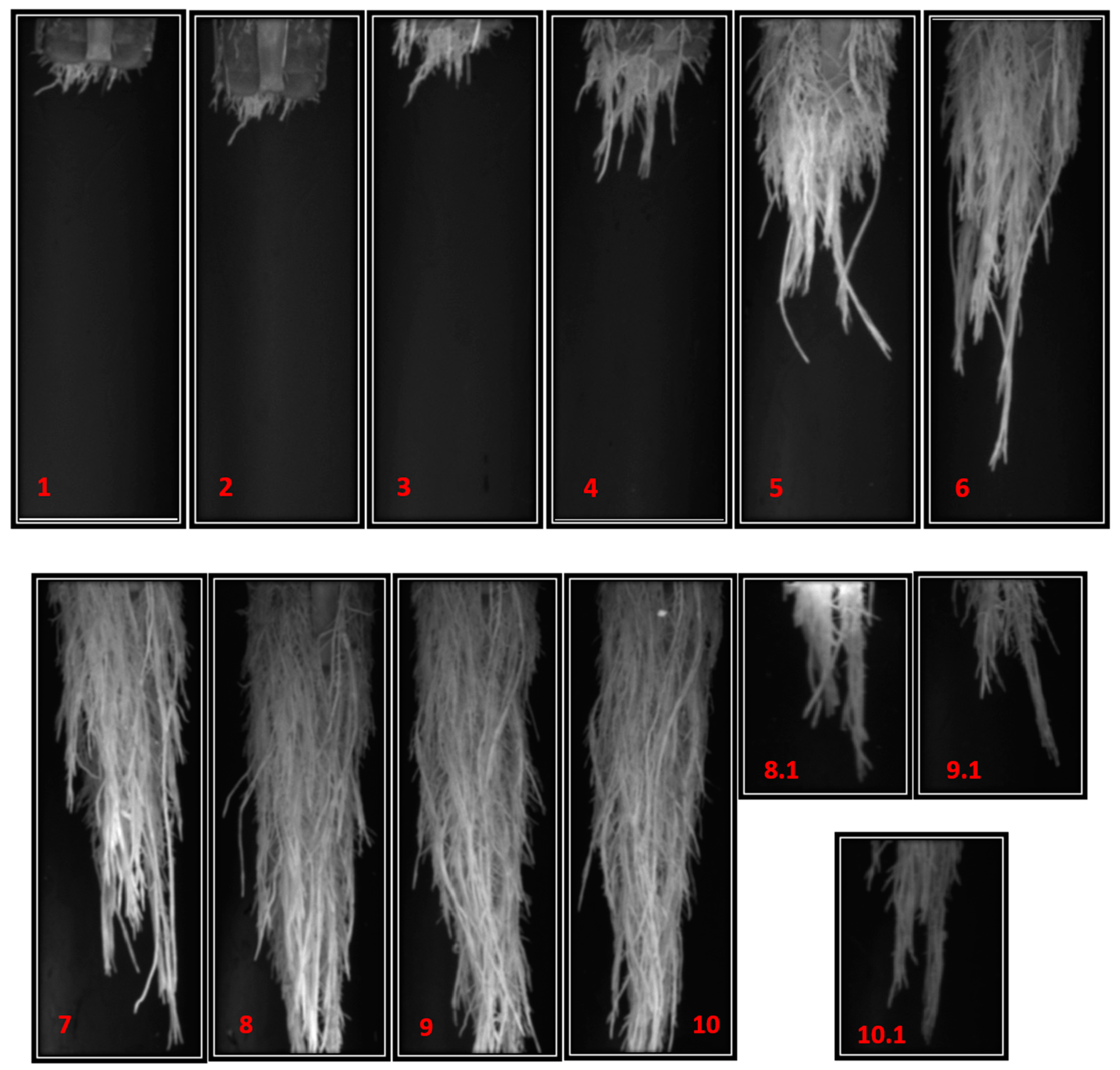
To measure the morphometric parameters of the root, it was necessary to capture more than one image per root starting from session 8. The analysis of the morphometric characteristics of each image was analyzed separately to later add the results.
Figure 13 shows the growth of the root system of a jalapeno pepper plant during the 10 sessions captured.
3.6. Data Analysis
Using the Shapiro-Wilk test with α = 0.05, the normal distribution of the morphometric and thermal parameters of the four crops were confirmed. Subsequently, the data were analyzed with the one-way ANOVA and Tukey test with the Microsoft Office Excel program version 2013 (Microsoft Corporation, Microsoft Way, Redmond, WA, USA) and the leaf and root morphometric data were considered significantly different if p ≤ 0.05. In
Table 5, it can be seen that in crops 2, 3 and 4 there was no significant difference for the parameters: height, area, perimeter and width from the side view, area and perimeter from the top view.
Table 6. The root growth parameters are shown. The results indicate that for the length and area of crops 2, 3 and 4 there is no significant difference between their means for p ≤ 0.05.
4. Conclusions
In this study, the feasibility of developing aeroponic crops of jalapeño peppers has been evaluated and it has been shown that this cultivation technique presents repeatability by taking care of the fertigation times and the moments in which the nutrient solution is changed depending on the phenological stage in which the plant is located. At the same time, it established that in the final stage of cultivation the plants consume 25% more nutrient solution than at the time of transplanting. The aeroponic system was thermally characterized, contrasting the ambient temperature against the internal temperature of the system, it was verified that in the hours of the day when there are higher temperatures in the greenhouse, the internal temperature of the aeroponic system remains optimal for the development of the roots.
The growth curve for the morphometric parameters analyzed from image processing for the plant and its root of the four crops was recorded.
The statistical analysis applied to the morphometric parameters from the digital processing of multispectral images indicates that it is possible to non-invasively monitor the growth of aeroponic crops. The Tukey test shows that there is no significant difference between the means of crops 2, 3 and 4, these crops are those that were developed with similar fertigation conditions. Thermal analysis of plants in optimal condition and plants with water stress were carried out. Additionally, plants with stress were monitored when a high peak of ambient temperature occurred and at normal temperatures. It was concluded that a plant that presents water stress and is also exposed to high temperatures has an average leaf temperature of 3.7 to 5 °C above a plant in optimal condition, while a plant with stress at normal temperatures showed temperature 1.3 °C higher than the plant without stress.
6. Future Works
The objectives of this research focus on establishing the operating characteristics of an aeroponic system for the cultivation of jalapeño peppers in a greenhouse and performing thermal and morphometric characterization through non-invasive multispectral image processing.
The results of the research suggest the versatility of an aeroponic system to work with other types of crops, analyzing the characteristics of each one and raises the possibility of carrying out an experiment with different treatments and the harvesting of fruits that could be done by cuts.
7. Patents
The patent for the Aeroponic System is pending at the Mexican Institute of Industrial Property (IMPI) with file number MX/a/2023/007957, successfully passing the formal examination.
Author Contributions
Conceptualization, J.P.O. and C.M.-N.; methodology, J.J.M.N., M.S.M. and J.A.P.-M.; software, C.M.-N., J.A.P.-M. and A.I.B.-G.; validation, J.A.P.-M. and M.S.M.; formal analysis, C.M.-N. and J.A.P.-M.; investigation, J.J.M.N and M.S.M..; resources, J.P.O. and A.I.B.-G.; data writing—review and editing, C.M.-N., J.A.P.-M. and A.I.B.-G.; visualization, R.G.G.-G. and J.A.P.-M.; supervision, J.A.P.-M. and J.J.M.N.; project administration, J.A.P.-M.; funding acquisition, C.M.-N. and J.A.P.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONAHCyT with a scholarship from one of the authors with number 736294.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- M. M. Islam et al., “DeepCrop: Deep learning-based crop disease prediction with web application,” J Agric Food Res, vol. 14, Dec. 2023. [CrossRef]
- R. Sandoval-Oliveros, L. Guevara-Olvera, J. P. Beltrán, C. Gómez-Mena, and G. Acosta-García, “Developmental landmarks during floral ontogeny of jalapeño chili pepper (Capsicum annuum L.) and the effect of gibberellin on ovary growth,” Plant Reprod, vol. 30, no. 3, pp. 119–129, Sep. 2017. [CrossRef]
- S. R. Delwiche, J. R. Stommel, M. S. Kim, B. T. Vinyard, and C. Esquerre, “Hyperspectral fluorescence imaging for shelf life evaluation of fresh-cut Bell and Jalapeno Pepper,” Sci Hortic, vol. 246, pp. 749–758, Feb. 2019. [CrossRef]
- M. Ramírez-Meraz et al., “Experimental races of Capsicum annuum cv. jalapeño: Chemical characterization and classification by 1H NMR/machine learning,” Food Research International, vol. 138, Dec. 2020. [CrossRef]
- J. M. Pichardo-González et al., “Effect of gibberellins on the yield of jalapeño pepper (Capsicum annuum L.).”.
- J. Santillano-Cázares et al., “Assessment of intercropping and plastic mulch as tools to manage heat stress, productivity and quality of jalapeño pepper,” Agronomy, vol. 8, no. 12, Dec. 2018. [CrossRef]
- H. A. Corzo-Ariyama et al., “Phylogroups, pathotypes, biofilm formation and antimicrobial resistance of Escherichia coli isolates in farms and packing facilities of tomato, jalapeño pepper and cantaloupe from Northern Mexico,” Int J Food Microbiol, vol. 290, pp. 96–104, Feb. 2019. [CrossRef]
- K. Bacon, R. Boyer, C. Denbow, S. O’Keefe, A. Neilson, and R. Williams, “Antibacterial activity of jalapeño pepper (Capsicum annuum var. annuum) extract fractions against select foodborne pathogens,” Food Sci Nutr, vol. 5, no. 3, pp. 730–738, May 2017. [CrossRef]
- P. A. Y. Ampim, E. Obeng, and E. Olvera-Gonzalez, “Indoor Vegetable Production: An Alternative Approach to Increasing Cultivation,” Plants, vol. 11, no. 21. MDPI, Nov. 01, 2022. [CrossRef]
- T. Moon, T. I. Ahn, and J. E. Son, “Long short-term memory for a model-free estimation of macronutrient ion concentrations of root-zone in closed-loop soilless cultures,” Plant Methods, vol. 15, no. 1, May 2019. [CrossRef]
- P. A. Putra and H. Yuliando, “Soilless Culture System to Support Water Use Efficiency and Product Quality: A Review,” Agriculture and Agricultural Science Procedia, vol. 3, pp. 283–288, 2015. [CrossRef]
- G. E. Barrett, P. D. Alexander, J. S. Robinson, and N. C. Bragg, “Achieving environmentally sustainable growing media for soilless plant cultivation systems – A review,” Scientia Horticulturae, vol. 212. Elsevier B.V., pp. 220–234, Nov. 22, 2016. [CrossRef]
- D. Savvas and N. Gruda, “Application of soilless culture technologies in the modern greenhouse industry - A review,” European Journal of Horticultural Science, vol. 83, no. 5. International Society for Horticultural Science, pp. 280–293, Oct. 01, 2018. [CrossRef]
- N. Mannerheim, C. H. Blessing, I. Oren, J. M. Grünzweig, C. Bachofen, and N. Buchmann, “Carbon allocation to the root system of tropical tree Ceiba pentandra using 13C pulse labelling in an aeroponic facility,” Tree Physiol, vol. 40, no. 3, pp. 350–366, Mar. 2020. [CrossRef]
- H. Chajra et al., “Plant milking technology : An innovative and sustainable process to produce highly active extracts from plant roots,” Molecules, vol. 25, no. 18, Sep. 2020. [CrossRef]
- E. G. Tafesse, M. K. Aidoo, N. Lazarovitch, and S. Rachmilevitch, “Aeroponic systems: A unique tool for estimating plant water relations and NO3 uptake in response to salinity stress,” Plant Direct, vol. 5, no. 4, Apr. 2021. [CrossRef]
- M. H. Rahman, M. J. Islam, M. O. K. Azad, M. S. Rana, B. R. Ryu, and Y. S. Lim, “Led light pre-treatment improves pre-basic seed potato (Solanum tuberosum l. cv. golden king) production in the aeroponic system,” Agronomy, vol. 11, no. 8, Aug. 2021. [CrossRef]
- E. Leibar-Porcel, M. R. McAinsh, and I. C. Dodd, “Phytohormone profiles of lettuce and pepper grown aeroponically with elevated root-zone carbon dioxide concentrations,” Agronomy, vol. 10, no. 5, May 2020. [CrossRef]
- T. M. K. Mohamed, J. Gao, M. E. Abuarab, M. Kassem, E. Wasef, and W. El-Ssawy, “Applying Different Magnetic Water Densities as Irrigation for Aeroponically and Hydroponically Grown Strawberries,” Agriculture (Switzerland), vol. 12, no. 6, Jun. 2022. [CrossRef]
- O. Åström, H. Hedlund, and A. Sopasakis, “Machine-Learning Approach to Non-Destructive Biomass and Relative Growth Rate Estimation in Aeroponic Cultivation,” Agriculture (Switzerland), vol. 13, no. 4, Apr. 2023. [CrossRef]
- J. Pasch, S. Appelbaum, H. W. Palm, and U. Knaus, “Growth of Basil (Ocimum basilicum) in Aeroponics, DRF, and Raft Systems with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics (s.s.),” AgriEngineering, vol. 3, no. 3, pp. 559–574, Sep. 2021. [CrossRef]
- J. B. Silva Filho, P. C. R. Fontes, J. F. S. Ferreira, P. R. Cecon, and E. Crutchfield, “Optimal Nutrient Solution and Dose for the Yield of Nuclear Seed Potatoes under Aeroponics,” Agronomy, vol. 12, no. 11, Nov. 2022. [CrossRef]
- S. I. Hassan, M. M. Alam, U. Illahi, M. A. Al Ghamdi, S. H. Almotiri, and M. M. Su’ud, “A Systematic Review on Monitoring and Advanced Control Strategies in Smart Agriculture,” IEEE Access, vol. 9. Institute of Electrical and Electronics Engineers Inc., pp. 32517–32548, 2021. [CrossRef]
- F. Ferrini et al., “Yield, characterization, and possible exploitation of cannabis sativa l. Roots grown under aeroponics cultivation,” Molecules, vol. 26, no. 16, Aug. 2021. [CrossRef]
- J. He, S. Y. Leng, and L. Qin, “Growth, Physiology and Nutritional Quality of C4 Halophyte Portulaca oleracea L. Grown Aeroponically in Different Percentages of Artificial Seawater under Different Light-Emitting Diode Spectral Qualities,” Plants, vol. 12, no. 18, Sep. 2023. [CrossRef]
- D. M. Cao, P. T. B. Vu, M. T. T. Hoang, A. L. Bui, and P. N. D. Quach, “Developing a sufficient protocol for the enhancement of α-glucosidase inhibitory activity by Urena lobata L. Aeroponic hairy roots using exogenous factors, a precursor, and an elicitor,” Plants, vol. 9, no. 4, Apr. 2020. [CrossRef]
- L. Wimmerova, Z. Keken, O. Solcova, L. Bartos, and M. Spacilova, “A Comparative LCA of Aeroponic, Hydroponic, and Soil Cultivations of Bioactive Substance Producing Plants,” Sustainability (Switzerland), vol. 14, no. 4, Feb. 2022. [CrossRef]
- E. Leibar-Porcel, M. R. McAinsh, and I. C. Dodd, “Elevated root-zone dissolved inorganic carbon alters plant nutrition of lettuce and pepper grown hydroponically and aeroponically,” Agronomy, vol. 10, no. 3, 2020. [CrossRef]
- C. Bambang Dwi Kuncoro, T. Sutandi, C. Adristi, and Y. Der Kuan, “Aeroponics root chamber temperature conditioning design for smart mini-tuber potato seed cultivation,” Sustainability (Switzerland), vol. 13, no. 9, 2021. [CrossRef]
- J. Calleja-Cabrera, M. Boter, L. Oñate-Sánchez, and M. Pernas, “Root Growth Adaptation to Climate Change in Crops,” Frontiers in Plant Science, vol. 11. Frontiers Media S.A., May 08, 2020. [CrossRef]
- V. Singh, N. Sharma, and S. Singh, “A review of imaging techniques for plant disease detection,” Artificial Intelligence in Agriculture, vol. 4. KeAi Communications Co., pp. 229–242, Jan. 01, 2020. [CrossRef]
- J. Gómez-Camperos, H. Jaramillo, and G. Guerrero-Gómez, “Técnicas de procesamiento digital de imágenes para detección de plagas y enfermedades en cultivos: una revisión,” INGENIERÍA Y COMPETITIVIDAD, vol. 24, no. 1, Oct. 2021. [CrossRef]
- K. E. Song, S. S. Hong, H. R. Hwang, S. H. Hong, and S. I. Shim, “Effect Analysis of Hydrogen Peroxide Using Hyperspectral Reflectance in Sorghum [Sorghum bicolor (L.) Moench] under Drought Stress,” Plants, vol. 12, no. 16, Aug. 2023. [CrossRef]
- J. Zhao et al., “Simultaneous Quantification and Visualization of Photosynthetic Pigments in Lycopersicon esculentum Mill. under Different Levels of Nitrogen Application with Visible-Near Infrared Hyperspectral Imaging Technology,” Plants, vol. 12, no. 16, Aug. 2023. [CrossRef]
- S. Paulus and A. K. Mahlein, “Technical workflows for hyperspectral plant image assessment and processing on the greenhouse and laboratory scale,” Gigascience, vol. 9, no. 8, Aug. 2020. [CrossRef]
- M. I. A. Abenina, J. M. Maja, M. Cutulle, J. C. Melgar, and H. Liu, “Prediction of Potassium in Peach Leaves Using Hyperspectral Imaging and Multivariate Analysis,” AgriEngineering, vol. 4, no. 2, pp. 400–413, Jun. 2022. [CrossRef]
- L. Wang et al., “LeafSpec: An accurate and portable hyperspectral corn leaf imager,” Comput Electron Agric, vol. 169, Feb. 2020. [CrossRef]
- A. Piron, V. Leemans, F. Lebeau, and M. F. Destain, “Improving in-row weed detection in multispectral stereoscopic images,” Comput Electron Agric, vol. 69, no. 1, pp. 73–79, Nov. 2009. [CrossRef]
- S. Lohumi, B. K. Cho, and S. Hong, “LCTF-based multispectral fluorescence imaging: System development and potential for real-time foreign object detection in fresh-cut vegetable processing,” Comput Electron Agric, vol. 180, Jan. 2021. [CrossRef]
- Y. Lan et al., “Comparison of machine learning methods for citrus greening detection on UAV multispectral images,” Comput Electron Agric, vol. 171, Apr. 2020. [CrossRef]
- D. Rong, H. Wang, Y. Ying, Z. Zhang, and Y. Zhang, “Peach variety detection using VIS-NIR spectroscopy and deep learning,” Comput Electron Agric, vol. 175, Aug. 2020. [CrossRef]
- Z. Zhou, Y. Majeed, G. Diverres Naranjo, and E. M. T. Gambacorta, “Assessment for crop water stress with infrared thermal imagery in precision agriculture: A review and future prospects for deep learning applications,” Computers and Electronics in Agriculture, vol. 182. Elsevier B.V., Mar. 01, 2021. [CrossRef]
- N. S. Chandel, Y. A. Rajwade, K. Dubey, A. K. Chandel, A. Subeesh, and M. K. Tiwari, “Water Stress Identification of Winter Wheat Crop with State-of-the-Art AI Techniques and High-Resolution Thermal-RGB Imagery,” Plants, vol. 11, no. 23, Dec. 2022. [CrossRef]
- I. A. Lakhiar, J. Gao, T. N. Syed, F. A. Chandio, and N. A. Buttar, “Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics,” J Plant Interact, vol. 13, no. 1, pp. 338–352, Jan. 2018. [CrossRef]
- J. He, C. Tan, and L. Qin, “Root-Zone Heat Priming Effects on Maximum Quantum Efficiency of PSII, Productivity, Root Morphology and Nutritional Quality of Two Aeroponically Grown Leafy Greens in a Tropical Greenhouse,” Plants, vol. 11, no. 13, Jul. 2022. [CrossRef]
- M. J. Gómez-Bellot, P. A. Nortes, M. J. Sánchez-Blanco, and M. F. Ortuño, “Sensitivity of thermal imaging and infrared thermometry to detect water status changes in Euonymus japonica plants irrigated with saline reclaimed water,” Biosyst Eng, vol. 133, pp. 21–32, May 2015. [CrossRef]
Figure 1.
Aeroponic system for growing jalapeño pepper in a greenhouse.
Figure 1.
Aeroponic system for growing jalapeño pepper in a greenhouse.
Figure 2.
Jalapeno pepper plant fruits: (a) Fruits on the plant; (b) Fruit size.
Figure 2.
Jalapeno pepper plant fruits: (a) Fruits on the plant; (b) Fruit size.
Figure 3.
Growth curves obtained from image processing: (a) Height; (b) Leaf area; (c) Leaf perimeter (side view); (d) Leaf perimeter (top view); (e) Leaf area (top view); (f) Leaf width (top view); (g) Root perimeter; (h) Root area; (i) Root Length.
Figure 3.
Growth curves obtained from image processing: (a) Height; (b) Leaf area; (c) Leaf perimeter (side view); (d) Leaf perimeter (top view); (e) Leaf area (top view); (f) Leaf width (top view); (g) Root perimeter; (h) Root area; (i) Root Length.
Figure 4.
Samples of thermal image captures (IR): (a) Leaf system in optimal state; (b) Leaf system with water stress and (c) Root system with water stress.
Figure 4.
Samples of thermal image captures (IR): (a) Leaf system in optimal state; (b) Leaf system with water stress and (c) Root system with water stress.
Figure 5.
Schematic diagram of this study.
Figure 5.
Schematic diagram of this study.
Figure 6.
Aeroponic growth chamber for growing jalapeno pepper in a greenhouse.
Figure 6.
Aeroponic growth chamber for growing jalapeno pepper in a greenhouse.
Figure 7.
Environmental variables recorded during the growth of the four crops: (a) Crop 1; (b) Crop 2; (c) Crop 3 ; (d) Crop 4.
Figure 7.
Environmental variables recorded during the growth of the four crops: (a) Crop 1; (b) Crop 2; (c) Crop 3 ; (d) Crop 4.
Figure 8.
Analysis/Thermal behavior of temperature and relative humidity of the environment and internal parts of the growth chamber.
Figure 8.
Analysis/Thermal behavior of temperature and relative humidity of the environment and internal parts of the growth chamber.
Figure 9.
Image acquisition system.
Figure 9.
Image acquisition system.
Figure 10.
Pseudocode diagram for multispectral image processing.
Figure 10.
Pseudocode diagram for multispectral image processing.
Figure 11.
Thermal images (IR): (a) Upper side of leaf; (b) Lower side of leaf; (c) Side of fruit; (d) Top view of leaf and (e) Side of root.
Figure 11.
Thermal images (IR): (a) Upper side of leaf; (b) Lower side of leaf; (c) Side of fruit; (d) Top view of leaf and (e) Side of root.
Figure 12.
Sample captures in the VIS spectrum.
Figure 12.
Sample captures in the VIS spectrum.
Figure 13.
NIR images of the root system of jalapeño pepper.
Figure 13.
NIR images of the root system of jalapeño pepper.
Table 1.
Distribution of yield and number of fruits per square meter for the four crops.
Table 1.
Distribution of yield and number of fruits per square meter for the four crops.
| Crop |
Yield
(Kg.m-2) |
No. of fruits
(m-2) |
Length
(cm) |
| 1 |
1.24 |
8.36 |
6.40 |
| 2 |
3.96 a |
19.66 a |
8.52 a |
| 3 |
4.94 a |
23.23 a |
9.35 a b |
| 4 |
5.34 a |
25.46 a |
9.88 b |
Table 2.
Thermal behavior of plant with water stress and normal ambient temperature, Ta = 23.3 °C and Tc = 23.6 °C.
Table 2.
Thermal behavior of plant with water stress and normal ambient temperature, Ta = 23.3 °C and Tc = 23.6 °C.
| Plant Condition |
Water stress |
| Th(°C) |
Tr(°C) |
| Normal |
23 |
21.5 |
| Stress |
24.6 |
23.7 |
Table 3.
Thermal behavior of plant with water stress and elevated ambient temperature, Ta = 41.1 °C and Tc = 34.3 °C.
Table 3.
Thermal behavior of plant with water stress and elevated ambient temperature, Ta = 41.1 °C and Tc = 34.3 °C.
| Plant Condition |
Water stress |
| Th(°C) |
Tr(°C) |
| Normal |
25.5 - 25.8 |
23.6 |
| Stress |
29.2 - 30.8 |
22 |
Table 4.
Images obtained per capture session.
Table 4.
Images obtained per capture session.
| Spectrum |
Part of Plant |
Number of samples |
| Visible (VIS) |
Leaf |
135 |
| Root |
45 |
| Far Infrared (FIR) |
Leaf |
75 |
| Root |
45 |
| Near Infrared (NIR) |
Root |
45 |
| |
Total |
345 |
Table 5.
Plant growth parameters.
Table 5.
Plant growth parameters.
| Crop |
Plant |
| Side view |
Top view |
| Heigth (cm) |
P |
Area (cm2/
plant) |
P |
Perimeter (cm /plant) |
P |
Width (cm) |
P |
Area (cm2) |
P |
Perimeter (cm/plant) |
P |
| 1 |
19.40 |
*** |
131.94 |
*** |
170.37 |
*** |
20.89 |
*** |
265.37 |
*** |
143.83 |
*** |
| 2 |
56.11 |
n.s. |
289.33 |
n.s. |
440.05 |
n.s. |
49.08 |
n.s. |
1099.84 |
n.s. |
505.76 |
n.s. |
| 3 |
52.56 |
n.s. |
359.77 |
n.s. |
449.48 |
n.s. |
46.81 |
n.s. |
860.05 |
n.s. |
459.48 |
n.s. |
| 4 |
58.22 |
n.s |
326.9 |
n.s. |
485.25 |
n.s. |
55.09 |
n.s. |
810.24 |
n.s. |
439.47 |
n.s. |
Table 6.
Root growth parameters.
Table 6.
Root growth parameters.
| Crop |
Root |
| |
|---|
| Length (cm) |
P |
Area (cm2) |
P |
Perimeter (cm/plant) |
P |
| 1 |
6.77 |
*** |
10.09 |
*** |
31.04 |
n.s. |
| 2 |
17.82 |
n.s. |
36.77 |
n.s. |
31.16 |
n.s. |
| 3 |
19.36 |
n.s. |
39.67 |
n.s. |
42.5 |
n.s. |
| 4 |
23.96 |
n.s. |
44.08 |
n.s. |
50.6 |
n.s. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).