Submitted:
25 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Pathophysiology and Pathogenesis
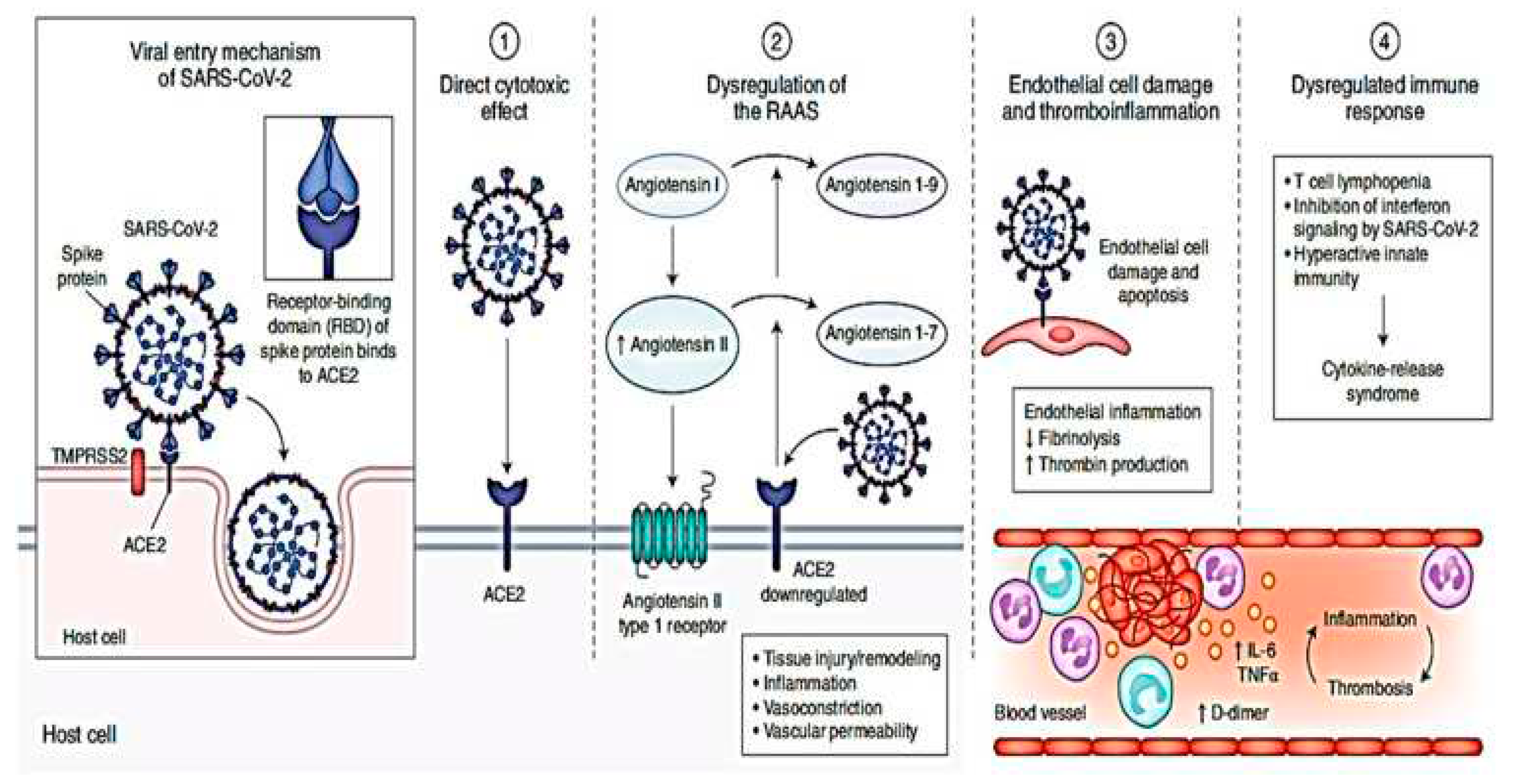
2.1. Interactions and entry of the SARS-CoV-2 into the cell
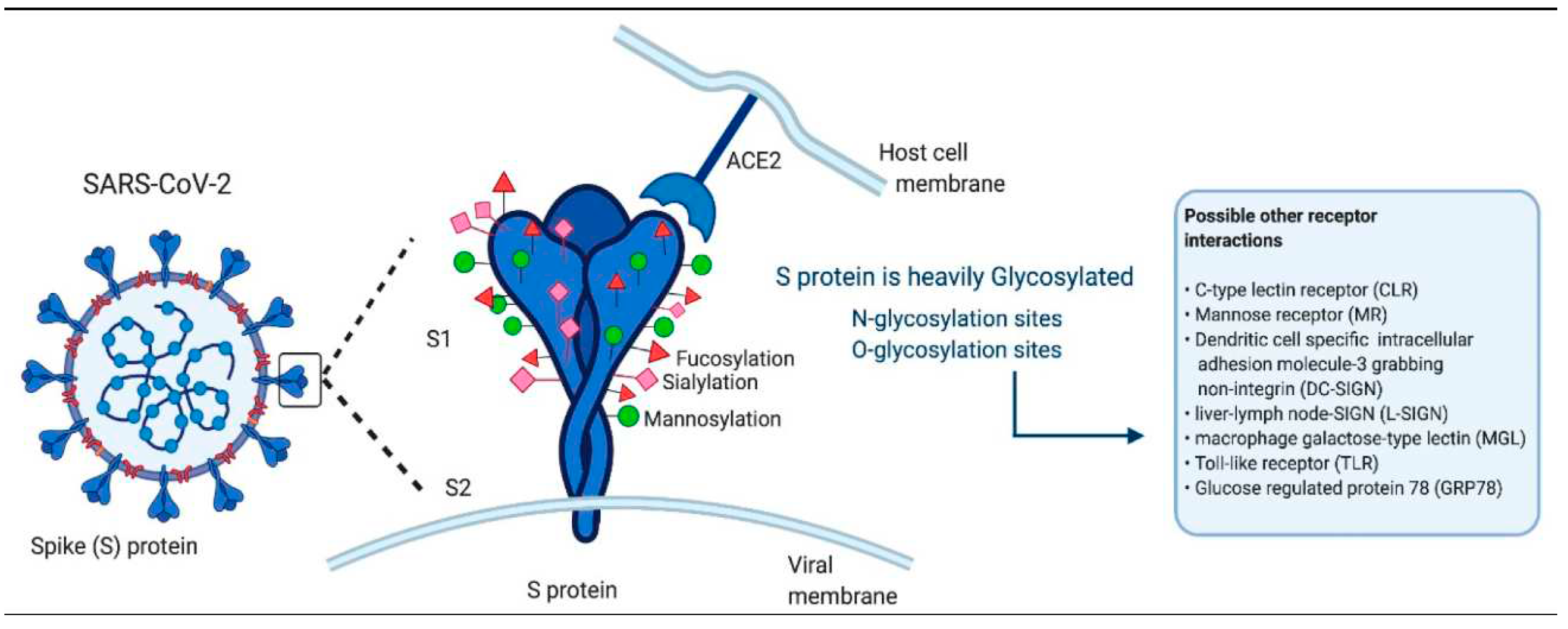

2.2. Long COVID
3. Recent Advances in Pathophysiology of COVID -19
3.1. Role of Soluble PD-L1 in the Course of Severe and Non-Severe COVID-19
3.2. Molecular Dynamics Simulations Suggest SARS-CoV-2 3CLpro Mutations in Beta and Omicron Variants Do Not Alter Binding Affinities for Cleavage Sites of Non-Structural Proteins
3.3. Growth Arrest of Alveolar Cells in Response to Cytokines from Spike S1-Activated Macrophages: Role of IFN-γ
3.4. Molecular Dynamics Simulations Suggest SARS-CoV-2 3CLpro Mutations in Beta and Omicron Variants Do Not Alter Binding Affinities for Cleavage Sites of Non-Structural Proteins
3.5. S-Peptide RBD 484–508 Induces IFN-γ T-Cell Response in Naïve-to-Infection and Unvaccinated Subjects with Close Contact with SARS-CoV-2-Positive Patients
3.6. New COVID Variants and their Implications
3.7. Variants of Interest (VOI)
| WHO label | Lineage + additional mutations | Country first detected (community) | Spike mutations of interest | Year and month first detected | Impact on transmissibility | Impact on immunity | Impact on severity | Transmission in EU/EEA |
| Omicron | BA.2.75 (x) | India | (y) | May 2022 | Unclear (1) | Similar to Baseline (2-4) | No evidence | Community |
| Omicron | XBB.1.5-like (a) | United States | N460K, S486P, F490S | n/a | Similar to Baseline (5, 6) | Reduced (v) (5, 7) | Similar to Baseline (8) | Community |
| Omicron | XBB.1.5-like + F456L (b) (e.g. EG.5, FL.1.5.1, XBB.1.16.6, and FE.1) |
n/a | F456L, N460K, S486P, F490S | n/a | Baseline | Baseline (9) | Baseline | Dominant |
| Omicron | BA.2.86 | n/a | I332V, D339H, R403K, V445H, G446S, N450D, L452W, N481K, 483del, E484K, F486P | n/a | Unclear (10) | Unclear (10-12) | No evidence | Community |
3.8. Variants under monitoring.
| WHO label | Lineage + additional mutations | Country first detected (community) | Spike mutations of interest | Year and month first detected | Impact on transmissibility | Impact on immunity | Impact on severity | Transmission in EU/EEA |
| Omicron | XBB.1.16 | n/a | E180V, T478R, F486P | n/a | No evidence | No evidence | No evidence | Detected (a) |
| Omicron | DV.7.1 | n/a | K444T, L452R, L455F | n/a | No evidence | No evidence | No evidence | Detected (a) |
| Omicron | XBB.1.5-like + L455F + F456L (b) | n/a | L455F, F456L, N460K, S486P, F490S | n/a | No evidence | No evidence | No evidence | Detected (a |
3.9. Pathology and Postmortem Changes.
|
Lung |
Severe squamous metaplasia with atypia, interstitial and intra-alveolar oedema, type 2 pneumocyte hyperplasia, capillary congestion, hyaline membranes, pneumocytic necrosis, and exudative and proliferative widespread alveolar damage |
|
Liver |
Pathological lesions, such as cardiac hypertrophy, atherosclerosis, general interstitial fibrosis, mild myocardial edoema, and atypical, minor, localised, and perivascular interstitial fibrosis, were seen in the livers of COVID-19-related deaths. |
|
Brain |
Microthrombi and acute infarcts, hypoxic alterations without any specific disease, and perivascular lymphocytic infiltration in the brainstem |
|
Coagulation Abnormality |
Disseminated intravascular coagulopathy (DIC), PE, deep vein thrombosis (DVT), arterial thrombosis, hypercoagulable coagulopathy, and intra-catheter thrombosis, among other thrombotic and/or thromboembolic complications. |
|
Kidneys |
Diffuse proximal tubule damage with brush boundary loss, non-isometric vacuolar degeneration, and frank necrosis were observed in the kidneys of COVID-19 patients. |
3.10. Diagnosis
| Types of diagnostic tests | Mechanism of detection | Source of samples | Result Interpretation |
|---|---|---|---|
| Nucleic acid amplification | Real Time PCR and NGS sequencing by using gene specific primer such as N,S,E and RdRP genes two independent sequences need to be detected | Nasal Swab, throat Swab, Bronchoalveolar lavage, blood faces and endotracheal aspirate | SARS-CoV2 Infection |
| Antibody based immunoassay | SARS-CoV2 IgM and IgG antibodies detection by ELISA | Serum | Immunity/Overall infection |
| Antigen based immunoassay | SARS-CoV2 detection protein | Nasal Swab, throat Swab, Bronchoalveolar lavage, blood faces and endotracheal aspirate | Confirm current SARS-CoV2 |
| CT- Imaging | Clinical symptoms (Fever/Cough, epidemiological history imaging CT) | Radiological features | Trade to identify for further target |
3.11. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)
4. Recent Therapy in Management of COVID -19
4.1. High-Affinity Neutralizing DNA Aptamers against SARS-CoV-2 Spike Protein Variants
4.2. BREATHOX® Device Inhalation on Acute Symptoms Associated with COVID-19
4.3. Role of Selective Digestive Decontamination in the Prevention of Ventilator-Associated Pneumonia in COVID-19 Patients
4.4. Tocilizumab
4.5. Baricitinib
| Drug candidate | Description | Existing disease approval |
|---|---|---|
| Ritonavir | Anti-HIV Drug | Investigational combination |
| Lopinavir | Anti-viral | Investigational combination |
| Favipiravir | Antiviral agent against influenza | Influenza |
| Remdesivir | Viral RNA-dependent RNA polymerase | Broad spectrum anti-viral drug |
| Prezcobix | HIV-1 protease inhibitor | HIV infection |
| Galidesivir | Viral replication inhibitor | Antiviral against RNA viruses |
| Danoprevir | Inhibitors of NS3/4A | HCV Protease inhibitor |
| Umifenovir | Replication inhibitors | Anti-viral used for Influenza |
| Baloxavir marboxil (BXM) | Polymerase acidic endonuclease inhibitor | Anti-viral used for Influenza |
| Levovir | polymerase inhibitor | Anti-viral used for hepatitis B Virus |
| Dexamethasone | Anti-inflammatory | Rheumatoid arthritis |
| Oseltamivir | Neuraminidase inhibitor | Prevent Influenza A and B |
4.6. Antiviral therapies
4.7. Remdesivir
4.8. Molnupiravir
4.9. Antithrombotic therapies
4.10. Neutralizing antibody therapies
4.11. Therapies targeting the RAAS
4.12. Development of SARS-CoV-2 Immunoglobulin based treatments option.
| Leading candidate | Description |
| Convalescent plasma | Passively transfer antibodies (Immunoglobulin) |
| STI-5656 (Abivertinib) | Tyrosine kinase inhibitor |
| PRO 140 (Leronlimab) | Monoclonal antibody targeted against CCR5 receptor |
| PTC299 | Dihydroorotate dehydrogenase inhibitors |
| CD24Fc | Immunomodulator (New drug) |
| Lenzilumab | Chronic Myelomonocytic leukemia |
| Tocilizumab | Immunosuppression |
| Sarilumab | Rheumatoid arthritis |
| Ravulizumab | Compliment inhibitors |
| Losmapimod | MAPK as potent suppressors of DUX4 expression |
| Pepcid H2 blocker | Mitigare (Colcrys) Anti-inflammatory agent |
4.13. Therapies for acute respiratory failure
4.14. Oxygen delivery
4.15. Prone positioning
4.16. Adjunctive therapy for hypoxemia
4.17. ECMO
4.18. Vaccines
| Vaccine candidate | Details |
|---|---|
| mRNA-1273 | mRNA-1273, a vaccine candidate based on previous study of SARS and MERS |
| Ad5-nCoV | Recombinant novel corona virus vaccine with adenovirus type 5 vector (Ad5) |
| ChAdOx1 | SARS-CoV-2, adenovirus vaccine vector MERS vaccine. |
| INO-4800 | DNA vaccine for SARS-CoV-2 |
| BNT162 | Modified mRNA-based, SARS-CoV-2 vaccine |
| NVX-CoV2373 | Recombinant nanoparticle vaccine candidates for SARS-CoV-2 |
| CureVac | mRNA-based SARS-CoV-2 vaccine |
| Vaxart | Oral recombinant SARS-CoV-2 vaccine; gene-based vaccine |
| DNA vaccine candidates | DNA-based vaccine for SARS-CoV-2 |
| mRNA vaccine | Repurposed SARS vaccine and mRNA vaccine candidate |
| DNA plasmid vaccine candidate | Modified vaccinia ankara virus like particles (MVA-VLP) vaccine candidate for SARS-CoV-2 |
| Adenovirus-based vector vaccine for SARS-CoV-2 | Adenovirus-based vector vaccine for SARS-CoV-2 |
| Modified avian coronavirus vaccine | Genetically similar avian coronavirus Infectious Bronchitis Virus |
| Gene-encoded antibody vaccine candidate | Next-generation, gene-encoded antibody vaccine for SARS-CoV-2 |
| DPX- SARS-CoV-2 | T-cell activating immunotherapy antigen vaccine |
| Intranasal DNA-based vaccine candidate | Stimulating an immune response in the nasal cavity |
| Single-dose patch delivery vaccine | Vaccine candidate for SARS-CoV-2 delivered through a single-dose patch |
4.19. Impact of new COVID 19 variants on vaccine
5. FUTURE TRENDS
5.1. Intranasal gene therapy to prevent infection by SARS-CoV-2 variants
5.2. Single-dose skin patch-delivered SARS-CoV-2 spike vaccine
5.3. Oral vaccination
5.4. Ethical considerations
6. Conclusion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395, 1054–62. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020, 382, 727–33. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.A. Properties of coronavirus and SARS-coV-2. Malays J Pathol 2020, 42, 3–11 PubMed Abstract | Google Scholar. [Google Scholar] [PubMed]
- Thakur, V.; Ratho, R.K. OMICRON (B.1.1.529): A new SARS-CoV-2 variant of concern mounting worldwide fear. J Med Virol 2022, 94, 1821–24. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://covid19.who.int/?mapFilter=cases.
- Dang, A.; Thakker, R.; Li, S.; Hommel, E.; Mehta, H.B.; Goodwin, J.S. Hospitalizations and mortality from non-SARS-coV-2 causes among medicare beneficiaries at US hospitals during the SARS-coV-2 pandemic. JAMA Netw Open 2022, 5, :e221754. [Google Scholar] [CrossRef] [PubMed]
- Sher, L. The impact of the COVID-19 pandemic on suicide rates. QJM 2020, 113, 707–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA 2020, 323, 1239–42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 2020, 81, e16–25. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–64. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; et al. Post-acute COVID-19 syndrome. Nat Med 2021, 27, 601–15. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Author Correction: Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun 2022, 13, 5104. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.; Lu, P.; Dhodapkar, R.M.; Gehlhausen, J.R.; et al. Distinguishing features of Long COVID identified through immune profiling. medRxiv ( 2022. [CrossRef] [PubMed]
- Hoehl, S.; Rabenau, H.; Berger, A.; Kortenbusch, M.; Cinatl, J.; Bojkova, D.; et al. Evidence of SARS-coV-2 infection in returning travelers from wuhan, China. N Engl J Med 2020, 382, 1278–80. [Google Scholar] [CrossRef]
- National Health Commission Of The People's Republic Of China. Protocol for prevention and control of COVID-19 (Trial edition 6); Available online: http://wwwnhcgovcn/yzygj/s7653p/202203/b74ade1ba4494583805a3d2e40093d88shtml.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; et al. SARS-coV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–80 e8. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. bioRxiv ( 2020. [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020, 26, 681–87. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020, 14, 185–92. [Google Scholar] [CrossRef]
- Chen, R.; Fu, J.; Hu, J.; Li, C.; Zhao, Y.; Qu, H.; et al. Identification of the immunodominant neutralizing regions in the spike glycoprotein of porcine deltacoronavirus. Virus Res 2020, 276, 197834. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-coV-2. Am J Respir Crit Care Med 2020, 202, 756–59. [Google Scholar] [CrossRef] [PubMed]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022, 606, 585–93. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020, 26, 672–75. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med J 2021, 97, 312–20. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020, 323, 1406–07. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, S.; Lou, J.; Chen, Y. Familial cluster of COVID-19 infection from an asymptomatic. Crit Care 2020, 24, 119. [Google Scholar] [CrossRef] [PubMed]
- Ganyani, T.; Kremer, C.; Chen, D.; Torneri, A.; Faes, C.; Wallinga, J. Estimating the generation interval for coronavirus disease (COVID-19) based on symptom onset data, March 2020. Euro Surveill 2020, 25. [Google Scholar] [CrossRef]
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; et al. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand J Immunol 2021, 93, e12998. [Google Scholar] [CrossRef]
- Yoshida, M.; Worlock, K.B.; Huang, N.; Huang, N.; Lindeboom, R.G.H.; Butler, C.R.; Kumasaka, N.; et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature 2022, 602, 321–27. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–24. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 324, 782–93. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Deutschman, C.S.; Legrand, M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med 2020, 46, 1105–08. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020, 8, 420–22. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Netea, M.G.; van Deuren, M.; van der Meer, J.W.; de Mast, Q.; Bruggemann, R.J.; et al. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife 2020, 9. [Google Scholar] [CrossRef]
- Singh, S.P.; Pritam, M.; Pandey, B.; Yadav, T.P. Microstructure, pathophysiology, and potential therapeutics of COVID-19: A comprehensive review. J Med Virol 2021, 93, 275–99. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-coV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin 2020, 35, 266–71. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020, 395, 1407–09. [Google Scholar] [CrossRef]
- Garbers, C.; Rose-John, S. Genetic IL-6R variants and therapeutic inhibition of IL-6 receptor signalling in COVID-19. Lancet Rheumatol 2021, 3, e96–7. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet 2020, 395, 1517–20. [Google Scholar] [CrossRef]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020, 9, 727–32. [Google Scholar] [CrossRef]
- Ochani, R.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; et al. COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med 2021, 29, 20–36. [Google Scholar] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, evaluation, and treatment of coronavirus (COVID-19); StatPearls: Treasure Island, FL.
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020, 46, 1089–98. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, D.; Sperhake, J.P.; Lutgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; et al. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann Intern Med 2020, 173, 268–77. [Google Scholar] [CrossRef]
- McGonagle, D.; Bridgewood, C.; Ramanan, A.V.; Meaney, J.F.M.; Watad, A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol 2021, 3, e224–e33. [Google Scholar] [CrossRef] [PubMed]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020, 17, 543–58. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchie, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 2021, 21, 319–29. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Moller, R.; et al. Imbalanced host response to SARS-coV-2 drives development of COVID-19. Cell 2020, 181, 1036–45 e9. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity 2020, 53, 248–63. [Google Scholar] [CrossRef]
- Cui, L.; Wang, H.; Ji, Y.; Yang, J.; Xu, S.; Huang, X.; et al. The nucleocapsid protein of coronaviruses acts as a viral suppressor of RNA silencing in mamMalian cells. J Virol 2015, 89, 9029–43. [Google Scholar] [CrossRef]
- Hurst, K.R.; Koetzner, C.A.; Masters, P.S. Characterization of a critical interaction between the coronavirus nucleocapsid protein and nonstructural protein 3 of the viral replicase-transcriptase complex. J Virol 2013, 87, 9159–72. [Google Scholar] [CrossRef] [PubMed]
- Walz, L.; Cohen, A.J.; Rebaza, A.P.; Vanchieri, J.; Slade, M.D.; Dela Cruz, C.S.; et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis 2021, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Cicco, S.; Cicco, G.; Racanelli, V.; Vacca, A. Neutrophil extracellular traps (NETs) and damage-associated molecular patterns (DAMPs): two potential targets for COVID-19 treatment. Mediators Inflammation 2020, 2020, 7527953. [Google Scholar] [CrossRef]
- Day, J.D.; Park, S.; Ranard, B.L.; Singh, H.; Chow, C.C.; Vodovotz, Y. Divergent COVID-19 disease trajectories predicted by a DAMP-centered immune network model. Front Immunol 2021, 12, 754127. [Google Scholar] [CrossRef] [PubMed]
- Canaday, D.H.; Oyebanji, O.A.; White, E.; Keresztesy, D.; Payne, M.; Wilk, D.; et al. Significantly elevated antibody levels and neutralization titers in nursing home residents after SARS-CoV-2 BNT162b2 mRNA booster vaccination. medRxiv ( 2021. [CrossRef]
- Lucas, C.; Klein, J.; Sundaram, M.E.; Liu, F.; Wong, P.; Silva, J.; et al. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat Med 2021, 27, 1178–86. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, L.; Chang, D.; Wang, J.; Hu, Y.; Chen, H.; et al. The kinetics of humoral response and its relationship with the disease severity in COVID-19. Commun Biol 2020, 3, 780. [Google Scholar] [CrossRef]
- Xie, C.; Li, Q.; Li, L.; Peng, X.; Ling, Z.; Xiao, B.; et al. Association of early inflammation with age and asymptomatic disease in COVID-19. J Inflammation Res 2021, 14, 1207–16. [Google Scholar] [CrossRef] [PubMed]
- Therapeutics and COVID-19: living guideline, 22 april 2022; WHO/2019-nCoV/therapeutics/20223; World Health Organization: Geneva, 2022.
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.O.; Watson, L.R.; et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc Diabetol 2022, 21, 148. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 2021, 12, 698169. [Google Scholar] [CrossRef]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–69. [Google Scholar] [CrossRef] [PubMed]
- Zubchenko, S.; Kril, I.; Nadizhko, O.; Matsyura, O.; Chopyak, V. Herpesvirus infections and post-COVID-19 manifestations: a pilot observational study. Rheumatol Int 2022, 42, 1523–30. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Weiss, M.; Krone, B.; Cohen, S.; Ilani, G.; Vinker, S.; et al. Clinical and socio-demographic variables associated with the diagnosis of long COVID syndrome in youth: A population-based study. Int J Environ Res Public Health. [CrossRef]
- Renz-Polster, H.; Tremblay, M.E.; Bienzle, D.; Fischer, J.E. The pathobiology of myalgic encephalomyelitis/chronic fatigue syndrome: the case for neuroglial failure. Front Cell Neurosci 2022, 16, 888232. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–95 e20. [Google Scholar] [CrossRef]
- Choutka, J.; Jansari, V.; Hornig, M.; Iwasaki, A. Unexplained post-acute infection syndromes. Nat Med 2022, 28, 911–23. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.E.; Tydeman, F.; Miners, A.; Pyper, K.; Martineau, A.R. Short-term and long-term impacts of COVID-19 on economic vulnerability: a population-based longitudinal study (COVIDENCE UK). BMJ Open 2022, 12, e065083. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zai, J.; Zhao, Q.; Nie, Q.; Li, Y.; Foley, B.T.; et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol 2020, 92, 602–11. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; et al. Aerosol and surface stability of SARS-coV-2 as compared with SARS-coV-1. N Engl J Med 2020, 382, 1564–67. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Opu, R.R.; Ahmed, N.; Talukder, A.; et al. Protective measures are associated with the reduction of transmission of COVID-19 in Bangladesh: A nationwide cross-sectional study. PloS One 2021, 16, e0260287. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-coV-2) based on SARS-coV immunological studies. Viruses ( 2020. [CrossRef]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, immunogenicity and safety of COVID-19 vaccines: A systematic review and meta-analysis. Front Immunol 2021, 12, 714170. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Seyahi, E.; Bakhdiyarli, G.; Oztas, M.; Kuskucu, M.A.; Tok, Y.; Sut, N.; et al. Antibody response to inactivated COVID-19 vaccine (CoronaVac) in immune-mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int 2021, 41, 1429–40. [Google Scholar] [CrossRef] [PubMed]
- Sonani, B.; Aslam, F.; Goyal, A.; Patel, J.; Bansal, P. COVID-19 vaccination in immunocompromised patients. Clin Rheumatol 2021, 40, 797–98. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.C.; Zhang, H.W.; Yu, J.; Xu, H.J.; Chen, H.; Luo, S.P.; et al. CT imaging and differential diagnosis of COVID-19. Can Assoc Radiol J 2020, 71, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Fistera, D.; Hartl, A.; Pabst, D.; Manegold, R.; Holzner, C.; Taube, C.; et al. What about the others: differential diagnosis of COVID-19 in a German emergency department. BMC Infect Dis 2021, 21, 969. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Kumar, B.K.; Deekshit, V.K.; Karunasagar, I.; Karunasagar, I. Detection technologies and recent developments in the diagnosis of COVID-19 infection. Appl Microbiol Biotechnol 2021, 105, 441–55. [Google Scholar] [CrossRef] [PubMed]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas Lopez, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–57. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; et al. Remdesivir for the treatment of covid-19 - final report. N Engl J Med 2020, 383, 1813–26. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–78. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med 2022, 386, 509–20. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med 2022, 386, 1397–408. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; et al. Bamlanivimab plus etesevimab in mild or moderate covid-19. N Engl J Med 2021, 385, 1382–92. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N Engl J Med 2021, 384, 238–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; et al. SARS-coV-2 neutralizing antibody LY-coV555 in outpatients with covid-19. N Engl J Med 2021, 384, 229–37. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Nirula, A.; Mulligan, M.J.; Novak, R.M.; Marovich, M.; Yen, C.; et al. Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: A randomized clinical trial. JAMA 2021, 326, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, P.O.; Quirk, D.; Furtado, R.H.; Maia, L.N.; Saraiva, J.F.; Antunes, M.O.; et al. Tofacitinib in patients hospitalized with covid-19 pneumonia. N Engl J Med 2021, 385, 406–15. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wei, J.; Zou, L.; Jiang, T.; Wang, G.; Chen, L.; et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020, 146, 137–46 e3. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020, 323, 1582–89. [Google Scholar] [CrossRef]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vazquez, C.; et al. A randomized trial of convalescent plasma in covid-19 severe pneumonia. N Engl J Med 2021, 384, 619–29. [Google Scholar] [CrossRef]
- A living WHO guideline on drugs for covid-19. BMJ 2022, 377, o1045. [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Woolner, E.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 2020, 295, 6785–97. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.G.; et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med 2021, 21, 167–79. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Arias, L. Decoding molnupiravir-induced mutagenesis in SARS-CoV-2. J Biol Chem 2021, 297, 100867. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Tchesnokov, E.P.; SChinazi, R.F.; Gotte, M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem 2021, 297, 100770. [Google Scholar] [CrossRef] [PubMed]
- Kabinger, F.; Stiller, C.; Schmitzova, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol 2021, 28, 740–46. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, R.; Foo, C.S.; De Jonghe, S.; Maes, P.; Weynand, B.; Neyts, J. Molnupiravir inhibits replication of the emerging SARS-coV-2 variants of concern in a hamster infection model. J Infect Dis 2021, 224, 749–53. [Google Scholar] [CrossRef]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; et al. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res 2022, 198, 105252. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lu, T.L.; Lin, L. Real-world clinical outcomes of molnupiravir for the treatment of mild to moderate COVID-19 in adult patients during the dominance of the omicron variant: A meta-analysis. Antibiotics (Basel). [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–93. [Google Scholar] [CrossRef]
- Sevrioukova, I.F.; Poulos, T.L. Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci U.S.A. 2010, 107, 18422–7. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–19. [Google Scholar] [CrossRef] [PubMed]
- Klank, D.; Hoffmann, M.; Claus, B.; Zinke, F.; Bergner, R.; Paschka, P. Monoclonal antibodies for the prevention and treatment of COVID-19 disease in patients with hematological Malignancies: two case reports and a literature review. Hemasphere 2021, 5, e651. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, M.P.; Forleo-Neto, E.; Musser, B.J.; Isa, F.; Chan, K.C.; Sarkar, N.; et al. Subcutaneous REGEN-COV antibody combination to prevent covid-19. N Engl J Med 2021, 385, 1184–95. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, D.F.; Diella, L.; Solimando, A.G.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; et al. Bamlanivimab and Etesevimab administered in an outpatient setting for SARS-CoV-2 infection. Pathog Glob Health 2022, 116, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Jenks, J.D.; Aslam, S.; Horton, L.E.; Law, N.; Bharti, A.; Logan, C.; et al. Early monoclonal antibody administration can reduce both hospitalizations and mortality in high-risk outpatients with coronavirus disease 2019 (COVID-19). Clin Infect Dis 2022, 74, 752–53. [Google Scholar] [CrossRef] [PubMed]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Anti-interleukin-6 therapies for hospitalized patients with COVID-19: a protocol for a prospective meta-analysis of randomized trials. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-PMA_protocols-anti-IL-6-2021.1 (accessed on 10 June 2021).
- Group WHOREAfC-TW, Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A meta-analysis. JAMA 2021, 326, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Study to assess the efficacy and safety of ruxolitinib in patients with COVID-19 associated cytokine storm (RUXCOVID); ClinicalTrials.gov National Library of Medicine: Bethesda, MD, USA; Available online: https://clinicaltrials.gov/ct2/show/results/NCT04362137 (accessed on 04 January 2022).
- Fragoulis, G.E.; McInnes, I.B.; Siebert, S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatol (Oxford) (2019) 58(Suppl 1):i43–54. [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O'Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discovery 2017, 16, 843–62. [Google Scholar] [CrossRef] [PubMed]
- Alhazzani, W.; Moller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med 2020, 46, 854–87. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Chotirmall, S.H.; Rello, J.; Alba, G.A.; Ginns, L.C.; Krishnan, J.A.; et al. Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020). Eur Respir Rev 2020, 29. [Google Scholar] [CrossRef]
- Calzetta, L.; Aiello, M.; Frizzelli, A.; Rogliani, P.; Chetta, A. Dexamethasone in patients hospitalized with COVID-19: whether, when and to whom. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Jamaati, H.; Hashemian, S.M.; Farzanegan, B.; Malekmohammad, M.; Tabarsi, P.; Marjani, M.; et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: A preliminary report of a randomized clinical trial. Eur J Pharmacol 2021, 897, 173947. [Google Scholar] [CrossRef]
- Roback, J.D.; Guarner, J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA 2020, 323, 1561–62. [Google Scholar] [CrossRef] [PubMed]
- Ankcorn, M.; Gallacher, J.; Ijaz, S.; Taha, Y.; Harvala, H.; Maclennan, S.; et al. Convalescent plasma therapy for persistent hepatitis E virus infection. J Hepatol 2019, 71, 434–38. [Google Scholar] [CrossRef]
- van Griensven, J.; Edwards, T.; de Lamballerie, X.; Semple, M.G.; Gallian, P.; Baize, S.; et al. Evaluation of convalescent plasma for ebola virus disease in Guinea. N Engl J Med 2016, 374, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhong, N.; Guan, Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med 2007, 357, 1450–1. [Google Scholar] [CrossRef] [PubMed]
- Lamikanra, A.; Nguyen, D.; Simmonds, P.; Williams, S.; Bentley, E.M.; Rowe, C.; et al. Comparability of six different immunoassays measuring SARS-CoV-2 antibodies with neutralizing antibody levels in convalescent plasma: From utility to prediction. Transfusion 2021, 61, 2837–43. [Google Scholar] [CrossRef]
- O'Donnell, M.R.; Grinsztejn, B.; Cummings, M.J.; Justman, J.E.; Lamb, M.R.; Eckhardt, C.M.; et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Haagmans, B.L.; Noack, D.; Okba, N.M.A.; Li, W.; Wang, C.; Bestebroer, T.; et al. SARS-coV-2 neutralizing human antibodies protect against lower respiratory tract disease in a hamster model. J Infect Dis 2021, 223, 2020–28. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, S. Physiology, blood volume; StatPearls: Treasure Island, FL, 2022. [Google Scholar]
- Siemieniuk, R.A.; Bartoszko, J.J.; Diaz Martinez, J.P.; Kum, E.; Qasim, A.; Zeraatkar, D.; et al. Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis. BMJ 2021, 374, n2231. [Google Scholar] [CrossRef]
- Cheung, J.C.; Ho, L.T.; Cheng, J.V.; Cham, E.Y.K.; Lam, K.N. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med 2020, 8, e19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





