Submitted:
23 November 2023
Posted:
24 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. The extraction processes
2.2. Total flavonoids, phenols, and anthocyanins quantification
- column - Kinetex C18 (150 x 4.6 mm, 100 Å);
- mobile phase consisting of a mixture of type A/B obtained automatically during the analysis with the help of the quaternary pump: acetonitrile (A), aqueous solution of acetic acid 0.1% (B) with a gradient of 10 to 65 A in B;
- initial and final flow rate 1 mL/min, 0.8 mL during the determinations;
- injected volume comprised between 5 -10 µL samples diluted in acidified hydroalcoholic solution and standards dissolved in the same solvent;
- simultaneous detection at three wavelengths 275 nm (flavonoids), 330 nm (polyphenolic acids), 520 nm (anthocyanins);
- elution time 25 minutes;
2.3. Iron Chelation Capacity Assessment
- Mix 0.2 mL of the test sample solution in ultrapure water, 0.74 mL of 0.1 M acetate buffer (pH 5.25), and 0.02 mL of 2 mM iron sulfate in 0.2 M hydrochloric acid. After 10-15 seconds of agitation, add 0.04 mL of 5 mM ferrozine solution.
- After 10 minutes of incubation in the dark, measure the absorbance of the solution at 562 nm against a control prepared under the same conditions as the sample (ultrapure water was used instead of iron sulfate solution).
- Simultaneously, prepare the control solution and its control: the control contains 0.2 mL of ultrapure water, 0.74 mL of 0.1 M acetate buffer (pH 5.25), 0.02 mL of 2 mM iron sulfate in 0.2 M hydrochloric acid, and 0.04 mL of 5 mM ferrozine solution.
- Gallic acid was used as a reference substance, and gallic acid solutions in DMSO were processed under the same conditions as the methanolic extract.
- All determinations were carried out in triplicate, and results are expressed as the mean of three determinations ± standard deviation.
2.4. Determination of Hydroxyl Radical Scavenging Capacity
2.5. Determining the scavenging capacity of the superoxide radical anion
2.6. Determination of the lipoxygenase inhibition capacity of Perilla frutescens extracts
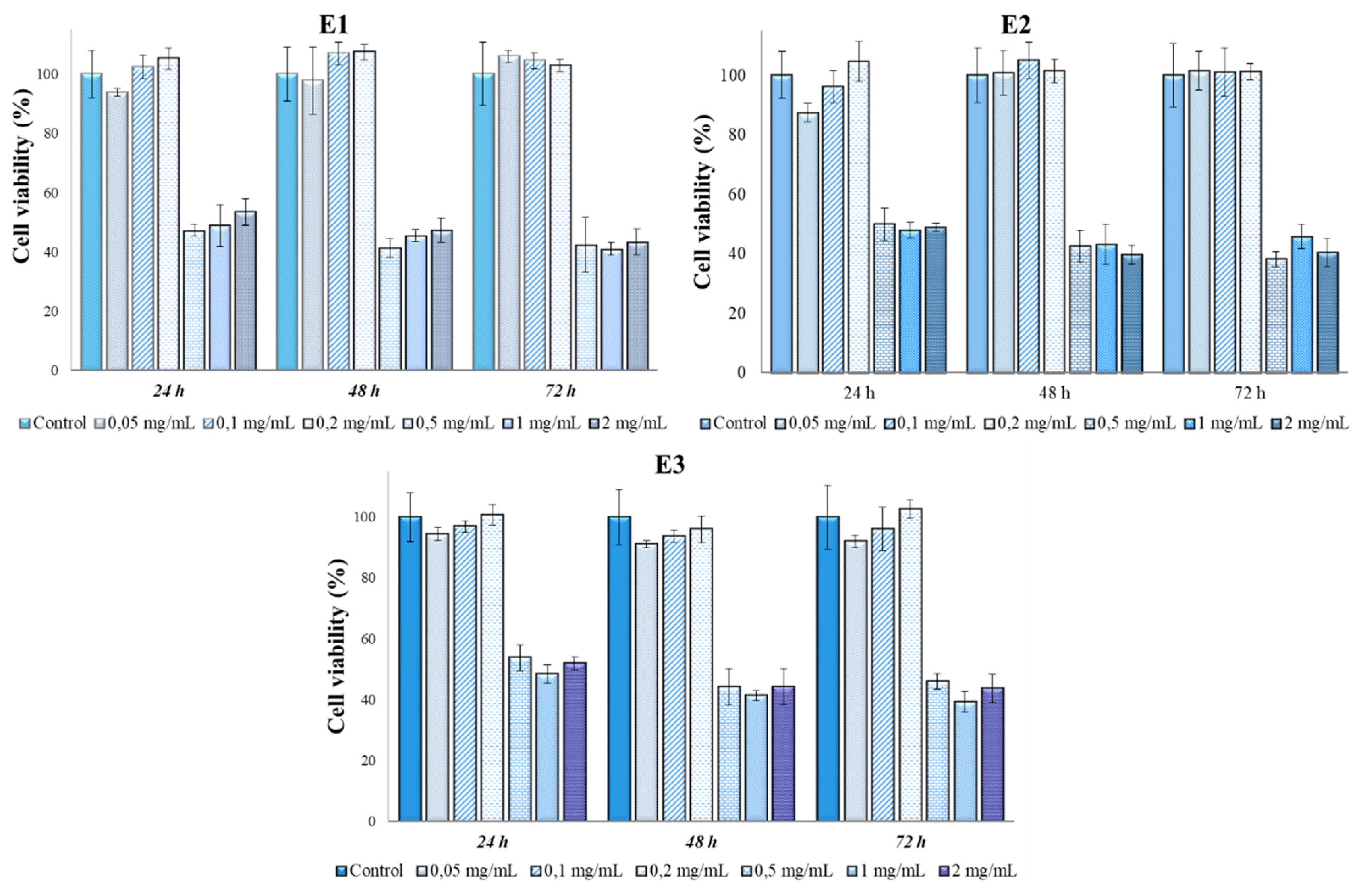
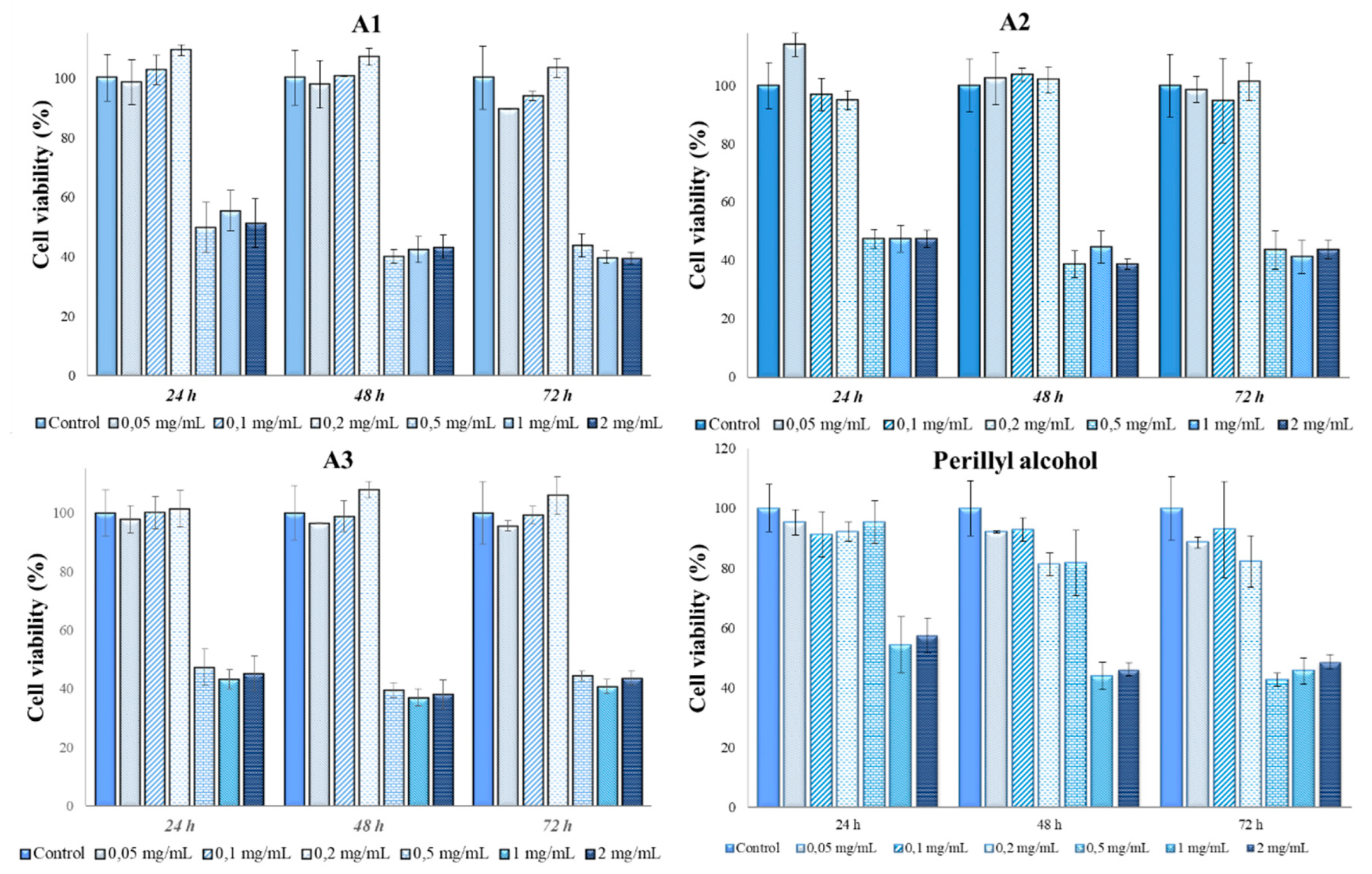
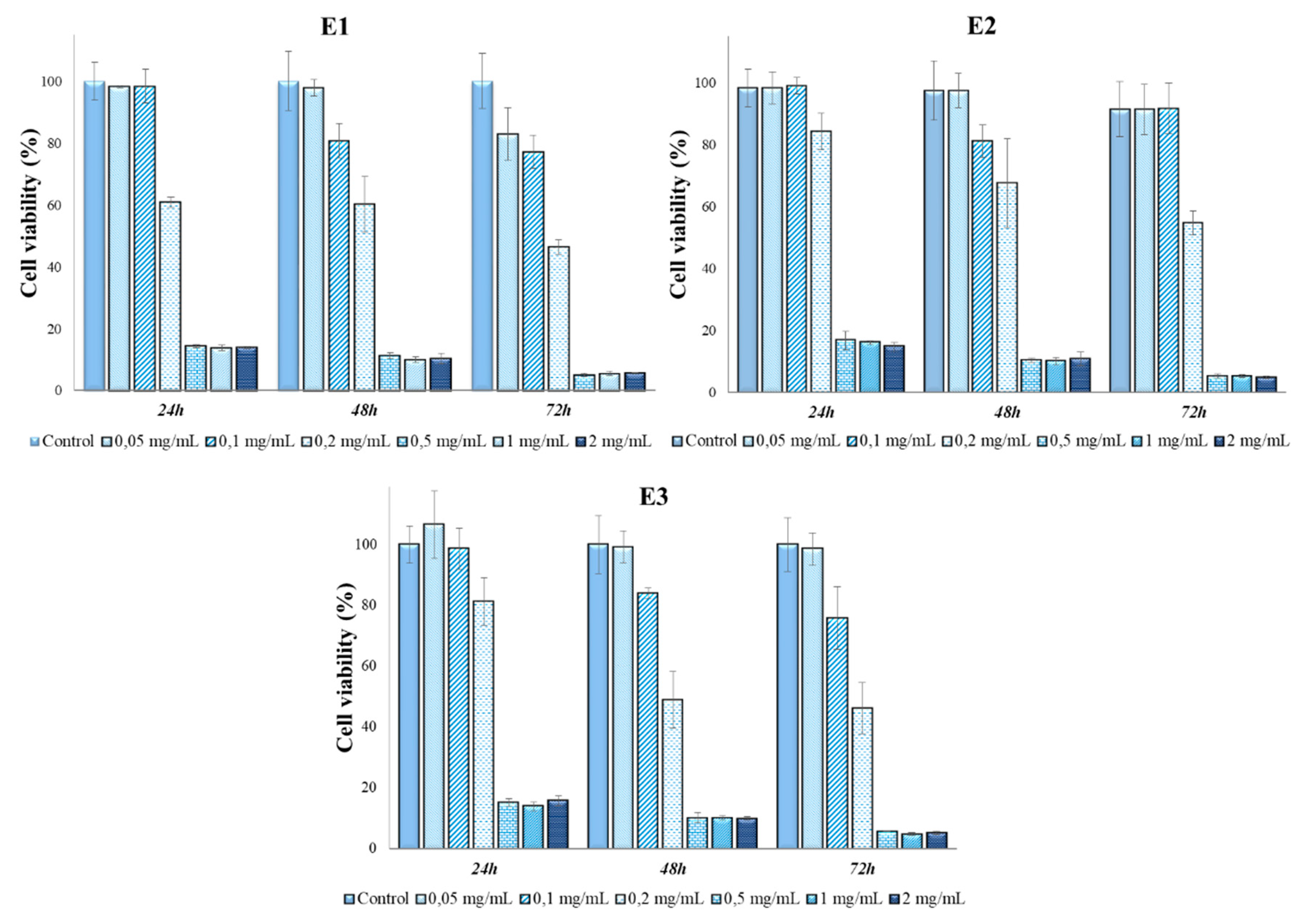
2.7. In vitro cytotoxicity tests and antitumor action of Perilla leaves extracts
3. Results
3.1. Quantification of the total phenols, flavonoids and anthocyanins from ethanolic and ethanol: acetone extracts of Perilla frutescens.
3.2. Determination of the in vitro antioxidant action of Perilla frutescens extracts
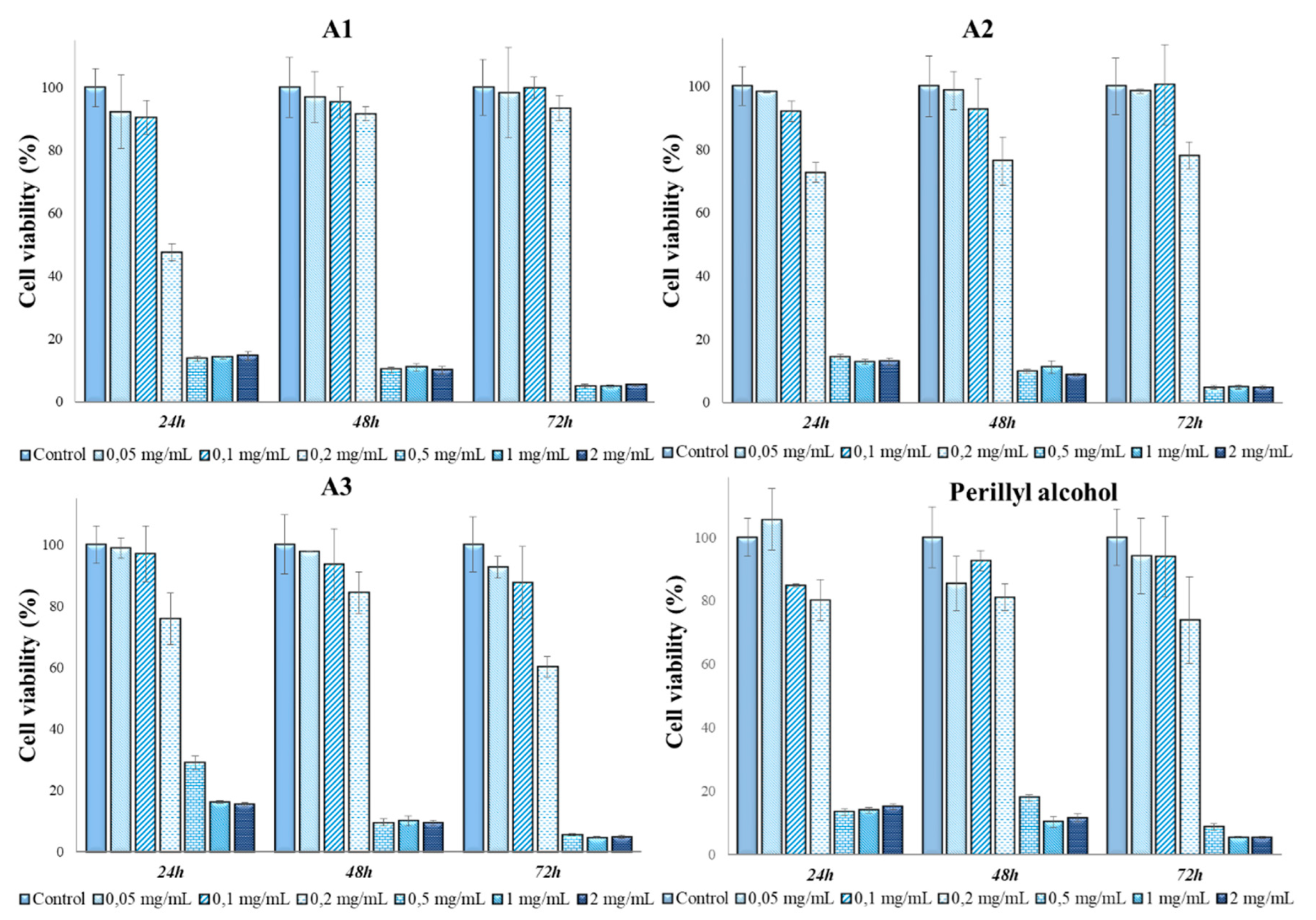
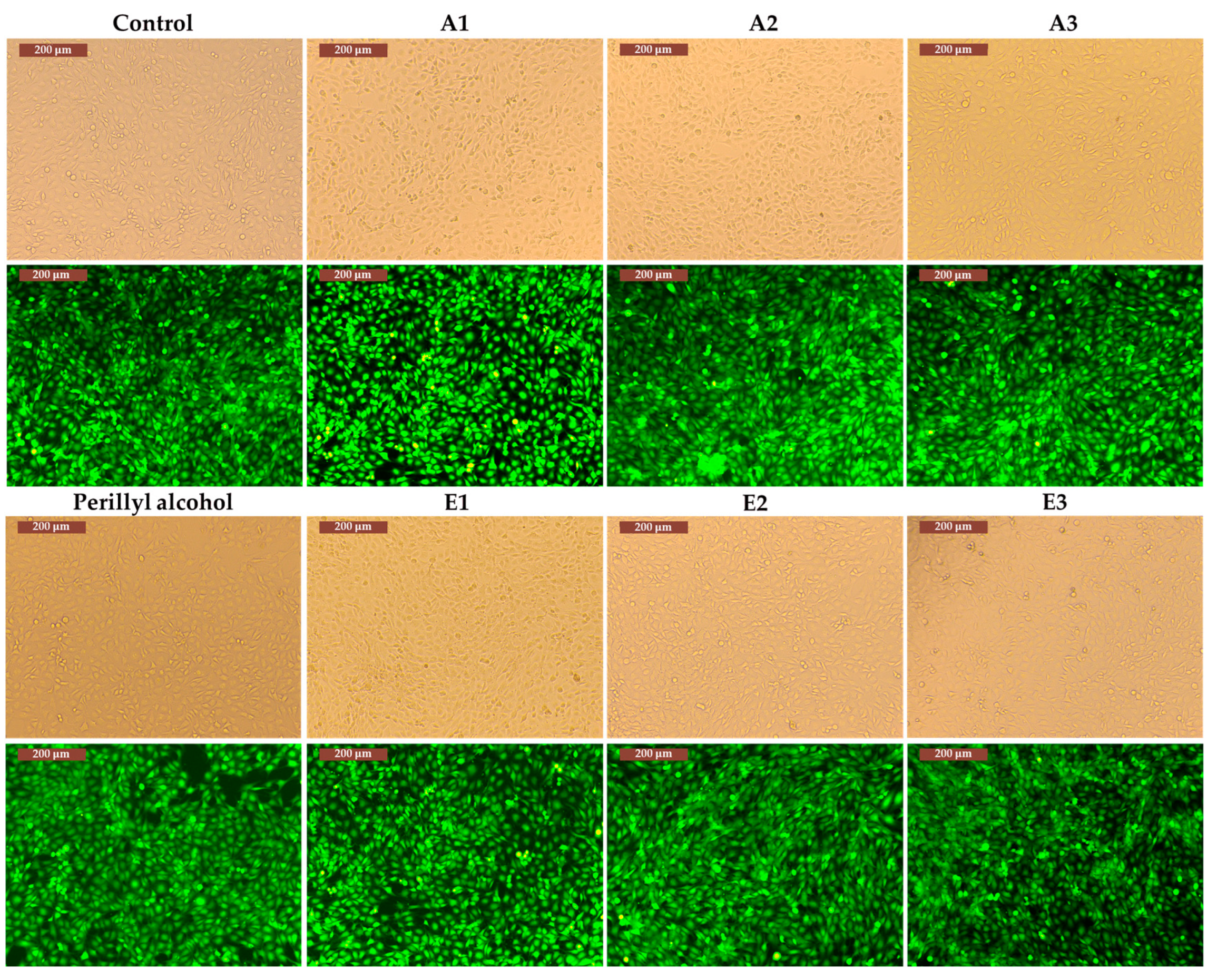
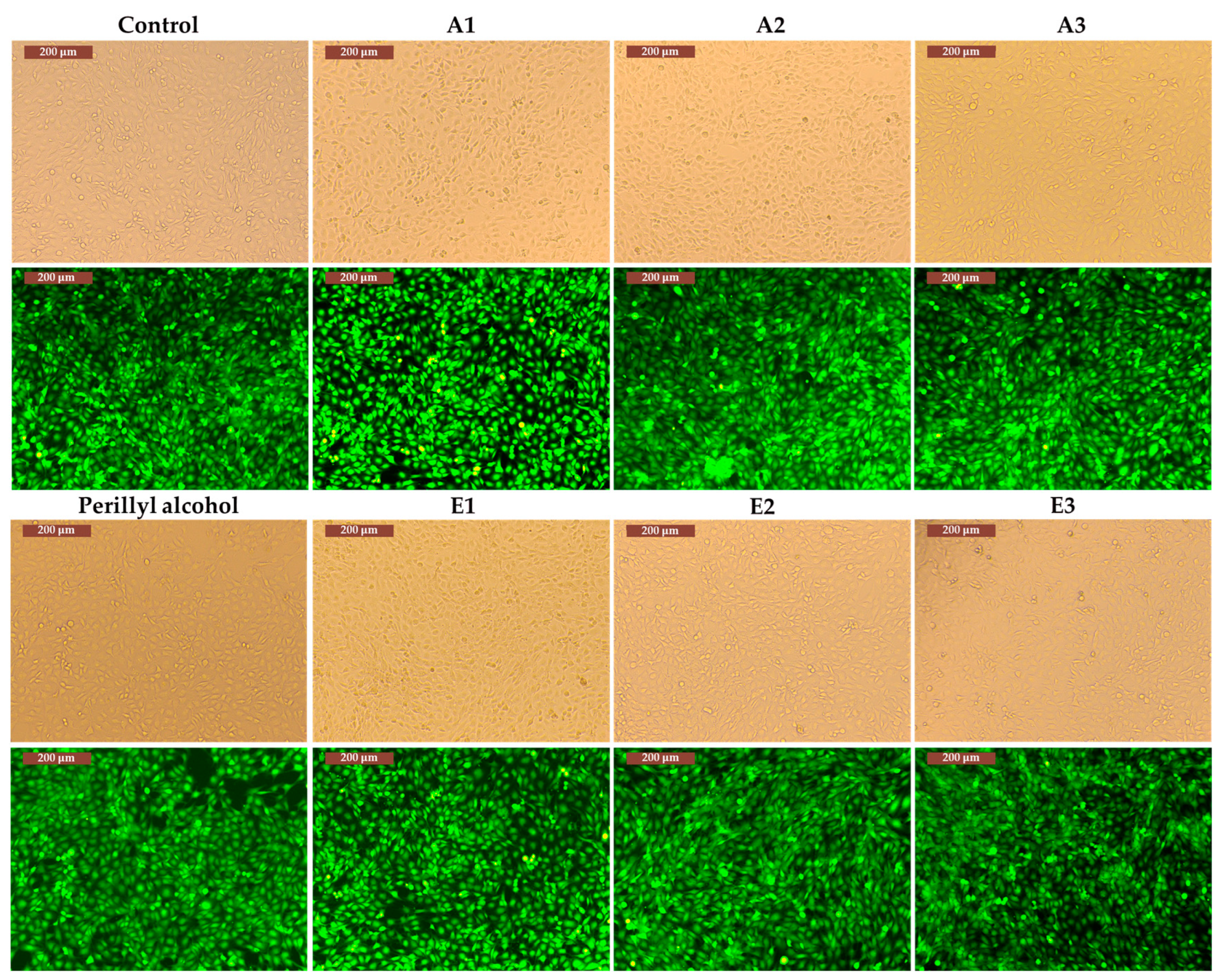
3.3. Determination of in vitro cytotoxicity and antitumor effects of Perilla frutescens extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adam, G.; Robu, S.; Flutur, M.M.; Cioanca, O.; Vasilache, I.A.; Adam, A.M.; et al. Applications of Perilla frutescens Extracts in Clinical Practice. Antioxidants (Basel). 2023, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Adam, A.M.; Robu, S.; Harabor, V.; Harabor, A.; Nechita, A.; et al. The Effects of Perilla frutescens Extracts on IgA Nephropathy: A Systematic Review and Meta-Analysis. Pharmaceuticals (Basel). 2023, 16, 988. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Nan, Y.; Chen, G.; Ning, N.; Du, Y.; Lu, D.; et al. The Role and Mechanism of Perilla frutescens in Cancer Treatment. Molecules. 2023, 28, 5883. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.L.; Shin, Y.S.; Choi, S.H.; Oh, S.; Kim, K.; Jeong, H.S.; et al. Extracts of Perilla frutescens var. Acuta (Odash.) Kudo Leaves Have Antitumor Effects on Breast Cancer Cells by Suppressing YAP Activity. Evid Based Complement Altern. Med. 2021, 2021, 5619761. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.F.; Gaydou, E.M.; Li, B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules. 2008, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, N.; Yeo, J.Y.; Seo, D.G.; Kim, S.; Lee, J.S.; et al. Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla frutescens Leaves against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules. 2019, 24, 4297. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, B.; Kim, S.; Kim, M.S.; Kim, H.; Hwang, S.R.; et al. Characterization of metabolite profiles from the leaves of green perilla (Perilla frutescens) by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry and screening for their antioxidant properties. J Food Drug Anal. 2017, 25, 776–788. [Google Scholar]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Batir Marin, D.; Cioanca, O.; Apostu, M.; Tuchilus, C.; Mircea, C.; Robu, S.; et al. The Comparative Study of Equisetum pratense, E. sylvaticum, E. telmateia: Accumulation of Silicon, Antioxidant and Antimicrobial Screening. Rev. De Chimie. 2019, 70, 2519–2523. [Google Scholar] [CrossRef]
- Aron, N.; Bogdan-Goroftei, R.-E.; Boev, M.; Marin, D.; Ramos-Villarroel, A.; Iancu, A.-V. Innovative Fermented Soy Drink with the Sea Buckthorn Syrup and the Probiotics Co-Culture of Lactobacillus Paracasei ssp. Paracasei (L. Casei® 431) and Bifidobacterium Animalis ssp. Lactis (Bb-12®). Fermentation 2023, 9, 806. [Google Scholar] [CrossRef]
- Russo, D. Flavonoids and the structure-antioxidant activity relationship. J Pharmacogn Nat Prod. 2018, 4. [Google Scholar] [CrossRef]
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta. 2003, 76, 55–61. [Google Scholar]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod.. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; et al. Antioxidant activity and healthy benefits of natural pigments in fruits: A review. Int. J. Mol. Sci.. 2021, 22, 4945. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Hazra, B.; Sarkar, R.; Biswas, S.; Mandal, N. Assessment of the antioxidant and reactive oxygen species scavenging activity of methanolic extract of Caesalpinia crista leaf. Evidence-Based Complementary and Alternative Medicine. 2011, 2011, 173768. [Google Scholar] [CrossRef] [PubMed]
- Humulescu, I.; Flutur, M.-M.; Cioanca, O.; Mircea, C.; Robu, S.; Marin-Batir, D.; et al. Comparative chemical and biological activity of selective herbal extracts. Farmacia. 2021, 69. [Google Scholar] [CrossRef]
- LUNGUII.; MARIN-BATÎRD.; PANAINTEA.; MIRCEAC.; TUCHILUȘ, C.; ȘTEFANACHEA; et al CATECHIN-ZINC-COMPLEX: SYNTHESIS.; CHARACTERIZATION AND BIOLOGICAL ACTIVITY ASSESSMENT. Farmacia. 2023, 71. [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lim, D.W.; Choi, J. Assessment of Size-Dependent Antimicrobial and Cytotoxic Properties of Silver Nanoparticles. Adv. Mater. Sci. Engineering. 2014, 2014, 763807. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr. Polymers. 2007, 68, 54–58. [Google Scholar] [CrossRef]
- Malterud, K.E.; Rydland, K.M. Inhibitors of 15-lipoxygenase from orange peel. J Agric Food Chem. 2000, 48, 5576–5580. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhang, M.; Hu, J.; Zhang, Y.; Yang, L.; Hou, X. Chemical Compositions, Extraction Optimizations, and In Vitro Bioactivities of Flavonoids from Perilla Leaves (Perillae folium) by Microwave-Assisted Natural Deep Eutectic Solvents. Antioxidants. 2023, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Wu, C.H.; Hung, L.S.; Lin, B.F. Ethanol Extract of Perilla frutescens Suppresses Allergen-Specific Th2 Responses and Alleviates Airway Inflammation and Hyperreactivity in Ovalbumin-Sensitized Murine Model of Asthma. Evid Based Complement Altern. Med. 2015, 2015, 324265. [Google Scholar]
- Zhao, Y.; Li, H.; Zhang, Z.; Ren, Z.; Yang, F. Extraction, preparative monomer separation and antibacterial activity of total polyphenols from Perilla frutescens. Food Funct. 2022, 13, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, Y.; Ito, M. Caffeic acid and rosmarinic acid contents in genus Perilla. J Nat Med. 2020, 74, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kono, M.; Ito, A.; Ito, M. Anthocyanins in perilla plants and dried leaves. Phytochemistry. 2018, 147, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wang, Y.; Li, X.; Zhou, L.; Yang, J.; et al. Metabolites and chemometric study of Perilla (Perilla frutescens) from different varieties and geographical origins. J Food Sci. 2022, 87, 5240–5251. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ye, J.; Kong, J. Determination of phenolic compounds in Perilla frutescens L. by capillary electrophoresis with electrochemical detection. J Agric Food Chem. 2005, 53, 8141–8147. [Google Scholar] [CrossRef] [PubMed]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Hydroxyl radical and hypochlorous acid scavenging activity of small centaury (Centaurium erythraea) infusion. A comparative study with green tea (Camellia sinensis). Phytomedicine. 2003, 10, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agriculture. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Lee, J.H.; Moon, K.D.; Jeong, I.Y.; Ahn, D.U.; Lee, M.K.; et al. Induction of apoptosis by isoegomaketone from Perilla frutescens L. in B16 melanoma cells is mediated through ROS generation and mitochondrial-dependent, -independent pathway. Food Chem Toxicol. 2014, 65, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.O.; Jin, C.H.; Park, Y.D.; Ryu, H.W.; Byun, M.W.; Seo, K.I.; et al. Isoegomaketone induces apoptosis through caspase-dependent and caspase-independent pathways in human DLD1 cells. Biosci Biotechnol Biochem. 2011, 75, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
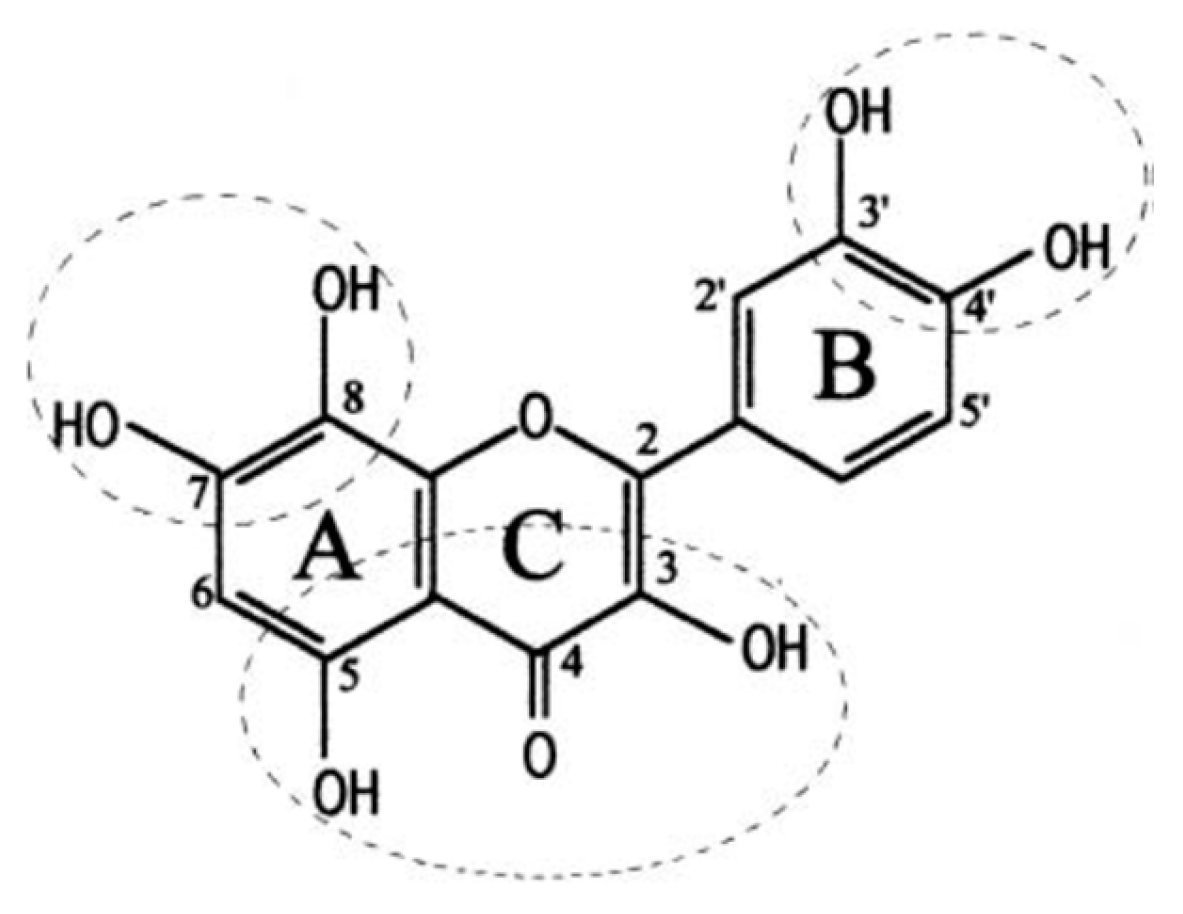
| Solvent | Perilla frutescens Species Variety | Extract Code | Extraction Yield |
|---|---|---|---|
| 70% Ethanol | Perilla frutescens var. crispa f. purpurea | E1 | 58.22% |
| 70% Ethanol | Perilla frutescens var. frutescens f. viridis | E2 | 58.79% |
| 70% Ethanol | Perilla frutescens var. frutescens f. crispidiscolor | E3 | 48.32% |
| Acetone:Ethanol (7:3) + Citric Acid | Perilla frutescens var. crispa f. purpurea | A1 | 38.51% |
| Acetone:Ethanol (7:3) + Citric Acid | Perilla frutescens var. frutescens f. viridis | A2 | 34.84% |
| Acetone:Ethanol (7:3) + Citric Acid | Perilla frutescens var. frutescens f. crispidiscolor | A3 | 44.47% |
| Sample | Total Phenols (µg gallic acid /mL extract) |
P value | Total Flavonoids (µg rutoside /mL extract) | P value | Anthocyanins (µg cyanidol/mL extract) | P value |
|---|---|---|---|---|---|---|
| E1 | 2253 | 0.002 | 194.8621 | 0.35 | 103 | 0.79 |
| E2 | 1475 | 98.7271 | 8.8 | |||
| E3 | 2142 | 125.7352 | 84 | |||
| A1 | 350 | 128.0261 | 98 | |||
| A2 | 174 | 84.5071 | - | |||
| A3 | 301 | 109.8420 | 64 |
| Compound | Identified compound (µg/mL extract) | Pvalue | |||||
| E1 | E2 | E3 | A1 | A2 | A3 | ||
| gallic acid | 0.249 | 0.235 | 0.095 | - | 0.225 | 0.103 | 0.72 |
| catechin | 1.526 | 2.025 | 1.672 | 1.388 | 1.355 | 1.581 | 0.14 |
| chlorogenic acid | 1.15 | 0.388 | 4.448 | 0.376 | 0.633 | 0.331 | 0.28 |
| caffeic acid | 109.714 | 55.12 | 81.306 | 8.582 | 12.022 | 7.108 | 0.01 |
| epi-catechin | 1.014 | 0.870 | 0.912 | 0.769 | 0.653 | 1.030 | 0.39 |
| syringic acid | 129.323 | 244.033 | 232.475 | 20.57 | 35.109 | 19.603 | 0.008 |
| p-coumaric acid | 11.163 | 10.069 | 7.097 | 2.222 | 2.25 | 3.788 | 0.007 |
| ferulic acid | 2.423 | 2.429 | 2.551 | - | 0.538 | 0.722 | 0.007 |
| ellagic acid | 9.930 | 5.865 | 4.807 | 3.421 | 3.677 | 2.731 | 0.08 |
| cinnamic acid | 70.465 | - | 22.698 | 16.544 | 11.494 | 25.218 | 0.56 |
| rosmarinic acid | 67.665 | 10.351 | 14.871 | 1.098 | 3.177 | 9.004 | 0.22 |
| quercitin | 19.663 | 3.912 | 16.828 | - | - | 12.243 | 0.21 |
| kaemferol | 36.45 | 38.12 | 41.09 | 22.05 | 26.15 | 19.47 | 0.002 |
| isorhamnetin | 1.194 | 0.805 | 0.745 | 0.138 | 0.101 | 0.263 | 0.007 |
| apigenin | 3.400 | 8.361 | 10.656 | 2.677 | 4.061 | 1.313 | 0.10 |
| pinostrobin | 31.827 | 28.35 | 24.081 | 23.601 | - | 24.320 | 0.21 |
| pinocembrin | 2.323 | 0.972 | 2.302 | 0.387 | 0.447 | 0.424 | 0.03 |
| crysin | 1.11 | 4.214 | 3.239 | 1.449 | 1.354 | 0.56 | 0.14 |
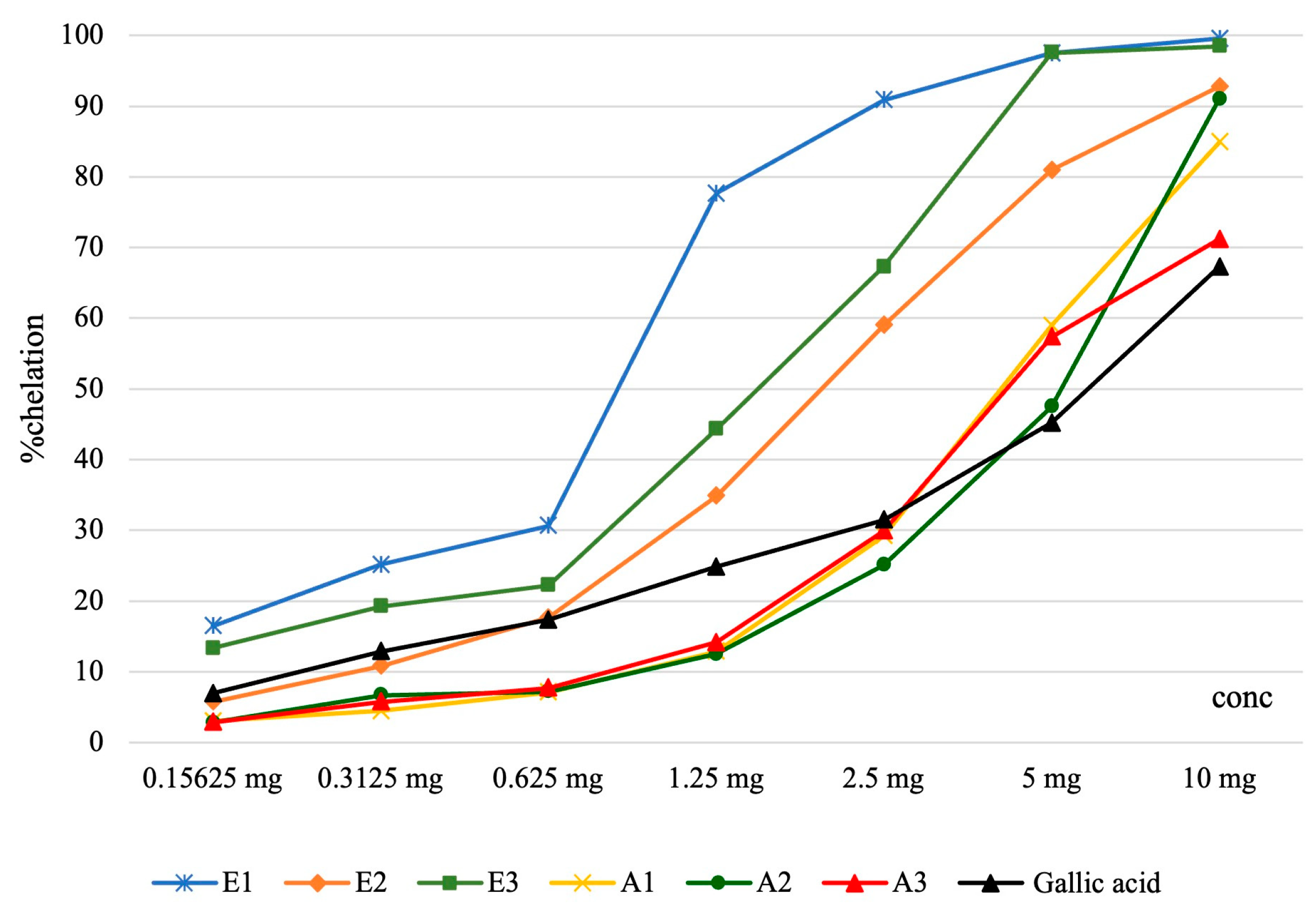
| Iron Chelation Capacity Assessment | |||||||||
| Samples | Concentration of the solution | Pvalue | |||||||
| 0.15625 mg | 0.3125 mg | 0.625 mg | 1.25 mg | 2.5 mg | 5 mg | 10 mg | IC50 (μg/mL final solution) | ||
| E1 | 16.42 ± 0.11 | 25.10 ± 0.02 | 30.60 ± 0.12 | 77.64 ± 0.01 | 90.83 ± 0.01 | 97.50 ± 0.02 | 99.53 ± 0.01 | 166.36 ± 0.18 | <0.001 |
| E2 | 5.76 ± 0.06 | 10.79 ± 0.04 | 17.61 ± 0.06 | 34.84 ± 0.05 | 58.99 ± 1.31 | 80.92 ± 0.02 | 92.78 ± 0.03 | 386.69 ± 9.36 | |
| E3 | 13.30 ± 0.01 | 19.22 ± 0.11 | 22.20 ± 0.01 | 44.29 ± 0.03 | 67.26 ± 0.05 | 97.55 ± 0.04 | 98.45 ± 0.06 | 297.01 ± 0.31 | |
| A1 | 3.03 ± 0.08 | 4.46 ± 0.08 | 7.11 ± 0.08 | 12.82 ± 0.08 | 29.18 ± 0.14 | 58.95 ± 0.04 | 84.90 ± 0.02 | 811.88 ± 1.32 | |
| A2 | 2.81 ± 0,06 | 6.68 ± 0.11 | 7.20 ± 0.09 | 12.50 ± 0.05 | 25.05 ± 0.07 | 47.48 ± 0.01 | 91.01 ± 0.02 | 1040.93 ± 0.17 | |
| A3 | 2.87 ± 0,09 | 5.74 ± 0.04 | 7.69 ± 0.31 | 14.14 ± 0.06 | 29.92 ± 0.01 | 57.35 ± 0.05 | 71.79 ± 0.03 | 830.49 ± 0.82 | |
| Gallic acid | 6.91 ± 0.07 | 12.86 ± 0.11 | 17.29 ± 0.09 | 24.82 ± 0.12 | 31.45 ± 0.28 | 45.19 ± 0.07 | 67.25 ± 0.08 | 1163.15±2.63 | |
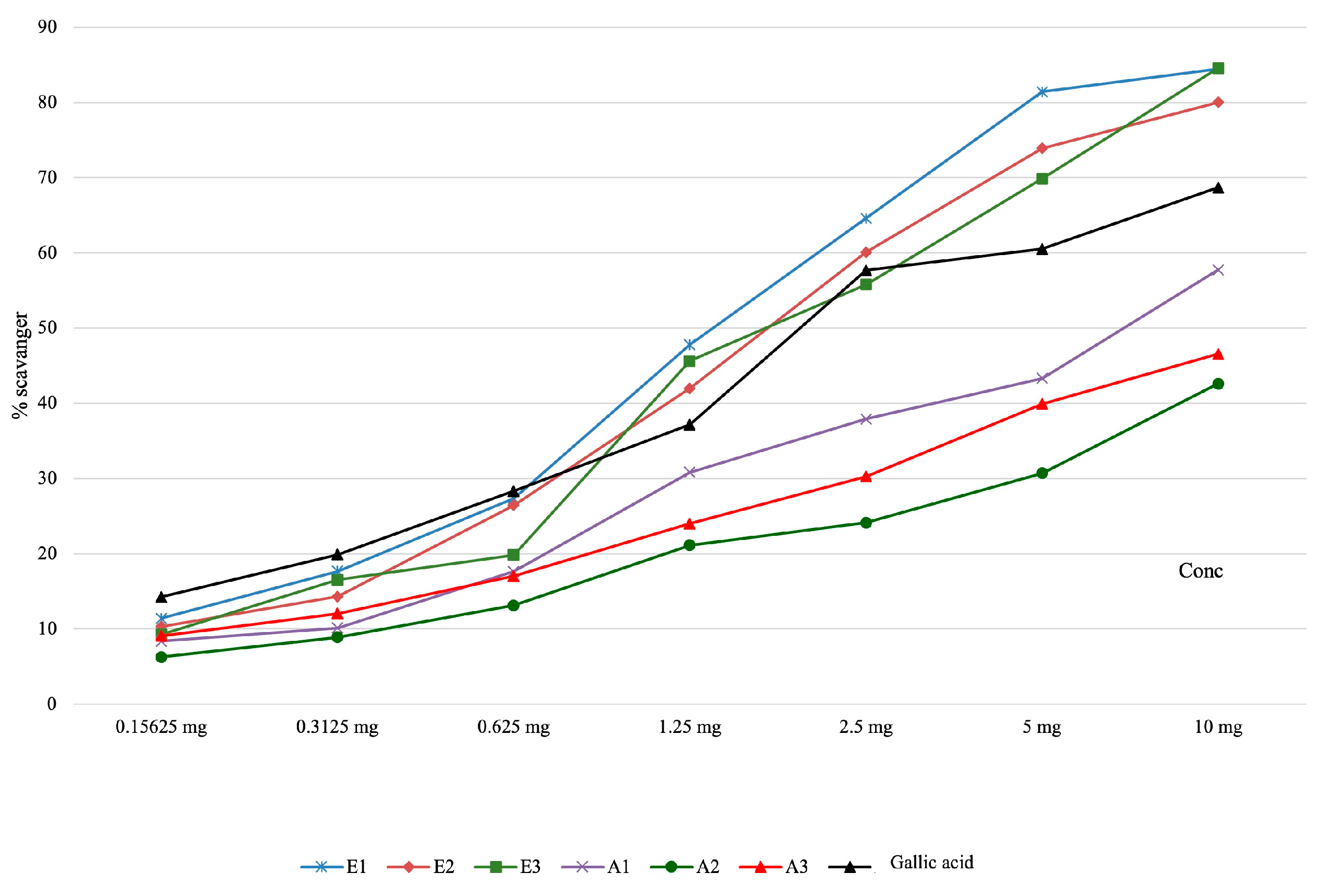
| Determination of Hydroxyl Radical Scavenging Capacity | |||||||||
| Samples | Concentration of the solution | Pvalue | |||||||
| 0.15625 mg | 0.3125 mg | 0.625 mg | 1.25 mg | 2.5 mg | 5 mg | 10 mg | IC50 (μg/mL final solution) | ||
| E1 | 11.41 ± 0.41 | 17.69 ± 0.35 | 27.39 ± 0.26 | 47.79 ± 0.11 | 64.61 ± 0.31 | 81.40 ± 0.26 | 84.86 ± 0.15 | 122.62±0.74 | <0.001 |
| E2 | 10.34 ± 0.53 | 14.31 ± 0.05 | 26.46 ± 0.19 | 42.00 ± 0.10 | 60.10 ± 0.03 | 73.91 ± 0.02 | 80.02 ± 0.08 | 154.58±0.41 | |
| E3 | 9.27 ± 0.64 | 16.55 ± 0.12 | 19.84 ± 0.08 | 45.64 ± 0.08 | 55.79 ± 0.28 | 69.87 ± 0.14 | 84.55 ± 0.16 | 153.16±1.11 | |
| A1 | 8.39 ± 0.34 | 10.15 ± 0.07 | 17.63 ± 0.13 | 30.83 ± 0.08 | 37.90 ± 0.12 | 43.31 ± 0.69 | 57.76 ± 0.15 | 156.96±3.57 | |
| A2 | 6.28 ± 0.17 | 8.89 ± 0.11 | 13.14 ± 0.62 | 21.13 ± 0.56 | 24.14 ± 0.43 | 30.71 ± 0.64 | 42.60 ± 1.05 | - | |
| A3 | 9.09 ± 0.15 | 12.06 ± 0.14 | 17.04 ± 0.52 | 24.00 ± 0.69 | 30.27 ± 0.15 | 39.93 ± 0.74 | 46.55 ±0.47 | - | |
| Gallic acid | 14.26 ± 0.18 | 19.89 ± 0.08 | 28.32 ± 0.21 | 37.14 ± 0.09 | 57.69 ± 0.11 | 60.54 ± 0.15 | 68.69 ± 0.20 | 177.29±0.66 | |
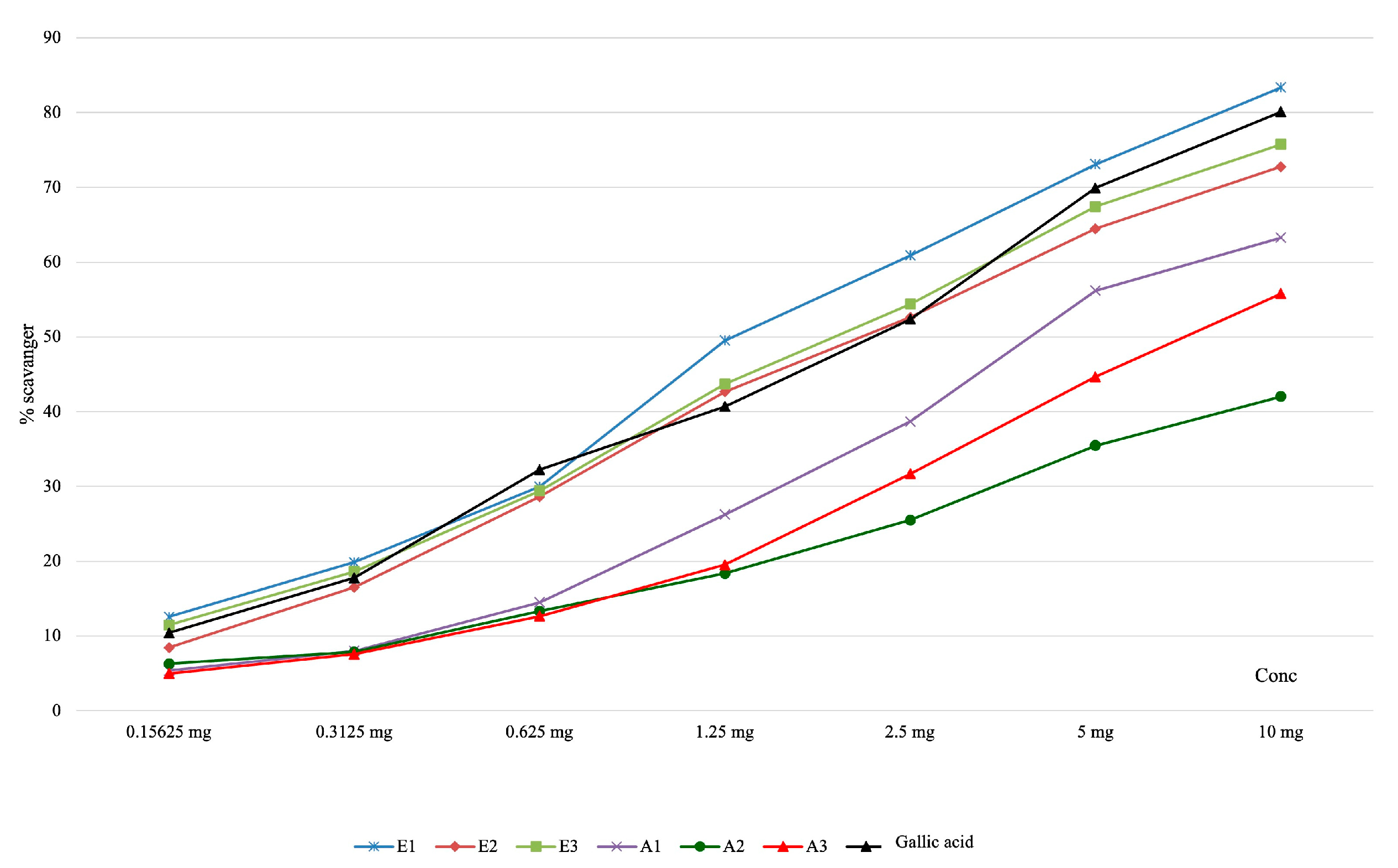
| Determination of Superoxide Anion Scavenging Capacity | |||||||||
| Samples | Concentration of the solution | Pvalue | |||||||
| 0.15625 mg | 0.3125 mg | 0.625 mg | 1.25 mg | 2.5 mg | 5 mg | 10 mg | IC50 (μg/mL final solution) | ||
| E1 | 12.58 ± 0,09 | 19.87 ± 0.25 | 29.97 ± 0.66 | 49.53 ± 0.47 | 60.90 ± 0.36 | 73.10 ± 0.75 | 83.37 ± 0.51 | 321.60±9.12 | <0.001 |
| E2 | 8.44 ± 0.19 | 16.52 ± 0.34 | 28.64 ± 0.17 | 42.66 ± 0.43 | 52.63 ± 0.48 | 64.46 ± 0.45 | 72.76 ± 0.74 | 520.79±16.90 | |
| E3 | 11.48 ± 0.06 | 18.64 ± 0.11 | 29.43 ± 0.42 | 43.71 ± 0.24 | 54.39 ± 0.24 | 67.43 ± 0.14 | 75.76 ± 0.62 | 470.08±7.32 | |
| A1 | 5.38 ± 0.29 | 8.02 ± 0.23 | 14.50 ± 0.39 | 26.24 ± 0.53 | 38.70 ± 1.50 | 56.19 ± 0.75 | 63.28 ± 0.89 | 977.47±39.37 | |
| A2 | 6.31 ± 0.09 | 7.86 ± 0.29 | 13.32 ± 0.32 | 18.37 ± 0.31 | 25.50 ± 0.41 | 35.46 ± 0.42 | 42.03 ± 0.79 | - | |
| A3 | 4.98 ± 0.47 | 7.58 ± 0.28 | 12.66 ± 0.44 | 19.54 ± 0.26 | 31.71 ± 0.19 | 44.68 ± 0.28 | 55.80 ± 0.27 | 1741.66±29.87 | |
| Gallic acid | 10.43 ± 0.42 | 17.78 ± 0.11 | 32.24 ± 0.14 | 40.70 ± 0.43 | 52.36 ± 0.49 | 69.92 ± 0.73 | 80.13 ± 0.76 | 543.38±15.43 | |
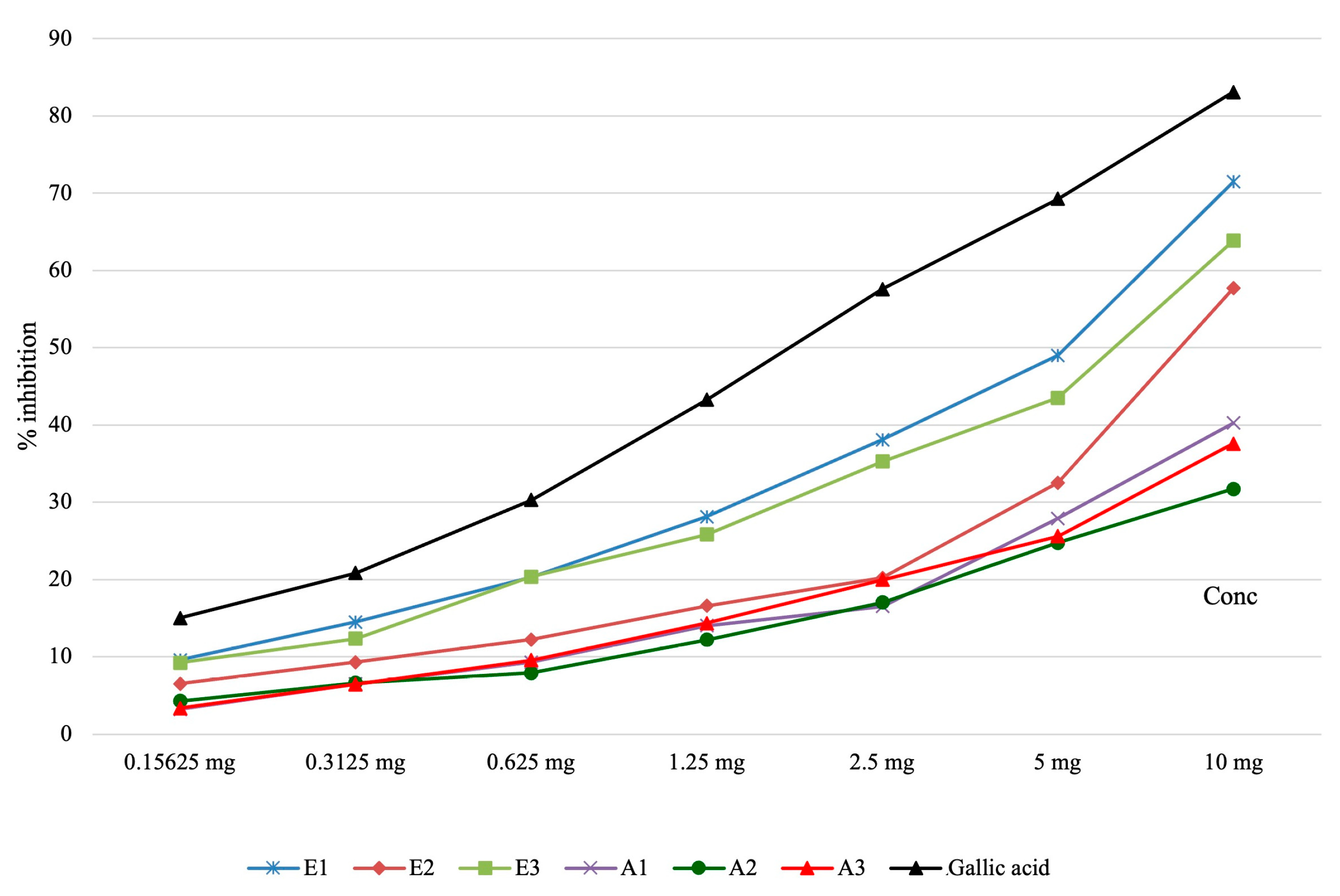
| Determination of the lipoxygenase inhibition capacity | |||||||||
| Samples | Concentration of the solution | Pvalue | |||||||
| 0.15625 mg | 0.3125 mg | 0.625 mg | 1.25 mg | 2.5 mg | 5 mg | 10 mg | IC50 (μg/mL final solution) | ||
| E1 | 9.58 ± 0.38 | 14.50 ± 0.18 | 20.31 ± 0.58 | 28.11 ± 0.38 | 38.08 ± 0.77 | 48.99 ± 0.68 | 71.47 ± 1.11 | 85.99±1.85 | <0.001 |
| E2 | 6.51 ± 0.16 | 9.28 ± 0.99 | 12.21 ± 0.52 | 16.59 ± 0.56 | 20.21 ± 1.03 | 32.54 ± 1.15 | 57.70 ± 0.46 | 134.77±2.49 | |
| E3 | 9.24 ± 0.46 | 12.34 ± 0.65 | 20.35 ± 0.57 | 25.81 ± 0.79 | 35.27 ± 1.16 | 43.50 ± 1.34 | 63.85 ± 1.61 | 104.10±5.05 | |
| A1 | 3.21 ± 0.14 | 6.49 ± 0.36 | 9.28 ± 0.11 | 14.01 ± 0.76 | 16.52 ± 1.12 | 27.90 ± 0.47 | 40.27 ±0.59 | - | |
| A2 | 4.28 ± 0.16 | 6.62 ± 0.27 | 7.90 ± 0.17 | 12.20 ± 0.42 | 17.04 ± 0.21 | 24.74 ± 0.34 | 31.71 ± 0.97 | - | |
| A3 | 3.37 ± 0.40 | 6.45 ± 0.46 | 9.56 ± 0.28 | 14.34 ± 0.30 | 19.95 ± 0.57 | 25.60 ± 0.75 | 37.58 ± 0.49 | - | |
| Gallic acid | 15.02 ± 0.25 | 20.84 ± 0.19 | 30.29 ± 0.78 | 43.26 ± 0.69 | 57.59 ± 0.38 | 69.25 ± 0.54 | 83.06 ± 0.95 | 28.85±0.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





