Submitted:
24 November 2023
Posted:
24 November 2023
You are already at the latest version
Abstract
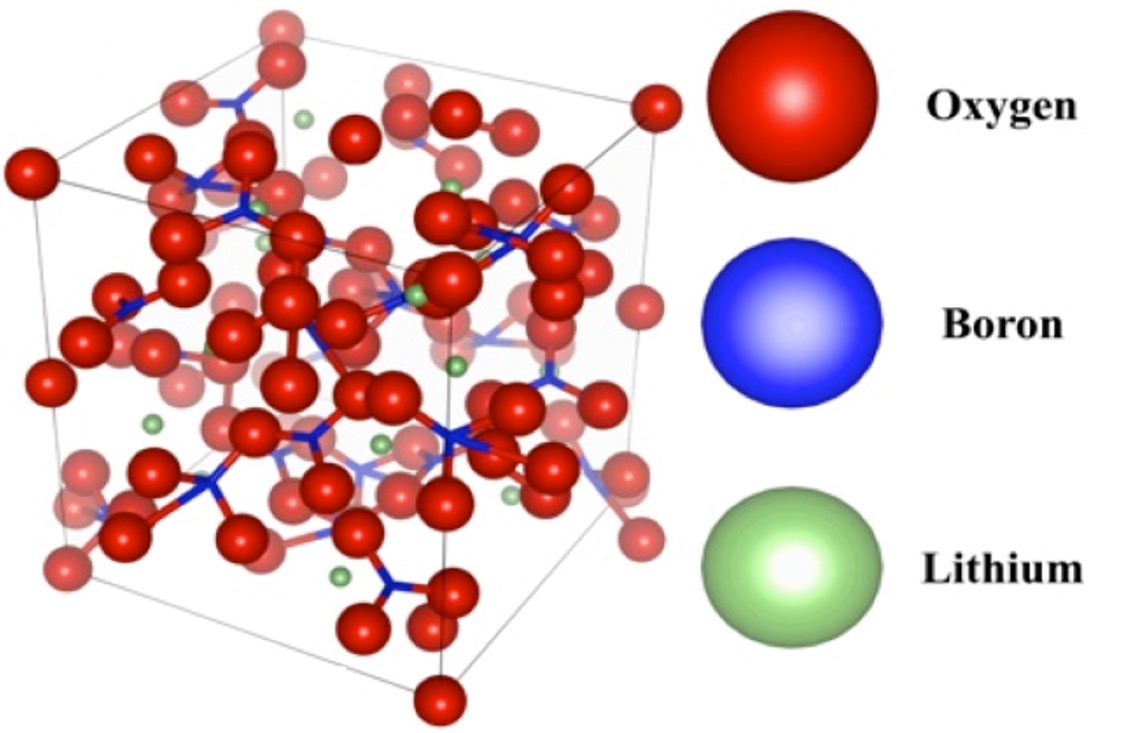
Keywords:
1. Introduction
2. Lithium tetraborate based scintillation detectors
- 10B + n → 7Li (0.84 MeV) + 4He (1.47 MeV) + γ (0.48 MeV) (94%)
- 10B + n → 7Li (1.015 MeV) + 4He (1.78 MeV) (6%)
- 6Li + n → 3H + 4He + (4.8 MeV)
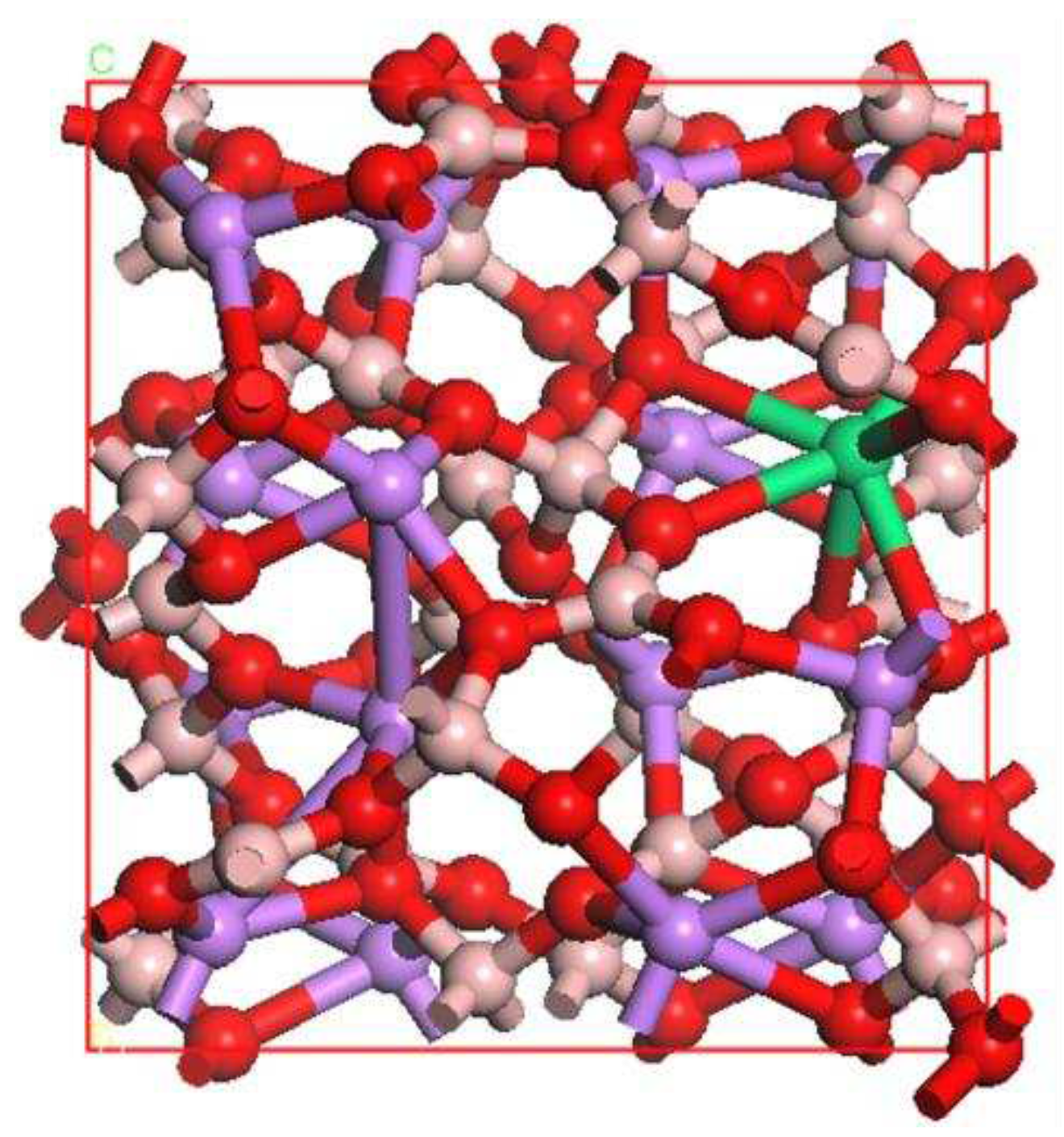
3. The Optical Characteristics of Li2B4O7

4. Factors Affecting Charge Production
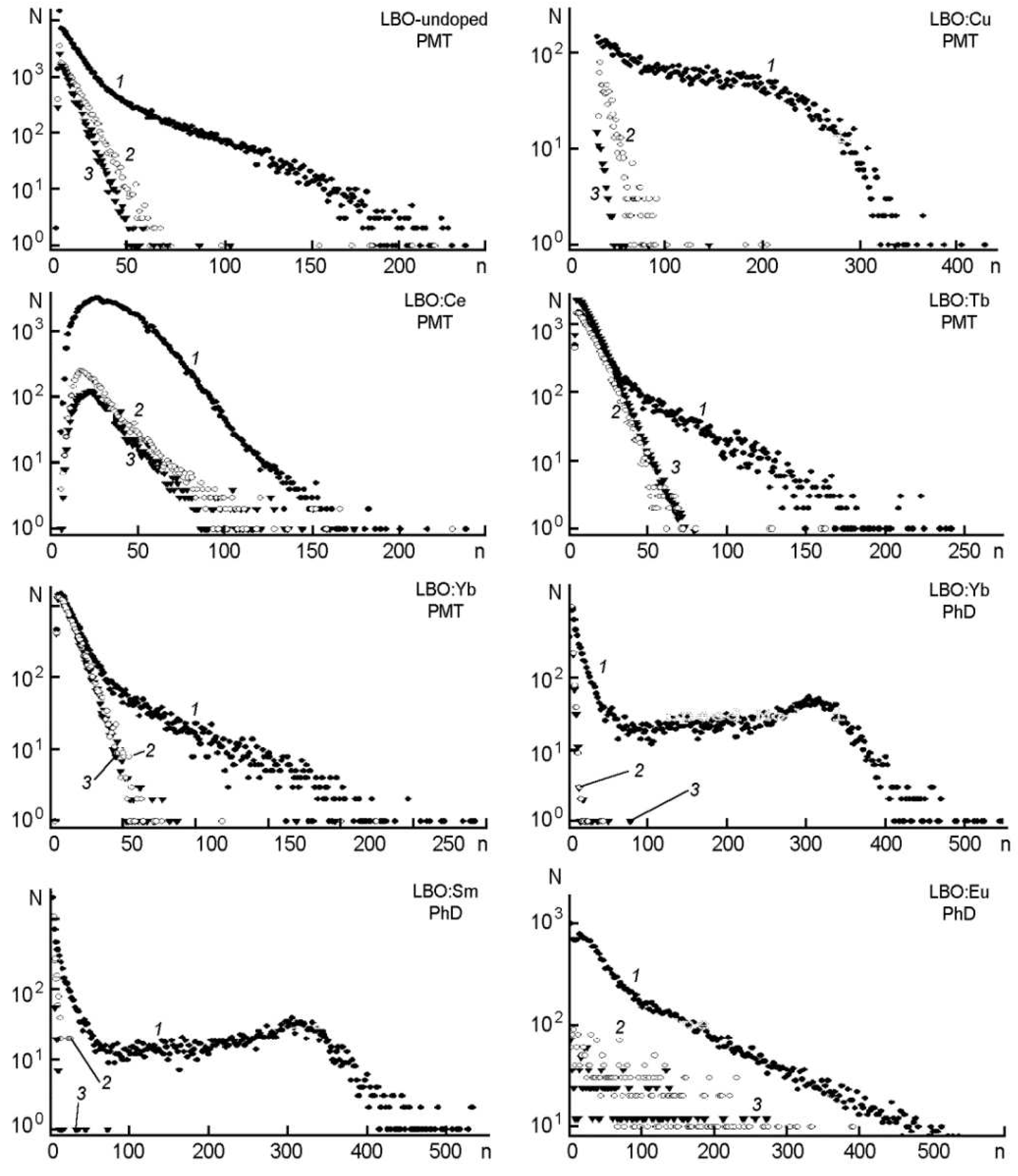
5. Factors affecting light production
6. Conclusions
Acknowledgments
References
- Kouzes, R.T. Detecting Illicit Nuclear Materials: The Installation of Radiological Monitoring Equipment in the United States and Overseas Is Helping Thwart Nuclear Terrorism. Am Sci 2005, 93, 422–427. [Google Scholar] [CrossRef]
- Kouzes, R.T.; Ely, J.H.; Erikson, L.E.; Kernan, W.J.; Lintereur, A.T.; Siciliano, E.R.; Stephens, D.L.; Stromswold, D.C.; Van Ginhoven, R.M.; Woodring, M.L. Neutron Detection Alternatives to 3He for National Security Applications. Nucl Instrum Methods Phys Res A 2010, 623, 1035–1045. [Google Scholar] [CrossRef]
- Seymour, R.S.; Richardson, B.; Morichi, M.; Bliss, M.; Craig, R.A.; Sunberg, D.S. Scintillating-Glass-Fiber Neutron Sensors, Their Application and Performance for Plutonium Detection and Monitoring. J Radioanal Nucl Chem 2000, 243, 387–388. [Google Scholar] [CrossRef]
- van der Ende, B.M.; Li, L.; Godin, D.; Sur, B. Stand-off Nuclear Reactor Monitoring with Neutron Detectors for Safeguards and Non-Proliferation Applications. Nat Commun 2019, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Eijk, C.W.E. van Inorganic Scintillators in Medical Imaging. Phys Med Biol 2002, 47, R85–R106. [Google Scholar] [CrossRef] [PubMed]
- Bird, K.; Sherwin, M.J. American Prometheus: The Triumph and Tragedy of J. Robert Oppenheimer; Alfred A. Knopf: New York, 2005;
- Caruso, A.N. The Physics of Solid-State Neutron Detector Materials and Geometries. Journal of Physics: Condensed Matter 2010, 22, 443201. [Google Scholar] [CrossRef] [PubMed]
- Frangville, C.; Grabowski, A.; Dumazert, J.; Montbarbon, E.; Lynde, C.; Coulon, R.; Venerosy, A.; Bertrand, G.H.V.; Hamel, M. Nanoparticles-Loaded Plastic Scintillators for Fast/Thermal Neutrons/Gamma Discrimination: Simulation and Results. Nucl Instrum Methods Phys Res A 2019, 942, 162370. [Google Scholar] [CrossRef]
- Leo, W.R. Techniques for Nuclear and Particle Physics Experiments; Springer Berlin Heidelberg: Berlin, Heidelberg, 1994; ISBN 978-3-540-57280-0. [Google Scholar]
- Burak, Y.V.; Adamiv, V.T.; Teslyuk, I.M.; Shevel, V.M. Optical Absorption of Isotopically Enriched Li2B4O7 Single Crystals Irradiated by Thermal Neutrons. In Proceedings of the Radiation Measurements; Pergamon, August 1 2004; Vol. 38, pp. 681–684.
- Zadneprovski, B.I.; Eremin, N.V.; Paskhalov, A.A. New Inorganic Scintillators on the Basis of LBO Glass for Neutron Detection. Functional Materials 2005, 12, 261–268. [Google Scholar]
- Seidl, E.; Schwertführer, W. Lithiumborat-Einkristalle Als Neutronendetektoren. Atomkernenergie 1966, 11, 155–162. [Google Scholar]
- Vinograd, E.L.; Vydai, Y.T.; Zagariy, L.B.; Kosmyna, M.B.; Kudin, A.M.; Levin, A.B.; Nazarenko, B.P.; Tarasov, V.A.; Chernikov, V.V. Scintillation Parameters of Single Crystals Containing Lithium: Li2B4O7, LiTaO3, LiNbO3. Functional Materials 1994, 1, 152–154. [Google Scholar]
- Bhalla, A.S.; Cross, L.E.; Whatmore, R.W. Pyroelectric and Piezoelectric Properties of Lithium Tetraborate Single Crystal. Jpn J Appl Phys 1985, 24, 727. [Google Scholar] [CrossRef]
- Adamiv, Volodymyr.T.; Burak, Yaroslav.V.; Wooten, David.J.; McClory, J.; Petrosky, J.; Ketsman, I.; Xiao, J.; Losovyj, Y.B.; Dowben, P.A. The Electronic Structure and Secondary Pyroelectric Properties of Lithium Tetraborate. Materials 2010, 3, 4550–4579. [CrossRef]
- Ketsman, I.; Wooten, D.; Xiao, J.; Losovyj, Ya.B.; Burak, Ya.V.; Adamiv, V.T.; Sokolov, A.; Petrosky, J.; McClory, J.; Dowben, P.A. The Off-Axis Pyroelectric Effect Observed for Lithium Tetraborate. Phys Lett A 2010, 374, 891–895. [Google Scholar] [CrossRef]
- Wooten, D.; Ketsman, I.; Xiao, J.; Losovyj, Y.B.; Petrosky, J.; McClory, J.; Burak, Y.V.; Adamiv, V.T.; Brown, J.M.; Dowben, P.A. The Electronic Structure of Li2B4O7(110) and Li2B4O7(100). EPJ Applied Physics 2010, 52, 1–8. [Google Scholar] [CrossRef]
- Baumer, V.N.; Chernikov, V.V.; Dubovik, M.F.; Gavrylyuk, V.P.; Grinyov, B.V.; Grin, L.A.; Korshikova, T.I.; Shekhovtsov, A.N.; Sysoeva, E.P.; Tolmachev, A. V; et al. Comparative Analysis of Scintillation Parameters Peculiarities of Li2B4O7, LaB3O6, Li6Gd(BO3)3 Single Crystals. Functional Materials 2001, 8, 736–741. [Google Scholar]
- Özdemir, Z.; Özbayoğlu, G.; Yilmaz, A. Investigation of Thermoluminescence Properties of Metal Oxide Doped Lithium Triborate. J Mater Sci 2007, 42, 8501–8508. [Google Scholar] [CrossRef]
- Ogorodnikov, I.N.; Pustovarov, V.A.; Kruzhalov, A.V.; Isaenko, L.I.; Kirm, M.; Zimmerer, G. Self-Trapped Excitons in LiB3O5 and Li2B4O7 Lithium Borates: Time-Resolved Low-Temperature Luminescence VUV Spectroscopy. Physics of the Solid State 2000, 42, 464–472. [Google Scholar] [CrossRef]
- Podgórska, D.; Kaczmarek, S.M.; Drozdowski, W.; Berkowski, M.; Worsztynowicz, A. Growth and Optical Properties of Li2B4O7 Single Crystals Pure and Doped with Yb, Co and Mn Ions for Nonlinear Applications. Acta Phys Pol A 2005, 107, 507–518. [Google Scholar] [CrossRef]
- Dubovik, M.F.; Tolmachev, A.V.; Grinyov, B.V.; Grin, L.A.; Dolzhenkova, E.F.; Dobrotvorskaya, M. V Luminescence and Radiation-Induced Defects in Li₂B₄O₇:Eu Single Crystals. Quantum Electronics and Optoelectronics 2000, 3, 420–422. [Google Scholar] [CrossRef]
- Kindrat, I.I.; Padlyak, B.V.; Lisiecki, R.; Adamiv, V.T. Spectroscopic and Luminescent Properties of the Lithium Tetraborate Glass Co-Doped with Tm and Ag. J Lumin 2020, 225, 117357. [Google Scholar] [CrossRef]
- Kelly, T.D.; Petrosky, J.C.; McClory, J.W.; Adamiv, V.T.; Burak, Y.V.; Padlyak, B.V.; Teslyuk, I.M.; Lu, N.; Wang, L.; Mei, W.-N.; et al. Rare Earth Dopant (Nd, Gd, Dy, and Er) Hybridization in Lithium Tetraborate. Front Phys 2014, 2. [Google Scholar] [CrossRef]
- Kindrat, I.I.; Padlyak, B.V.; Lisiecki, R.; Adamiv, V.T. Spectroscopic and Luminescent Properties of the Lithium Tetraborate Glass Co-Doped with Nd and Ag. J Alloys Compd 2021, 853, 157321. [Google Scholar] [CrossRef]
- Nagirnyi, V.; Kotlov, A.; Corradi, G.; Watterich, A.; Kirm, M. Electronic Transitions in Li2B4O7:Cu Single Crystals. physica status solidi c 2007, 4, 885–888. [Google Scholar] [CrossRef]
- Patra, G.; Singh, A.; Tiwari, B.; Singh, S.; BARe, D.D. 2015, undefined Development of Thermal Neutron Detector Based on Lithium Tetraborate (LTB) Single Crystal. barc.gov.in 2015.
- Chen, Y.; Huang, Y.; Luo, Z. Spectroscopic Properties of Yb3+ in Bismuth Borate Glasses. Chem Phys Lett 2003, 382, 481–488. [Google Scholar] [CrossRef]
- Danilyuk, P.S.; Popovich, K.P.; Puga, P.P.; Gomonai, A.I.; Primak, N.V.; Krasilinets, V.N.; Turok, I.I.; Puga, G.D.; Rizak, V.M. Optical Absorption Spectra and Energy Levels of Er3+ Ions in Glassy Lithium Tetraborate Matrix. Opt Spectrosc 2014, 117, 759–763. [Google Scholar] [CrossRef]
- Ignatovych, M.; Holovey, V.; Watterich, A.; Vidóczy, T.; Baranyai, P.; Kelemen, A.; Chuiko, O. Luminescence Characteristics of Cu- and Eu-Doped Li2B4O7. Radiat Meas 2004, 38, 567–570. [Google Scholar] [CrossRef]
- Ishii, M.; Kuwano, Y.; Asaba, S.; Asai, T.; Kawamura, M.; Senguttuvan, N.; Hayashi, T.; Koboyashi, M.; Nikl, M.; Hosoya, S.; et al. Luminescence of Doped Lithium Tetraborate Single Crystals and Glass. Radiat Meas 2004, 38, 571–574. [Google Scholar] [CrossRef]
- Jayasankar, C.K.; Babu, P. Optical Properties of Sm3+ Ions in Lithium Borate and Lithium Fluoroborate Glasses. J Alloys Compd 2000, 307, 82–95. [Google Scholar] [CrossRef]
- Kaczmarek, S.M. Li2B4O7 Glasses Doped with Cr, Co, Eu and Dy. Opt Mater (Amst) 2002, 19, 189–194. [Google Scholar] [CrossRef]
- Kassab, L.; Tatumi, S.; Morais, A.; Courrol, L.; Wetter, N.; Salvador, V. Spectroscopic Properties of Lead Fluoroborate Glasses Doped with Ytterbium. Opt Express 2001, 8, 585. [Google Scholar] [CrossRef]
- Kelly, T.D.; Kong, L.; Buchanan, D.A.; Brant, A.T.; Petrosky, J.C.; McClory, J.W.; Adamiv, V.T.; Burak, Y.V.; Dowben, P.A. EXAFS and EPR Analysis of the Local Structure of Mn-Doped Li2B4O7. physica status solidi (b) 2013, 250, 1376–1383. [Google Scholar] [CrossRef]
- Kenyon, A. Recent Developments in Rare-Earth Doped Materials for Optoelectronics. Prog Quantum Electron 2002, 26, 225–284. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishii, M.; Senguttuvan, N. Scintillation Characteristics of Undoped and Cu+-Doped Li2B4O7 Single Crystals. 2015.
- Lin, H.; Yang, D.; Liu, G.; Ma, T.; Zhai, B.; An, Q.; Yu, J.; Wang, X.; Liu, X.; Yue-Bun Pun, E. Optical Absorption and Photoluminescence in Sm3+- and Eu3+-Doped Rare-Earth Borate Glasses. J Lumin 2005, 113, 121–128. [Google Scholar] [CrossRef]
- Padlyak, B.; Ryba-Romanowski, W.; Lisiecki, R.; Adamiv, V.; Burak, Y.; Teslyuk, I.; Banaszak-Piechowska, A. Optical Spectra and Luminescence Kinetics of the Sm3+ and Yb3+ Centres in the Lithium Tetraborate Glasses. Optica Applicata 2010, 40, 427–438. [Google Scholar]
- Padlyak, B.; Ryba-romanowski, W.; Lisiecki, R.; Pieprzyk, B.; Drzewiecki, A.; Adamiv, V.; Burak, Y.; Teslyuk, I. ; Li, or Synthesis and Optical Spectroscopy of the Lithium Tetraborate Glasses, Doped with Terbium and Dysprosium. Optica Applicata 2012, XLII. [Google Scholar] [CrossRef]
- Vivien, D.; Georges, P. Crystal Growth, Optical Spectroscopy and Laser Experiments on New Yb3+-Doped Borates and Silicates. Opt Mater (Amst) 2003, 22, 81–83. [Google Scholar] [CrossRef]
- Polisadova, E.F.; Valiev, D.T.; Belikov, K.N.; Egorova, N.L. Scintillation Lithium-Phosphate-Borate Glasses Doped by REI. Glass Physics and Chemistry 2015, 41, 98–103. [Google Scholar] [CrossRef]
- Rivera, V.A.G.; Ferri, F.A.; Marega, E. Localized Surface Plasmon Resonances: Noble Metal Nanoparticle Interaction with Rare-Earth Ions. In Plasmonics - Principles and Applications; InTech, 2012.
- Shimizugawa, Y.; Umesaki, N.; Qiu, J.; Hirao, K. Local Structure around Europium Ions Doped in Borate Glasses. J Synchrotron Radiat 1999, 6, 624–626. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Pisarska, J.; Dominiak-Dzik, G.; Ryba-Romanowski, W. Visible and Infrared Spectroscopy of Pr3+ and Tm3+ Ions in Lead Borate Glasses. Journal of Physics: Condensed Matter 2004, 16, 6171–6184. [Google Scholar] [CrossRef]
- Senguttuvan, N.; Ishii, M.; Shimoyama, M.; Kobayashi, M.; Tsutsui, N.; Nikl, M.; Dusek, M.; Shimizu, H.M.; Oku, T.; Adachi, T.; et al. Crystal Growth and Luminescence Properties of Li2B4O7 Single Crystals Doped with Ce, In, Ni, Cu and Ti Ions. Nucl Instrum Methods Phys Res A 2002, 486, 264–267. [Google Scholar] [CrossRef]
- Santos, C.; Lima, A.F.; Lalic, M.V. First-Principles Study of Structural, Electronic, Energetic and Optical Properties of Substitutional Cu Defect in Li2B4O7 Scintillator. J Alloys Compd 2018, 735, 756–764. [Google Scholar] [CrossRef]
- Rzyski, B.M.; Morato, S.P. Luminescence Studies of Rare Earth Doped Lithium Tetraborate. Nuclear Instruments and Methods 1980, 175, 62–64. [Google Scholar] [CrossRef]
- Saisudha, M.B.; Ramakrishna, J. Effect of Host Glass on the Optical Absorption Properties of Nd3+, Sm3+, and Dy3+ in Lead Borate Glasses. Phys Rev B 1996, 53, 6186–6196. [Google Scholar] [CrossRef]
- Pekpak, E.; Yilmaz, A.; Ozbayoglu, G. An Overview on Preparation and TL Characterization of Lithium Borates for Dosimetric Use. The Open Mineral Processing Journal 2010, 3, 14–24. [Google Scholar] [CrossRef]
- Knoll, G.F. Radiation Detection and Measurement; 3rd ed.; John Wiley & Sons Inc: New York, 1999;
- Chaminade, J.P.; Viraphong, O.; Guillen, F.; Fouassier, C.; Czirr, B. Crystal Growth and Optical Properties of New Neutron Detectors Ce3+:Li6R(BO3)3 (R=Gd,Y). IEEE Trans Nucl Sci 2001, 48, 1158–1161. [Google Scholar] [CrossRef]
- Chernikov, V.V.; Dubovik, M.F.; Gavrylyuk, V.P.; Grinyov, B.V.; Griǹ, L.A.; Korshikova, T.I.; Shekhovtsov, A.N.; Sysoeva, E.P.; Tolmachev, A.V.; Zelenskaya, O.V. Peculiarities of Scintillation Parameters of Some Complex Composition Borate Single Crystals. Nucl Instrum Methods Phys Res A 2003, 498, 424–429. [Google Scholar] [CrossRef]
- Ishii, M.; Kuwano, Y.; Asai, T.; Asaba, S.; Kawamura, M.; Senguttuvan, N.; Hayashi, T.; Kobayashi, M.; Nikl, M.; Hosoya, S.; et al. Boron Based Oxide Scintillation Glass for Neutron Detection. Nucl Instrum Methods Phys Res A 2005, 537, 282–285. [Google Scholar] [CrossRef]
- Burak, Ya.V.; Adamiv, V.T.; Teslyuk, I.M.; Antonyak, O.T.; Malynych, S.Z.; Pidzyrailo, M.S. Thermoluminescence in Doped Single Crystals Li2B4O7:A (A = Cu, Ag). Ukrayins’kij Fyizichnij Zhurnal (Kiev) 2005, 50, 1153–1158. [Google Scholar]
- Islam, M.M.; Maslyuk, V.V.; Bredow, T.; Minot, C. Structural and Electronic Properties of Li2B4O7. Journal of Physical Chemistry B 2005, 109, 13597–13604. [Google Scholar] [CrossRef]
- Wooten, D.; Ketsman, I.; Xiao, J.; Losovyj, Ya.B.; Petrosky, J.; McClory, J.; Burak, Ya.V.; Adamiv, V.T.; Dowben, P.A. The Surface Core Level Shift for Lithium at the Surface of Lithium Borate. Physica B Condens Matter 2010, 405, 461–464. [Google Scholar] [CrossRef]
- Sugawara, T.; Komatsu, R.; Uda, S. Linear and Nonlinear Optical Properties of Lithium Tetraborate. Solid State Commun 1998, 107, 233–237. [Google Scholar] [CrossRef]
- Borlido, P.; Schmidt, J.; Huran, A.W.; Tran, F.; Marques, M.A.L.; Botti, S. Exchange-Correlation Functionals for Band Gaps of Solids: Benchmark, Reparametrization and Machine Learning. NPJ Comput Mater 2020, 6, 96. [Google Scholar] [CrossRef]
- Lentz, L.C.; Kolpak, A.M. Predicting HSE Band Gaps from PBE Charge Densities via Neural Network Functionals. Journal of Physics: Condensed Matter 2020, 32, 155901. [Google Scholar] [CrossRef]
- Perdew, J.P. Density Functional Theory and the Band Gap Problem. Int J Quantum Chem 1985, 28, 497–523. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yang, W.; Burke, K.; Yang, Z.; Gross, E.K.U.; Scheffler, M.; Scuseria, G.E.; Henderson, T.M.; Zhang, I.Y.; Ruzsinszky, A.; et al. Understanding Band Gaps of Solids in Generalized Kohn–Sham Theory. Proceedings of the National Academy of Sciences 2017, 114, 2801–2806. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Deilmann, T.; Thygesen, K.S. Towards Fully Automated GW Band Structure Calculations: What We Can Learn from 60.000 Self-Energy Evaluations. NPJ Comput Mater 2021, 7, 22. [Google Scholar] [CrossRef]
- Kwon, T.Y.; Ju, J.J.; Kim, H.K.; Cha, J.W.; Kim, J.N.; Cha, M.; Yun, S.I. Linear Optical Properties and Characteristics of Critically Phase-Matched Second Harmonic Generation of a Li2B4O7 Crystal Grown by the Czochralski Method. Mater Lett 1996, 27, 317–321. [Google Scholar] [CrossRef]
- Petrov, V.; Rotermund, F.; Noack, F.; Komatsu, R.; Sugawara, T.; Uda, S. Vacuum Ultraviolet Application of Li2B4O7 Crystals: Generation of 100 Fs Pulses down to 170 Nm. J Appl Phys 1998, 84, 5887–5892. [Google Scholar] [CrossRef]
- Burak, Ya.V.; Adamiv, V.T.; Teslyuk, I.M.; Moroz, I.Ye.; Malynych, S.Z. What Is the True Value of Bulk Band Gap of Lithium Tetraborate Single Crystal? Physics and Chemistry of Solid State 2022, 23, 113–119. [Google Scholar] [CrossRef]
- Wang, L.; Mei, W.N.; Dowben, P.A. The Surface States of Lithium Tetraborate. Journal of Physics Condensed Matter 2013, 25. [Google Scholar] [CrossRef]
- Antonyak, O.T.; Burak, Y.V.; Lyseiko, I.T.; Pidzyrailo, N.S.; Khapko, Z.A. Luminescence of Li2B4O7 Crystals. Opt Spectrosc 1986, 61, 550–553. [Google Scholar]
- Wettlaufer, J.S.; Jackson, M.; Elbaum, M. A Geometric Model for Anisotropic Crystal Growth. J Phys A Math Gen 1994, 27, 5957–5967. [Google Scholar] [CrossRef]
- Moustafa, Y.M.; Hassan, A.K.; El-Damrawi, G.; Yevtushenko, N.G. Structural Properties of V2O5:Li2O:B2O3 Glasses Doped with Copper Oxide. J Non Cryst Solids 1996, 194, 34–40. [Google Scholar] [CrossRef]
- Klein, C.A. Bandgap Dependence and Related Features of Radiation Ionization Energies in Semiconductors. J Appl Phys 1968, 39, 2029–2038. [Google Scholar] [CrossRef]
- Bussolati, C.; Fiorentini, A.; Fabri, G. Energy for Electron-Hole Pair Generation in Silicon by Electrons and α Particles. Physical Review 1964, 136, A1756–A1758. [Google Scholar] [CrossRef]
- Emery, F.E.; Rabson, T.A. Average Energy Expended Per Ionized Electron-Hole Pair in Silicon and Germanium as a Function of Temperature. Physical Review 1965, 140, A2089–A2093. [Google Scholar] [CrossRef]
- Wooten, D.; Ketsman, I.; Xiao, J.; Losovyj, Ya.B.; Petrosky, J.C.; McClory, J.; Burak, Ya.; Adamiv, V.; Dowben, P.A. Differences in the Surface Charging at the (100) and (110) Surfaces of Li2B4O7. MRS Proceedings 2009, 1164, 1164–L04-04. [Google Scholar] [CrossRef]
- Xiao, J.; Lozova, N.; Losovyj, Ya.B.; Wooten, D.; Ketsman, I.; Swinney, M.W.; Petrosky, J.; McClory, J.; Burak, Ya.V.; Adamiv, V.T.; et al. Surface Charging at the (100) Surface of Cu Doped and Undoped Li2B4O7. Appl Surf Sci 2011, 257, 3399–3403. [Google Scholar] [CrossRef]
- Brant, A.T.; Kananan, B.E.; Murari, M.K.; McClory, J.W.; Petrosky, J.C.; Adamiv, V.T.; Burak, Ya. V.; Dowben, P.A.; Halliburton, L.E. Electron and Hole Traps in Ag-Doped Lithium Tetraborate (Li2B4O7) Crystals. J Appl Phys 2011, 110, 093719. [Google Scholar] [CrossRef]
- Brant, A.T.; Buchanan, D.A.; McClory, J.W.; Dowben, P.A.; Adamiv, V.T.; Burak, Ya.V.; Halliburton, L.E. EPR Identification of Defects Responsible for Thermoluminescence in Cu-Doped Lithium Tetraborate (Li2B4O7) Crystals. J Lumin 2013, 139, 125–131. [Google Scholar] [CrossRef]
- Swinney, M.W.; McClory, J.W.; Petrosky, J.C.; Yang, S.; Brant, A.T.; Adamiv, V.T.; Burak, Y.V.; Dowben, P.A.; Halliburton, L.E. Identification of Electron and Hole Traps in Lithium Tetraborate (Li2B4O7) Crystals: Oxygen Vacancies and Lithium Vacancies. J Appl Phys 2010, 107, 113715. [Google Scholar] [CrossRef]
- Wojtowicz, A.J. Scintillation Mechanism: The Significance of Variable Valence and Electron-Lattice Coupling in RE-Activated Scintillators. In Proceedings of the Proc. of SCINT- 95; University Press: Delft, Netherlands, 1996; pp. 95–103. [Google Scholar]
- Puga, P.P.; Puga, G.D.; Popovich, K.P.; Kel’man, V.A.; Krasylynec, V.N.; Turok, I.I.; Prymak, M.V.; Danyliuk, P.S. Optical Absorption and X-Ray Luminescence of Glassy Lithium Tetraborate Activated by Terbium Oxide. Glass Physics and Chemistry 2012, 38, 190–195. [Google Scholar] [CrossRef]
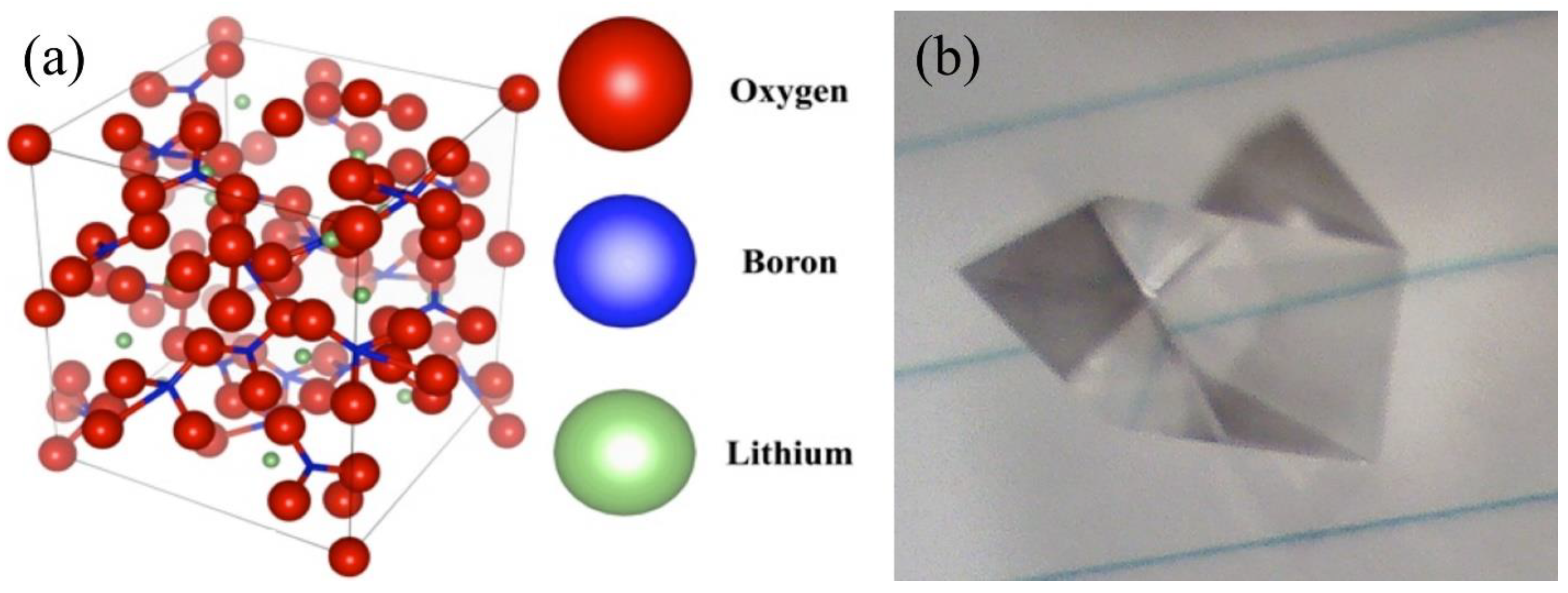
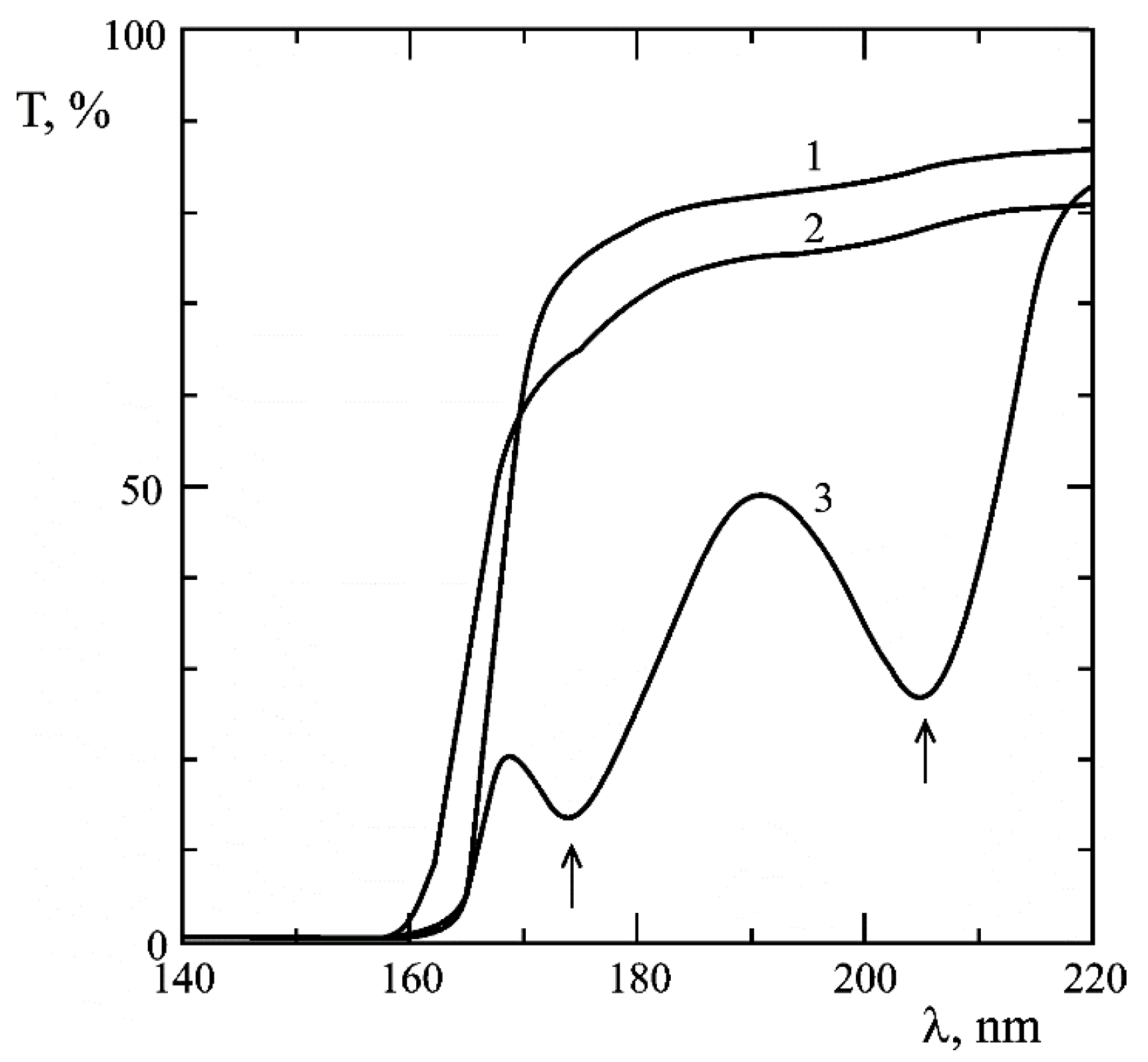
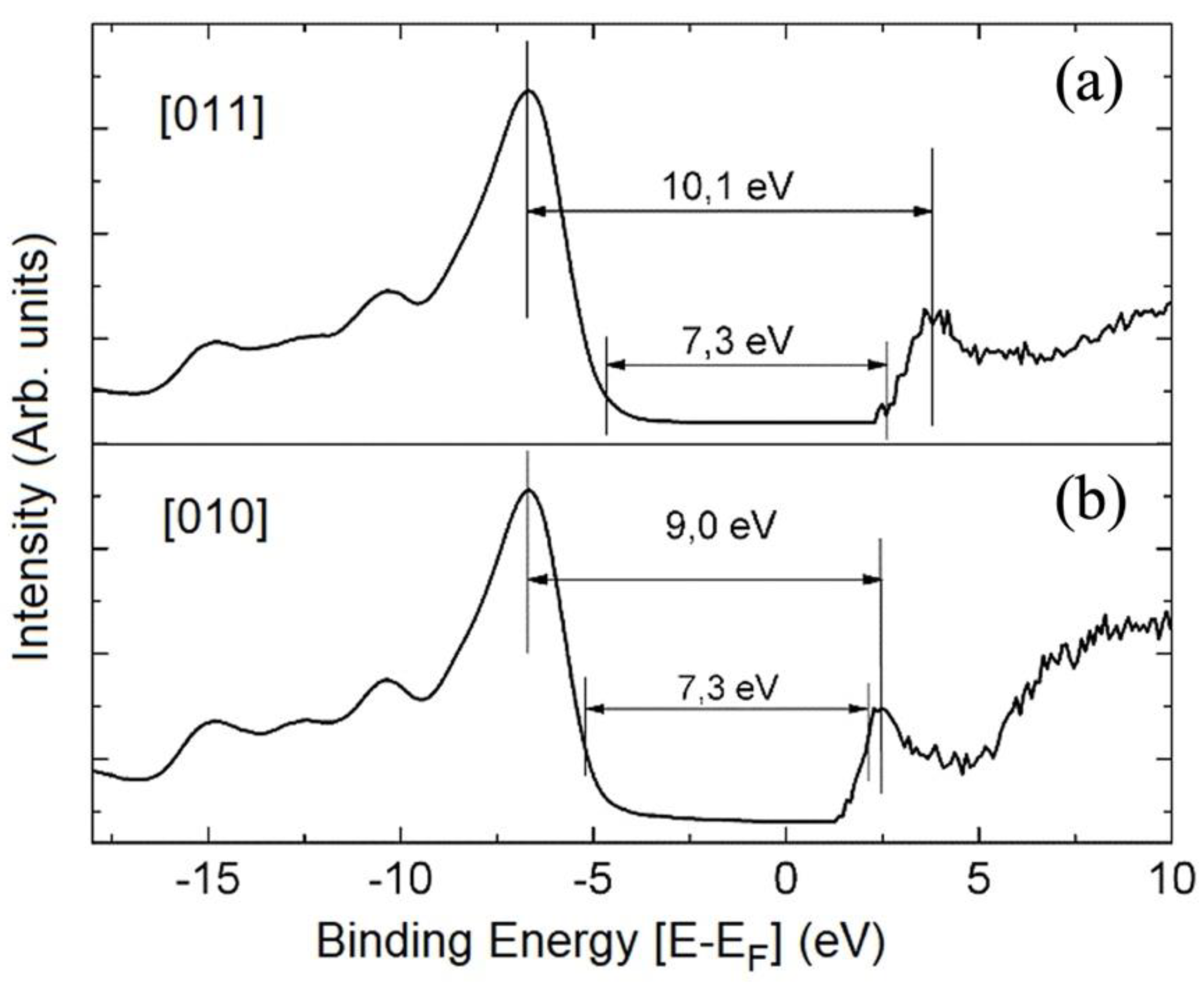
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




