1. Introduction
Gene deletions or mutations affecting the survival motor neuron (SMN1) gene cause spinal muscular atrophy (SMA), a rare autosomal recessive neuromuscular disorder. It is characterized by progressive symmetrical muscle weakness and atrophy due to loss of lower motor neuron in the brainstem and spinal cord [
1]. Based on the age at which symptoms first appear, the greatest motor milestone acquired, and the extent of the symptoms, SMA can be divided into five types: 0 – prenatal/foetal, 1 – non-sitters, 2 – sitters, 3 and 4 – walkers. Clinical phenotype widely ranges from death within weeks of birth to only mild proximal weakness developing during adulthood [
2,
3]. Growing knowledge on the pathophysiology of SMA alongside breakthroughs in genetic therapeutics have led to the discovery of gene replacement agents, antisense oligonucleotides, and splicing modifiers that improve life span, motor function and decrease morbidity, even among patients with most sever clinical forms [
2,
3,
4,
5].
Nowadays, after discovery of new disease modifying drugs (DMDs) on top of nusinersen (as a piooner option with intrathecal route of administration), especially due to availability of options with peroral and intravenous application, there is a possibility for patients to switch treatments. Switch may be indicated due to inadequate clinical response or more frequently due to easiness of applications and unwanted side effects from the medicine or its administration. Furthermore, intrathecal nusinersen administration could be challenging or even impossible in patients with reduced spinal access due to severe scoliosis or scoliosis surgery-related spinal fusion [
6,
7]. Thus, from safety and effectiveness perspective, collecting more scientific evidence and experience on a switch from nusinersen to other therapies is of high importance. [
5,
8].
Risdiplam is the first and only orally administered SMA DMD; a SMN2-directed splicing modifier, which consequently boosts its effect via producing full-length and functional SMN protein. Due to results pointing toward favourable benefit to risk ratio accompanied by improvement of motor function in both infantile-onset and late-onset SMA (FIREFISH and SUNFISH clinical trials, respectively), it is approved by FDA (in 2020) and EMA (in 2021) and available on the market for the treatment [
9,
10,
11]. Up to now, real-world findings (although scarce) on effectiveness, safety and tollerability of risdiplam are predominantly congruent with the data obtained from pivotal clinical trials [
12].
Our previous research in real-world setting confirmed the effectiveness and safety of nusinersen in Croatian paediatric and adult SMA patients [
13]. Data on combination or sequential treatment of SMA with DMDs are missing and the latter field is poorly understood. The currently available data of patients on risdiplam previously treated with nusinersen are coming from exploratory research mainly focused on safety [the examples can be seen from JEWELFISH (NCT03032172) study and and Kwon et al. expanded access program report] [
14,
15]. To the best of our knowledge, our study is a pioneer one aiming to investigate real-world effectiveness (hypothesizing non-inferiority) and safety profile of risdiplam in paediatric and adult nusinersen-risdiplam SMA switch cohort.
2. Results
During the study period, from 30 SMA patients in total, 17 patients met inclusion criteria [58.9% female; median age 12.75 (3.0-44.5) yr.]. Baseline (at the time of nusinersen-risdiplam switch) demographic and clinical characteristics are presented in
Table 1. Overall, three patients required MV, and three required BiPAP (all SMA type 1). According to feeding support data, two patients required PEG and two required NGT (all SMA type 1).
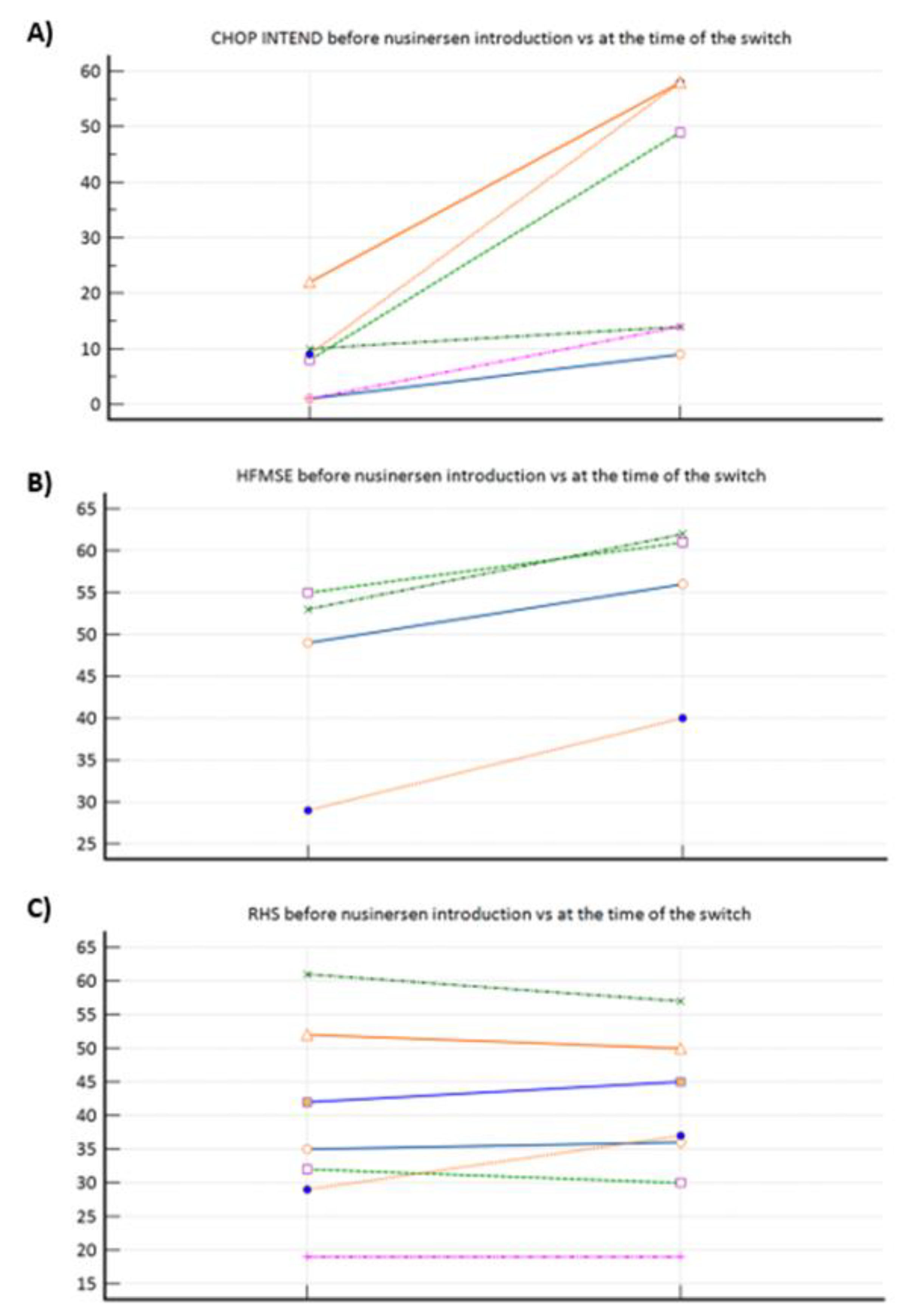
Figure 1a–c demonstrated motor function progress after nusinersen treatment completion (pre-treatment vs at the time of the nusinersen-risdiplam switch. To elaborate, significant improvement was observed among SMA 1 patients (
Figure 1a), positive trend among SMA 3p subpopulation (
Figure 1b), whilst there was practically no change when nusinersen was introduced in SMA 3 patients after the age of 18 yr. (
Figure 1c).
In total, there were 33 positive risdiplam reimbursement requests. The reasons for the nusinersen-risdiplam switch were difficulty performing a lumbar puncture /pain (41.2% ; n=7), post-dural puncture headache (11.8% ; n=2), application preference (parents’ request) (5.9% ; n=1), and not specified (41.2% ; n=7).
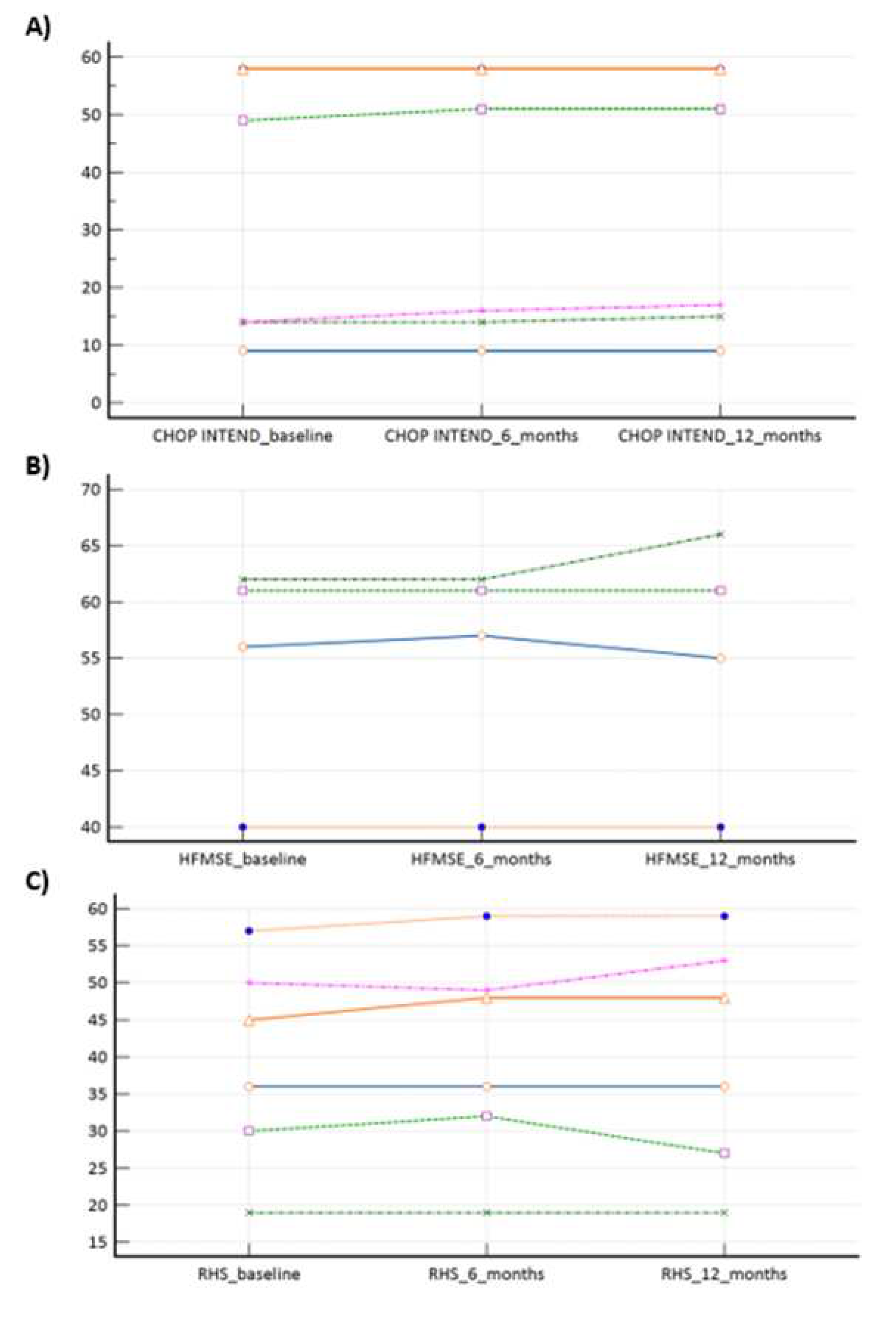
In our ‘switch’ cohort, we have demonstrated a non-inferiority of risdiplam to nusinersen, in SMA 1, SMA 3p and SMA 3a subpopulations, during a one year follow-up period (
Table 2,
Figure 2a–c). There were no reports on respiratory function worsening (defined as respiratory support introduction or switch from BiPAP to MV), feeding worsening (defined as introduction of PEG or NGT), and there were no lethal events during the study period.
Safety profile was in accordance with the already known from the summary of product characteristics. New potential ADR ‘signal’ which has been notices is weight gain – one SMA 3p patient increased his body weight by 5 kg, while one SMA 3a patient got 10 kg after 1 yr. of risdiplam treatment. At the end of follow-up there were 2 cases of re-switch –they were connected with notable weight gain as well as unsatisfactory motor function dynamics.
3. Discussion
A definite forte of our research is the pioneer demonstration of risdiplam’s real-world non-inferiority and safety in SMA patients previously treated with nusinersen. This is important both from safety and effectiveness perspective, since a portion of patients may experience ADRs or drug administration barriers (e.g., scoliosis / spinal fusion) and thus demanding a new DMD with peroral (risdiplam) or intravenous (onasemnogene abeparvovec) route of administration.
FIREFISH study (patients aged 1-7 months at enrolment, with a genetically confirmed SMA and two SMN2 copies), which was a multicentre, open-label, two-part trial aiming towards demonstrating safety and efficacy of risdiplam in SMA type 1, i.e., 12 infants (29%) were able to sit without support for at least 5 seconds after 1 year of treatment. Furhtermore, 56% of patients within risdiplam group as compared with 17% among historical controls achieved CHOP-INTEND score of 40 or higher, whilst 90% as compared with 17% increased at least 4 points from baseline in the CHOP-INTEND score. The results were also promising in terms of survival, event-free survival, as well as in terms of respiratory and feeding domains [
9].
In SUNFISH trial (phase 3, randomised, double-blind, placebo-controlled study, enrolling patients aged 2–25 years with confirmed 5q autosomal recessive SMA type 2 or 3), the primary motor endpoint, defined as change from baseline in the 32-item Motor Function Measure total score at month 12, was met in a significantly greater number of patients receiving risdiplam compared to placebo. Risdiplam group also reached statistical significance for secondary objectives such as RULM, HFMSE, and SMA Independence Scale. Adverse events that were reported in at least 5% more patients who received risdiplam than those who received placebo were pyrexia, diarrhoea, mouth and aphthous ulcers, urinary tract infection, and arthralgias. No significant difference in terms of serious adverse events rate was observed [
10].
As we are waiting for JEWELFISH to be finished, which is a multicenter, exploratory, non-comparative, open-label study evaluating safety, tolerability and PK/PD relationship of risdiplam in pediatric and adult patients with SMA (6 months to 60 yr. of age at screening) who have been previously treated with other approved SMA DMD [
14], our pivotal reports are of great importance.
We have again demonstrated nusinersen’s effectiveness (motor function improvement) in SMA 1 and SMA 3a, whilst its effectiveness was again questionable in SMA 3p subpopulation, where we can talk about motor function preservance only (
Figure 1a–c). Those observations confirmed our previous conclusions provided from our bigger real-word nusinersen cohort, that we recently published in Journal of Clinical Medicine [
13]. Our study clearly demonstrated risdiplam as a non-inferior treatment option, when compared to nusinersen in SMA 1, SMA 3p, and SMA 3a subpopulation (
Table 2). When a more in-depth look is given to SMA 1 subpopulation results (
Table 2,
Figure 1a), there is a potential positive trend in favour of risdiplam motor effectiveness (e.g., since p value is very close to significance on a relatively small sample). The latter is in concordance with data and conclusions provided by Ribero et al., who positioned risdiplam as a superior option, compared to nusinersen, solely for SMA type 1 (and probably non-inferior for other types) [
16]. Potential explanations is in the fact that risdiplam, on contrary to nusinersen, increases functional SMN protein levels in both the CNS and peripheral tissues; thus, not solely targeting the motor neurons of the spinal cord [
17]. Also, as per exploratory observations from risdiplam’s RCTs, motor function generally seems to improve more in younger individuals, whilst in older individuals it is predominantly stabilised while on DMD, possibly due to (ir)reversibility of motor neuron deterioration.
By presenting this data we are once again supporting SMA orphan drugs’ efficacy and safety on wider, heterogenous sample (compared to RCT models in rare diseases), as well as gaining and providing further insights with DMT individualization, switch, and comparison [
13]. Besides being important for regulatory issues (pricing, reimbursement, and potential shift of available financial resources), by providing experience and concrete evidence it also upgrades SMA patients’ clinical care, quality of life, options for management if/when ineffectiveness or ADRs of primary introduced DMT arise.
On top what is already known and mentioned in risdiplam’s summary of product characteristics, we here report on a new potential pharmacovigilance ‘signal’, which is weight gain that arose in one patient with SMA 3p (5 kg) and one with SMA 3a (10 kg). To the best of our knowledge, no evidence on this was reported within in-human trials; only animal study by Porier et al. exploratory reported on increased body weight in mice model [
17]. We have sent a formal request to check for potential pharmacovigilance reports on the latter, but no such confirmed cases were identified within Agency for Medicinal Products and Medicinal Devices (HALMED) and Eudravigilance database nor through searching periodic safety update reports. Thus, one may find our pivotal observations interesting, and some focus on weight monitoring (or even body composition monitoring by bioelectrical impedance) during risdiplam treatment may be put both for clinicians and scientists for further research.
Although the importance of this study, which is presenting pivotal findings real-world effectiveness (non-inferiority) and safety of risdiplam in paediatric and adult nusinersen-risdiplam SMA switch cohort over a one year period, is indisputable, it is not without limitations. Since this was a retrospective observational study using already collected and aggregated data within CHIF database and standardized reimbursement documentation, the reported results should be reported accordingly. Unfortunately, due to high prevalence of scoliosis and spinal fusion among SMA 2 patients (as it can also be seen from our previous report) they were only occasionally candidates for nusinersen and/or started risdiplam through compassionate use very early, so they did not meet our prespecified inclusion criteria from several reasons; thus we here only provide scientific evidence for SMA 1, SMA 3p, and SMA 3a [
13]. However, since we have proven non-inferiority in SMA 1 and SMA 3, bearing in mind the clinical and pathophysiological characteristics, we are confident that our safety and effectiveness conclusions can be easily extrapolated to SMA 2 subpopulation also. Among limitations is definitely a fact that some adverse events that potentially arose during the study period were not included medical records, which depends on the leading physician’s/assessor’s quality of evaluation of ADR probability and documentation management habits. However, all patients were strictly followed in the same medical centre (National Referral Centre for SMA). Standard motor scales were used for treatment effectiveness estimation, which was at all times estimated by starting/initial well-trained assessor to obtain uniformity. Besides novelty of findings another advantage is the presentation of detailed genotype data for entire cohort as well as conduction of individual motor effectiveness subanalyses for subpopulations such as SMA 3p and SMA 3a.
4. Materials and Methods
Croatian Health Insurance Fund (CHIF) database and associated reimbursement documentation were used to identify all Croatian SMA patients switched from nusinersen to risdiplam (reimbursed by CHIF) up to September 2023.
The indication for risdiplam introduction (which also corresponds to the CHIF reimbursement criteria) is the diagnosis of 5q SMA 1-3 or one to four copies of SMN2. In order to obtain a reimbursement for risdiplam by CHIF, the Drug and Therapeutic Committee of the National Referral Centre for SMA needs to submit the formal request accompanied by relevant clinical documentation. The request and reimbursement applies for the period of next six months, after which the procedure should be repeated.
Under a de-identified personal code we have retrospectively collected the following: age when nusineresen-risdiplam switch was implemented, gender, SMA type and stage, genotype (SMN1, SMN2, and NAIP copy numbers), dose of risdiplam and number of reimbursement requests, motor function pre-treatment, at the time of switch (baseline) and during the follow-up (1 year time), need for respiratory (mechanical ventilation – MV or bi-level positive airway pressure – BiPAP) and feeding support (percutaneous endoscopic gastrostomy – PEG or nasogastric tube – NGT), and adverse drug reactions (ADRs).
Motor function evaluations were performed as per standardized procedures using validated scales: i) CHOP INTEND (Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders) for non-sitting patients and those who were <2 yr. old; ii) HFMSE (Hammersmith Functional Motors Scales Expanded) for all sitters, and for walkers younger than 18 yr. of age (SMA 3p); iii) RHS (Revised Hammersmith Scale) for all adult walkers (SMA 3a).
The following inclusion criteria were set: i) risdiplam reimbursed by CHIF; ii) patient received at least 6 doses of nusinersen before switch to risdiplam; iii) there was no relevant pause between the latter DMDs; iv) availability of all prespecified studied data and parameters. After applying the latter criteria, eligible patients were included in further clinical-demographic overview, motor effectiveness and safety (ADR frequency) analyses.
After collection, data was imported from patient-specific forms to Microsoft Excel 2016 (Microsoft Office) and MedCalc v12.1.3 (MedCalc Software bvba, Ostend, Belgium) for statistical analyses. The Kolmogorov-Smirnov test was used to assess the normality of the distribution. Variable values were described by absolute and relative frequencies, measures of central tendency, and measures of spread. In order to present the initial clinical effectiveness of nusinersen, the Wilcoxon matched pairs signed rank test was used to compare the motor function pre-treatment versus at the time of the switch (baseline). Furthermore, the changes over time in terms of motor function (baseline vs 6 months after switch vs 12 months after switch) were analysed using Friedman test for each SMA type separately. All statistical tests were two-tailed and with a 95% CI. The criterion for statistical significance was set at p<0.05.
The study was conducted in accordance with the Declaration of Helsinki. The data was anonymized and already recorded, and because of the retrospective nature and design a waiver of informed consent was applied.
5. Conclusions
There is an obvious clinical (effectiveness and safety) and regulatory (e.g., reimbursement and pricing decisions, and shift of financial resources for indications with proven benefit) need for continuous regional and local data collection by national health insurance providers, at the national healthcare level as well as through disease registry networks, especially in the setting of rare disease where expensive orphan therapeutic options are available. Hence, we have reported a pivotal real-world findings on switching SMA patients from nusinersen to risdiplam and demonstrated its effectiveness (non-inferiority), safety and tolerability in a heterogenous paediatric and adult ‘switch’ cohort, which will further increase the quality and standards of care as well as safety of a notable portion of SMA patients; especially for those who demand switch from nusinersen to other DMD from clinical or personal reasons.
Author Contributions
All authors contributed substantially and fulfilled the ICMJE criteria of co-authorship. A.B.: Conceptualization, Methodology, Data collection, Data analysis, Writing; T.S.: Conceptualization, Data collection, Supervision; M.K.Š.: Writing; D.V.: Conceptualization, Methodology, Data analysis, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Bearing in mind the retrospective nature of the study and its design, as well as the fact that used data had already been anonymized and recorded, a waiver of informed consent was approved.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Darras, B.T. Spinal muscular atrophies. Pediatr Clin North Am 2015, 62, 743–766. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H. New and Developing Therapies in Spinal Muscular Atrophy: From Genotype to Phenotype to Treatment and Where Do We Stand. Int J Mol Sci 2020, 21, 3297. [Google Scholar] [CrossRef] [PubMed]
- Talbot, K.; Tizzano, E.F. The clinical landscape for SMA in a new therapeutic era. Gene Ther 2017, 24, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Tizzano, E.F.; Finkel, R.S. Spinal muscular atrophy: A changing phenotype beyond the clinical trials. Neuromuscul Disord 2017, 27, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.A.; Carey, K.A.; Paguinto, S.G.; Kasparian, N.A.; De Abreu Lourenço, R. "The Whole Game is Changing and You've Got Hope": Australian Perspectives on Treatment Decision Making in Spinal Muscular Atrophy. Patient 2020, 13, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, J.; Weiss, D.; Schlenker, F.; Groth, M.; Denecke, J. Intrathecal Administration of Nusinersen in Pediatric SMA Patients with and without Spine Deformities: Experiences and Challenges over 3 Years in a Single Center. Neuropediatrics 2021, 52, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Stolte, B.; Totzeck, A.; Kizina, K.; Bolz, S.; Pietruck, L.; Mönninghoff, C.; Guberina, N.; Oldenburg, D.; Forsting, M.; Kleinschnitz, C.; et al. Feasibility and safety of intrathecal treatment with nusinersen in adult patients with spinal muscular atrophy. Ther Adv Neurol Disord 2018, 11, 1756286418803246. [Google Scholar] [CrossRef] [PubMed]
- Agosto, C.; Benedetti, F.; Salamon, E.; Mercante, A.; Papa, S.; Giacomelli, L.; Santini, A.; Benini, F. How children and caregivers viewed the change from nusinersen to risdiplam for treating spinal muscular atrophy. Acta Paediatr 2023, 112, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Darras, B.T.; Masson, R.; Mazurkiewicz-Bełdzińska, M.; Rose, K.; Xiong, H.; Zanoteli, E.; Baranello, G.; Bruno, C.; Vlodavets, D.; Wang, Y.; et al. Risdiplam-Treated Infants with Type 1 Spinal Muscular Atrophy versus Historical Controls. N Engl J Med 2021, 385, 427–435. [Google Scholar] [CrossRef]
- Mercuri, E.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Oskoui, M.; Saito, K.; Vuillerot, C.; Baranello, G.; Boespflug-Tanguy, O.; Goemans, N.; et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol 2022, 21, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Paik, J. Risdiplam: A Review in Spinal Muscular Atrophy. CNS Drugs 2022, 36, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Boccanegra, B.; Vitturi, G.; Trifirò, G.; De Luca, A. Pharmacological Therapies of Spinal Muscular Atrophy: A Narrative Review of Preclinical, Clinical-Experimental, and Real-World Evidence. Brain Sci 2023, 13, 1446. [Google Scholar] [CrossRef] [PubMed]
- Belančić, A.; Strbad, T.; Kučan Štiglić, M.; Vitezić, D. Effectiveness of Nusinersen in Type 1, 2 and 3 Spinal Muscular Atrophy: Croatian Real-World Data. J Clin Med 2023, 12, 2839. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, C.A.; Bruno, C.; Duong, T.; Fischer, D.; Mercuri, E.; Kirschner, J.; Kostera-Pruszczyk, A.; Jaber, B.; Gorni, K.; Kletzl, H.; et al. Risdiplam in Patients Previously Treated with Other Therapies for Spinal Muscular Atrophy: An Interim Analysis from the JEWELFISH Study. Neurol Ther 2023, 12, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.M.; Arya, K.; Kuntz, N.; Phan, H.C.; Sieburg, C.; Swoboda, K.J.; Veerapandiyan, A.; Assman, B.; Bader-Weder, S.; Dickendesher, T.L.; et al. An expanded access program of risdiplam for patients with Type 1 or 2 spinal muscular atrophy. Ann Clin Transl Neurol 2022, 9, 810–818. [Google Scholar] [CrossRef]
- Ribero, V.A.; Daigl, M.; Martí, Y.; Gorni, K.; Evans, R.; Scott, D.A.; Mahajan, A.; Abrams, K.R.; Hawkins, N. How does risdiplam compare with other treatments for Types 1-3 spinal muscular atrophy: a systematic literature review and indirect treatment comparison. J Comp Eff Res 2022, 11, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Poirier, A.; Weetall, M.; Heinig, K.; Bucheli, F.; Schoenlein, K.; Alsenz, J.; Bassett, S.; Ullah, M.; Senn, C.; Ratni, H.; et al. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol Res Perspect 2018, 6, e00447. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).