1. Introduction
Blueberries, known as the "king of berries", have rich nutritional value and medical care functions [
1,
2,
3]. The cultivation of blueberries was introduced to China in 1989 [
4] and had since experienced rapid growth in both planting area and yield [
5]. However, blueberry cultivation faces various challenges due to its strict requirements for soil conditions [
6]. Therefore, studying soil improvement methods for blueberry cultivation is crucial for enhancing yield and quality and promoting the development of the blueberry industry.
Blueberries require high soil organic matter to support their normal growth and development. Additionally, maintaining a suitable pH level in the soil, typically between 4.5 and 5.5, is essential for blueberry growth [
7]. However, most of the soil in Chinese does not meet these conditions [
8]. To address these issues, many researchers conducted extensive studies on soil improvement technologies aimed at enhancing soil physical and chemical properties, microbial environment, fertility, and water retention capacity [
5,
9,
10]. Currently, the common soil improvement materials include sulfur, peat, rice husk (straw), mushroom bran [
11,
12]. Previous study has shown that acidic fertilizers such as aluminum sulfate, sulfur powder and ferrous sulfate are commonly used to adjust soil pH, with sulfur powder being the most stable and long-lasting option [
13]. However, long-term application of sulfur will lead to blueberry soil pollution, and decreases the number of soil microorganisms [
14]. Peat, containing a large amount of organic matter, provides nutrients for blueberry growth [
15]. In the process of blueberry cultivation, it can also improve the soil aggregate structure, make the soil loose and have strong water absorption and nitrogen absorption, thus promoting the growth and development of blueberry plants [
16]. In addition, peat contains a lot of organic acids that have lower soil pH level [
17]. Rice husk powder, rich in carbohydrates, can be metabolized by microorganisms under specific conditions, converting them into organic acid [
18]. Mushroom bran, abundant in organic matter and acidic substances such as lactic acid and acetic acid, can reduce the soil pH value after decomposition [
19]. Previous studies have concluded that mushroom bran significantly enhances the plant growth and dry matter accumulation and production [
20,
21]. However, long-term application of mushroom bran may lead to nutrient deficiency in the soil. At present, research comparing different soil improvement methods is lacking, and the optimal method for blueberry growth has not been identified.
Bacterial communities play an important role in soil health and plant nutrient acquisition [
22].The structure and biodiversity of bacteria populations can largely reflect the status and changes of soil fertility [
23]. Particularly, the interaction between microorganisms and blueberries is vital for the growth of blueberry plants, especially in rhizosphere soil where microbial diversity is higher compared to other soil regions [
24]. On one hand, microorganisms can promote the growth of blueberries by improving soil structure, soil fertility and promoting plant growth; On the other hand, blueberries also provide a habitat and nutrients for microorganisms, establishing a mutually beneficial and symbiotic relationship [
5,
24,
25]. Furthermore, endophytes exist in plants that coexist with host plants without causing harm. These endophytes can enhance mineral absorption and provide metabolites, such as hormones necessary for plant growth, thus improving the stress resistance of plants [
26,
27]. Previous study have shown that Proteobacteria, Acidobacteria, and Bacteroidetes are the dominant bacterial phyla in blueberry soil, and the soil organic matter affects the composition of soil microbial communities and promotes the production of amino acid metabolites in blueberry soil [
28]. In recent years, extensive research has been conducted on the plant growth-promoting rhizobacteria (PGPR), a diverse group of bacteria found in soil, renowned for their multifaceted roles in enhancing plant growth, improving nutrient uptake, and conferring resistance to various biotic and abiotic stresses [
29,
30]. A lot of studies have concluded that PGPR influence various plant hormones such as 3-acetic acid (IAA), gibberellins, cytokinins and abscisic acid [
31,
32]. In addition, several bacteria involved in N cycle, including
Magnetospirillum,
Herbaspirillum,
Burkholderia,
Azospira,
Rhodopseudomonas,
Bradyrhizobium Pseudomonas,
Azospirillum,
Klebsiella,
Pantoea,
Rhizobium,
Sphingomonas and
Ottowia, have been identified [
33,
34,
35,
36]. Therefore, it is of great significance to study and understand the influence mechanism of microorganisms on blueberry growth.
However, there are few reports on the changes of blueberry plant rhizosphere soil community environment after soil improvement, especially the effect of changes in rhizosphere and endophytic bacterial community diversity and metabolism function. Hence, we analyzed the response of microbial community composition, microbial function and N cycle (including the functional bacteria and N cycle at the module level) using high throughput sequencing. We aimed to (ⅰ) explore the effects of different soil improvement methods on bacterial community composition, structure and function. (ⅱ) To explore the response of N cycle to different soil improvement methods. (ⅲ) To investigate the best soil improvement at the microbial perspective.
2. Materials and Methods
2.1. Experimental design
Samples were collected from the soil and water conservation experimental field of Jilin Agricultural University in 2022, located in Changchun City, Jilin Province (E 125°24′55″, N 43°48′34″). The average annual temperature is 4.8 ℃, with an accumulated temperature of 2880℃ ≥ 10℃, the frost-free period is 140-155 days, and the annual average rainfall is 579mm. The majority rainfall occurs from June to September each year, accounting for about 70% of the annual precipitation. The soil type of the experimental plot is black soil. The cultivation object is 2-year-old semi-tall cluster of "Hokuriku" blueberries. Four treatments were implemented: peat combined with sulfur (T1), peat combined with acidified rice husk (T2), mushroom ban(T3), peat combined with mushroom ban (T4). Each process was repeated three times. The row spacing was 1.5 m x 2.0 m, and the planting holes measured 35cm x 35cm x 40cm. The methods adopted for the measurements of soil properties, i.e., soil pH, total N (TN), available phosphorus (AP) and available potassium (AK) were described in Li et al [
37]. The soil properties are listed in
Table A1.
2.2. Determination method
Total genomic DNA was isolated from 0.25g of rhizosphere soil and 5g of root samples using PowerSoil® DNA Isolation kit (allwegene Technology Company, Beijing, China) according to the manufacturer's instructions. The bacterial community was analyzed using 16S rDNA amplicon sequencing. The V3-V4 region of the 16S rDNA gene was targeted for sequencing. Overlap paired reads from paired-end sequencing were merged into a single sequence using Pandaseq Software. Sequences with low quality (contain ambiguous base ‘N’> 3, and average base quality score < 20) were filtered out. Finally, the length of the reads was ranged from 220 to 500 bp.
2.3. Data analysis
We used analysis of variance (ANOVA) to analyze the differences among means, followed by least significant different (LSD) test if the difference was significant. Spearman's test method was used to perform correlation network analysis on the top 20 genera based on absolute abundance of all samples. A p-value greater than 0.05 or a correlation value less than 0.6 were excluded from the analysis. The similarities and differences in bacterial community composition between berry rhizosphere and root among the four soil improvement methods were described using a tree branch structure. PICUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) software was used to predict the function of 16s high-throughput sequencing data, and information from the KEGG database was compared to obtain N cycle at the module level.
3. Results
3.1. Bacterial community diversity and composition
A total of 78225 and 8767 operational taxonomic units (OTUs) were obtained from the berry rhizosphere soil and roots after 16s rDNA high throughput sequencing. Regardless of whether it was in the berry rhizosphere soil or roots, the highest number of OTUs was found in the T4 treatment, followed by the T2 treatment. In addition, we analyzed the alpha diversity of the soil bacteria community. The results revealed that the Chao 1 index and PD whole tree were highest in the T4 treatment, followed by the T2 treatment. These findings were consistent with the results obtained from the analysis of the root samples. On the other hand, the Shannon and Simpson indices were lowest in the T1 treatment in the blueberry rhizosphere soil, but there is no significantly difference observed in the blueberry root samples (
Table 1).
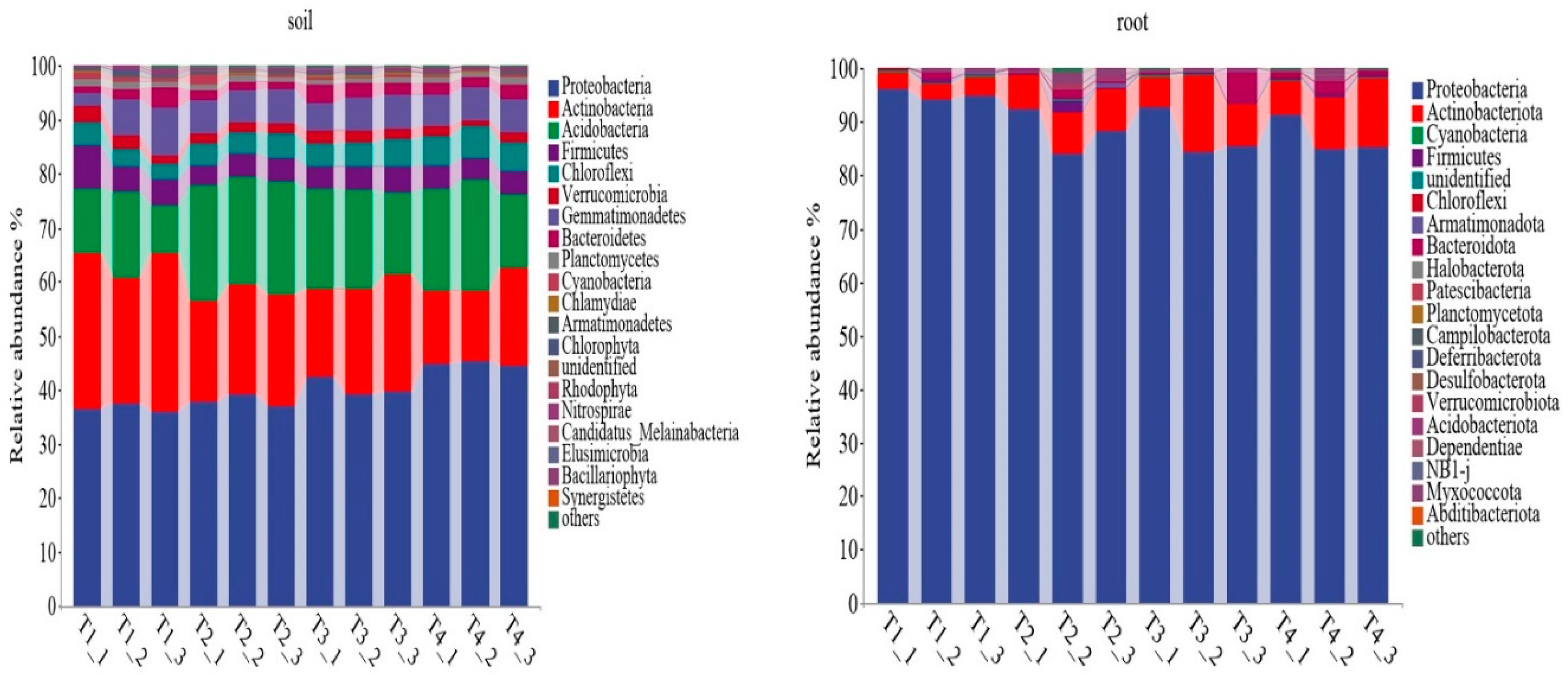
In terms of phyla, a total of 36 were identified in the berry rhizosphere soil. The dominant phylum was Proteobacteria (36.02-45.38%), followed by Actinobacteria (13.01-29.42%) and Acidobacteria (11.84-21.29%). Notably, significant changes were observed in the abundance of Proteobacteria, Actinobacteria, Acidobacteria, Chloroflexi and Nitrospirae under the four soil improvement methods. The relative abundance of Proteobacteria and Chloroflexi was higher in the T4 treatment compared to the other treatment, while the lowest abundance of Actinobacteria was found in the T4 treatment. Additionally, the T1 treatment increased the relative abundance of Actinobacteria, but decreased Acidobacteria and Nitrospirae. Furthermore, the T2 treatment resulted in an increase in the abundance of Acidobacteria and Elusimicrobia. In terms of blueberry root sample, a total of 29 phyla were identified. The dominant phylum was Proteobacteria, with the highest relative abundance observed in the T1 treatment (95.06%). This was followed by Actinobacteriota (2.96-14.04%), which showed highest relative abundance in the T1 treatment. No significant changes were observed in the relative abundance of other phyla under the four treatments (
Figure 1).
The dominant genus in the blueberry rhizosphere soil varied under the four soil improvement methods. The highest relative abundance of
Gaiella (7.21%),
Acidobacterium (6.67%) was observed in the T1 and T2 treatments, respectively.
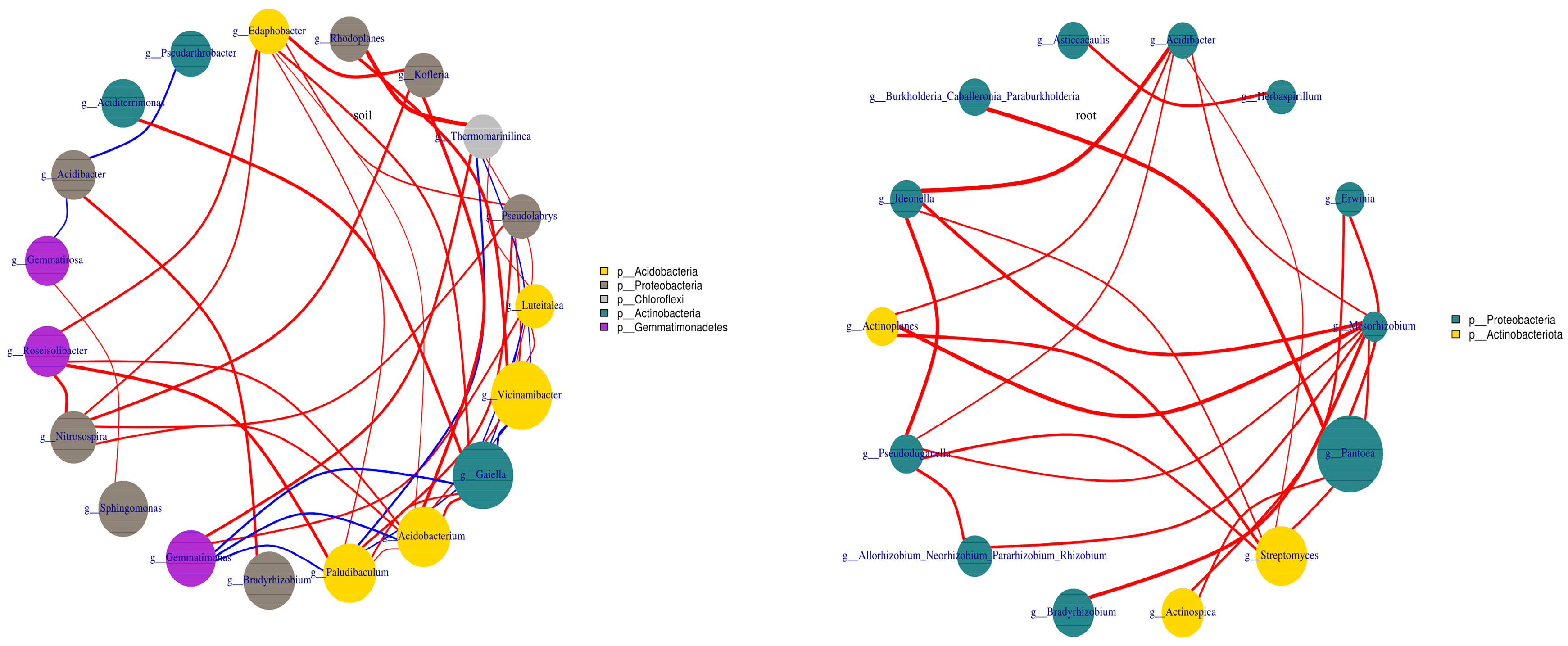
Vicinamibacter was the dominant genus in the T3 (7.11%) and T4 (9.70%) treatments. The correlation network analysis revealed that
Acidobacterium and
Paludibaculum both belong to Acidobacteria, had the strongest correlation with nine other bacteria at the genus level. This was followed by
Edaphobacter and
Gaiella, which exhibited strong correlations with eight and seven genera, respectively (
Figure 2). In blueberry roots, significant changes were observed in the relative abundance of genera under the four improvement methods. The dominant genus in the T1 treatment was
Pantoea, with a relative abundance of 37.17%.
Pseudomonas was the dominant genus in the T2, T3 and T4 treatments, with highest relative abundance found in the T4 treatment (55.27%). The correlation network analysis revealed that
Mycobacterium, belong to Proteobacteria had a significant correlation with nine other genera. In addition,
Pantoea,
Pseudomonas,
Streptomyces and
Acidibacter exhibited significant correlations with five genera (
Figure 2).
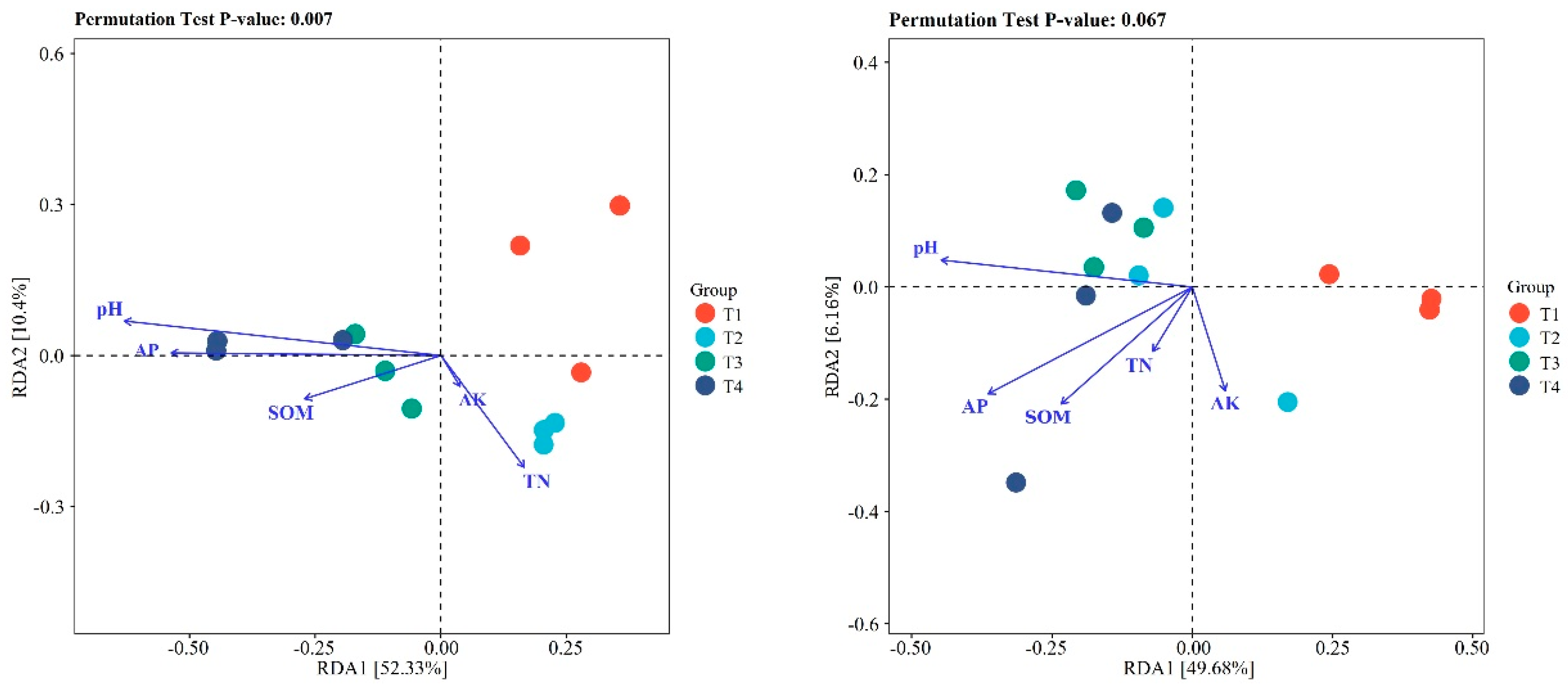
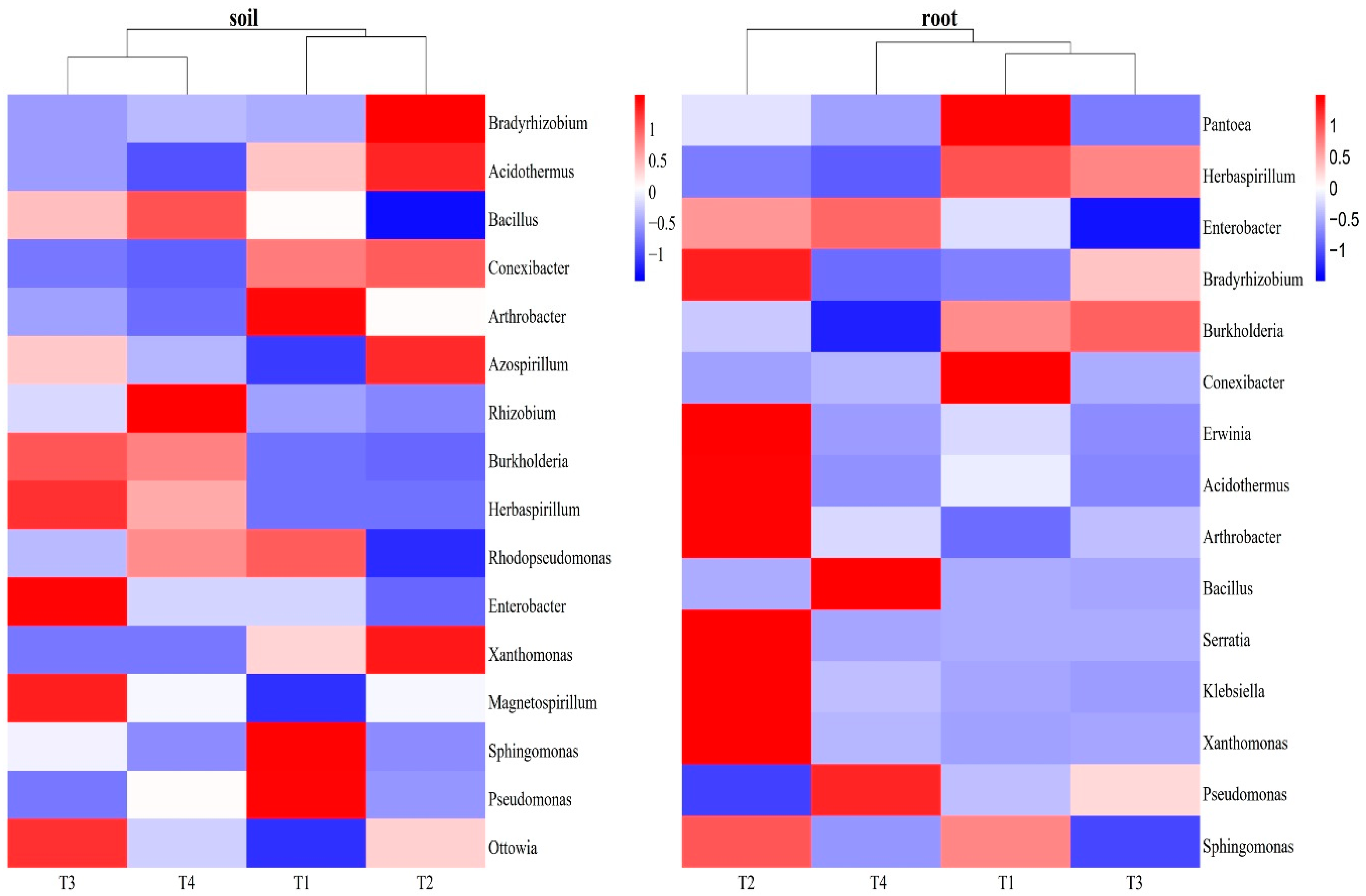
Furthermore, we analyzed the correlation between soil physical-chemical properties and the top 20 genera. The results indicated that soil physical-chemical properties were significant correlated with the bacterial community in blueberry rhizosphere soil (p=0.007), but only showed a weak correlation with the bacterial community in blueberry root samples (p=0.067). Therein, pH had the highest influence on the soil bacterial community, whereas soil organic matter (SOM) had the highest influence on the root bacterial community (
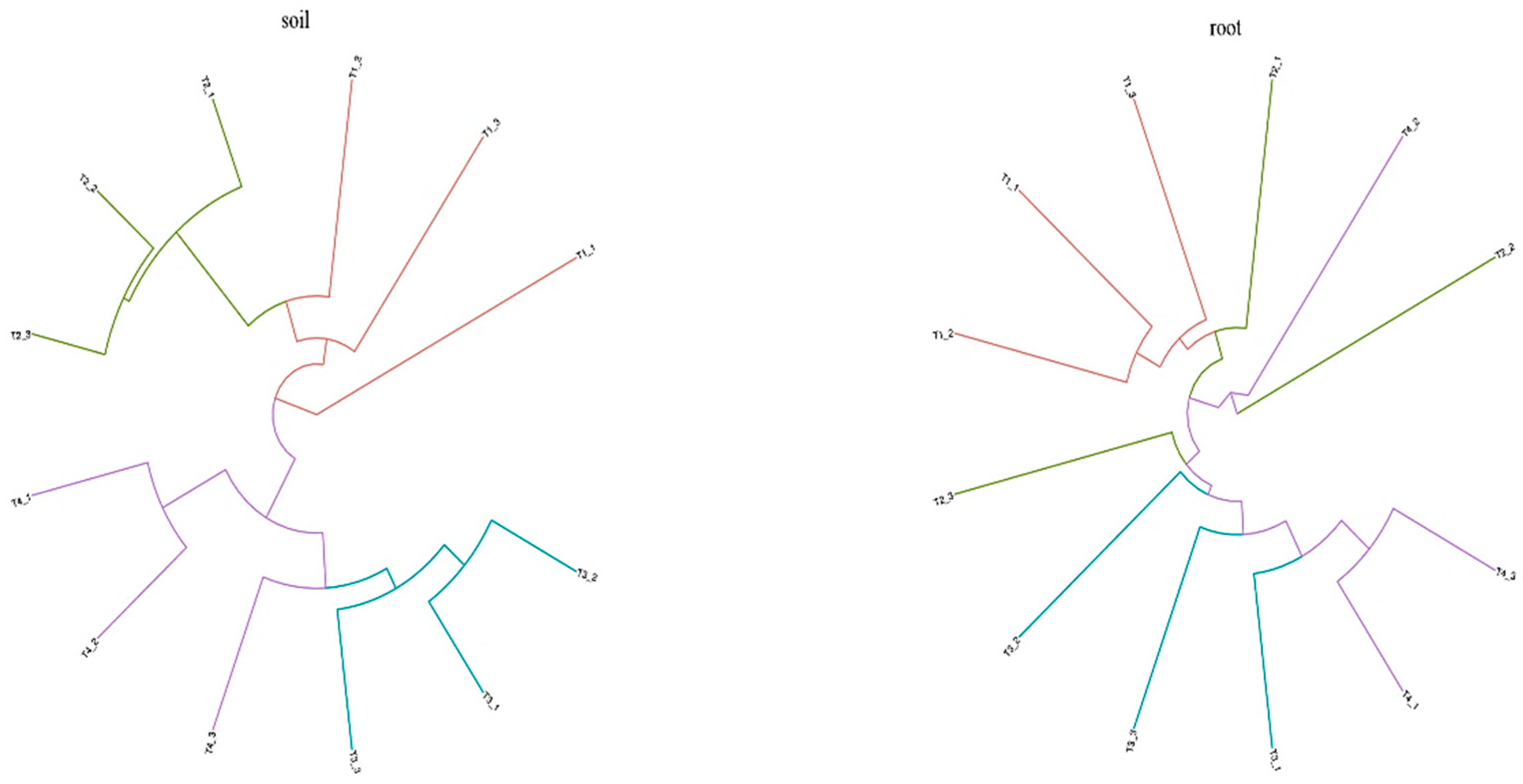
Figure A1). Cluster analysis showed that the improved blueberry soils could be classified into two categories, T1 and T2 were grouped together, whereas T3 and T4 were grouped together. However, the classification characteristics of microorganisms in blueberry roots were not distinct, and only the T1 and T3 treatments are divided into different categories. The differences within the other treatments were greater than the differences observed between groups, which further supports the findings shown in
Figure 1. Interestingly, the four soil improvement methods had no significant effects on the endophytic microbial community in blueberry roots (
Figure 3).
3.2. Impact of different soil improvement methods on microbial function in the rhizosphere and root of blueberry
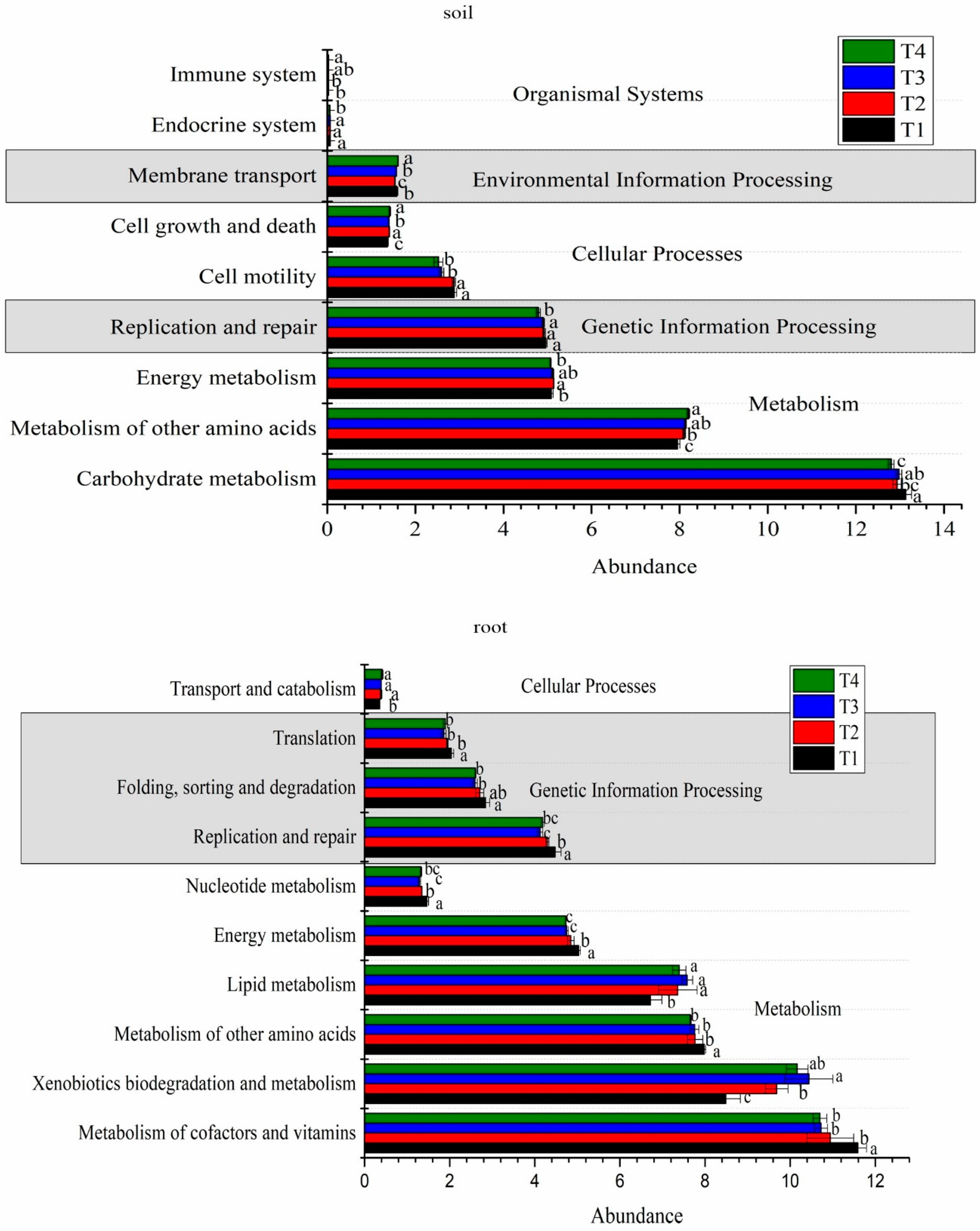
In the paper, we analyzed the effects different soil improvement methods on the microbial function in the blueberry rhizosphere soil and roots. The results showed that carbohydrate metabolism and amino acid metabolism were the primary pathways involved in the metabolism processes in the blueberry rhizosphere soil. Interesting, the carbohydrate metabolism significant varied among different treatments, with the highest abundance observed in T1 treatment and the lowest in the T4 treatment. The opposite trend was observed for the metabolism of the other amino acids energy metabolism, which showed the highest abundance in the T4 treatment. Furthermore, the T2 treatment showed significant improvement in energy metabolism.
Regarding genetic information processing, the highest relative abundance pathway was replication and repair, accounting for 4.79-4.92% of the total. This pathway displayed the lowest abundance in the T4 treatment. In terms of cellular processes, cell motility was the dominant pathway, with higher abundance observed in the T1 and T2 treatments compared to the T3 and T4 treatments. Conversely, the relative abundance of cell growth and death pathway was lowest in the T1 treatment. As for environmental information processes, the membrane transport pathway exhibited the highest relative abundance, which increased under the T4 treatment. In addition, we also analyzed the organismal systems, the dominant pathway was environmental adaptation, accounting for 0.16%. Moreover, the T4 treatment increased the abundance of the immune system while decreasing the endocrine system (
Figure 4, Tabel A2).
Similar to the rhizosphere soil, the dominant pathways in the blueberry roots were also related to carbohydrate metabolism and amino acid metabolism, with no significant changes observed among the different treatments. However, some specific pathways showed higher activity levels in the T1 treatment compared to the other treatments. These pathways included metabolism of cofactors and vitamins, metabolism of other amino acids, energy metabolism and nucleotide metabolism in metabolism processes and the replication and repair, folding, sorting and degradation and translation pathways in genetic information processing. In addition, the T1 treatment significantly decreased the abundance of transport and catabolism and xenobiotics biodegradation and metabolism pathways (
Figure 4, Tabel A2).
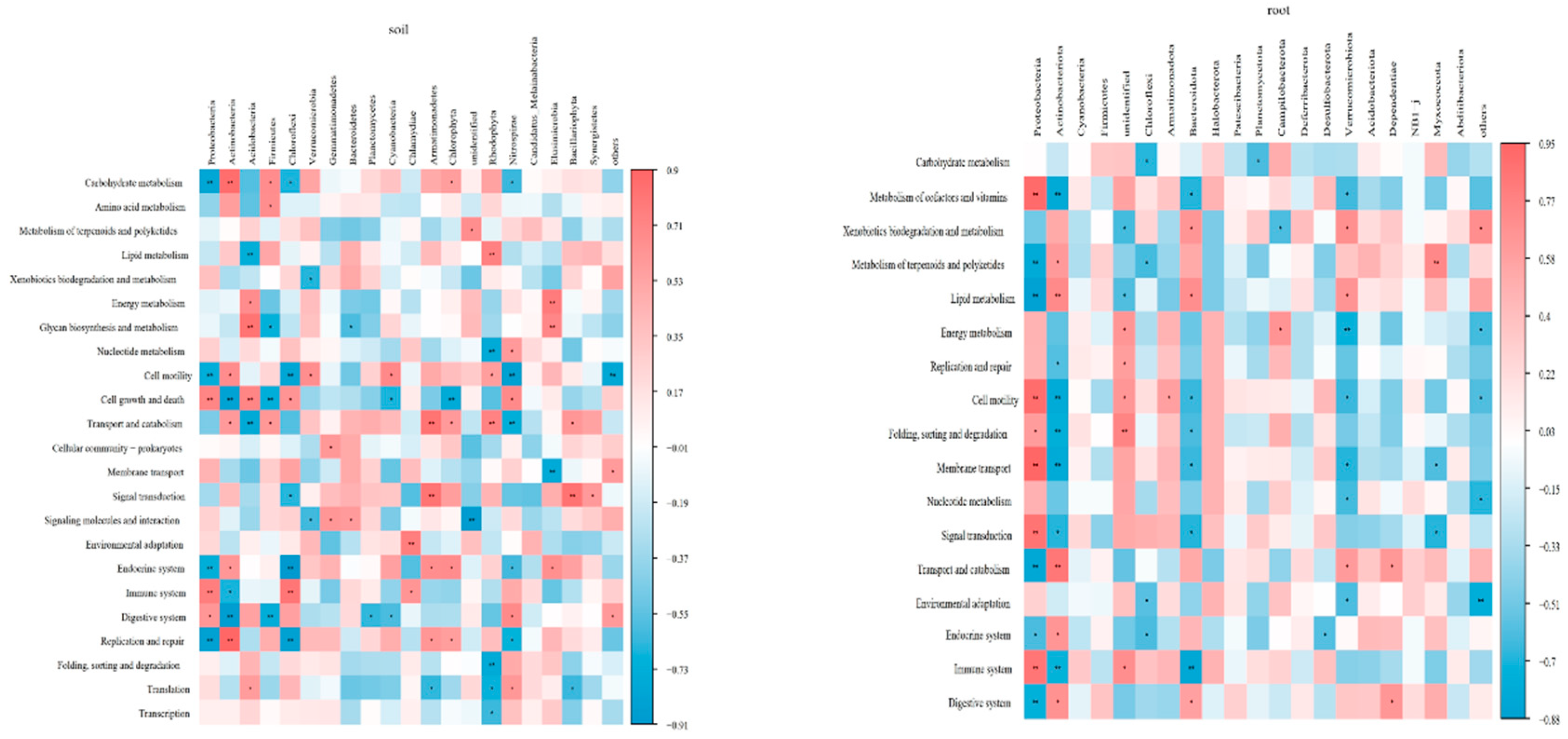
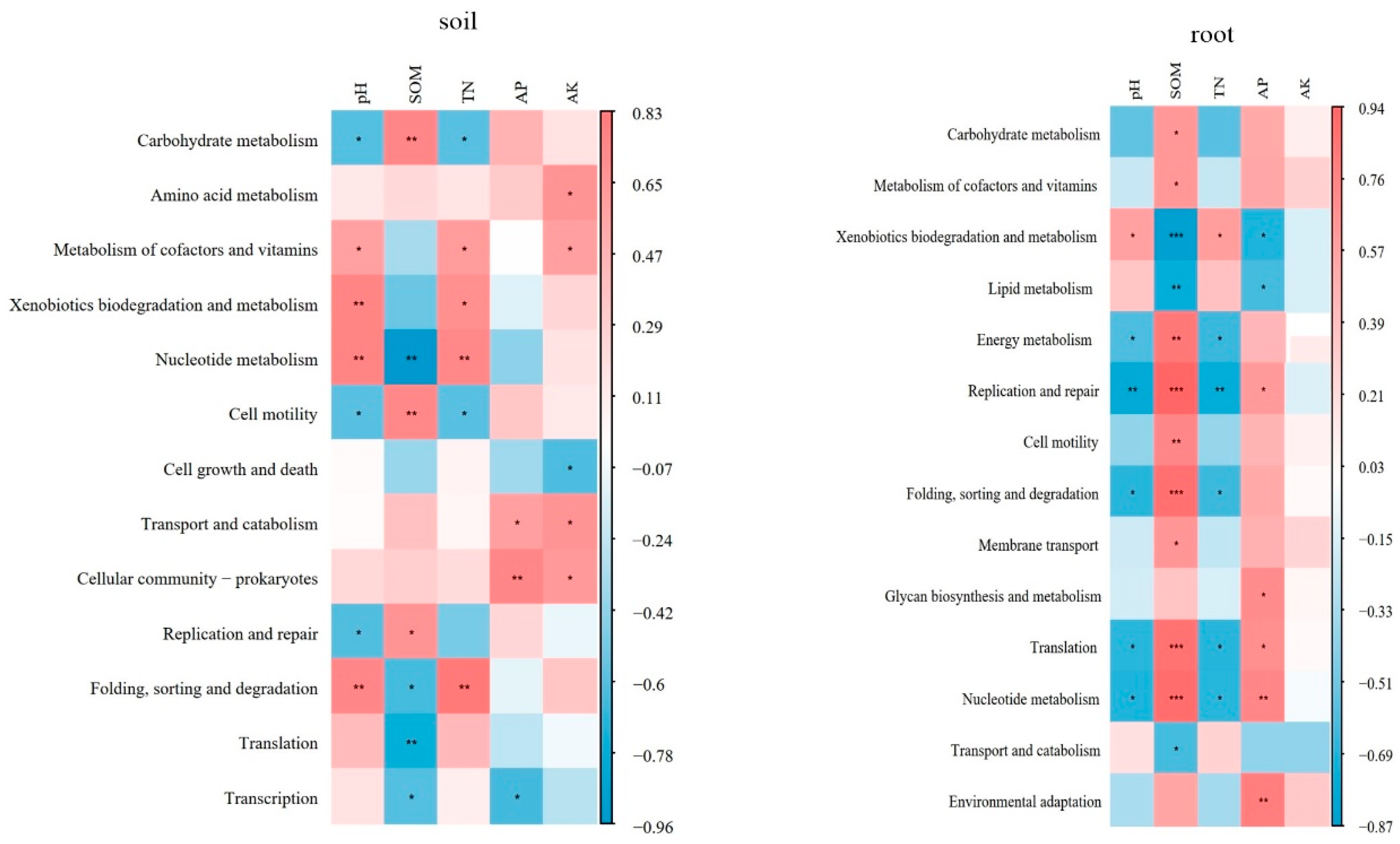
Interestingly, the metabolism pathways were found to be significantly correlated with the soil physical-chemical properties. In the blueberry rhizosphere soil, soil pH and total nitrogen (TN) showed a significant negative correlation with carbohydrate metabolism and cell motility, while exhibiting a positive correlation with metabolism of cofactors and vitamins, xenobiotics biodegradation and metabolism, nucleotide metabolism and folding, sorting and degradation (p<0.05). SOM showed a positive correlation with carbohydrate metabolism, cell motility and replication and repair, negative correlation with nucleotide metabolism, folding, sorting and degradation, translation and transcription (p<0.05). In addition, available phosphorus (AP) and available potassium (AK) exhibited strong correlations with transport and catabolism and cellular community-prokaryotes (p<0.05). In blueberry roots, xenobiotics biodegradation and metabolism, energy metabolism, replication and repair, folding, sorting and degradation, translation and nucleotide metabolism displayed significant negative correlations with soil pH and TN (p<0.05), while showing a positive correlation with SOM (p<0.01). AP showed a negative correlation with xenobiotics biodegradation and metabolism and lipid metabolism, but had a positive correlation with replication and repair, glycan biosynthesis and metabolism, translation, nucleotide metabolism and environmental adaptation (p<0.05) (
Figure 5).
3.3. Functional bacteria and modules involved in the N cycle
3.3.1. Influences of soil improvement on functional bacteria in the blueberry rhizosphere soil and roots
There was a total of 13 bacteria participated in N fixation pathway in the blueberry rhizosphere soil. The dominant genus among these bacteria was
Bradyrhizobium, accounting for 2.43-3.33%, followed by
Acidothermus (0.58-1.29%). These two genera showed highest abundance under the T2 treatment.
Bacillus (0.24-1.07%) and
Rhizobium (0.03-0.40%) exhibited the highest relative abundance in the T4 treatment and the lowest in T2 treatment. The T2 treatment also increased the abundance of
Azospirillum. In blueberry root, we also obtained 13 bacteria related to N fixation. the dominant genus among these bacteria was
Pantoea (2.17-37.17%), which reached its highest abundance in the T1 treatment.
Herbaspirillum and
Bradyrhizobium also showed significant differences among the four treatments. Interesting, both
Sphingomonas and
Pseudomonas participated in nitrification and denitrification, were founded both in rhizosphere soil and root. However,
Sphingomonas (1.74-4.52%) and
Pseudomonas (28.58-55.27%) were the dominant genera in the rhizosphere soil and roots, respectively (
Table 2). Correlation analysis revealed that soil pH and TN displayed significant positive relations with
Burkholderia,
Bacillus,
Rhizobium and
Herbaspirillum in the blueberry rhizosphere soil, as well as
Pseudomonas in the root. Moreover, pH and TN exhibited negative associations with
Pantoea and
Sphingomonas in the root. In addition, SOM showed an opposite effect compared to pH and TN. It demonstrated a significant negative influence on
Burkholderia,
Rhizobium,
Herbaspirillum,
Magnetospirillum and
Ottowia in the blueberry rhizosphere soil, but a positive effect on
Pantoea and
Sphingomonas in the root. Furthermore, AP displayed a positive correlation with
Pseudomonas in the soil and
Pantoea in the root, while having a negative correlation with
Azospirillum and
Ottowia in the rhizosphere soil. (
Figure 6,
Table A3).
3.3.2. The effect of soil improvement on N cycle at the module level in the blueberry rhizosphere soil and roots
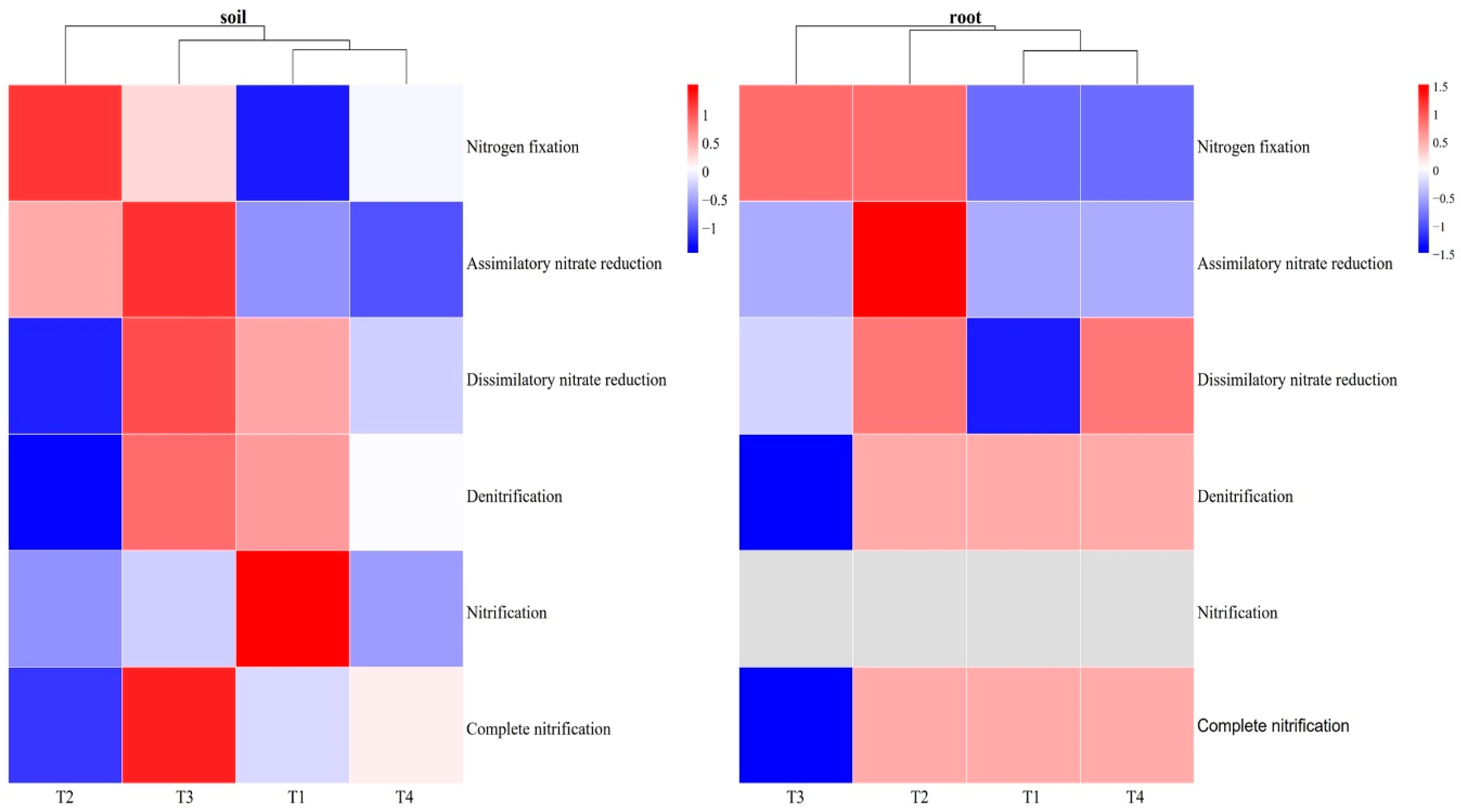
In order to further analyze the influence of different improvement methods on the N cycle, we analyzed the N metabolism at module level. This includes processes such as N fixation, degradation, nitrification and denitrification, assimilatory nitrate reduction (ANRA) and dissimilatory nitrate reduction (DNRA) and complete nitrification. In blueberry rhizosphere soil, the dominant metabolism pathway in the N cycle was DNRA (0.27%), followed by denitrification (0.12%) and ANRA (0.11%). The T2 treatment had a significant impact on the N cycle compared to other treatments, enhancing microbial N fixation while decreasing the microbial DNRA, denitrification, and complete nitrification. The abundance of ANRA, DNRA, denitrification and complete nitrification was highest in the T3 treatment. The T1 treatment increased the nitrification but decreased the N fixation, while the T4 treatment has the lowest abundance of ANRA. In blueberry roots, DNRA was also the dominant pathway in the N cycle (0.37%), with higher abundance in the T2 and T4 treatment and lower abundance in the T1 treatment. Denitrification (0.11%) and ANRA (0.09%) were the next most abundance pathways, with the highest relative abundance in the T2 treatment. N fixation was more abundant in the T2 and T3 treatment than in T1 and T4 treatment (
Figure 7).
The nitrogen metabolism pathways in the blueberry rhizosphere were significantly related to soil properties. DNRA showed a significant positive correlation with pH, TN and AK (p<0.05). AK also had a significant influence on nitrification and denitrification, with denitrification strongly correlated with soil pH and TN (p<0.05). In addition, complete nitrification showed a signification correlation with pH, SOM, TN and AK (p<0.05). The correlation between N metabolism pathway in blueberry roots and soil properties was weaker than that observed in rhizosphere soil. However, there were still significant correlation between DNRA and AK, as well as complete nitrification and SOM (p<0.05). N fixation showed a strong correlation with AP (p<0.01) (
Table 2).
4. Discussion
4.1. Differences of microbial community diversity and structure in blueberry rhizosphere and root under different soil improvement methods
Blueberry plants thrive in moist, acidic and high soil organic matter soil with good aeration and drainage [
38]. However, most soils in China do not meet these conditions. Therefore, applying peat, mushroom bran, rice husk, and sulfur to the soil can adjust soil pH, increase organic matter and improve soil permeability. These applications promote the growth and development of blueberries and increase fruit yield and quality. In this paper, peat and acidified rice husk resulted in the lowest soil pH value (5.53), followed by peat and sulfur treatment (5.76) (
Table A1). Previous studies have shown that blueberry prefers soil conditions with low pH (4.4-5.5), and soil pH impact on plant growth and development including photosynthetic characteristics, root development, and fruit quality, through affecting blueberry plant N absorption and utilization efficiency, and amino acid synthesis [
8,
39]. Therefore, the T2 treatment, which resulted in the best pH value, is the most effective. Pervious study had shown that the suitable soil organic matter content for blueberry growth is 8-12%. And all treatments, except T3, had organic matter content higher than 85g/kg (
Table A1), which is suitable for blueberry growth [
40]. Peat has an organic matter content higher than 30%, so its application effectively increases organic matter content. In this experiment, T1 treatment had significantly higher SOM content than other treatments (93.5 g/kg), due to the application of a larger amount of peat. Different soil improvement methods have significant effects on soil pH and nutrient content, so these changes in physical and chemical properties are bound to have a certain impact on soil microorganisms.
Soil microbes play a crucial role in the soil ecosystem, influencing plant life forms, community functioning and nutrient cycling [
41]. In this paper, the quantity and diversity of soil microbes were observed to be highest in the T4 treatment and lowest in T1 (
Table 1). This could be attributed to the significant interaction between the diversity and composition of rhizosphere bacteria communities and soil pH and SOM [
42]. The physical and chemical properties in the T4 treatment favorable conditions for most microbial activities. Whereas the T1 treatment had the highest SOM, but the pH was not suitable for microorganisms that decompose organic matter [
43]. Endophytic bacteria are closely with plants, and reside in living plant tissue without causing symptoms of disease [
44]. In this paper, the number of endophytic bacterial community was highest in the T4 treatment, and lowest in T1 treatment. Pervious study has confirmed that most endophytic bacteria migrate from the rhizosphere soil [
45], and the highest number of OTUs and diversity in blueberry rhizosphere soil were observed in the T4 treatment, while the lowest were observed in the T1 treatment. There were no significant differences in the Shannon and Simpson indices among different treatments in blueberry roots. It might because that the diversity of endophytic bacteria communities was also influenced by plant genotype [
46].
Proteobacteria, Actinobacteria, and Acidobacteria were identified as dominant phyla in the soil microbial community (
Figure 1), consistent with major trends observed in dominant bacteria in forest ecosystems [
47,
48]. The relative abundance of Proteobacteria, Actinobacteria, Acidobacteria was highest in the T4, T1 and T2 treatments, respectively. It might because that different improvement soil methods changed the soil properties, indirectly influenced the soil microbial community (
Figure A1). It was similarly in blueberry root endophyte bacterial community, Proteobacteria and Actinobacteria were also the dominant phyla. And the highest relative abundance of Proteobacteria and Actinobacteria was observed in the T1 and T4 treatments. This confirms that the endophytic microorganisms migrate from the soil again. The community composition of bacterial community at the genus level differed among different soil improvement methods in blueberry rhizosphere soil.
Mycobacterium, a well-known PGPR, which produces indole-3-acetic acid (IAA) to promote plant growth and seed germination exhibited the highest relative abundance in the T4 treatment.
Gaiella and
Acidobacterium were the dominant genera in the T1 and T2 treatments, respectively, while
Vicinamibacter dominated in the T3 and T4 treatments (
Figure 2). These observations might because soil pH had significant influence on the bacterial community blueberry rhizosphere soil (
Figure A1). In addition, the network analysis resulted that
Acidobacteria was the main factor, and the cluster analysis showed that T1 and T2 treatment were clustered into one category, T3 and T4 treatment were clustered into the other category. These results showed that pH was the main factor affecting the microbial community in blueberry rhizosphere soil.
4.2. The function of microbial community in blueberry rhizosphere soil and root
Functional prediction indicated that bacterial metabolism in blueberry rhizosphere was found to primarily involve carbohydrate metabolism, amino acid metabolism, replication and repair, cell motility, membrane transport pathway and environmental adaptation (
Table A2), these findings were consistent with previous studies [
38,
47]. In these paper, different soil improvement methods had varying effects on microbial metabolism. For example, carbohydrate metabolism significantly differed among the treatments, with the highest found in the T1 treatment and the lowest in the T4 treatment. Carbohydrate metabolism was associated with Actinobacteria, Firmicutes, and Chlorophyta, with the highest abundance in the T1 treatment and the lowest in the T4 treatment. In addition, carbohydrate metabolism also had significant negative correlation with soil pH and TN, and positive correlation with SOM (
Figure 4,
Figure A2). And the T2 treatment increased the abundance of energy metabolism, it was because that the T2 treatment promoted the abundance of Acidobacteria and Elusimicrobia, which were associated with energy metabolism (
Figure A2). The function of blueberry rhizosphere microbial was significantly correlated with soil properties, which had been reported in previous study [
6].
In blueberry roots, the dominant pathways were consistent with those observed in the rhizosphere soil, and these pathways did not show significant changes among the different treatments (
Table A2). The result confirmed that different soil improvement methods did not alter the dominant function of endophytic microbial. However, most pathways were influenced by the T1 treatment due to the significant correlation between endophytic microbial function and SOM, which could provide carbon source for microbial development [
49,
50]. The SOM content in the T1 treatment was significantly higher than that in other treatments. In addition, Proteobacteria and Actinobacteria were involved in most pathways, and their relative abundance differed from those observed in other treatments (
Figure 1,
Figure A2).
4.3. Effects of Different Soil Improvements on N Cycle
N is crucial for plant growth and development, influencing processes such as plant protein synthesis, cell division, and photosynthesis [
51,
52,
53]. Previous studies have confirmed that the PGPR can benefit saffron growers by enhancing corm growth, increasing stigma and biomass yield, as well as elevating the levels of secondary metabolites [
29,
54]. In this paper, we investigated the effects of different soil improvement methods on N fixation functional bacteria. And the T2 treatment had a notably positive influence on the majority of N fixation function bacteria both in the blueberry rhizosphere soil and roots. In the blueberry rhizosphere soil,
Bradyrhizobium was found to be the dominant N fixation bacteria, and its abundance was higher in the T2 treatment compared to other treatments. Additionally, the T2 treatment promoted the relative abundance of
Conexibacter and
Acidothermus, which had been reported to contribute to the host's health and stress resistance through sulfate shuttle and cellulolytic activity, in addition to their ability to fix N [
47]. Moreover, the T2 treatment was also found to facilitate N fixation through Xanthomonas in both the blueberry rhizosphere soil and roots. In blueberry roots, the T2 treatment exhibited the highest relative abundance of N fixation functional bacteria including
Bradyrhizobium,
Arthrobacter,
Erwinia,
Acidothermus,
Serratia and
Klebsiella (
Figure 6). These bacteria also positively influenced root development and nutrient availability through IAA and siderophore production, which promoted root hair development and enhanced plant stress resistance [
55,
56]. Moreover, these bacteria played a role in mobilizing inorganic phosphorus in the soil [
57,
58,
59]. The T2 treatment stood out from the other treatments as it also promoted the growth of PGPR, mainly because that the addition of rice husk into blueberry soil. Previous study has confirmed that rice husk is rich in bioactive compounds such as phenolic acids and flavonoids, which have various biological effects and can benefit plant growth [
60]. Furthermore, the addition of rice husks improves soil aeration and promotes microbial growth and development [
61,
62,
63]. In this paper, compared to other treatments, the T2 treatment promoted N fixation functional microbial community in both the blueberry rhizosphere soil and root endophytes. This suggests that the acidified application of rice husks is more conducive to promoting microbial activity during blueberry growth and development, while also enhancing microbial N fixation ability. Besides, the acidified application of rice husks influenced the PGPR, while the addition of mushroom chaff affected it differently. The T3 treatment increased the relative abundance of
Burholderia and
Herbaspirillum, which were lowest in the T2 treatment in both blueberry rhizosphere soil and root. That might because that
Burholderia and
Herbaspirillum were the diazotrophic bacteria [
64], and the TN content was highest in the T3 treatment. In blueberry roots,
Pantoea was the dominate N fixation functional bacteria, and its relative abundance was highest in the T1 treatment, which had the highest SOM content. Pervious study has concluded that high organic matter can promote the growth of microorganisms [
65,
66].
Pantoea was the main dominant endophytic bacteria, and had significant positive correlation with soil SOM, negative correlation with pH and TN (
Table A3).
At the module level, similar results were obtained, with the relative abundance of the fixation N module being highest in the T2 treatment in the blueberry rhizosphere soil. Additionally, the T2 treatment led to a decrease in the microbial DNRA, denitrification, and complete nitrification (
Figure 7). This suggests that the addition of acidified rice husk can increase the content of nitrogen in soil and reduce nitrate leaching loss and N
2O emission. These findings are consistent with previous studies [
67,
68,
69]. In contrast, the single application of mushroom bran promoted ANRA, DNRA, denitrification and complete nitrification. These metabolic processes were significantly positive correlated with soil pH and AK, which were highest in the T3 treatment. Furthermore, the T1 treatment promoted the nitrification, and significantly increased nitrate content. This could be attributed to the presence of
Sphingomonas and
Pseudonocardia, which are involved in nitrification and exhibited the highest relative abundance in the T1 treatment. In blueberry roots, the T2 treatment had a greater impact on the N cycle compared with other treatments, including N fixation, ANRA, DNRA, denitrification and completed nitrification. It indicates that the addition of acidified rice husk can promote N metabolism in blueberry root endophytic microorganism. In addition, the T3 treatment exerted different effect on the N cycle in rhizosphere compared to the root. In the blueberry root, the T3 treatment decreased the relative abundance of denitrification and complete nitrification. Although both rice husk and mushroom bran could increase microbial activity, they had different effects on the N cycle. These two soil improvement methods could induce changes in soil properties and plant secondary metabolism. Therefore, we conclude that the addition of acidified rice husk can promote microbial N fixation and reduce the greenhouse emission in the blueberry rhizosphere soil. In blueberry roots, the addition of acidified rice husk enhances N metabolism.
5. Conclusions
In this paper, the application of different substances was found to have varying effects on the number and diversity of microorganisms in the blueberry rhizosphere soil and root. Specifically, the T4 treatment resulted in the highest number and diversity of microorganisms, followed by the T2 treatment. On the other hand, the T1 treatment had the lowest impact on the microbial populations. Although the dominant phyla remained unchanged across all treatment, their relative abundance were altered. In the rhizosphere soil, the dominant phyla were identified as Proteobacteria and Actinobacteria. It was observed that the relative abundance of Proteobacteria was highest in the T4 treatment, while Actinobacteria showed the highest abundance in the T1 treatment. Interestingly, this trend was reversed when examining the roots. The T2 treatment had a significant impact on Acidobacteria in the rhizosphere soil, where its relative abundance was higher compared to other treatments. This finding suggests that the T2 treatment could be beneficial for the growth of blueberry plants. Additionally, the correlation network analysis resulted that Acidobacterium and Paludibaculum, both belonging to the Acidobacteria phylum, had the highest influence on the microbial community structure in the rhizosphere soil. Again, the T2 treatment exhibited the highest influence in this regard. In the blueberry root, the dominant influential genera were Mycobacterium, and its highest relative abundance observed in the T4 treatment. Furthermore, the pH and SOM were found to have the highest influence on the bacterial communities in the rhizosphere soil and roots, respectively. The microbial community structure of blueberry rhizosphere soil was divided into two distinct categories: one class wasT1 and T2 treatments, and other was T3 and T4 treatments.
Different soil improvement methods have not changed the main functions of microbes, but have altered the relative abundance of most functions. In the rhizosphere soil, carbohydrate metabolism was highest in the T1 treatment, while it was lowest in the T4 treatment. The T2 treatment promoted the energy metabolism, cell motility and cell growth and death. In the blueberry root, the most changes were observed in the T1 treatment. The dominant N fixation functional bacteria were Bradyrhizobium and Acidothermus, with the highest relative abundance observed in the T2 treatment in the rhizosphere soil. Moreover, the T2 treatment also had the highest number and relative abundance of N fixation functional bacteria in the blueberry root. Additionally, The T2 treatment increased the relative abundance of N fixation module and decreased the ANRA, DNRA, denitrification and completed nitrification in blueberry rhizosphere soil. However, it promoted the ANRA in blueberry root. Overall, the addition of acidified rice husk could promote microbial N metabolism, especially N fixation. Although the T2 treatment did not exhibit the highest microbial diversity, it enhanced energy metabolism and increased microbial N fixation function. Based on the effects of four improvement methods on soil pH, it is recommended to use a combination of peat and acidified rice husk for the optimal improvement of blueberry soil. However, this paper only compared the effects of different soil improvement on the blueberry rhizosphere soil and root endophytic microbial communities. The effects on the above-ground plant changes and yield under these four improvement methods need further analysis.
Author Contributions
Conceptualization, Shuxia Liu and Lin Wu; methodology, Dongmei Wang, Qi Li and Chengyu Wang; software, Yanan Li, Dongmei Wang and Chengyu Wang; resources, Shuxia Liu and Lin Wu; data curation, Yanan Li; writing—original draft preparation, Yanan Li and Shuxia Liu; writing—review and editing, Yanan Li and Shuxia Liu; visualization, Shuxia Liu; supervision, Qi Li and Chengyu Wang; project administration, Shuxia Liu; funding acquisition, Shuxia Liu and Lin Wu. All authors have read and agreed to the published version of the manuscript.
Funding
This work is a contribution to Natural Science Foundation of Jilin Province (20210101100JC), Changchun Science and Technology Bureau Project (21ZGN10), Jilin province science and technology development plan project (20200402083NC) and Jingyu County Science and Technology Development Plan Project (XBJ202208).
Data Availability Statement
The data presented in this study are available in the articles.
Acknowledgments
We thanks to Jialu Sun for soil collecting and sample pretreatment.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Table A1.
Soil properties under different improvement methods.
Table A1.
Soil properties under different improvement methods.
| pH |
SOM g/kg |
TN g/kg |
AP g/kg |
AK g/kg |
| 5.73±0.01c |
93.5±3.80a |
3.99±0.11bc |
33.60±4.90a |
152.53±3.00a |
| 5.53±0.01d |
87.73±1.33b |
3.89±0.05c |
23.33±2.02b |
118.80±6.45c |
| 6.15±0.00a |
78.70±0.60c |
4.32±0.01a |
23.07±3.05b |
154.27±6.72a |
| 6.13±0.00b |
86.12±0.13b |
4.12±0.11b |
24.20±2.10b |
139.00±3.00b |
Table A2.
The top metabolism pathway in blueberry rhizosphere soil and root.
Table A2.
The top metabolism pathway in blueberry rhizosphere soil and root.
| |
soil |
root |
| |
T1 |
T2 |
T3 |
T4 |
T1 |
T2 |
T3 |
T4 |
| Carbohydrate metabolism |
13.13±0.13a |
12.93±0.10bc |
12.98±0.06ab |
12.80±0.06c |
13.22±0.36a |
13.11±0.27a |
12.71±0.27a |
12.81±0.24a |
| Amino acid metabolism |
13.01±0.14a |
12.90±0.09a |
12.96±0.08a |
12.92±0.02a |
13.31±0.39a |
13.53±0.33a |
13.88±0.33a |
13.19±0.24a |
| Replication and repair |
4.96±0.03a |
4.92±0.03a |
4.91±0.03a |
4.79±0.04b |
4.48±0.04a |
4.29±0.06a |
4.12±0.06a |
4.46±0.04a |
| Cell motility |
2.88±0.06a |
2.87±0.04a |
2.59±0.05b |
2.52±0.10b |
3.99±0.46a |
3.59±0.15a |
3.53±0.15a |
3.83±0.41a |
| Membrane transport |
1.57±0.03b |
1.54±0.01c |
1.57±0.00b |
1.61±0.01a |
2.76±0.27a |
2.36±0.11a |
2.38±0.11a |
2.50±0.16a |
| Environmental adaptation |
0.16±0.00a |
0.16±0.00a |
0.16±0.00a |
0.16±0.00a |
0.19±0.01a |
0.16±0.02a |
0.15±0.02a |
0.29±0.00a |
Table A3.
The correlation between soil physical-chemical properties and bacterial community in blueberry rhizosphere soil and root.
Table A3.
The correlation between soil physical-chemical properties and bacterial community in blueberry rhizosphere soil and root.
| |
pH |
SOM |
TN |
AP |
AK |
| soil |
| Bradyrhizobium |
-0.58 |
0.15 |
-0.54 |
-0.29 |
-0.54 |
| Burkholderia |
0.81**
|
-0.76**
|
0.82**
|
-0.20 |
0.20 |
| Bacillus |
0.63*
|
-0.34 |
0.51 |
0.01 |
0.22 |
| Azospirillum |
-0.20 |
-0.39 |
-0.13 |
-0.63*
|
-0.54 |
| Rhizobium |
0.77**
|
-0.59*
|
0.68*
|
-0.09 |
0.29 |
| Herbaspirillum |
0.88**
|
-0.81**
|
0.88**
|
-0.31 |
0.34 |
| Rhodopseudomonas |
0.24 |
0.18 |
0.17 |
0.54 |
0.44 |
| Magnetospirillum |
0.48 |
-0.72**
|
0.56 |
-0.37 |
-0.02 |
| Enterobacter |
0.33 |
-0.32 |
0.18 |
-0.28 |
0.30 |
| Arthrobacter |
-0.50 |
0.62*
|
-0.46 |
0.30 |
0.11 |
| Xanthomonas |
-0.57 |
0.25 |
-0.44 |
-0.16 |
-0.41 |
| Klebsiella |
0.11 |
0.18 |
-0.05 |
0.50 |
0.71**
|
| Conexibacter |
-0.72**
|
0.57 |
-0.63*
|
0.17 |
-0.20 |
| Acidothermus |
-0.69*
|
0.60*
|
-0.54 |
0.34 |
-0.24 |
| Sphingomonas |
0.26 |
0.27 |
0.19 |
0.48 |
0.74**
|
| Pseudomonas |
-0.02 |
0.50 |
0.06 |
0.87**
|
0.55 |
| Ottowia |
0.37 |
-0.63*
|
0.50 |
-0.63*
|
-0.13 |
| root |
| Pantoea |
-0.78**
|
0.97**
|
-0.77**
|
0.59*
|
-0.13 |
| Burkholderia |
0.40 |
-0.17 |
0.42 |
0.08 |
0.66*
|
| Bradyrhizobium |
-0.39 |
-0.01 |
-0.32 |
-0.46 |
-0.30 |
| Herbaspirillum |
-0.03 |
0.27 |
-0.05 |
0.34 |
0.37 |
| Bacillus |
0.01 |
-0.16 |
0.07 |
-0.26 |
-0.56 |
| Enterobacter |
-0.53 |
0.31 |
-0.54 |
-0.26 |
-0.82**
|
| Erwinia |
-0.98**
|
0.77**
|
-0.95**
|
0.10 |
-0.59*
|
| Serratia |
-0.55 |
0.22 |
-.058*
|
-0.45 |
-0.38 |
| Arthrobacter |
-0.25 |
-0.34 |
-0.21 |
-0.55 |
-0.59*
|
| Xanthomonas |
-0.29 |
-0.01 |
-0.17 |
-0.46 |
-0.76**
|
| Klebsiella |
-0.36 |
0.25 |
-0.37 |
-0.32 |
-0.49 |
| Conexibacter |
0.08 |
0.42 |
0.03 |
0.23 |
0.40 |
| Acidothermus |
-0.57 |
0.19 |
-0.55 |
0.05 |
-0.36 |
| Pseudomonas |
0.72**
|
-0.55 |
0.69*
|
-0.10 |
0.11 |
| Sphingomonas |
-0.87**
|
0.83**
|
-0.76**
|
0.29 |
-0.51 |
Figure A1.
The correlation between soil physical-chemical properties and microbial communities in blueberry rhizosphere soil and root using redundancy analysis.
Figure A1.
The correlation between soil physical-chemical properties and microbial communities in blueberry rhizosphere soil and root using redundancy analysis.
Figure A2.
The correlation between metabolism pathways and bacterial phyla. ‘*’ indicates a significant difference (p<0.05) and ‘**’ indicates a significant difference (p<0.01) among different samples for each treatment (n=12) using the spearman method.
Figure A2.
The correlation between metabolism pathways and bacterial phyla. ‘*’ indicates a significant difference (p<0.05) and ‘**’ indicates a significant difference (p<0.01) among different samples for each treatment (n=12) using the spearman method.
References
- Delpino, F. M.; Figueiredo, L. M.; Gonçalves da Silva, T.; Flores, T. R. Effects of blueberry and cranberry on type 2 diabetes parameters in individuals with or without diabetes: A systematic review and meta-analysis of randomized clinical trials. Nutrition, Metabolism and Cardiovascular Diseases 2022, 32, 1093–1109. [Google Scholar] [CrossRef]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J. L.; Lyons, T. J.; Alexander, J. M. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. The Journal of Nutrition 2021, 151, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Stote, K. S.; Wilson, M. M.; Hallenbeck, D.; Thomas, K.; Rourke, J. M.; Sweeney, M. I.; Gottschall-Pass, K. T.; Gosmanov, A. R. Effect of Blueberry Consumption on Cardiometabolic Health Parameters in Men with Type 2 Diabetes: An 8-Week, Double-Blind, Randomized, Placebo-Controlled Trial. Current Developments in Nutrition 2020, 4, nzaa030. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, S.; Gao, J.; Cheng, K.; Yuan, F. Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. LWT 2019, 109, 233–240. [Google Scholar] [CrossRef]
- He, L.; Jing, G.; Zhao, N.; Lu, Q.; Zhang, Z.; Chen, Z.; Huang, B.; Ding, X. Soil nutrients and the responses of microbial community structure to pine bark and vinegar residues in blueberry cultivation. Applied Soil Ecology 2023, 189, 104907. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Y.; Shao, T.; Long, X.; Gao, X.; Zhou, Z. Relationship between rhizosphere soil properties and disease severity in highbush blueberry (Vaccinium corymbosum). Applied Soil Ecology 2019, 137, 187–194. [Google Scholar] [CrossRef]
- Chen, J.; Ren, R.; Wang, H.; Luo, T.; Yao, X.; Zhang, Z.; Hu, K. Effect of Lou soil pH change on selenium forms and availability. Northwest. Geol 2020, 53, 254–260. [Google Scholar]
- Xu, J.; Fang, Y.; Tavakkoli, E.; Pan, X.; Liao, F.; Chen, W.; Guo, W. Preferential ammonium: nitrate ratio of blueberry is regulated by nitrogen transport and reduction systems. Scientia Horticulturae 2021, 288. [Google Scholar] [CrossRef]
- Zeng, Q.; Jiang, Y.; Wei, J.; Yu, H. Common problems in the process of blueberry cultivation-soil organic matter improvement is not in place. Fruit growers' Friend 2017, 6, 25–26. [Google Scholar]
- Wei, H.; Li, G.; Ding, Q. Common problems and solutions of blueberry planting soil management. Fruit growers' Friend 2014, 10, 19. [Google Scholar]
- Ma, Y.; Guo, C.; Xu, C. Progress in research on function, utilization and organic cultivation of blueberry. Journal of Jinling Institute of Technology 2009, 25, 49–54. [Google Scholar]
- Sun, J.; Dong, L.; Xu, G.; Shao, H. Effects of furfural and it biocha additions on physcial-chemical characteristics of a sline soil. Journal of Agro-Environment Science 2014, 33, 532–538. [Google Scholar]
- Zhou, X.; Tang, Z.; Bai, H.; Li, J.; Mo, L. Research status and prospect of soil environment on blueberry plant growth and development. Journal of Jilin Normal University (Natural Science Edition) 2019, 40, 100–105. [Google Scholar]
- Liu, Y.; Li, H. Research on Blueberry Cultivation for Soil Improvement in Shaanxi. Shaanxi Forest Science and Technology 2019, 47, 48–51. [Google Scholar]
- Ochmian, I.; Malinowski, R.; Kubus, M.; Malinowska, K.; Sotek, Z.; Racek, M. The feasibility of growing highbush blueberry (V. corymbosum L.) on loamy calcic soil with the use of organic substrates. Scientia Horticulturae 2019, 257, 108690. [Google Scholar] [CrossRef]
- Kang, B. Effects of mixed application of natural soil improvement materials and PAM on soil physcial and chemical properties. Northweat A&F University, 2014.
- Dymov, A. A.; Gorbach, N. M.; Goncharova, N. N.; Karpenko, L. V.; Gabov, D. N.; Kutyavin, I. N.; Startsev, V. V.; Mazur, A. S.; Grodnitskaya, I. D. Holocene and recent fires influence on soil organic matter, microbiological and physico-chemical properties of peats in the European North-East of Russia. CATENA 2022, 217, 106449. [Google Scholar] [CrossRef]
- Tian, X. Effects of soil compaction on sulfur metabolism of yong apple tree and improvement of rice husk biochar. Shangsong Agricultural University, 2023.
- Chen, H. Research on the recycle unilization of spent mushroom substrates. Chinese Academy of Agricultural Sciences Dissertation, 2009.
- Chen, Y.; Xie, Y.; Zhou, H.; Chen, F.; Liu, Y.; Chen, Q. Effects of different organic materials mulching on jasmine flower and its soil. Contemporary Horticulture 2022, 45, 10–11. [Google Scholar]
- Lin, B. Effecting of spent mushroom sunstrates (SMS) and biogas residue on improving navel orange production and its fruit quality. Fujian Journal of Agricultural Sciences 2006, 21, 293–195. [Google Scholar]
- Yurgel, S. N.; Douglas, G. M.; Dusault, A.; Percival, D.; Langille, M. G. I. Dissecting Community Structure in Wild Blueberry Root and Soil Microbiome. Frontiers in Microbiology 2018, 9. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, J.; Li, Y.; Koziol, L.; Podzikowski, L.; Delgado-Baquerizo, M.; Wang, G.; Zhang, J. Relationships between soil biodiversity and multifunctionality in croplands depend on salinity and organic matter. Geoderma 2023, 429, 116273. [Google Scholar] [CrossRef]
- Guo, X.; Wan, Y.; Shakeel, M.; Wang, D.; Xiao, L. Effect of mycorrhizal fungi inoculation on bacterial diversity, community structure and fruit yield of blueberry. Rhizosphere 2021, 19. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, R.; Zhu, W.; Shi, Y.; Ni, S.; Wu, C.; Li, T. Impact of dielectric barrier discharge cold plasma on the quality and phenolic metabolism in blueberries based on metabonomic analysis. Postharvest Biology and Technology 2023, 197, 112208. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Zheng, H. Research progress on plant endophytic nirtogen-fixing bacteri and their nitrogen fixation mechanism. current biotechnology 2022, 12, 17–26. [Google Scholar]
- Orozco-Mosqueda, M. d. C.; Santoyo, G. Plant-microbial endophytes interactions: Scrutinizing their beneficial mechanisms from genomic explorations. Current Plant Biology 2021, 25, 100189. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, J.; He, Y.; Yu, X.; Chen, S.; Penttinen, P.; Liu, S.; Yang, Y.; Zhao, K.; Zou, L. Organic Fertilizers Shape Soil Microbial Communities and Increase Soil Amino Acid Metabolites Content in a Blueberry Orchard. Joutnal of Microbial Ecology 2022. [Google Scholar] [CrossRef] [PubMed]
- Chamkhi, I.; Sbabou, L.; Aurag, J. Improved growth and quality of saffron (Crocus sativus L.) in the field conditions through inoculation with selected native plant growth-promoting rhizobacteria (PGPR). Industrial Crops and Products 2023, 197. [Google Scholar] [CrossRef]
- Khan, A.; Bano, A.; Khan, R. A.; Khan, N. Role of PGPR in suppressing the growth of Macrophomina phaseolina by regulating antioxidant enzymes and secondary metabolites in Vigna radiata (L.) R. Wilczek. South African Journal of Botany 2023, 158, 443–451. [Google Scholar] [CrossRef]
- Castillo, P.; Molina, R.; Andrade, A.; Vigliocco, A.; Alemano, S.; Cassán, F. D.: Phytohormones and Other Plant Growth Regulators Produced by PGPR: The Genus Azospirillum. In Handbook for Azospirillum: Technical Issues and Protocols; Cassán, F. D., Okon, Y., Creus, C. M., Eds.; Springer International Publishing: Cham, 2015; pp 115-138. [CrossRef]
- Khan, A. L.; Halo, B. A.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J.; Al-Harrasi, A.; Lee, I.-J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electronic Journal of Biotechnology 2016, 21, 58–64. [Google Scholar] [CrossRef]
- Gou, Z.; Zheng, H.; He, Z.; Su, Y.; Chen, S.; Chen, H.; Chen, G.; Ma, N. L.; Sun, Y. The combined action of biochar and nitrogen-fixing bacteria on microbial and enzymatic activities of soil N cycling. Environmental Pollution 2023, 317, 120790. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, X.; Zhu, L.; Wu, J.; Mohamed, T. A.; Zhang, X.; Chen, X.; Zhao, Y.; Wei, Z. Elucidating the negative effect of denitrification on aromatic humic substance formation during sludge aerobic fermentation. Journal of Hazardous Materials 2020, 388, 122086. [Google Scholar] [CrossRef]
- Wang, K.; Wu, Y.; Wang, Z.; Wang, W.; Ren, N. Insight into effects of electro-dewatering pretreatment on nitrous oxide emission involved in related functional genes in sewage sludge composting. Bioresource Technology 2018, 265, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, M. H.; Mustapha, N. A.; Maeda, T.; Ramli, N.; Sakai, K.; Hassan, M. Biochar enhanced the nitrifying and denitrifying bacterial communities during the composting of poultry manure and rice straw. Waste Management 2020, 106, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Wu, J.; Zhang, Y.; Li, Q.; Liu, S.; Gao, Y. The Effects of Localized Plant-Soil-Microbe Interactions on Soil Nitrogen Cycle in Maize Rhizosphere Soil under Long-Term Fertilizers. Agronomy 2023, 13, 2114. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, X.; Gao, X.; Shao, T.; Long, X.; Rengel, Z. Effects of Soil Properties and Microbiome on Highbush Blueberry (Vaccinium corymbosum) Growth. Agronomy 2022, 12. [Google Scholar] [CrossRef]
- Darnell, R. L.; Casamali, B.; Williamson, J. G. Nutrient Assimilation in Southern Highbush Blueberry and Implications for the Field %J HortTechnology hortte. 2015, 25, 460-463. [CrossRef]
- Yan, J. Effects of different fertilizers on physiological and fruit quality of blueberry. Guizhou University, 2018.
- Aqeel, M.; Ran, J.; Hu, W.; Irshad, M. K.; Dong, L.; Akram, M. A.; Eldesoky, G. E.; Aljuwayid, A. M.; Chuah, L. F.; Deng, J. Plant-soil-microbe interactions in maintaining ecosystem stability and coordinated turnover under changing environmental conditions. Chemosphere 2023, 318, 137924. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, S.; Li, R.; Zhang, J.; Liu, Y.; Lv, L.; Zhu, H.; Wu, W.; Li, W. Plant cultivars imprint the rhizosphere bacterial community composition and association networks. Soil Biology and Biochemistry 2017, 109, 145–155. [Google Scholar] [CrossRef]
- Khan, A.; Ding, Z.; Ishaq, M.; Khan, I.; Ahmed, A. A.; Khan, A. Q.; Guo, X. Applications of beneficial plant growth promoting rhizobacteria and mycorrhizae in rhizosphere and plant growth:A review. International Journal of Agriculture and Bioengineering 2020, 199–208. [Google Scholar] [CrossRef]
- Robinson, R. J.; Fraaije, B. A.; Clark, I. M.; Jackson, R. W.; Hirsch, P. R.; Mauchline, T. H. Endophytic bacterial community composition in wheat (Triticum aestivum) is determined by plant tissue type, developmental stage and soil nutrient availability. Plant and Soil 2015, 405, 381–396. [Google Scholar] [CrossRef]
- Ma, Y.; Weisenhorn, P.; Guo, X.; Wang, D.; Yang, T.; Shi, Y.; Zhang, H.; Chu, H. Effect of long-term fertilization on bacterial communities in wheat endosphere. Pedosphere 2021, 31, 538–548. [Google Scholar] [CrossRef]
- Chen, J.; Dai, J.; Song, X.; Jiang, Q.; Zhao, C.; Sun, C.; Chen, C.; Chen, N.; Han, B. Endophytic microbiota comparison of Dendrobium huoshanense root and stem in different growth years. Planta Med. 2020, 86, 967–975. [Google Scholar] [CrossRef]
- Zhao, R.; Zheng, S.; Hu, Y.; Li, H.; Chen, Y.; Chun, Z. Endophytic bacterial diversity of the medicinal orchid Dendrobium nobile. South African Journal of Botany 2023, 158, 90–97. [Google Scholar] [CrossRef]
- Ma, S.; Verheyen, K.; Props, R.; Wasof, S.; Vanhellemont, M.; Boeckx, P.; Boon, N.; De Frenne, P. Plant and soil microbe responses to light, warming and nitrogen addition in a temperate forest. Functional ecology 2018, 32, 1293–1303. [Google Scholar] [CrossRef]
- Du, X.; Hu, H.; Wang, T.; Zou, L.; Zhou, W.; Gao, H.; Ren, X.; Wang, J.; Hu, S. Long-term rice cultivation increases contributions of plant and microbial-derived carbon to soil organic carbon in saline-sodic soils. Science of The Total Environment 2023, 904, 166713. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Jiang, T.; Thomas, B. W.; Chen, J.; Xie, J.; Hu, Y.; Kong, F.; Yang, Y.; Chen, X.; Zhang, Y.; Shi, X. Legume cover crops enhance soil organic carbon via microbial necromass in orchard alleyways. Soil and Tillage Research 2023, 234, 105858. [Google Scholar] [CrossRef]
- Mir, I. R.; Rather, B. A.; Sehar, Z.; Masood, A.; Khan, N. A. Nitric oxide in co-ordination with nitrogen reverses cadmium-inhibited photosynthetic activity by interacting with ethylene synthesis, strengthening the antioxidant system, and nitrogen and sulfur assimilation in mustard (Brassica juncea L.). Scientia Horticulturae 2023, 314, 111958. [Google Scholar] [CrossRef]
- Dey, S.; Kundu, R.; Gopal, G.; Mukherjee, A.; Nag, A.; Paul, S. Enhancement of nitrogen assimilation and photosynthetic efficiency by novel iron pulsing technique in Oryza sativa L. var Pankaj. Plant Physiology and Biochemistry 2019, 144, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Das, P. P.; Singh, K. R.; Nagpure, G.; Mansoori, A.; Singh, R. P.; Ghazi, I. A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ Res 2022, 214, 113821. [Google Scholar] [CrossRef]
- Cid-Pérez, T. S.; Nevárez-Moorillón, G. V.; Ochoa-Velasco, C. E.; Navarro-Cruz, A. R.; Hernández-Carranza, P.; Avila-Sosa, R. The Relation between Drying Conditions and the Development of Volatile Compounds in Saffron (Crocus sativus). molecules 2021, 26, 6954. [Google Scholar] [CrossRef]
- Hanif, M. K.; Malik, K. A.; Hameed, S.; Saddique, M. J.; Ayesha; Fatima, K.; Naqqash, T.; Majeed, A.; Iqbal, M. J.; Imran, A. Growth stimulatory effect of AHL producing Serratia spp. from potato on homologous and non-homologous host plants. Microbiological Research 2020, 238, 126506. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, S.; Banerjee, S.; Mukherjee, S.; Ganguli, A.; Mondal, S. Heavy metals bio-removal potential of the isolated Klebsiella sp TIU20 strain which improves growth of economic crop plant (Vigna radiata L.) under heavy metals stress by exhibiting plant growth promoting and protecting traits. Biocatalysis and Agricultural Biotechnology 2021, 38, 102204. [Google Scholar] [CrossRef]
- Benizri, E.; Kidd, P. S.: The Role of the Rhizosphere and Microbes Associated with Hyperaccumulator Plants in Metal Accumulation. In Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants; Van der Ent, A., Echevarria, G., Baker, A. J. M., Morel, J. L., Eds.; Springer International Publishing: Cham, 2018; pp 157-188. [CrossRef]
- Ghasemi, Z.; Ghaderian, S. M.; Rodríguez-Garrido, B.; Prieto-Fernández, Á.; Kidd, P. S. Plant species-specificity and effects of bioinoculants and fertilization on plant performance for nickel phytomining. Plant and Soil 2018, 425, 265–285. [Google Scholar] [CrossRef]
- Schwabe, R.; Dittrich, C.; Kadner, J.; Rudi Senges, C. H.; Bandow, J. E.; Tischler, D.; Schlömann, M.; Levicán, G.; Wiche, O. Secondary metabolites released by the rhizosphere bacteria Arthrobacter oxydans and Kocuria rosea enhance plant availability and soil–plant transfer of germanium (Ge) and rare earth elements (REEs). Chemosphere 2021, 285, 131466. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Ubeyitogullari, A. Extraction of phenolic compounds from rice husk via ethanol-water-modified supercritical carbon dioxide. Heliyon 2023, 9, e14196. [Google Scholar] [CrossRef]
- Alitaleshi, F.; Daghbandan, A.; Pendashteh, A. Performance of Rice Husk Biocarrier on Ammonia Nitrogen Removal in the MBBR Treating Aquaculture Wastewater Using Biological Attached Growth Process: Performance and Kinetic Study. Journal of Environmental Chemical Engineering 2023, 111446. [Google Scholar] [CrossRef]
- Saqib Rashid, M.; Liu, G.; Yousaf, B.; Song, Y.; Ahmed, R.; Rehman, A.; Arif, M.; Irshad, S.; Cheema, A. I. Efficacy of rice husk biochar and compost amendments on the translocation, bioavailability, and heavy metals speciation in contaminated soil: Role of free radical production in maize (Zea mays L.). Journal of Cleaner Production 2022, 330, 129805. [Google Scholar] [CrossRef]
- Duan, H.; Ji, M.; Chen, A.; Zhang, B.; Shi, J.; Liu, L.; Li, X.; Sun, J. Evaluating the impact of rice husk on successions of bacterial and fungal communities during cow manure composting. Environmental Technology & Innovation 2021, 24, 102084. [Google Scholar] [CrossRef]
- Montañez, A.; Blanco, A. R.; Barlocco, C.; Beracochea, M.; Sicardi, M. Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in vitro. Applied Soil Ecology 2012, 58, 21–28. [Google Scholar] [CrossRef]
- Li, Q.; Koyama, M.; Nakasaki, K. Effect of storage time on organic matter decomposition during composting by inoculating enriched microorganisms. Environmental Technology & Innovation 2023, 29, 102984. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Song, Z.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic fertilizer activates soil beneficial microorganisms to promote strawberry growth and soil health after fumigation. Environmental Pollution 2022, 295, 118653. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Ma, J.; Jiang, J.; You, X.; Lv, R.; Zhou, S.; Pan, C.; Liu, B.; Xu, Q.; Xie, Z. How does biochar influence soil nitrification and nitrification-induced N2O emissions? Science of The Total Environment 2023, 168530. [Google Scholar] [CrossRef]
- Iboko, M. P.; Dossou-Yovo, E. R.; Obalum, S. E.; Oraegbunam, C. J.; Diedhiou, S.; Brümmer, C.; Témé, N. Paddy rice yield and greenhouse gas emissions: Any trade-off due to co-application of biochar and nitrogen fertilizer? A systematic review. Heliyon 2023, e22132. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-I.; Park, H.-J.; Jeong, Y.-J.; Seo, B.-S.; Kwak, J.-H.; Yang, H. I.; Xu, X.; Tang, S.; Cheng, W.; Lim, S.-S.; Choi, W.-J. Biochar-induced reduction of N2O emission from East Asian soils under aerobic conditions: Review and data analysis. Environmental Pollution 2021, 291, 118154. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).