Submitted:
16 November 2023
Posted:
17 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
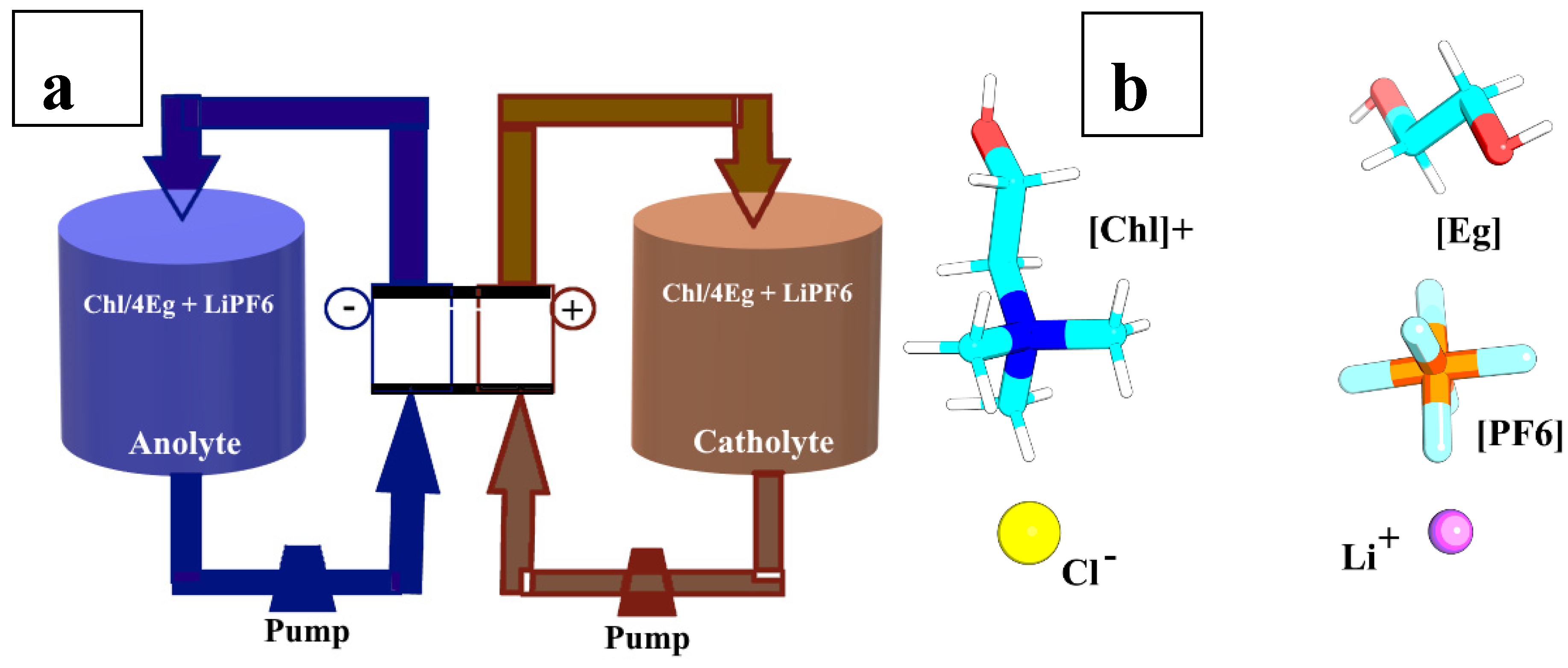
2.1. Experimental Section
Materials, Apparatus, and Procedure
2.2. Computational Section
Calculation of the Physicochemical Properties
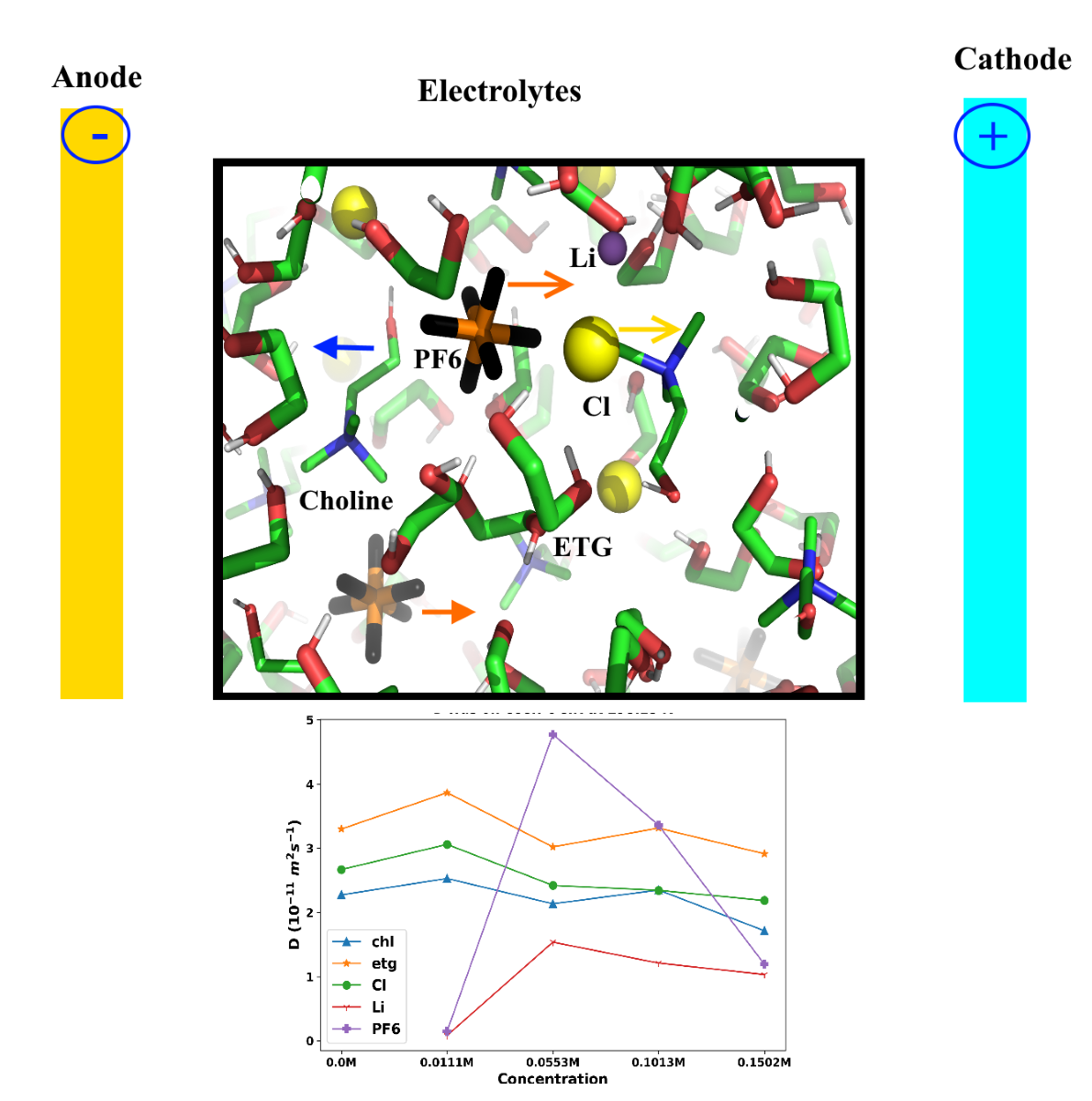
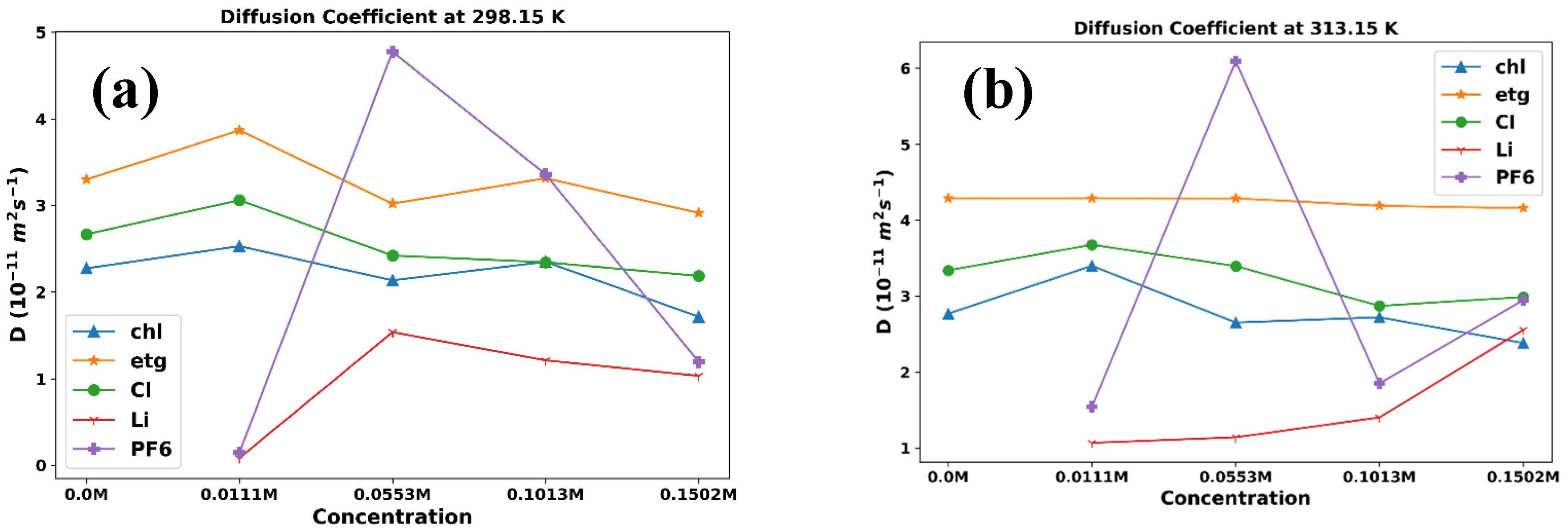
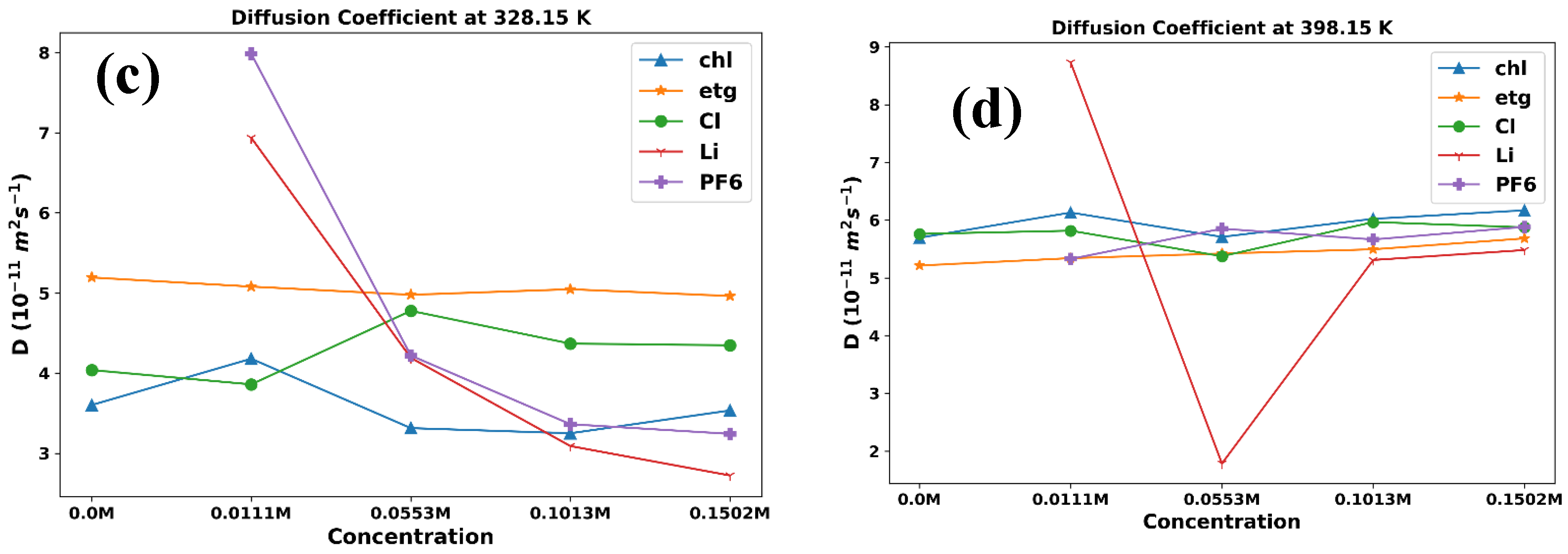
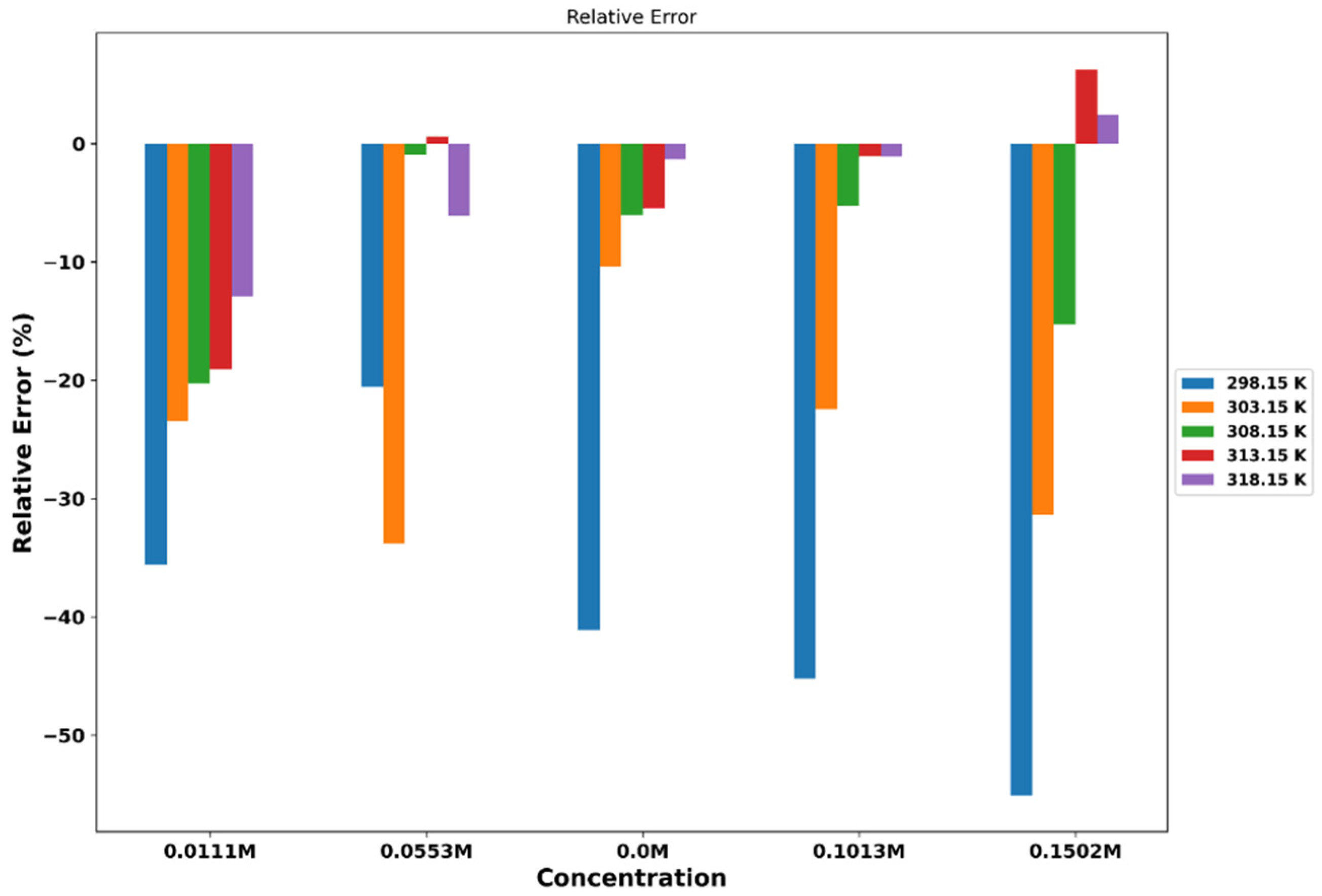
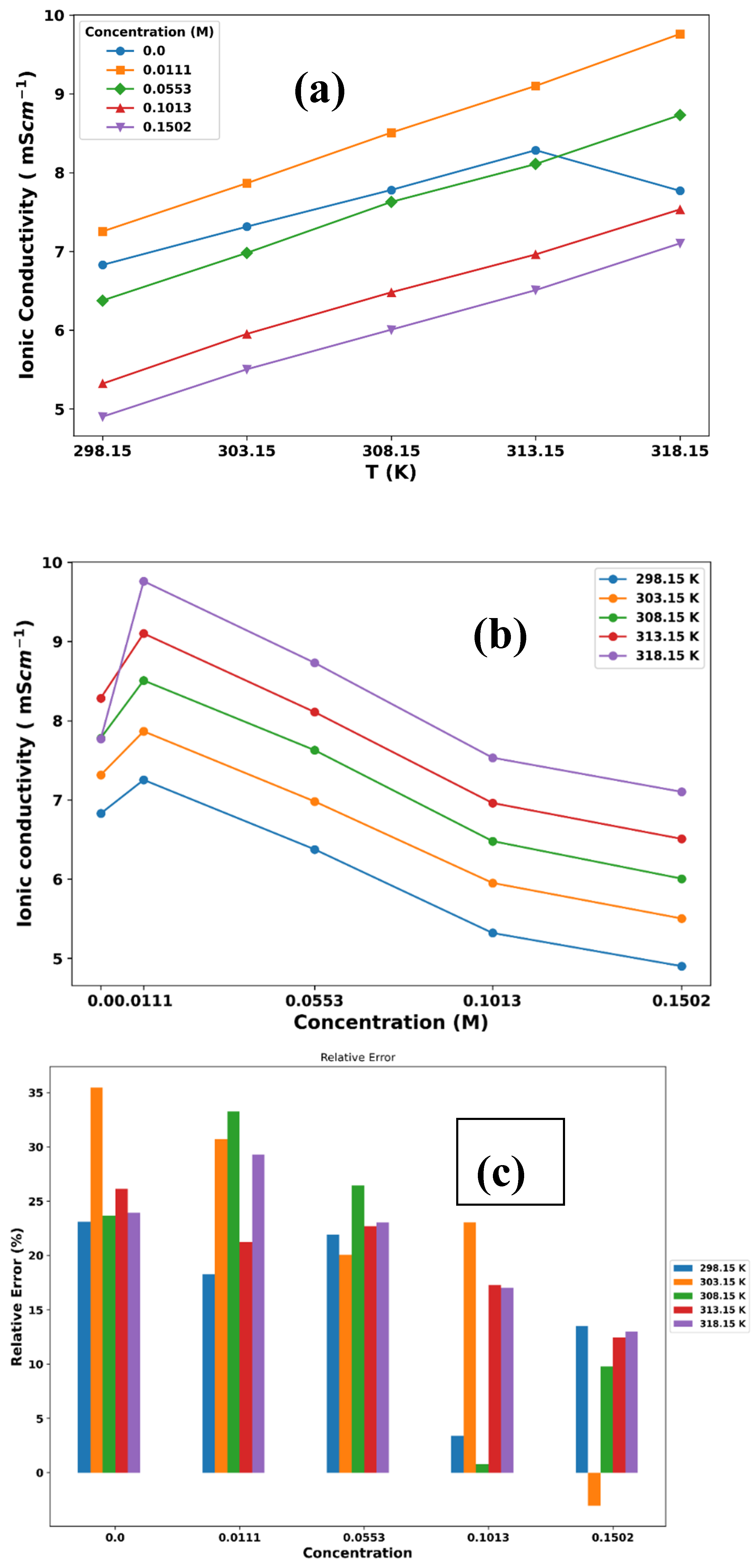
3. Results and Discussion
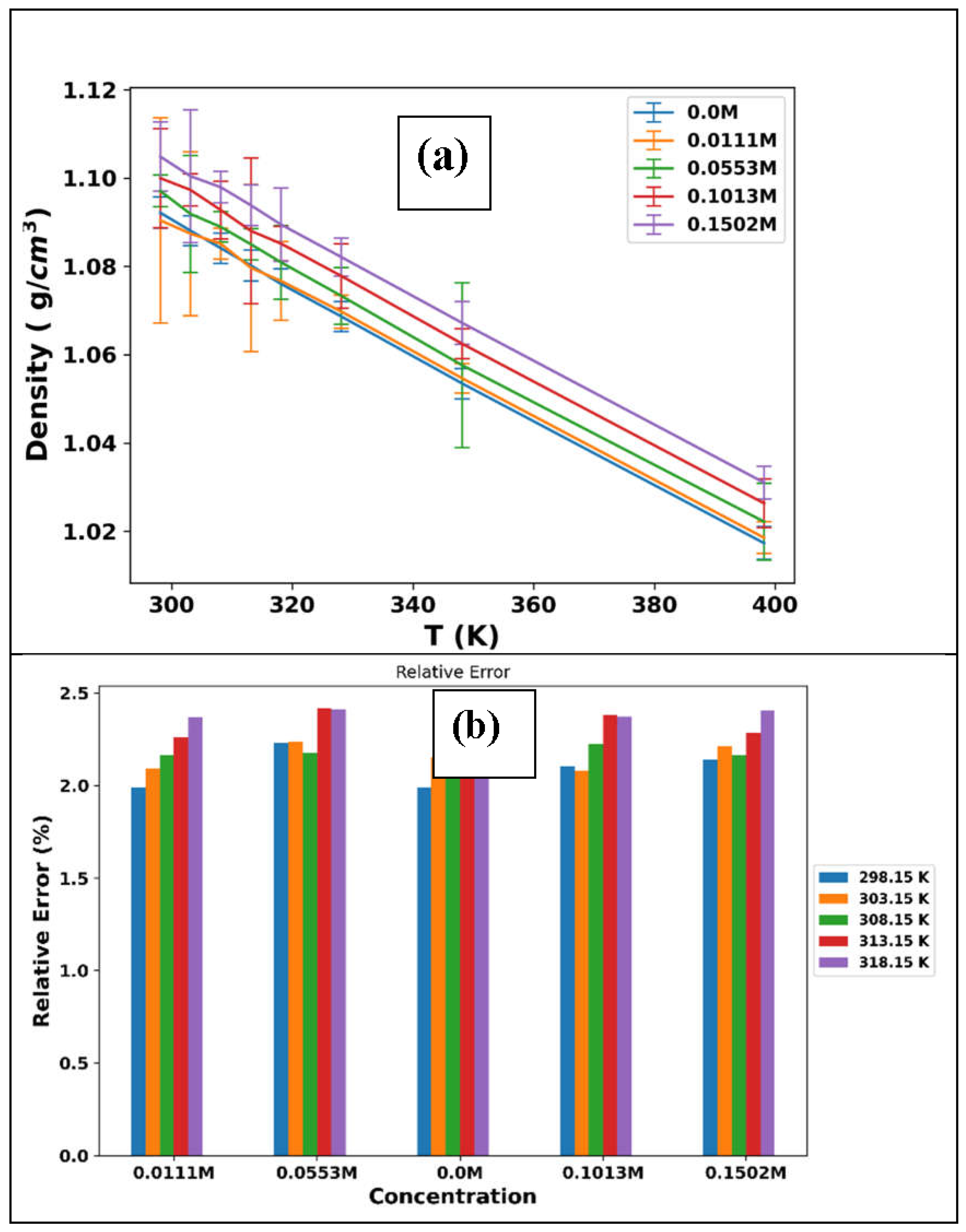
3.1. Thermophysical Properties
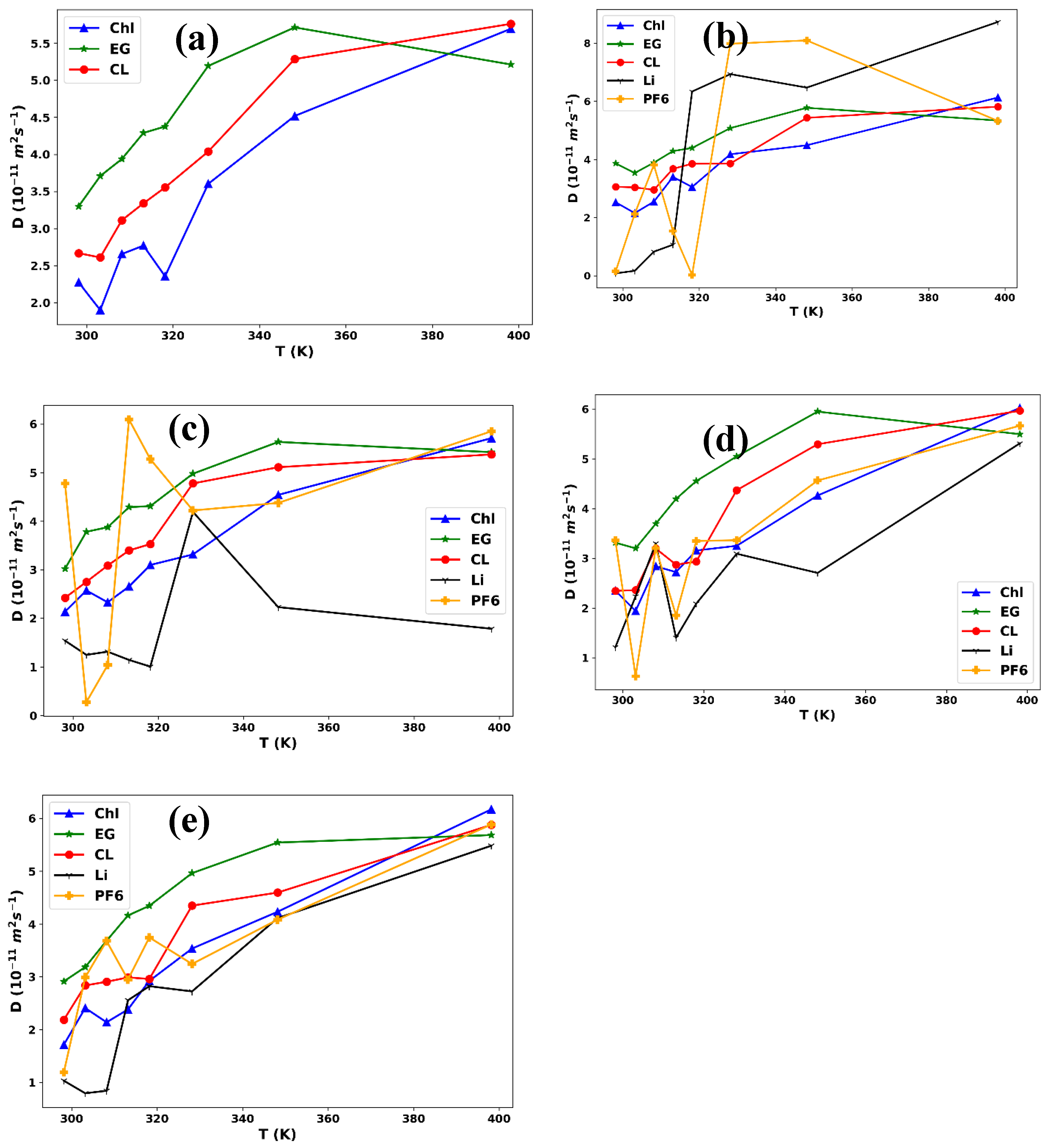
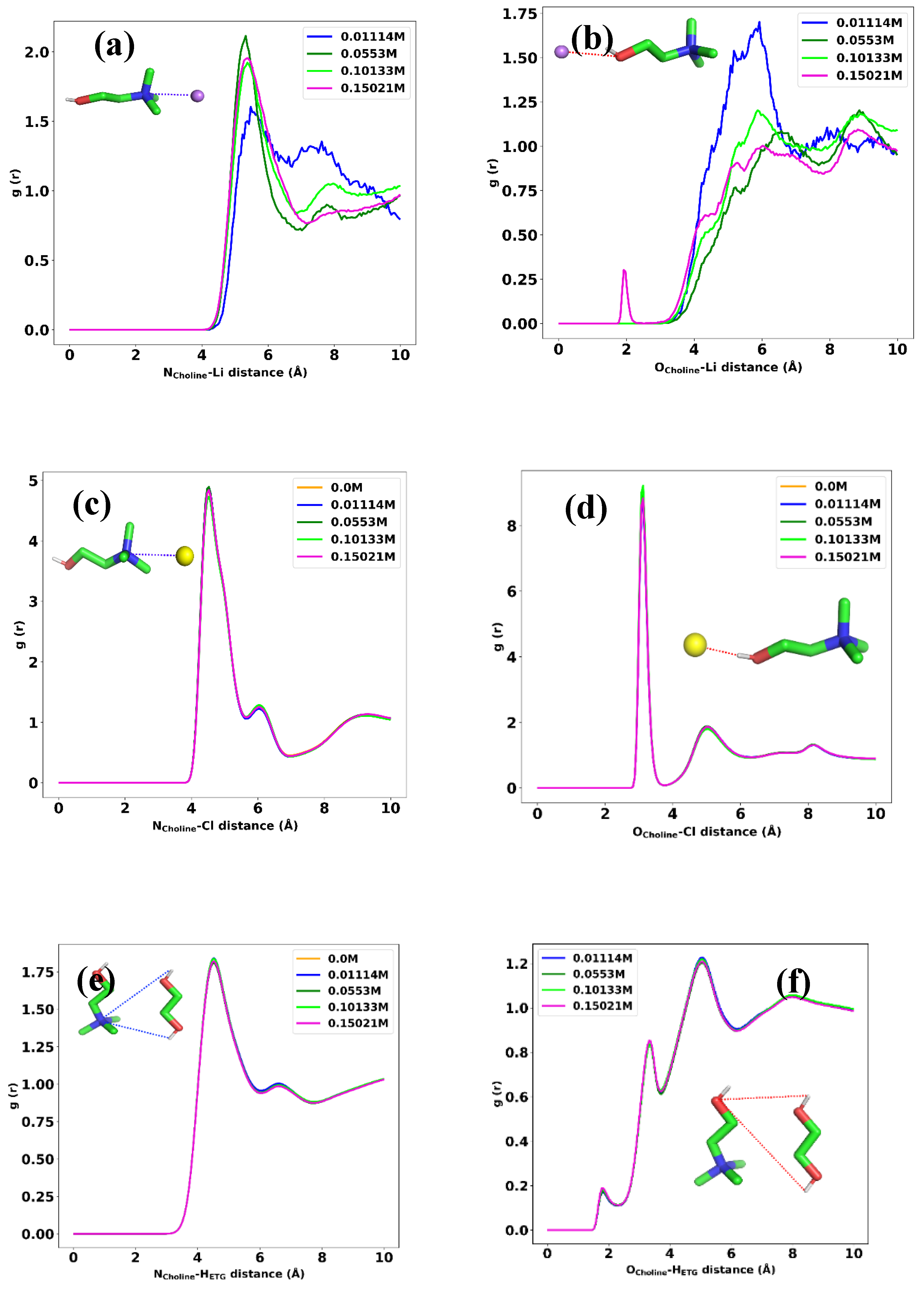
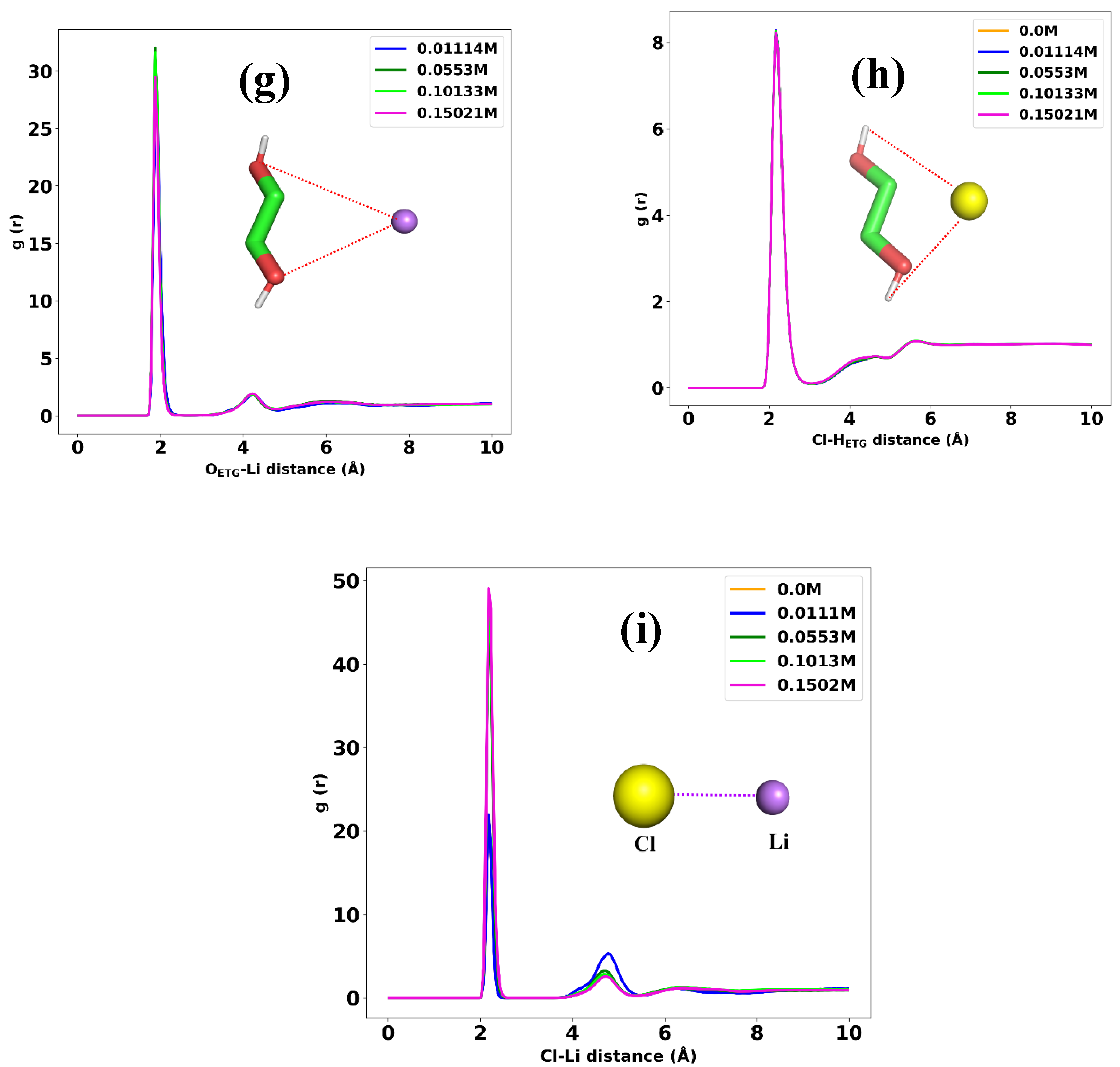
3.1. Structural Analysis
4. Conclusions
References
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lise, W.; van der Laan, J.; Nieuwenhout, F.; Rademaekers, K. Assessment of the required share for a stable EU electricity supply until 2050. Energy Policy 2013, 59, 904–913. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W. Battery technologies for grid-level large-scale electrical energy storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Li, Y.; Peng, L.; Byon, H.R.; Goodenough, J.B.; Yu, G. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 2015, 44, 7968–7996. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Xia, L.; Wu, C.; Ding, M.; Jia, C.; Wang, Q. Redox targeting-based flow batteries. J. Phys. D: Appl. Phys. 2019, 52, 443001. [Google Scholar] [CrossRef]
- Zhao, E.W.; Liu, T.; Jónsson, E.; Lee, J.; Temprano, I.; Jethwa, R.B.; Wang, A.; Smith, H.; Carretero-González, J.; Song, Q. In situ NMR metrology reveals reaction mechanisms in redox flow batteries. Nature 2020, 579, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.H.; Mjalli, F.S.; AlNashef, I.M.; Hashim, M.A.; Hussain, M.A.; Bahadori, L.; Low, C.T.J. Prospects of applying ionic liquids and deep eutectic solvents for renewable energy storage by means of redox flow batteries. Renew. Sustain. Energy Rev. 2014, 30, 254–270. [Google Scholar] [CrossRef]
- Miller, M.; Wainright, J.; Savinell, R. Communication—Iron ionic liquid electrolytes for redox flow battery applications. J. Electrochem. Soc. 2016, 163, A578. [Google Scholar] [CrossRef]
- Lloyd, D.; Vainikka, T.; Ronkainen, M.; Kontturi, K. Characterisation and application of the Fe (II)/Fe (III) redox reaction in an ionic liquid analogue. Electrochim. Acta 2013, 109, 843–851. [Google Scholar] [CrossRef]
- Lloyd, D.; Vainikka, T.; Kontturi, K. The development of an all copper hybrid redox flow battery using deep eutectic solvents. Electrochim. Acta 2013, 100, 18–23. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, L.; Ji, Y.; Leung, P.; Su, H.; Qiao, F.; Yang, W.; Shah, A.; Li, H. A deep eutectic solvent (DES) electrolyte-based vanadium-iron redox flow battery enabling higher specific capacity and improved thermal stability. Electrochim. Acta 2019, 293, 426–431. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Ding, Y.; Ramirez-Meyers, K.; Yu, G. A low-cost and high-energy hybrid iron-aluminum liquid battery achieved by deep eutectic solvents. Joule 2017, 1, 623–633. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Z.; Zheng, Q.; Zhang, C.; Ye, J.; Dai, G.; Zhao, Y.; Zhang, X. Zn-based eutectic mixture as anolyte for hybrid redox flow batteries. Sci. Rep. 2018, 8, 5740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, H. A green and cost-effective rechargeable battery with high energy density based on a deep eutectic catholyte. Energy Environ. Sci. 2016, 9, 2267–2272. [Google Scholar] [CrossRef]
- Miller, M.A.; Wainright, J.S.; Savinell, R.F. Iron electrodeposition in a deep eutectic solvent for flow batteries. J. Electrochem. Soc. 2017, 164, A796. [Google Scholar] [CrossRef]
- Shen, D.; Vukmirovic, M.B.; Akolkar, R. Understanding the Role of Complexation in the Charge-Transfer Kinetics of the Cu2++ e↔ Cu1+ Redox Reaction in Ethaline Deep Eutectic Solvent. J. Electrochem. Soc. 2019, 166, E526. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.D. vanderSpoel, E. Lindahl. J. Chem. Theory Comput 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, M.; Rahman, A. Polymorphic transítions in single crystals: A new molecular dynamics method polymorphic transítions in single crystals: A new molecular dynamics method. 2002.
- Hess, B.; Bekker, H.; Berendsen, H.; Fraaije, J. AIDJCC4. 3.0. CO: 1997.
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Van Gunsteren, W.F.; Berendsen, H.J. A leap-frog algorithm for stochastic dynamics. Mol. Simul. 1988, 1, 173–185. [Google Scholar] [CrossRef]
- Perkins, S.L.; Painter, P.; Colina, C.M. Molecular dynamic simulations and vibrational analysis of an ionic liquid analogue. J. Phys. Chem. B 2013, 117, 10250–10260. [Google Scholar] [CrossRef] [PubMed]
- Hess, B. Determining the shear viscosity of model liquids from molecular dynamics simulations. J. Chem. Phys. 2002, 116, 209–217. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.-C.; Hummer, G. System-size dependence of diffusion coefficients and viscosities from molecular dynamics simulations with periodic boundary conditions. J. Phys. Chem. B 2004, 108, 15873–15879. [Google Scholar] [CrossRef]
- Bittner, E.R. Chemical Dynamics in the Condensed Phases: Relaxation, Transfer, and Reactions in Condensed Molecular Systems By Abraham Nitzan (Tel Aviv University). Oxford University Press: Oxford, New York. 2006. xxii+ 720 pp. $89.50. ISBN 0-19-852979-1. ACS Publications: 2006.
- Moradi, H.; Farzi, N. Experimental and computational assessment of the physicochemical properties of choline chloride/ethylene glycol deep eutectic solvent in 1: 2 and 1: 3 mole fractions and 298.15–398.15 K. J. Mol. Liq. 2021, 339, 116669. [Google Scholar] [CrossRef]
- Moradi, H.; Farzi, N. The investigation of deep eutectic electrolyte based on Choline Chloride: Ethylene glycol in 1: 3 M ratio and lithium hexafluorophosphate salt for application in Lithium-Ion batteries. J. Mol. Liq. 2022, 360, 119476. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Voroshylova, I.V.; Pereira, C.M.; DS Cordeiro, M.N.l. Improved force field model for the deep eutectic solvent ethaline: Reliable physicochemical properties. J. Phys. Chem. B 2016, 120, 10124–10137. [Google Scholar] [CrossRef]
- Del Pópolo, M.G.; Voth, G.A. On the structure and dynamics of ionic liquids. J. Phys. Chem. B 2004, 108, 1744–1752. [Google Scholar] [CrossRef]
| Chemicals | Origin | CAS No. | Mass fraction (purity) | Structure |
|---|---|---|---|---|
| Ethylene glycol | Merck | 107-21-1 | >0.99 | |
| Choline Chloride | Merck | 67-48-1 | >0.99 | |
| Lithium hexafluorophosphate | Merck | >0.99 | LiPF6 |
| LiPF6 conc. | Box size (nm3) | No.Choline | No. Chloride | No. Ethylene glycol | No. Li | No.PF6 |
|---|---|---|---|---|---|---|
| 0. 0111M | 6.87413 | 553 | 553 | 2212 | 3 | 3 |
| 0.0553M | 6.87653 | 553 | 553 | 2212 | 12 | 12 |
| 0.1013M | 6.88713 | 553 | 553 | 2212 | 22 | 22 |
| 0.1502M | 6.89583 | 553 | 553 | 2212 | 33 | 33 |
| T/K | ρ/g.cm-3 | ||||
|---|---|---|---|---|---|
| 0.0 M | 0. 0111M | 0.0553M | 0.1013M | 0.1502M | |
| 298.15 | 1.1143 | 1.1153 | 1.1193 | 1.1236 | 1.1290 |
| 303.15 | 1.1113 | 1.1123 | 1.1159 | 1.1206 | 1.1253 |
| 308.15 | 1.1081 | 1.1093 | 1.1129 | 1.1176 | 1.1222 |
| 313.15 | 1.1052 | 1.1064 | 1.1099 | 1.1146 | 1.1194 |
| 318.15 | 1.1021 | 1.1033 | 1.1068 | 1.1115 | 1.1163 |
| T/K | ρ/g.cm-3 | ||||||||
| 0.0 M LiPF6 | 0. 0111 M LiPF6 | 0.0553 M LiPF6 | 0.1013 M LiPF6 | 0.1502 M LiPF6 | |||||
| 298.15 | 1.0922 | 1.0905 | 1.0971 | 1.0999 | 1.1049 | ||||
| 303.15 | 1.0881 | 1.0874 | 1.0919 | 1.0973 | 1.1005 | ||||
| 308.15 | 1.0842 | 1.0852 | 1.0890 | 1.0928 | 1.0980 | ||||
| 313.15 | 1.0802 | 1.0797 | 1.0850 | 1.0881 | 1.0939 | ||||
| 318.15 | 1.0761 | 1.0767 | 1.0809 | 1.0852 | 1.0895 | ||||
| 328.15 | 1.0687 | 1.0698 | 1.0733 | 1.0778 | 1.0821 | ||||
| 348.15 | 1.0535 | 1.0547 | 1.0576 | 1.0625 | 1.0672 | ||||
| 398.15 | 1.0173 | 1.0186 | 1.0222 | 1.0264 | 1.0310 | ||||
| ChlCl/Eg (1:4) | |||||||
|---|---|---|---|---|---|---|---|
| T/ K |
D Chl+ (1011 m2s-1) |
DEg (1011 m2s-1) |
D Cl- (1011 m2s-1) |
DLi+ (1011 m2s-1) |
DPF6 (1011 m2s-1) |
||
| 0.0 M LiPF6 | |||||||
| 298.15 | 2.27 | 3.30 | 2.67 | ||||
| 303.15 | 1.90 | 3.70 | 2.61 | ||||
| 308.15 | 2.65 | 3.94 | 3.11 | ||||
| 313.15 | 2.77 | 4.29 | 3.34 | ||||
| 318.15 | 2.36 | 4.37 | 3.56 | ||||
| 328.15 | 3.60 | 5.19 | 4.04 | ||||
| 348.15 | 4.52 | 5.71 | 5.29 | ||||
| 398.15 | 5.70 | 5.21 | 5.76 | ||||
| 0. 0111 M LiPF6 | |||||||
| 298.15 | 2.53 | 3.87 | 3.06 | 0.08 | 0.15 | ||
| 303.15 | 2.16 | 3.54 | 3.04 | 0.17 | 2.13 | ||
| 308.15 | 2.55 | 3.89 | 2.96 | 0.83 | 3.81 | ||
| 313.15 | 3.4 | 4.29 | 3.68 | 1.07 | 1.54 | ||
| 318.15 | 3.05 | 4.4 | 3.85 | 6.34 | 0.03 | ||
| 328.15 | 4.18 | 5.08 | 3.86 | 6.94 | 7.98 | ||
| 348.15 | 4.49 | 5.78 | 5.43 | 6.47 | 8.1 | ||
| 398.15 | 6.13 | 5.34 | 5.82 | 8.73 | 5.33 | ||
| 0.0553 M LiPF6 | |||||||
| 298.15 | 2.14 | 3.02 | 2.42 | 1.54 | 4.77 | ||
| 303.15 | 2.57 | 3.78 | 2.75 | 1.25 | 0.28 | ||
| 308.15 | 2.33 | 3.87 | 3.09 | 1.31 | 1.04 | ||
| 313.15 | 2.65 | 4.29 | 3.4 | 1.14 | 6.1 | ||
| 318.15 | 3.1 | 4.31 | 3.53 | 1 | 5.28 | ||
| 328.15 | 3.31 | 4.98 | 4.78 | 4.19 | 4.22 | ||
| 348.15 | 4.54 | 5.63 | 5.11 | 2.23 | 4.38 | ||
| 398.15 | 5.71 | 5.42 | 5.37 | 1.78 | 5.85 | ||
| 0.1013 M LiPF6 | |||||||
| 298.15 | 2.35 | 3.31 | 2.34 | 1.21 | 3.36 | ||
| 303.15 | 1.94 | 3.2 | 2.36 | 2.23 | 0.63 | ||
| 308.15 | 2.84 | 3.69 | 3.2 | 3.3 | 3.2 | ||
| 313.15 | 2.72 | 4.19 | 2.87 | 1.4 | 1.85 | ||
| 318.15 | 3.16 | 4.55 | 2.93 | 2.09 | 3.35 | ||
| 328.15 | 3.25 | 5.05 | 4.37 | 3.09 | 3.36 | ||
| 348.15 | 4.26 | 5.95 | 5.29 | 2.71 | 4.56 | ||
| 398.15 | 6.02 | 5.49 | 5.97 | 5.31 | 5.66 | ||
| 0.1502 M LiPF6 | |||||||
| 298.15 | 1.71 | 2.91 | 2.19 | 1.03 | 1.19 | ||
| 303.15 | 2.41 | 3.18 | 2.84 | 0.8 | 2.99 | ||
| 308.15 | 2.14 | 3.68 | 2.91 | 0.84 | 3.68 | ||
| 313.15 | 2.38 | 4.16 | 2.99 | 2.56 | 2.95 | ||
| 318.15 | 2.92 | 4.34 | 2.96 | 2.82 | 3.75 | ||
| 328.15 | 3.54 | 4.96 | 4.35 | 2.72 | 3.24 | ||
| 348.15 | 4.23 | 5.54 | 4.59 | 4.11 | 4.08 | ||
| 398.15 | 6.17 | 5.68 | 5.88 | 5.48 | 5.88 | ||
| T/K | η/ mPa·s | ||||
| 0.0 M | 0. 0111M | 0.0553M | 0.1013M | 0.1502M | |
| 298.15 | 20.84 | 23.20 | 25.14 | 26.83 | 28.04 |
| 303.15 | 17.44 | 19.20 | 20.68 | 22.17 | 23.51 |
| 308.15 | 15.17 | 16.67 | 17.38 | 18.52 | 19.19 |
| 313.15 | 12.73 | 13.92 | 14.89 | 15.87 | 16.72 |
| 318.15 | 11.07 | 12.05 | 13.00 | 13.96 | 14.84 |
| T/K | ηTCCF(cP) | |||||||||
| 0.0 M LiPF6 | 0. 0149 M LiPF6 | 0.0553 M LiPF6 | 0.1013 M LiPF6 | 0.1502 M LiPF6 | ||||||
| 298.15 | 28.27 ± 5.3 | 27.99 ± 5.4 | 35.48 ± 6.9 | 38.97 ± 7.9 | 43.49 ± 9.1 | |||||
| 303.15 | 21.54 ± 3.7 | 25.69 ± 4.6 | 22.83 ± 4.0 | 27.16 ± 5.0 | 30.89 ± 6.1 | |||||
| 308.15 | 18.26 ± 3.2 | 16.84 ± 2.6 | 18.44 ± 3.0 | 19.49 ± 3.2 | 22.13 ± 3.8 | |||||
| 313.15 | 15.16 ± 2.3 | 13.84 ± 2.2 | 15.71 ± 2.4 | 16.04 ± 2.6 | 15.68 ± 2.5 | |||||
| 318.15 | 12.51 ± 1.8 | 12.79 ± 2.0 | 13.18 ± 1.8 | 14.12 ± 2.2 | 14.48 ± 2.1 | |||||
| 328.15 | 9.51 ± 1.2 | 10.40 ± 1.5 | 8.51 ± 0.8 | 9.18 ± 1.0 | 10.51 ± 1.5 | |||||
| 348.15 | 11.15 ± 1.5 | 11.20 ± 1.4 | 9.44 ± 1.4 | 9.52 ± 1.5 | 9.89 ± 1.5 | |||||
| 398.15 | 2.23 ± 3.6 | 2.70 ± 1.3 | 5.63 ± 1.7 | 7.84 ± 1.3 | 7.78 ± 1.4 | |||||
| T/K | σexp (mS cm-1) | ||||
|---|---|---|---|---|---|
| 0.0 M | 0. 0111M | 0.0553M | 0.1013M | 0.1502M | |
| 298.15 | 6.828 | 7.253 | 6.376 | 5.320 | 4.901 |
| 303.15 | 7.314 | 7.866 | 6.981 | 5.952 | 5.503 |
| 308.15 | 7.780 | 8.508 | 7.628 | 6.480 | 6.006 |
| 313.15 | 8.285 | 9.101 | 8.109 | 6.961 | 6.508 |
| 318.15 | 7.769 | 9.761 | 8.731 | 7.533 | 7.102 |
| T/K | σNE (mS cm-1) | ||||||||
| 0.0 M LiPF6 | 0. 0149M LiPF6 | 0.0553M LiPF6 | 0.1013M LiPF6 | 0.1502M LiPF6 | |||||
| 298.15 | 5.25 | 5.93 | 4.98 | 5.14 | 4.24 | ||||
| 303.15 | 4.72 | 5.45 | 5.58 | 4.58 | 5.67 | ||||
| 308.15 | 5.94 | 5.68 | 5.61 | 6.43 | 5.42 | ||||
| 313.15 | 6.12 | 7.17 | 6.27 | 5.76 | 5.70 | ||||
| 318.15 | 5.91 | 6.90 | 6.72 | 6.25 | 6.18 | ||||
| 328.15 | 7.39 | 7.82 | 7.96 | 7.56 | 7.88 | ||||
| 348.15 | 8.95 | 9.09 | 8.88 | 8.92 | 8.40 | ||||
| 398.15 | 9.13 | 9.56 | 8.94 | 9.86 | 10.04 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





