Submitted:
16 November 2023
Posted:
17 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
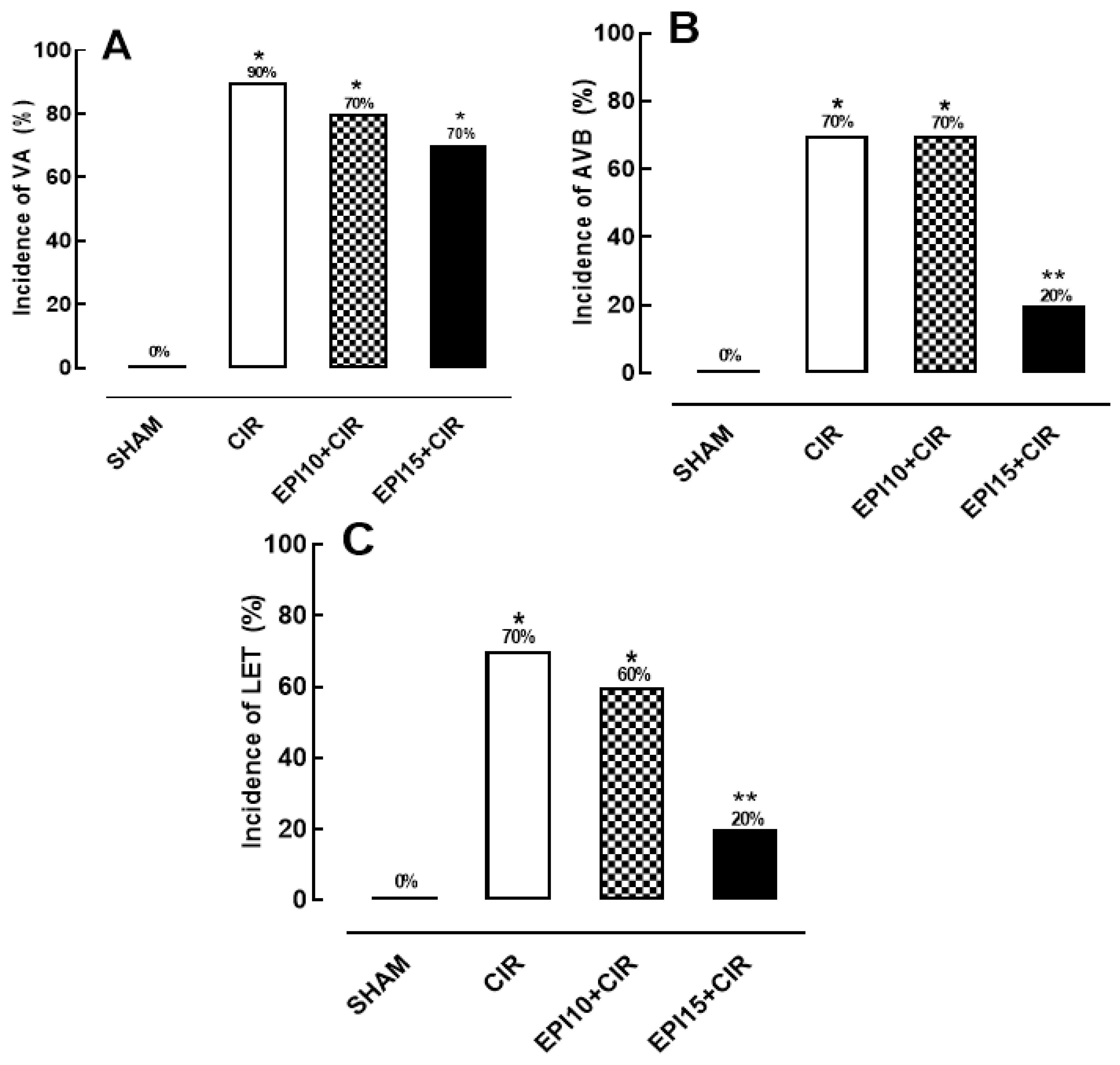
2.1. Incidence of VA, AVB and LET induced by CIR
2.2. Effects of the EPI on the incidence of VA, AVB and LET induced by CIR
2.3. Effects of the EPI on the biomarker’s levels of cardiac injury total creatine kinase and CK-M in animals submitted to CIR
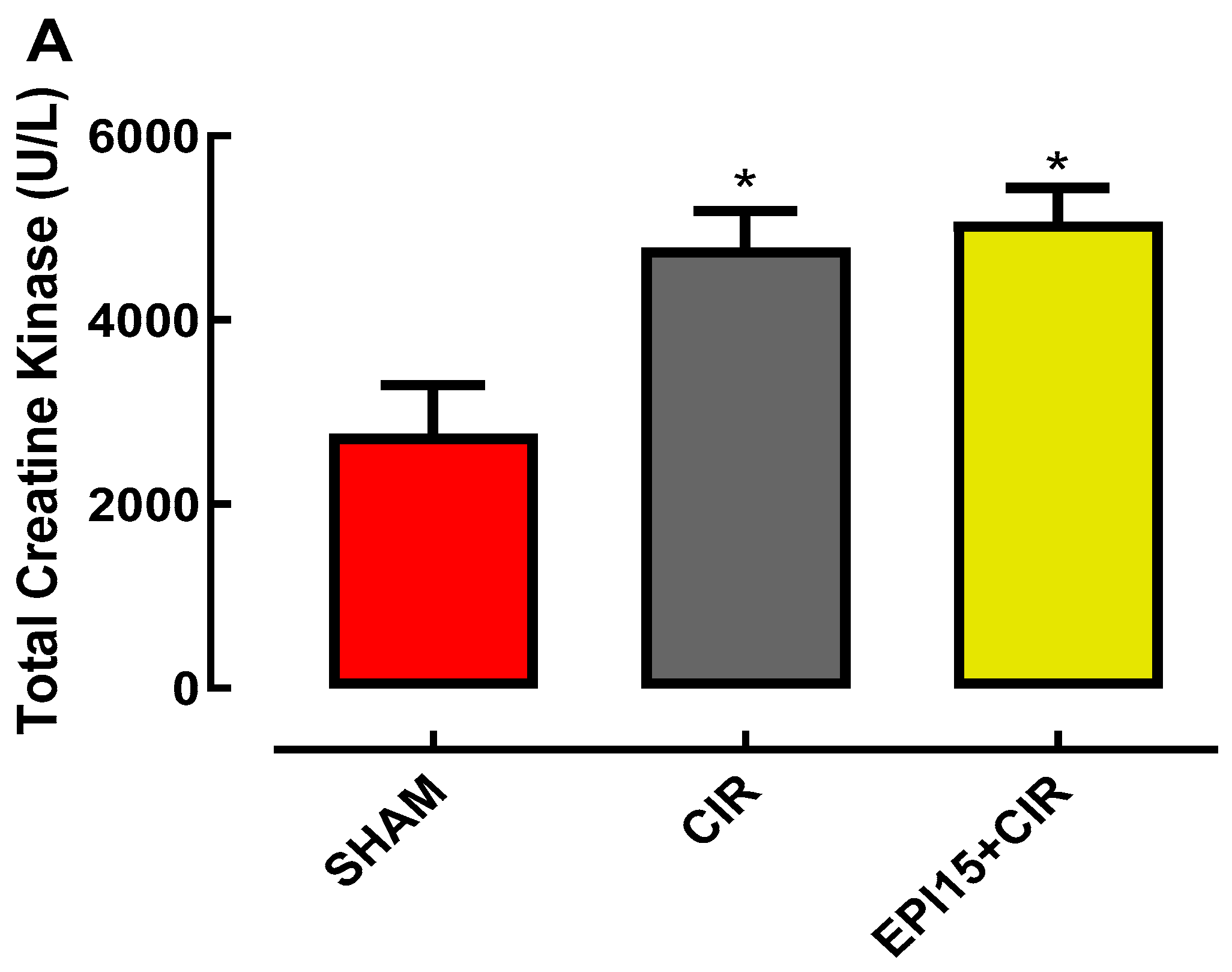
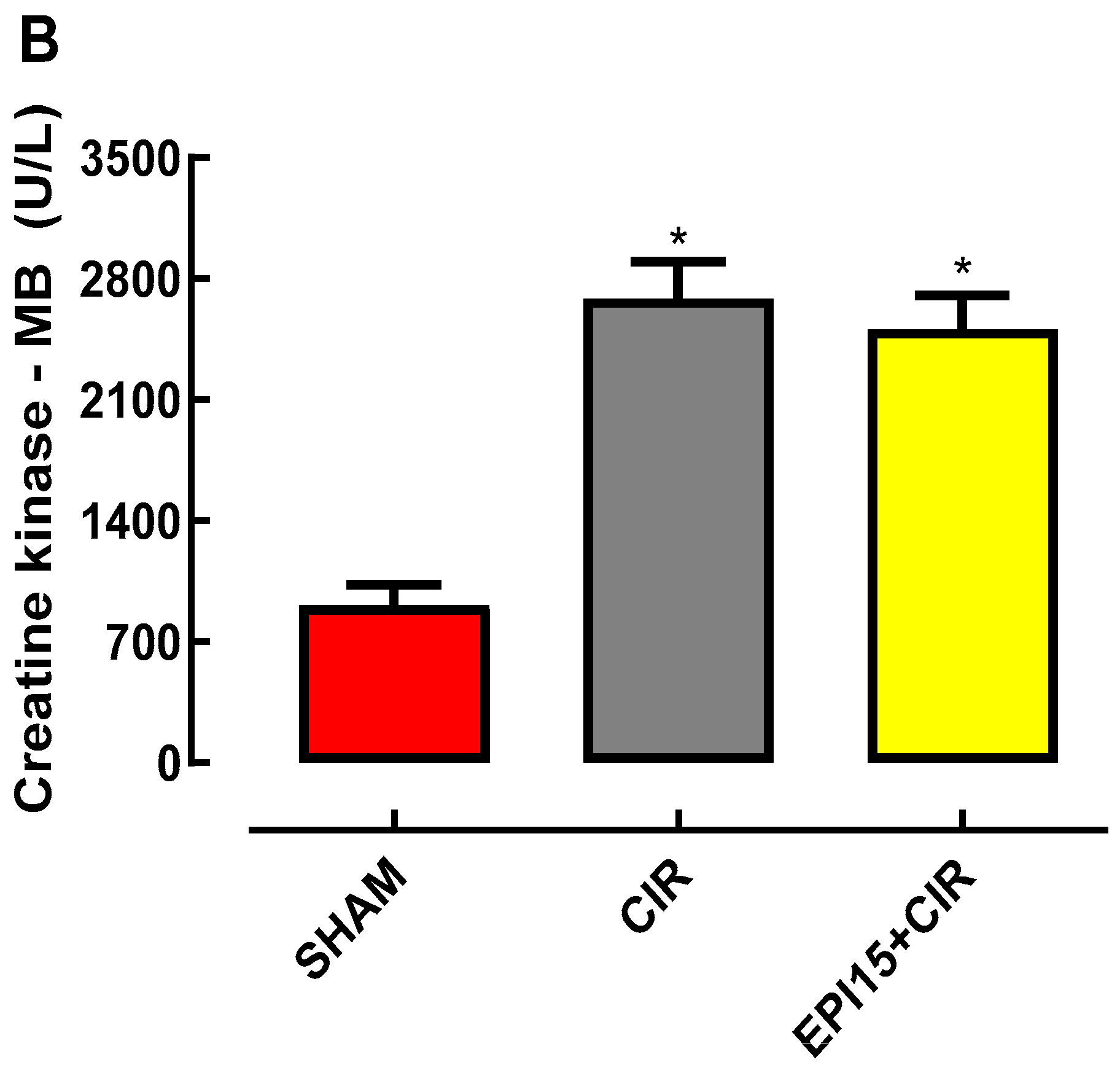
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cardiac ischemia and reperfusion (CIR) induction
4.3. Assessment of cardiac activity during CIR
4.4. Biochemical assessment of heart lesions’ biomarkers
4.5. Pharmacological Treatments
4.6. Statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics--2015 Update: A Report from the American Heart Association. Circulation 2015, 131, e29-322.
- Writing Group Members; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38-360.
- Rutledge, T.; Reis, V.A.; Linke, S.E.; Greenberg, B.H.; Mills, P.J. Depression in Heart Failure a Meta-Analytic Review of Prevalence, Intervention Effects, and Associations with Clinical Outcomes. J. Am. Coll. Cardiol. 2006, 48, 1527–1537.
- Frampton, J.; Ortengren, A.R.; Zeitler, E.P. Arrhythmias After Acute Myocardial Infarction. Yale J. Biol. Med. 2023, 96, 83–94.
- Naryzhnaya, N. V; Maslov, L.N.; Derkachev, I.A.; Ma, H.; Zhang, Y.; Prasad, N.R.; Singh, N.; Fu, F.; Pei, J.-M.; Sarybaev, A.; et al. The Effect of an Adaptation to Hypoxia on Cardiac Tolerance to Ischemia/Reperfusion. J. Biomed. Res. 2022, 1–25. [CrossRef]
- Panuccio, G.; Carabetta, N.; Torella, D.; De Rosa, S. Clinical impact of coronary revascularization over medical treatment in chronic coronary syndromes: a systematic review and meta-analysis. Hellenic J Cardiol. 2023, S1109-9666, 00194-X. [CrossRef]
- Menezes-Rodrigues, F.S.; Tavares, J.G.P.; Vasques, E.R.; Errante, P.R.; Araújo, E.A. de; Pires-Oliveira, M.; Scorza, C.A.; Scorza, F.A.; Taha, M.O.; Caricati-Neto, A. Cardioprotective Effects of Pharmacological Blockade of the Mitochondrial Calcium Uniporter on Myocardial Ischemia-Reperfusion Injury. Acta Cir. Bras. 2020, 35, e202000306. [CrossRef]
- Menezes-Rodrigues, F.S.; de Oliveira, M.P.; Araújo, EA.; Ferraz, HB.; Finsterer, J.; Olszewer, E.; Taha, M.O.; Scorza, C.A.; Caricati-Neto, A.; Scorza, F.A. Role of cardiac β1-adrenergic and A1-adenosine receptors in severe arrhythmias related to Parkinson’s disease. Clinics. 2023, 78, 100243.
- Menezes-Rodrigues, F.S.; Errante, P.R.; Tavares, J.G.P.; Ferraz, R.R.N.; Gomes, W.J.; Taha, M.O.; Scorza, C.A.; Scorza, F.A.; Caricati-Neto, A. Pharmacological Modulation of B-Adrenoceptors as a New Cardioprotective Strategy for Therapy of Myocardial Dysfunction Induced by Ischemia and Reperfusion. Acta Cir. Bras. 2019, 34, e201900505. [CrossRef]
- Filho, C.E.B.; Barbosa, A.H.P.; Nicolau, L.A.D.; Medeiros, J.V.R.; Pires-Oliveira, M.; Dos Santos Póvoa, R.M.; Govato, T.C.P.; Júnior, H.J.F.; de Carvalho, R.G.; Luna-Filho, B.; Sabia Tallo, F.; de Araújo, E.A.; Padrão Tavares, J.G.; Arida, R.M.; Caricati-Neto, A.; Menezes-Rodrigues, F.S. Pharmacological Modulation by Low Molecular Weight Heparin of Purinergic Signaling in Cardiac Cells Prevents Arrhythmia and Lethality Induced by Myocardial Infarction. J Cardiovasc Dev Dis 2023, 10, 103. [CrossRef]
- Tallo FS, de Santana PO, Pinto SAG, Lima RY, de Araújo EA, Tavares JGP, Pires-Oliveira M, Nicolau LAD, Medeiros JVR, Taha MO, David AI, Luna-Filho B, Filho CEB, Barbosa AHP, Silva CMC, Wanderley AG, Caixeta A, Caricati-Neto A, Menezes-Rodrigues FS. Pharmacological Modulation of the Ca2+/cAMP/Adenosine Signaling in Cardiac Cells as a New Cardioprotective Strategy to Reduce Severe Arrhythmias in Myocardial Infarction. Pharmaceuticals (Basel). 2023, 16, 1473.
- Gersh, B.J. [The changing prognosis of myocardial infarction in the reperfusion era: implications for evaluation and management of ventricular arrhythmias]. Rev Esp Cardiol 2003, 56, 535-42.
- Santos, A.P.; Moreno, P. R. H. Pilocarpus spp.: A survey of its chemical constituents and biological activities. Brazilian Journal of Pharmaceutical Sciences 2004, 40, 116-137, 2004. [CrossRef]
- Pinheiro, C.U. Jaborandi (Pilocarpus sp., rutaceae): a wild species. Econ. Bot. 1997, 51, 49–58. [CrossRef]
- Andrade-Neto, M.; Mendes, P. H.; Silveira, E. R. An imidazole alkaloid and other constituents from Pilocarpus trachyllophus. Phytochemistry 1996, 42, 885−887. [CrossRef]
- Carvalho, W.A.; Lemônica, L. Molecular and Cellular Mechanisms of Inflammatory Pain. Peripheral Modulation and Therapeutic Advances. Revista Brasileira de Anestesiologia 1998, 48, 137-158.
- Veras,L.M.; Guimaraes, M.A.; Campelo, Y.D.; Vieira, M.M.; Nascimento, C.; Lima, D.F.; Vasconcelos, L.; Nakano, E.; Kuckelhaus, S.S.; Batista, M.C.; Leite, J.R.; Moraes, J.. Activity of epiisopiloturine against Schistosoma mansoni. Curr Med Chem 2012, 19, 2051-8. [CrossRef]
- Silva, V.G.; Silva, R.O.; Damasceno, S.R.B.; Carvalho, N.S.; Prudêncio, RS.; Aragão, KS.; Guimarães, M.A.; Campos, S.A.; Véras, L.M.C.; Godejohann, M.; Leite, J.R.S.A,; Barbosa, A.L.R.; Medeiros, J-V.R. Anti-inflammatory and antinociceptive activity of epiisopiloturine, an imidazole alkaloid isolated from Pilocarpus microphyllus. J Nat Prod 2013, 76, 1071-7. [CrossRef]
- Rocha, T.M.; Machado, N.J.; de Sousa, J.A.C.; Araujo, E.V.O.; Guimaraes, M.A.; Lima, D.F.; de Almeida Leite, J.R.S.; Leal, L.K.A.M. Imidazole alkaloids inhibit the pro-inflammatory mechanisms of human neutrophil and exhibit anti-inflammatory properties in vivo. Journal of Pharmacy and Pharmacology 2019, 71, 849–859.
- Silva, V.G.; Silva, R.O.; Damasceno, S.R.B.; Carvalho, N.S.; Prudêncio, R.S.; Aragão, K.S.; Guimarães, M.A.; Campos, S.A.; Véras, L.M.C.; Godejohann, M.; Leite, J.R.S.; Barbosa, A.L.R.; Medeiros, J-V.R. Anti-inflammatory and antinociceptive activity of epiisopiloturine, an imidazole alkaloid isolated from Pilocarpus microphyllus. J Nat Prod. 2013, 76, 1071-7. [CrossRef]
- de Sousa, J.A.C.; Azul, F.V.C.S.; de Araújo, A.B.; Tomé, R.C.; Silva, F.R.M.; de Vasconcelos, S.M.M.; Rios, F.J.; Leal, L.K.A.M. Epiisopiloturine, an Alkaloid from Pilocarpus microphyllus, Attenuates LPS-Induced Neuroinflammation by Interfering in the TLR4/NF-κB-MAPK Signaling Pathway in Microglial Cells. Oxid Med Cell Longev 2023, 2023, 4752502.
- Sousa, P.S.deA.; Nogueira, S.S.; Ayala, K.N.R.; Silva, P.C.; Santos, E.daS.; Sá, R.E.de.; Lima Neto, F.E.M.de.; LIMA, J.R.daC.; Rodrigues, K.A.da F.; Rocha, J.A.; Véras, L.M.C. Scientific and technological prospection of Pilocarpus microphyllus and the alkaloid epiisopiloturine with emphasis on antileishmanial activity. Research, Society and Development 2021, 10, e59810716984.
- de Carvalho, L.R.; de Brito, T.V.; Júnior , J.S.C.; Júnior, G.J.D.; Magalhãres, D.deA.; Sousa, S.G.; Silva, R.O.; da Silva, F.R.P.; Vasconcelos, D.S.P.; Véras, L.M.C.; Leite, J.R.deS.deA.; Martins, D.S.; Martins, C.daS.; de Oliveira, J.S.; Barbosa, A.L.D.R.. Epiisopiloturine, an imidazole alkaloid, reverses inflammation and lipid peroxidation parameters in the Crohn disease model induced by trinitrobenzenosulfonic acid in Wistar rats. Biomed Pharmacother 2018, 102, 278-285.
- Nicolau, L.A.D.; Carvalho, N.S.; Pacífico, D.M.; Lucetti, L.T.; Aragão, K.S.; Véras, L.M.C.; Souza, M.H.L.P.; Leite, J.R.S.A.; Medeiros, J.V.R. Epiisopiloturine hydrochloride, an imidazole alkaloid isolated from Pilocarpus microphyllus leaves, protects against naproxen-induced gastrointestinal damage in rats. Biomed Pharmacother 2017,87, 188-195. [CrossRef]
- Bers, D.M. Calcium Cycling and Signaling in Cardiac Myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [CrossRef]
- Wagner, S.; Maier, L.S.; Bers, D.M. Role of Sodium and Calcium Dysregulation in Tachyarrhythmias in Sudden Cardiac Death. Circ. Res. 2015, 116, 1956–1970. [CrossRef]
- Halestrap, A.P.; Clarke, S.J.; Khaliulin, I. The Role of Mitochondria in Protection of the Heart by Preconditioning. Biochim. Biophys. Acta 2007, 1767, 1007–1031. [CrossRef]
- Xie, L.-H.H.; Weiss, J.N. Arrhythmogenic Consequences of Intracellular Calcium Waves. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H997–H1002. [CrossRef]
- Peart, J.; Flood, A.; Linden, J.; Matherne, G.P.; Headrick, J.P. Adenosine-Mediated Cardioprotection in Ischemic-Reperfused Mouse Heart. J. Cardiovasc. Pharmacol. 2002, 39, 117–129. [CrossRef]
- Lasley, R.D.; Rhee, J.W.; Van Wylen, D.G.; Mentzer, R.M. Adenosine A1 Receptor Mediated Protection of the Globally Ischemic Isolated Rat Heart. J. Mol. Cell. Cardiol. 1990, 22, 39–47.
- Yu, H.J.; Ma, H.; Green, R.D.; Hai Jing Yu; Ma, H.; Green, R.D.; Yu, H.J.; Ma, H.; Green, R.D. Calcium Entry via L-Type Calcium Channels Acts as a Negative Regulator of Adenylyl Cyclase Activity and Cyclic AMP Levels in Cardiac Myocytes. Mol. Pharmacol. 1993, 44, 689–693.
- Sellers, Z.M.; Naren, A.P.; Xiang, Y.; Best, P.M. MRP4 and CFTR in the Regulation of cAMP and β-Adrenergic Contraction in Cardiac Myocytes. Eur. J. Pharmacol. 2012, 681, 80–87. [CrossRef]
- Muthal, A.P.; Kulkarni, R.; Kumar, D.; Bagul, C.; Mukherjee-Kandhare, A.A.; Kandhare, A.D.; Ambavade, S.D.; Wagh, V.; Bodhankar, S.L. Cyclic adenosine monophosphate: Recent and future perspectives on various diseases. Journal of Applied Pharmaceutical Science. 2022, 12, 001-015.
- Halls, M.L.; Cooper, D.M.F. Regulation by Ca2+-Signaling Pathways of Adenylyl Cyclases. Cold Spring Harb Perspect Biol 2011, 3, a004143. [CrossRef]
- Lasley, R.D.; Mentzer, R.M. Dose-Dependent Effects of Adenosine on Interstitial Fluid Adenosine and Postischemic Function in the Isolated Rat Heart. J. Pharmacol. Exp. Ther. 1998, 286, 806–811.
- Lasley, R.D.; Kristo, G.; Keith, B.J.; Mentzer, R.M. The A2a/A2b Receptor Antagonist ZM-241385 Blocks the Cardioprotective Effect of Adenosine Agonist Pretreatment in in Vivo Rat Myocardium. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H426-31. [CrossRef]
- Dhalla, A.K.; Shryock, J.C.; Shreeniwas, R.; Belardinelli, L. Pharmacology and Therapeutic Applications of A1 Adenosine Receptor Ligands. Curr. Top. Med. Chem. 2003, 3, 369–385. [CrossRef]
- Burnstock, G.; Pelleg, A. Cardiac Purinergic Signalling in Health and Disease. Purinergic Signal. 2015, 11, 1–46. [CrossRef]
- Belardinelli, L.; Lerman, B.B. Adenosine: Cardiac Electrophysiology. Pacing Clin. Electrophysiol. 1991, 14, 1672–1680. [CrossRef]
- Shryock, J.C.; Belardinelli, L. Adenosine and Adenosine Receptors in the Cardiovascular System: Biochemistry, Physiology, and Pharmacology. Am. J. Cardiol. 1997, 79, 2–10. [CrossRef]
- Zhan, E.; McIntosh, V.J.; Lasley, R.D. Adenosine A₂A and A₂B Receptors Are Both Required for Adenosine A₁ Receptor-Mediated Cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1183-9.
- Urmaliya, V.B.; Church, J.E.; Coupar, I.M.; Rose’Meyer, R.B.; Pouton, C.W.; White, P.J. Cardioprotection Induced by Adenosine A1 Receptor Agonists in a Cardiac Cell Ischemia Model Involves Cooperative Activation of Adenosine A2A and A2B Receptors by Endogenous Adenosine. J. Cardiovasc. Pharmacol. 2009, 53, 424–433. [CrossRef]
- Safran, N.; Shneyvays, V.; Balas, N.; Jacobson, K.A.; Nawrath, H.; Shainberg, A. Cardioprotective Effects of Adenosine A1 and A3 Receptor Activation during Hypoxia in Isolated Rat Cardiac Myocytes. Mol. Cell. Biochem. 2001, 217, 143–152. [CrossRef]
- Du, L.; Gao, Z.-G.; Nithipatikom, K.; Ijzerman, A.P.; Veldhoven, J.P.D. van; Jacobson, K.A.; Gross, G.J.; Auchampach, J.A. Protection from Myocardial Ischemia/Reperfusion Injury by a Positive Allosteric Modulator of the A₃ Adenosine Receptor. J. Pharmacol. Exp. Ther. 2012, 340, 210–217. [CrossRef]
- Okada, M.; Falcão, L.F.R.; Ferez, D.; Martins, J.L.; Errante, P.R.; Rodrigues, F.S.M.; Caricati-Neto, A.; Marinho, M.; Fenelon, G.; Oliveira-Júnior. I.S.; Effect of atenolol pre-treatment in heart damage in a model of intestinal ischemia-reperfusion. Acta Cir. Bras 2017, 32, 964-972. [CrossRef]
- Di Diego, J.M.; Antzelevitch, C. Cellular Basis for ST-Segment Changes Observed during Ischemia. J. Electrocardiol. 2003, 36 Suppl, 1–5.
- Yan, G.-X.; Lankipalli, R.S.; Burke, J.F.; Musco, S.; Kowey, P.R. Ventricular Repolarization Components on the Electrocardiogram: Cellular Basis and Clinical Significance. J. Am. Coll. Cardiol. 2003, 42, 401–409.
- Walker, M.J.; Curtis, M.J.; Hearse, D.J.; Campbell, R.W.; Janse, M.J.; Yellon, D.M.; Cobbe, S.M.; Coker, S.J.; Harness, J.B.; Harron, D.W. The Lambeth Conventions: Guidelines for the Study of Arrhythmias in Ischaemia Infarction, and Reperfusion. Cardiovasc. Res. 1988, 22, 447–455. [CrossRef]
- Huggins, C.E.; Bell, J.R.; Pepe, S.; Delbridge, L.M.D. Benchmarking Ventricular Arrhythmias in the Mouse--Revisiting the “Lambeth Conventions” 20 Years On. Heart. Lung Circ. 2008, 17, 445–450.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




