1. Introduction
The utilization of hand sanitizer products has become exceptionally prevalent worldwide throughout the coronavirus (COVID-19) pandemic time. Alcohol-based hand sanitizers are very effective and have been widely used for disinfecting microbes. The key mechanism in the disinfection process involves the denaturation of proteins and the disruption of the cell membrane of microbes by alcohol [
1] . Among alcohols, methanol has a very low antimicrobial activity whereas ethanol and isopropanol have the highest antimicrobial activities, and hence they are the most common alcohols present in hand sanitizer products [
2]. In addition to alcohol, other ingredients such as glycerol and hydrogen peroxide (H
2O
2) are also present in hand sanitizers [
3,
4] . To serve as a disinfectant, hand sanitizer should contain a certain concentration of alcohol. The typical concentration of alcohol in hand sanitizer ranges from 60% - 95%. According to World Health Organization (WHO) formulations for hand hygiene products, ethanol-based products typically contain 80% whereas isopropanol based products contain 75% concentration [
5] . Alcohol-based hand sanitizers are available in a variety of formats such as gel, liquid and foam. In addition to alcohol- based hand sanitizers, alcohol-free hand sanitizers are also effective in disinfecting microbes [
6,
7]. Benzalkonium chloride, a quaternary ammonium compound, is one of the main ingredients in alcohol-free hand sanitizer. The positively charged nitrogen atom in the quaternary ammonium compound interacts with the phosphate group of the phospholipids in the microbial cell membrane which leads to destabilization of the cell membrane and eventually causes the cell lysis of microbes [
7].
Besides glycerol and H
2O
2, other minor components present in the hand sanitizer are oxidative products of ethanol, isopropanol, and methanol. The oxidative products can be formed during the prolonged storage of alcohol-based hand sanitizer at room temperature or at an elevated temperature such as in an automobile that has been parked outside. Acetaldehyde and acetone are common products formed during the storage of hand sanitizers due to the oxidation of ethanol and isopropanol respectively. Acetaldehyde is considered as a toxic chemical for animals and humans whereas low concentrations of acetone exposure have no known health hazards. In the case of acetaldehyde, it is classified as a substance reasonably anticipated to be a human carcinogen in the 15th report on carcinogens by the US Department of Health and Human Services [
8]. In humans and mammals, acetaldehyde and acetone are generated in the body as a part of the main excretion mechanism of ingested alcohols, through the oxidation of ethanol to acetaldehyde and isopropanol to acetone by the enzyme alcohol dehydrogenase (ADH) [
9,
10,
11,
12]. Acetaldehyde is further metabolized into acetate by aldehyde dehydrogenase (ALDH) whereas acetone metabolism is a more complex mechanism that involves the generation of glucose, carbon dioxide and water [
9,
13]. A significant amount of acetone was detected through the excretion of CO
2 via breath [
13,
14]. Abnormalities in the ALDH enzyme or its inhibition can cause high levels of acetaldehyde buildup for an extended period which has been correlated with several diseases including liver disease [
10,
11]. Isopropanol intoxication is a common health problem, and it is partially due to the slow metabolism of isopropanol [
15,
16]. Acetone is CNS a depressant and has a longer elimination half-life than ethanol [
14]. Exposure to acetone at low levels is not associated with any health issues. However, acute or long-term exposure to acetone can cause problems, including skin irritation and respiratory issues. Acetone is found in several household items including disinfectants, cleaners, and nail polish removers.
Methanol is a toxic chemical found in several household items. It is a common contaminant in ethanol due to imperfect distillation or fermentation, which led to multiple accidental methanol poisonings through alcoholic beverages [
17,
18]. Apart from CNS depression and metabolic acidosis, vision impairment is a characteristic symptom of methanol poisoning [
19]. Methanol is metabolized into formaldehyde and formic acid by ALDH and ADH respectively.
The accurate concentration determination of alcohols and their oxidative products is very critical in forensics, toxicology, environmental science, and several other fields [
20,
21,
22]. Gas chromatography (GC), infrared spectroscopy (IR) and Raman spectroscopy techniques are very popular in forensic and environmental fields to determine trace amounts of chemicals [
23,
24,
25]. Analytical sensors based on the enzymatic reaction of alcohol dehydrogenase are also used in the quantification of alcohol in biological samples [
25,
26,
27]. Among all these methods, GC-based methods are the most reliable and robust to detect alcohols and other volatile organic compounds [
28,
29]. In most of the applications, GC is either coupled with a flame ionization (GC-FID) or mass spectrometry (GC-MS) detector. In certain cases, a combination of both detectors was employed [
30]. Alcohol and its metabolites in blood and other complex samples have been measured widely using GC-FID and GC-MS methods [
22,
30,
31,
32,
33,
34]. Although the sample can be introduced into the GC system via a liquid injection system, the headspace (HS) gaseous injection method is the popular choice for alcohols and their metabolites because of their volatile nature [
29]. A brief list of studies that determine the concentration of alcohols and their related compounds with their limit of detection (LOD) and limit of quantification (LOQ) are given is
Table 1.
In this study, a new GC-MS method is developed to determine the accurate concentration of alcohols and their metabolites using deuterium-labelled analytes. The use of isotopes as an internal standards (ISTD) not only enables the unambiguous identification of peaks within complex mixtures but also facilitate the accurate determination of the analyte concentration [
35]. The method has been successfully applied in the analysis of hand sanitizer samples to determine the presence of alcohols, acetaldehyde, and acetone. The primary objective of this study was to measure levels of the oxidation products of alcohol, acetaldehyde and acetone, and methanol. The effect of temperature and storage duration of hand sanitizer was also a focus of this study.
2. Materials and Methods
2.1. Chemicals
Commercially available methanol, ethanol, isopropanol, n-propanol, and acetone were obtained from Fisher Scientific, and acetaldehyde from Thermo Scientific. Heavy isotopes were obtained from the following sources; methanol-d4, ethanol-d6, acetone-d6 (Cambridge Isotopes), isopropanol-d8 (Thermo Chemicals) and acetaldehyde-d4 (Fisher Scientific).
2.2. Headspace Gas Chromatography /Mass Spectrometry (HS-GC/MS) Analysis
GC-MS analysis was performed on an Agilent 8860 GC attached to a 5977B MSD and 7697A headspace sampler. The GC column used for the analysis was an Agilent J&W DB-WAX ultra inert (30 m × 0.25 mm × 0.50 μm). An Agilent ultra inert split liner was used in the GC inlet for the split injection (50:1). The samples were injected from a 20 mL GC Headspace vial with a Teflon/silica crimp cap vial using the headspace autosampler. The headspace oven temperature, loop temperature, and transfer line temperatures were kept at 60 °C, 80 °C and 100 °C respectively. The sample vial was equilibrated in the headspace oven at 60 °C for 10 min prior to the injection and the sample was injected into the GC column over a period of 30 sec. The temperature of the GC inlet was kept at 120 °C. The GC-oven temperature program was started with a 2 min hold at 32 °C and then increased up to 110 °C at a rate of 10 °C /min and kept at 110 °C for 5 minutes. Helium was used as a carrier gas with a flow rate of 52.4 mL/min.
The mass spectrometer was operated in the electron impact (EI) mode. The MSD transfer line temperature and source temperature were set at 250 °C and 230 °C respectively.
2.3. Hand Sanitizer Sample Preparation
Commonly available gel-based hand sanitizer samples were purchased from local stores. Seven out of nine of them were ethanol-based hand sanitizers. Other types of samples used here are isopropanol-based and alcohol-free (benzalkonium chloride) hand sanitizers.
In ethanol-based hand sanitizers, the concentration of ethanol is significantly higher than the concentration of the other analytes. Therefore, two types of sample preparation were performed, one to quantify the ethanol content and the second to quantify all the other analytes except ethanol. The sample for ethanol quantification was prepared by using 1 mL of 1000 times diluted hand sanitizer (diluted with dI water) whereas the sample for the quantification of other analytes was prepared using 1 mL of the undiluted hand sanitizer. A similar approach was used in the preparation of isopropanol-based hand sanitizer too, one for the quantification of isopropanol (1.0 mL of 1000 times diluted hand sanitizer) and the second (1.0 mL of undiluted hand sanitizer) for the quantification of other analytes. In the case of non-alcohol-based hand sanitizer, the samples were prepared only by using 1 mL of the undiluted hand sanitizer. A known amount of the deuterium labelled compounds of the analytes, 5 μL methanol-d4 , 10 μL ethanol-d6, 5 μL isopropanol-d8, 5 μL acetaldehyde-d4, 5 μL acetone-d6 were also added as internal standards to each sample. The concentration of the internal standards were in the range of 0.041 mg/mL to 0.087 mg/mL. All the samples were prepared in a 20 mL crimp capped headspace GC glass vial.
For the temperature-dependent studies, the samples were sealed in GC vials after preparation and kept in a water bath at different temperature for 24 hours. Similarly, for time-dependent studies, the samples were placed in a 25 °C water bath for various durations of time.
3. Results and Discussion
3.1. GC-MS Analysis of Pure Alcohols, Acetone and Acetaldehyde
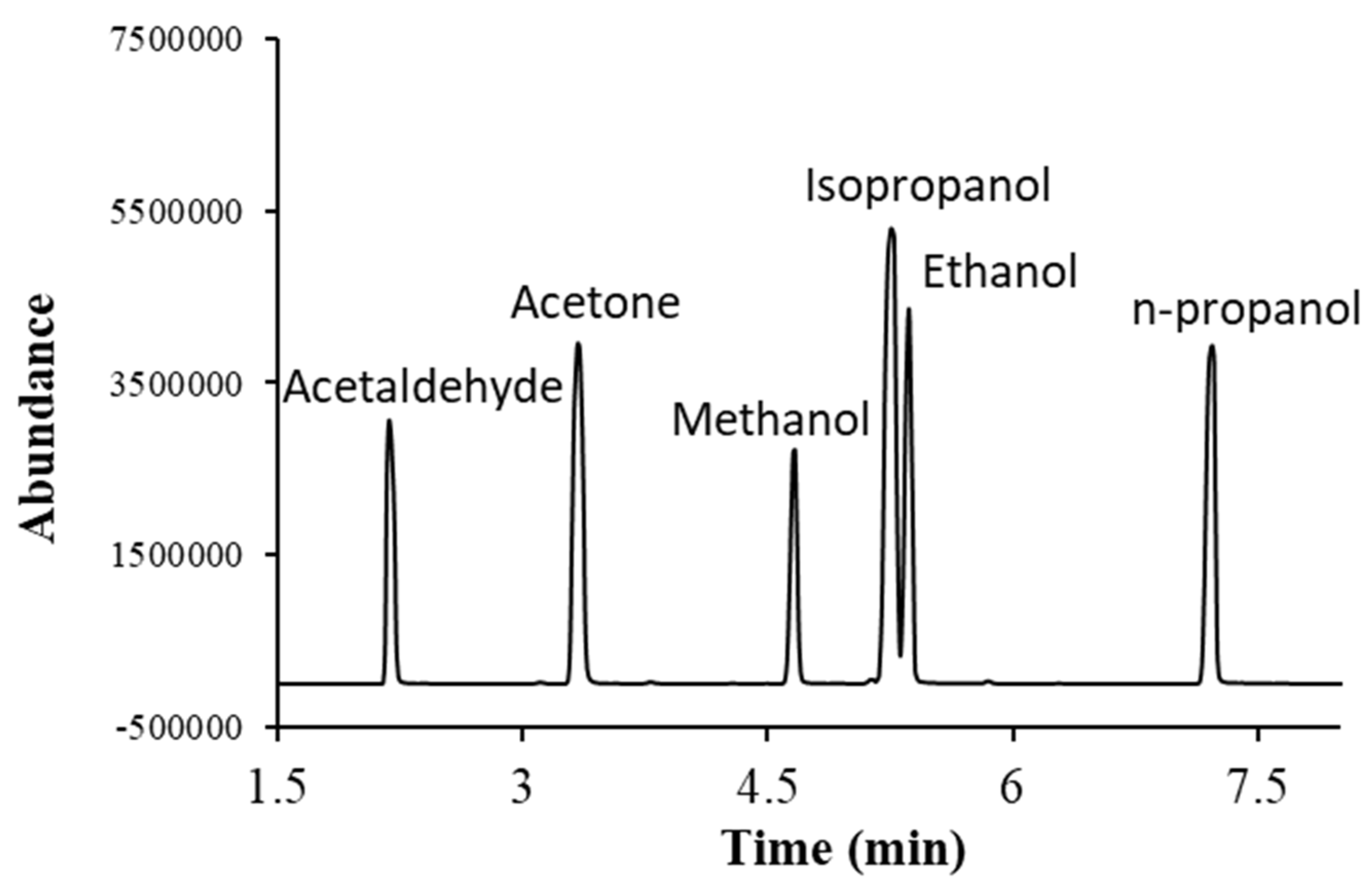
Commercially available alcohols, acetaldehyde and acetone were used to optimize the separation of analytes and their analysis conditions. Each compound was injected individually and as a mixture to confirm the chromatographic retention time and their mass spectrometric parameters. The GC-MS chromatogram obtained after the injection of a mixture of alcohols, acetone and acetaldehyde is given in
Figure 1. Acetaldehyde elutes at the earliest, whereas n-propanol elutes at the latest. Isopropanol and ethanol were eluting closely.
Initially, the mass spectrometry analysis was done in scan mode to identify the major characteristic ions (m/z) of each analyte. Similarly, isotopes (methanol-d4, ethanol-d6, acetone-d6, isopropanol-8 and aceladehyde-4) of the above were also injected to see the major ions in each compound. The characteristic ions of each compound found in the scan mode analysis were used for the quantification of analytes in hand sanitizers using the selected ion monitoring (SIM) method. For each compound, there was a qualifier ion and a quantifier ion. The quantifier ions used for the SIM mode analysis were as follows, methanol (m/z: 31, 33), ethanol (m/z: 31, 33), isopropanol (m/z: 45, 49), acetaldehyde (m/z: 44, 48), and acetone (m/z: 43, 46), where the lower m/z values are from the analyte and the higher m/z is from the isotope analogue of the analyte. The quantification was done by comparing the peak area of the analyte with the corresponding deuterated internal standard. A detailed list of major ions and fragment ions of each analyte is given in
Supplementary materials.
3.2. Method Validation
The method developed here has been validated by specificity, accuracy, precision, linearity, limit of detection (LOD) and limit of quantification (LOQ). Each analyte was injected in triplicate at a minimum of three different concentrations. The retention time of the analyte and the area of the corresponding peaks were analyzed.
Specificity: The chromatographic separation and characteristic mass to charge (m/z) peaks of each analyte were monitored to check if any other interfering peak was present at the retention time of the analyte by injecting both blank and standard samples. Each compound was injected individually and as a mixture to confirm the chromatographic retention time and their mass spectrometric parameters. The analytes used in this study had enough chromatographic separation and no interfering peak was observed. The GC-MS chromatogram obtained after the injection of a mixture of alcohols, acetone and acetaldehyde is given in
Figure 1.
Precision, and accuracy: The precision of the method was tested by preparing three sample replicates of standard solutions of each analyte and determining their concentration using the method described above. The concentration value obtained for each analyte from the triplicate samples exhibited a high degree of consistency. The relative standard deviation percentages obtained for each analyte are, methanol: 1.96%, ethanol: 1.64%, isopropanol: 1.97%, acetaldehyde: 1.95%, and acetone: 2.60%.
The accuracy was tested by injecting triplicate samples at concentrations of 0.001582 mg/mL, 0.001578 mg/mL, 0.00157 mg/mL, 0.00314 mg/mL and 0.000627 mg/mL, for methanol, ethanol, isopropanol, acetaldehyde, and acetone respectively. The observed concentration values were within the acceptance level.
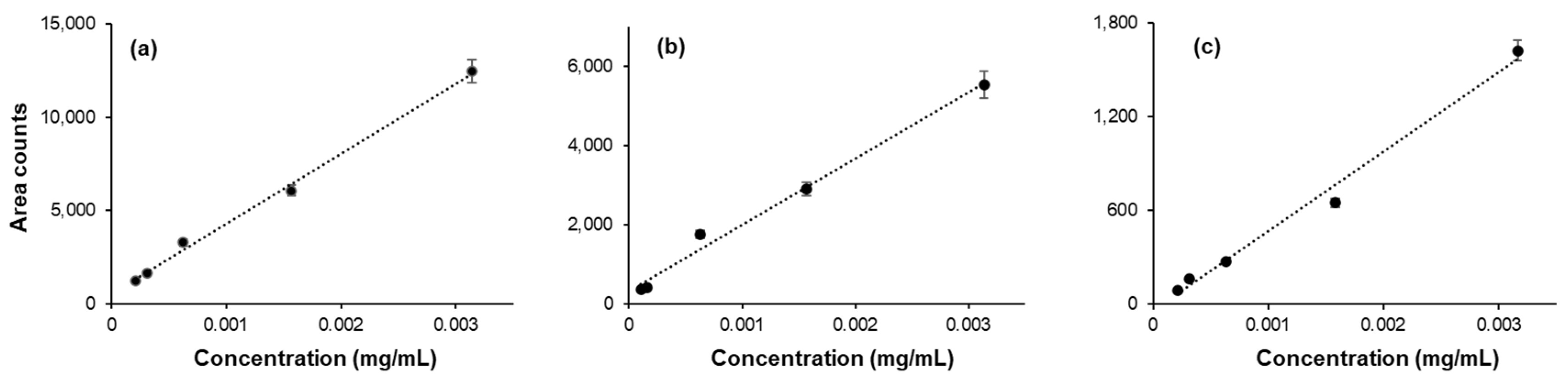
Linearity, limit of detection (LOD) and limit of quantification (LOQ): The GC-MS signal response to the concentration of analyte has been checked for each analyte by preparing a standard curve. The samples for the standard curve were prepared by adding a specified amount of the analyte (concentration range: 0.000001 -1.0 mg/mL) in a 20 mL glass vial. The total volume of the sample was adjusted to 1 mL by adding dI water. The standard curve obtained for acetaldehyde, acetone, and methanol are given in
Figure 2. Similar standard curves were obtained for ethanol, isopropanol and n-propanol . The limits of detection and limits of quantification were also determined. A wide range of LOD and LOQ values are reported in the literature. The LOD and LOQ values determined in this work, along with those from a few selected previous studies, are given in
Table 1.
3.3. GC-MS Analysis of Hand Sanitizers Kept in Common Storage Conditions and in Parked Cars
Most of the hand sanitizers selected in this study contain ethanol as the main ingredient. The concentration of alcohols, acetaldehyde and acetone present in each hand sanitizer was determined using the internal standard method described above. The ethanol-based hand sanitizers, HS1, HS2, HS3, HS4, and HS7 were stored at room temperature whereas HS6 and HS8 were stored in a parked car outside. The isopropanol-based hand sanitizer, HS9, and the non-alcohol based hand sanitizer, HS5, were stored at room temperature.
3.3.1. Analysis of Major Components in Hand Sanitizer
In all the ethanol-based hand sanitizers, the ethanol concentration was found to be in the range of 520- 560 mg/mL, which is in agreement with the expected values for ethanol-based hand sanitizers [
4,
43]. The amount of ethanol observed in the room-temperature samples was higher than that observed in the parked car samples. This is due to the high temperature inside the car during the parked stage. At elevated temperatures, ethanol evaporates from the hand sanitizers leading to a decrease in ethanol concentration in the parked car. In the isopropanol-based hand sanitizer (HS9), the major component was found to be isopropanol (540 mg/mL). In the alcohol-free hand sanitizers (HS5), no detectable amount of ethanol or other alcohols was determined. The main ingredient present in HS5 was benzalkonium chloride. No attempt was made to determine the concentration of benzalkonium chloride using GC-MS.
3.3.2. Quantification of Minor Components in Hand Sanitizer
The alcohol-related minor components found in the ethanol-based hand sanitizers were methanol, isopropanol, acetaldehyde and n-propanol (
Table 2). The highest concentration of methanol observed was 0.0152 mg/mL whereas the lowest concentration was 0.00014 mg/mL, which was observed in the HS7 and HS5 samples respectively. These levels of methanol were lower than the FDA approved level of 0.63 mg/mL[
44]. Methanol is a common impurity found in hand sanitizers as well as in other consumer products [
36,
43,
45]. Isopropanol was found as a minor component in all ethanol-based hand sanitizers selected here. The highest amount of isopropanol (13.84 mg/mL) was observed in HS2 hand sanitizer. Isopropanol was not detected in HS5 hand sanitizer. In most of the hand sanitizers, a small amount of n-propanol (0.0009-0.15 mg/mL) was also observed, except in HS1 and HS5. Here also, the amount of n-propanol observed was lower than the approved FDA level, which is 1.0 mg/mL. In all ethanol-based hand sanitizers, acetaldehyde was observed as a minor component. This is consistent with previous studies [
43]. The highest amount of acetaldehyde was observed in the HS4 sample, which was stored in room temperature for long time (2 months). Hand sanitizer samples from the parked cars (HS6 and HS8) have the lowest amount (0.001-0.002 mg/mL) of acetaldehyde. This could be due to the low boiling point of acetaldehyde, which must have evaporated out of the hand sanitizer bottle since it was equipped with a push down pump dispenser. In the case of acetone, a detectable amount was not found in ethanol or Benzalkylchloride -based hand sanitizers. However, in isopropanol-based hand sanitizer, acetone was found at a significant level.
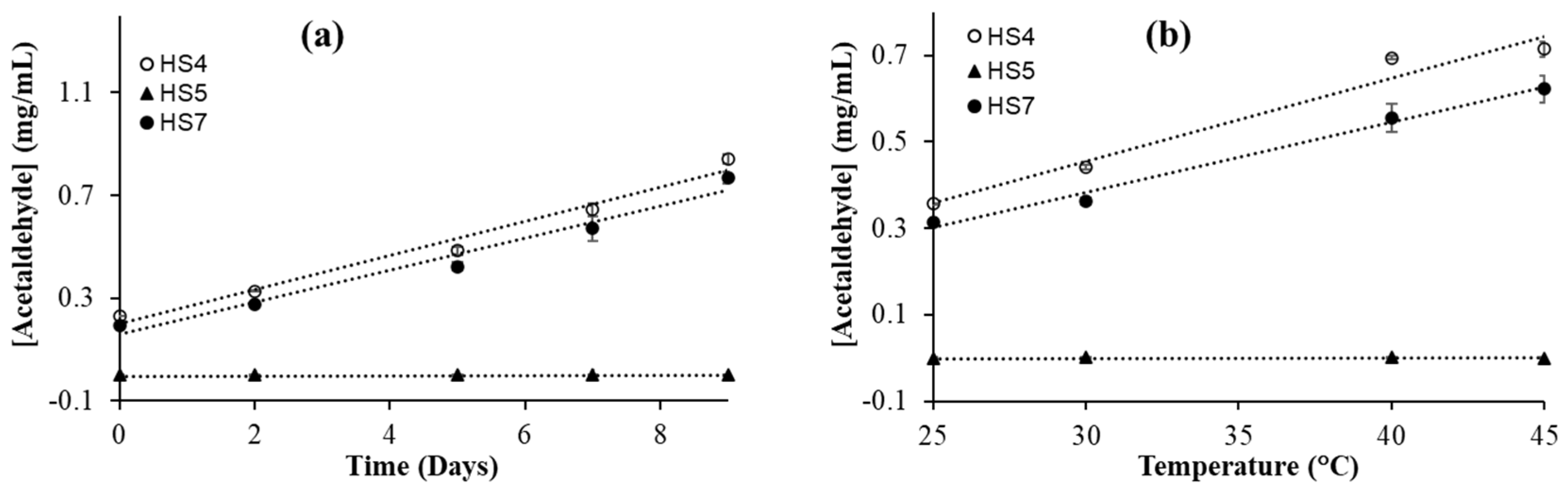
3.4. Effect of Storage Time on Acetaldehyde Formation in Ethanol-Based Hand Sanitizers
In all ethanol-based hand sanitizers, a small amount of acetaldehyde was observed. In HS4, and HS7, there was a significantly higher amount of acetaldehyde observed than in the other ethanol-based hand sanitizers. The lowest amount of acetaldehyde was found in the HS5, the alcohol-free hand sanitizer. Acetaldehyde is formed by the oxidation of ethanol at room temperature. Previous studies have shown that acetaldehyde can be formed in alcoholic beverages and other consumer products containing ethanol due to its long storage time [
43,
46,
47]. Acetaldehyde is a reactive compound, which can undergo reactions with other compounds as well. For example, in alcoholic beverages, especially wine, it reacts with flavonoids and produces other chemicals [
48].
To see the effect of storage time on the oxidation of alcohol, the hand sanitizer samples were kept in a sealed GC-MS vial for various durations of time at room temperature (25 °C) and analyzed the amount of acetaldehyde formed in the sample. It is observed that the concentration of acetaldehyde increases linearly with time (
Figure 3a). The concentration of acetaldehyde in HS4 and HS7 increased more than 200% over a period of seven days. In HS5, an alcohol-free hand sanitizer, no formation of acetaldehyde was observed. The HS5 hand sanitizer was used as a control for this study. This agrees with the expected result because acetaldehyde is formed from ethanol oxidation. The significant increase in acetaldehyde concentration in hand sanitizers may result from the presence of hydrogen peroxide, a common additive in hand sanitizers [
3,
4]. Hydrogen peroxide is a powerful oxidizing agent commonly used in various chemical reactions [
49]. The optimal condition for H
2O
2 - mediated oxidation is a slight acidic environment in the presence of transition metal ions. It was reported earlier that the oxidation of soft wood kraft pulp using H
2O
2 converts hydroxyl groups in carbohydrate to carbonyl groups, a reaction similar to the conversion of alcohols to acetaldehyde and acetone [
50]. The prolonged storage of hand sanitizer that contains a significant amount of H
2O
2 poses a higher chance of producing more acetaldehyde and can be a potential risk. Chemical oxidation of ethanol typically does not occur without the presence of oxidizing agents. This may explain why ethanol oxidation is minimal in alcoholic beverages since they may not contain significant concentration of H
2O
2. Also, some alcoholic beverages (e.g. wine) contain antioxidants [
51].
3.5. Effect of Temperature on Acetaldehyde Formation in Ethanol-Based Hand Sanitizers
Keeping hand sanitizers in cars and in other high-temperature places is a very common practice in daily life. The hand sanitizers HS6 and HS8 were kept in a car for two weeks before the analyses. As we can see in the
Table 2, acetaldehyde concertation was low in those samples compared with other samples. The bottles of HS6 and HS8 were equipped with push-pump system to deliver the hand sanitizer. Since it was not completely sealed, ethanol and acetaldehyde must have evaporated from the bottle. The ethanol concentration was also lower in these samples compared with the other samples stored at room temperature.
To check the effect of temperature on hand sanitizer, the hand sanitizer samples were prepared in sealed GC-vials for each temperature individually and kept at different temperatures for 24 hours. The highest temperature used was 45 °C, which is very close to the inside temperature of a parked car in the summer. For this experiment, two ethanol-based (HS4 and HS7) and a non-alcohol based hand sanitizer (HS5) were used. A significant increase in the concentration of acetaldehyde was observed in both ethanol-based hand sanitizers (
Figure 3b). However, no such increase was observed in HS5, which was expected. In both ethanol-based hand sanitizers, we observed a linear dependence on acetaldehyde formation with temperature. An increase of more than 100% in acetaldehyde concentration was observed in HS4 and HS7 when the sample was kept at 45 °C for 24 hours compared to the samples kept at 25 °C for the same duration. This is not very surprising, because temperature typically increases the rate of most reactions. Previous kinetic studies of the ethanol oxidation reaction, by the reaction between alpha hydroxyethyl radical and oxygen, showed the increased production of acetaldehyde at higher temperature [
52]. This is consistent with the results obtained here. As described earlier, H2O2 plays a role here as well. The efficacy studies of ethanol-based hand sanitizer against microbes have shown that their efficiency is optimal at 22 °C and decreases when the temperature is above 40 °C [
53] which could be due to the decrease in ethanol concentration via evaporation as well as the conversion to acetaldehyde.
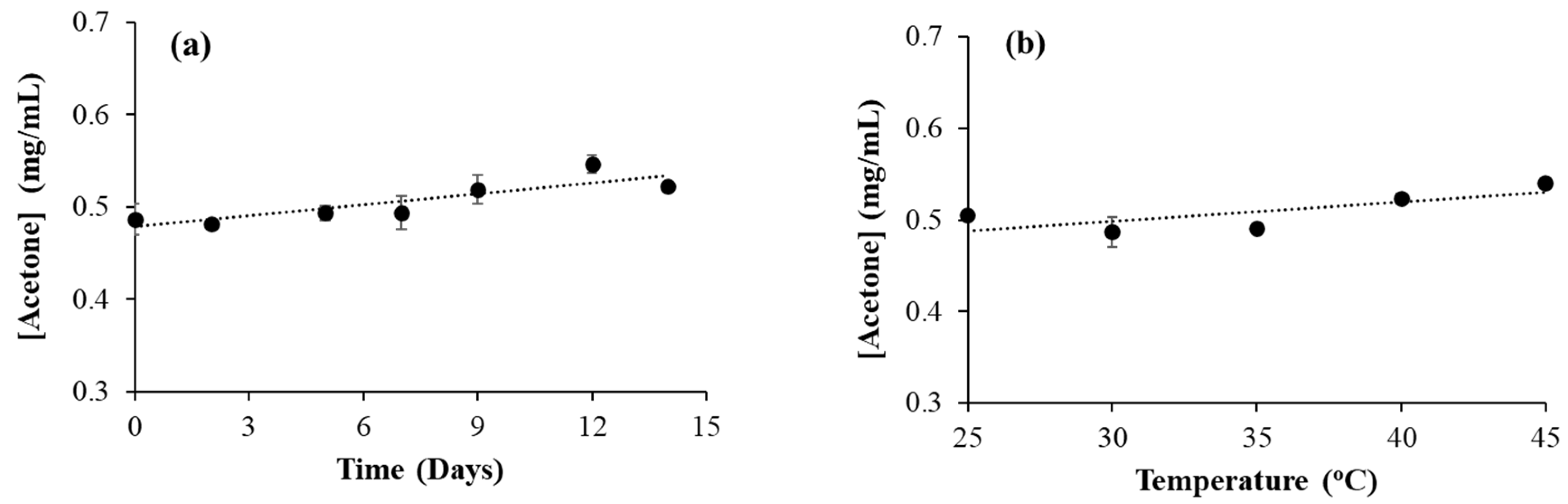
3.6. Effect of Time and Temperature on Acetone Formation in Isopropanol-Based Hand Sanitizer
Similar to ethanol, isopropanol can also undergo oxidation to form its oxidation product, acetone. In isopropanol-based hand sanitizer, HS9, a small amount of acetone was observed (
Table 2). In order to see the effect of time on oxidation, a time-dependent experiment was conducted with HS9 samples at 30°C . The samples were kept in a GC-MS vial, sealed and placed in a 30°C water bath for various time durations. However, no significant increase in acetone concentration was observed during this time (
Figure 4a). In seven days, the increase in acetone concentration was less than 10%. Similarly, the effect of temperature on acetone formation was also checked by heating the HS9 sample at various temperatures for 24 hours. Here also, there was no significant increase in acetone concentration observed (
Figure 4b).
This is different from what we observed with acetaldehyde formation in ethanol-based hand sanitizers. Acetaldehyde concentration increased with both time and temperature, whereas acetone concentration didn’t change significantly with time or temperature. This can be due to the difference in the nature of the OH functional groups in ethanol and isopropanol. The OH functional group in ethanol is a primary alcohol whereas in isopropanol it is a secondary alcohol. The oxidation of secondary alcohol is always more difficult than that of primary alcohol. Additionally, in enzymatic reactions, the secondary alcohol oxidizes slower than the primary alcohol [
54].
4. Conclusion
The headspace GC-MS method developed in this study proves to be a reliable and sensitive method to quantify trace amounts of impurities such as acetaldehyde, acetone, methanol and other alcohols present in hand sanitizer and similar samples. The incorporation of heavy-labelled isotopes assists in definitively identifying the target compound within a complex mixture. This method has a broad application in various sample types including body fluids and other complex mixtures, making it suitable for diverse fields such as forensics and toxicology. The oxidative products of alcohol formed in hand sanitizers (e.g. acetaldehyde) are toxic chemicals that can be formed very easily at room temperature conditions. An increase of 10-20 °C above room temperature, which is analogous to the temperature inside a parked car, can significantly elevate the generation of acetaldehyde in ethanol-based hand sanitizers. If the hand sanitizer bottle has a permanent opening, such as a push-down pump dispenser bottle, it can release the acetaldehyde vapors into the atmosphere inside the car, exposing passengers to it. Similarly, prolonged storage of hand sanitizer at room temperature in closed containers can result in the accumulation of high concentrations of acetaldehyde inside the bottle. The presence of H2O2 in hand sanitizers can enhance the generation of acetaldehyde. The amount of H2O2 can vary based on the manufacturers and can have a significant impact on the efficiency of hand sanitizer. The structural differences between ethanol and isopropanol influence their extent of oxidation. Since isopropanol-based hand sanitizers have slower oxidation rate than ethanol-based hand sanitizers, isopropanol-based hand sanitizer can be more efficient than ethanol-based hand sanitizers when they need to be stored for longer periods or exposed to higher temperatures.
Supplementary Materials
The following supporting information can be downloaded at: insert URL here. Table S1: GC-MS mass spectrometric ions and major fragment ions of alcohols, acetaldehyde and acetone.
Author Contributions
JT: Conceptualization, funding acquisition, investigation, methodology, project administration, data analysis, supervision and writing the manuscript. CT: methodology, data analysis, review and editing, investigation. All authors have read and agreed to the published version of the manuscript.
Funding
JT received financial support from UHD’s Office of Research and Sponsored Programs (ORCA), NS department, and Welch grant to conduct this project. CT received financial support from UHD Scholars academy, Welch scholarship and DOED MSEIP awards.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data availability statement
The data presented in this study are stored with the corresponding author available upon request. Additional data are available as
Supplementary Materials.
Acknowledgments
Authors acknowledge the contribution of Austin Harmon to this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Filipe, H. A. L.; Fiuza, S. M.; Henriques, C. A.; Antunes, F. E. Antiviral and Antibacterial Activity of Hand Sanitizer and Surface Disinfectant Formulations. Int. J. Pharm. 2021, 609, 121139. [CrossRef]
- Tilley, F. W.; Schaffer, J. M. Relation between the chemical constitution and germicidal activity of the monohydric alcohols and phenols. J. Bacteriol. 1926, 12 (5), 303–309. [CrossRef]
- Golin, A. P.; Choi, D.; Ghahary, A. Hand sanitizers: A review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am. J. Infect. Control 2020, 48 (9), 1062–1067. [CrossRef]
- Jing, J. L. J.; Pei Yi, T.; Bose, R. J. C.; McCarthy, J. R.; Tharmalingam, N.; Madheswaran, T. Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations. Int. J. Environ. Res. Public Health 2020a, 17 (9), 3326. [CrossRef]
- (WHO), W. H. O. WHO guidelines on hand hygiene in health care: First global patient safety challenge: clean care is safer care; World Health Organization, Patient Safety: Geneva, Switzerland, 2009.
- Pereira, B. M. P.; Tagkopoulos, I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019, 85 (13). [CrossRef]
- Mohapatra, S.; Yutao, L.; Goh, S. G.; Ng, C.; Luhua, Y.; Tran, N. H.; Gin, K. Y.-H. Quaternary Ammonium Compounds of Emerging Concern: Classification, Occurrence, Fate, Toxicity and Antimicrobial Resistance. J. Hazard. Mater. 2022, 130393. [CrossRef]
- Lunn, R. M.; Mehta S. S.; Jahnke G. D.; Wang A.; Wolfe M. S.; Berridge B. R. Cancer hazard evaluations for contemporary needs: Highlights from new national toxicology program evaluations and methodological advancements. J Natl Cancer Inst. 2022, 114(11), 1441-1448. [CrossRef]
- Ramchandani, V. A.; Bosron, W. F.; Li, T. K. Research advances in ethanol metabolism. Pathol. Biol. 2001, 49 (9), 676–682. [CrossRef]
- Mello, T.; Ceni, E.; Surrenti, C.; Galli, A. Alcohol induced hepatic fibrosis: Role of acetaldehyde. Mol. Asp. Med. 2008, 29 (1-2), 17–21. [CrossRef]
- Lieber, C. S. Ethanol metabolism, cirrhosis and alcoholism. Clin. Chim. Acta 1997, 257 (1), 59–84.
- Nordmann, R.; Ribiere, C.; Rouach, H.; Beauge, F.; Giudicelli, Y.; Nordmann, J. Metabolic pathways involved in the oxidation of isopropanol into acetone by the intact rat. Life Sci. 1973, 13 (7), 919–932. [CrossRef]
- Kalapos, M. P. On the mammalian acetone metabolism: from chemistry to clinical implications. Biochim. Biophys. Acta (BBA) - Gen. Subj. 2003, 1621 (2), 122–139. [CrossRef]
- Ross, J. A.; Borek, H. A.; Holstege, C. P.; King, J. D. Toxic Alcohol Poisoning. Emerg. Med. Clin. North Am. 2022, 40 (2), 327–341. [CrossRef]
- Daniel, D. R.; McAnalley, B. H.; Garriott, J. C. Isopropyl Alcohol Metabolism After Acute Intoxication in Humans. J. Anal. Toxicol. 1981, 5 (3), 110–112. [CrossRef]
- Jones, A. W. Elimination Half-Life of Acetone in Humans: Case Reports and Review of the Literature. J. Anal. Toxicol. 2000, 24 (1), 8–10. [CrossRef]
- Tomsia, M.; Głaz, M.; Nowicka, J.; Cieśla, J.; Sosnowski, M.; Chełmecka, E. Fatal Methanol Poisoning Caused by Drinking Industrial Alcohol: Silesia Region, Poland, April–June 2022. Toxics 2022, 10 (12), 800.
- HOVDA, K. E.; HUNDERI, O. H.; TAFJORD, A. B.; DUNLOP, O.; RUDBERG, N.; JACOBSEN, D. Methanol outbreak in Norway 2002-2004: epidemiology, clinical features and prognostic signs. J. Intern. Med. 2005, 258 (2), 181–190.
- Liberski, S.; Kaluzny, B. J.; Kocięcki, J. Methanol-induced optic neuropathy: a still-present problem. Arch. Toxicol. 2022, 96 (2), 431–451. [CrossRef]
- Cordell, R. L.; Pandya, H.; Hubbard, M.; Turner, M. A.; Monks, P. S. GC-MS analysis of ethanol and other volatile compounds in micro-volume blood samples—quantifying neonatal exposure. Anal. Bioanal. Chem. 2013, 405 (12), 4139–4147.
- Mead, R. N.; Cala, J. M.; Felix, J. D.; Shimizu, M. S.; Casas, M. S.; Lathrope, T.; Avery, G. B.; Kieber, R. J.; Willey, J. D. A static headspace GC-MS/MS method for the determination of ethanol, iso-butanol, and n -butanol at nanomolar concentrations in aqueous environmental samples. Limnol. Oceanogr. 2017, 15 (12), 1007–1014.
- Mihretu, L. D.; Gebru, A. G.; Mekonnen, K. N.; Asgedom, A. G.; Desta, Y. H. Determination of ethanol in blood using headspace gas chromatography with flameionization detector (HS-GC-FID): Validation of a method. Cogent Chem. 2020, 6 (1), 1760187. [CrossRef]
- Swift, R. Direct measurement of alcohol and its metabolites. Addiction 2003, 98, 73–80. [CrossRef]
- Ellis, D. I.; Muhamadali, H.; Xu, Y.; Eccles, R.; Goodall, I.; Goodacre, R. Rapid through-container detection of fake spirits and methanol quantification with handheld Raman spectroscopy. Analyst 2019, 144 (1), 324–330. [CrossRef]
- Enrico, P.; Diana, M. On the Accuracy of In Vivo Ethanol and Acetaldehyde Monitoring, a Key Tile in the Puzzle of Acetaldehyde as a Neuroactive Agent. Front. Behav. Neurosci. 2017, 11. [CrossRef]
- Kristoffersen, L.; Smith-Kielland, A. An Automated Alcohol Dehydrogenase Method for Ethanol Quantification in Urine and Whole Blood. J. Anal. Toxicol. 2005, 29 (5), 387–389.
- Church, A. S.; Witting, M. D. Laboratory testing in ethanol, methanol, ethylene glycol, and isopropanol toxicities. J. Emerg. Med. 1997, 15 (5), 687–692. [CrossRef]
- Sugaya, N.; Nakagawa, T.; Sakurai, K.; Morita, M.; Onodera, S. Analysis of Aldehydes in Water by Head Space-GC/MS. J. HEALTH SCI. 2001, 47 (1), 21–27. [CrossRef]
- Abrigo, N.; Ruzicka, C.; Faustino, P.; Stiber, N.; NguyenPho, A.; O’Connor, T.; Shakleya, D. Development and validation of a headspace GC-MS method to evaluate the interconversion of impurities and the product quality of liquid hand sanitizers. AAPS Open 2022, 8 (1). [CrossRef]
- Tiscione, N. B.; Alford, I.; Yeatman, D. T.; Shan, X. Ethanol Analysis by Headspace Gas Chromatography with Simultaneous Flame-Ionization and Mass Spectrometry Detection. J. Anal. Toxicol. 2011, 35 (7), 501–511. [CrossRef]
- Chun, H.-J.; Poklis, J. L.; Poklis, A.; Wolf, C. E. Development and Validation of a Method for Alcohol Analysis in Brain Tissue by Headspace Gas Chromatography with Flame Ionization Detector. J. Anal. Toxicol. 2016, 40 (8), 653–658. [CrossRef]
- Islek, D.; Ramadanoglu, S. Headspace-gas chromatography/mass spectrometry analysis of methanol in blood. Med. Sci. | Int. Med. J. 2017, 1.
- Xiao, H.-t.; He, L.; Tong, R.-s.; Yu, J.-y.; Chen, L.; Zou, J.; Li, J.-q.; Bian, Y.; Zhang, Y. Rapid and Sensitive Headspace Gas Chromatography-Mass Spectrometry Method for the Analysis of Ethanol in the Whole Blood. J. Clin. Lab. Anal. 2014, 28 (5), 386–390.
- Wunder, C.; Pogoda, W.; Paulke, A.; Toennes, S. W. Assay of ethanol and congener alcohols in serum and beverages by headspace gas chromatography/mass spectrometry. MethodsX 2021, 8, 101563.
- Patel, J. P.; Sowers, M. L.; Herring, J. L.; Theruvathu, J. A.; Emmett, M. R.; Hawkins, B. E.; Zhang, K.; DeWitt, D. S.; Prough, D. S.; Sowers, L. C. Measurement of Postreplicative DNA Metabolism and Damage in the Rodent Brain. Chem. Res. Toxicol. 2015, 28 (12), 2352–2363. [CrossRef]
- Jang, M.; Yang, H.; Shin, G.; Koo, J. M.; Hwang, S. Y.; Park, J.; X. Oh, D. Determination of Methanol in Commercialized Alcohol-based Hand Sanitizing and Other Similar Products using Headspace GC-MS. Curr. Anal. Chem. 2022, 18.
- Valavala, S.; Seelam, N.; Tondepu, S.; Jagarlapudi, V. S. K.; Sundarmurthy, V. Analytical Method Development and Validation for the Quantification of Acetone and Isopropyl Alcohol in the Tartaric Acid Base Pellets of Dipyridamole Modified Release Capsules by Using Headspace Gas Chromatographic Technique. J. Anal. Methods Chem. 2018, 2018, 1–10. [CrossRef]
- Rahman, Z.; Akhtar, S.; Siddiqui, A.; Ciavarella, A. B.; Nguyenpho, A.; Faustino, P. J.; Khan, M. A. A headspace-gas chromatography method for isopropanol determination in warfarin sodium products as a measure of drug crystallinity. Acta Pharm. 2018, 68 (1), 31–46. [CrossRef]
- Elzinga, S.; Dominguez-Alonzo, J.; Keledjian, R.; Douglass, B.; Raber, J. C. Acetone as Artifact of Analysis in Terpene Samples by HS-GC/MS. Molecules 2022, 27 (18), 6037. [CrossRef]
- Massick, S. Portable breath acetone measurements combine chemistry and spectroscopy. SPIE Newsroom 2007. [CrossRef]
- Dong, C.; Xing, X.; Chen, N.; Liu, X.; Wang, Y.; Synthesis of Hollow CuO Fibers for Low-Ppm-Level n-Propanol Detection via a Facile Solution Combustion Method. Sens Actuators B. 2016, 230, 1-8.
- Li, W.; Li, X.; Zong, Y.; Kong, L.; Zhu, W.; Xu, M., et al. Detection of n-Propanol Down to the Sub-ppm Level Using p-Type Delafossite AgCrO2 Nanoparticles, ACS Sens. 2023, 8, 289-96.
- Manuel, C. S.; Yeomans, D. J.; Williams, J. A.; Fricker, C.; Kucera, K.; Light, D. et al. Presence of unsafe chemical impurities, accelerated evaporation of alcohol, and lack of key labeling requirements are risks and concerns for some alcohol-based hand sanitizers and dispenser practices during the COVID-19 pandemic. PLoS One. 2022, 17. [CrossRef]
- Gloekler, L. E.; de Gandiaga, E. J.; Binczewski, N. R.; Steimel, K. G.; Massarsky, A.; Kozal, J. et al. Evaluation of the Safety and Efficacy of Hand Sanitizer Products Marketed to Children Available during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health, 2022, 19, 14424. [CrossRef]
- Nisbar, N. D. ; Jamal Khair, S. K.; Bujang, N. B.; Mohd Yusop, A. Y. Determination of ethanol, isopropyl alcohol and methanol in alcohol-based hand sanitiser to ensure product quality, safety and efficacy. Sci Rep. 2023, 13, 9478. [CrossRef]
- Moreira, M. T. G.; Pereira, P. R.; Aquino, A.; Conte-Junior, C. A.; Paschoalin, V. M. F. Aldehyde Accumulation in Aged Alcoholic Beer: Addressing Acetaldehyde Impacts on Upper Aerodigestive Tract Cancer Risks. Int. J. Mol. Sci. 2022, 23,14147.
- Shin, K. S.; Lee, J. H. Acetaldehyde contents and quality characteristics of commercial alcoholic beverages. Food Sci. Biotechnol. 2019, 28, 1027-36. [CrossRef]
- Han, G.; Webb, M. R.; Waterhouse, A. L. Acetaldehyde reactions during wine bottle storage. Food Chem. 2019, 290, 208-15. [CrossRef]
- Heitler, C.; Scaife, D. B.; Thompson, B. W. The oxidation of ethanol by hydrogen peroxide. Part I. Catalysis by ferric ion. J. Chem. Soc. A. 1967, 1967, 1409-13.
- Martinsson, A.; Hasani, M.; Theliander, H. The influence of transition metal ions on the oxidation of kraft pulp using hydrogen peroxide under mildly acidic conditions. Holzforschung. 2023, 77, 318-25.
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T. et al. Contribution of Red Wine Consumption to Human Health Protection. Molecules, 2018, 23, 1684. [CrossRef]
- da Silva, G.; Bozzelli, J. W.; Liang, L.; Farrell, J. T. Ethanol oxidation: kinetics of the alpha-hydroxyethyl radical + O2 reaction. J. Phys. Chem. A, 2009, 113, 8923-33. [CrossRef]
- Molom-Ochir S, Davis KM. Effects of Temperature on Hand Sanitizer Efficiency. J. Emerg. Invest. 2022, 5, 1. [CrossRef]
- Dalziel, K.; Dickinson, F. M. The kinetics and mechanism of liver alcohol dehydrogenase with primary and secondary alcohols as substrates. Biochem. J. 1966, 100, 34-46. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).