Background
Age-related macular degeneration (AMD) is a chronic degenerative disorder of the retina that leads to the progressive loss of central vision (
1). Neovascular AMD (nAMD), otherwise known as wet or exudative AMD, is characterized by aberrant angiogenesis originating from the choroidal or retinal circulation (
2). nAMD accounts for 7% of blindness worldwide (
3) and is responsible for more than 80% of AMD-related vision loss, despite only accounting for approximately 15% of all AMD cases (
4). As such, early diagnosis and treatment of nAMD are essential to prevent permanent vision loss in the affected eye (
5).
Intravitreal anti-vascular endothelial growth factor (VEGF) therapies, such as ranibizumab, aflibercept and unlicensed bevacizumab, are considered the gold standard for the treatment of nAMD, slowing the pathophysiological progression and reducing the severity of vision loss [6-8]. Frequent and continuous treatment with anti-VEGFs has been shown to be critical in maintaining visual acuity (VA) and preventing vision loss in patients with nAMD; however, the burden of regular clinic visits on patients and their caregivers is substantial (
9,
10). Regular visits are crucial in maintaining vision gained during the loading phase of treatment; however, these visits increase the treatment burden for patients, who tend to be elderly and have mobility issues or other comorbidities. It is also known that in chronic diseases the absence of persons, such as family members or nursing staff, accompanying patients to medical appointments adversely affects patients’ adherence (
11,
12). Variation in adherence to anti-VEGF treatment has previously been reported, and although there are reports of its association with functional outcomes (
13,
14,
15,
16,
17), there remains a need for a better understanding of the impact of patients’ adherence to treatment on visual outcomes in real-world clinical practice in some countries.
The aim of this retrospective, single centre cohort study was to investigate the impact of patient adherence to anti-VEGF treatment on visual outcomes in a real-world setting in Switzerland.
Methods
Patients and procedures
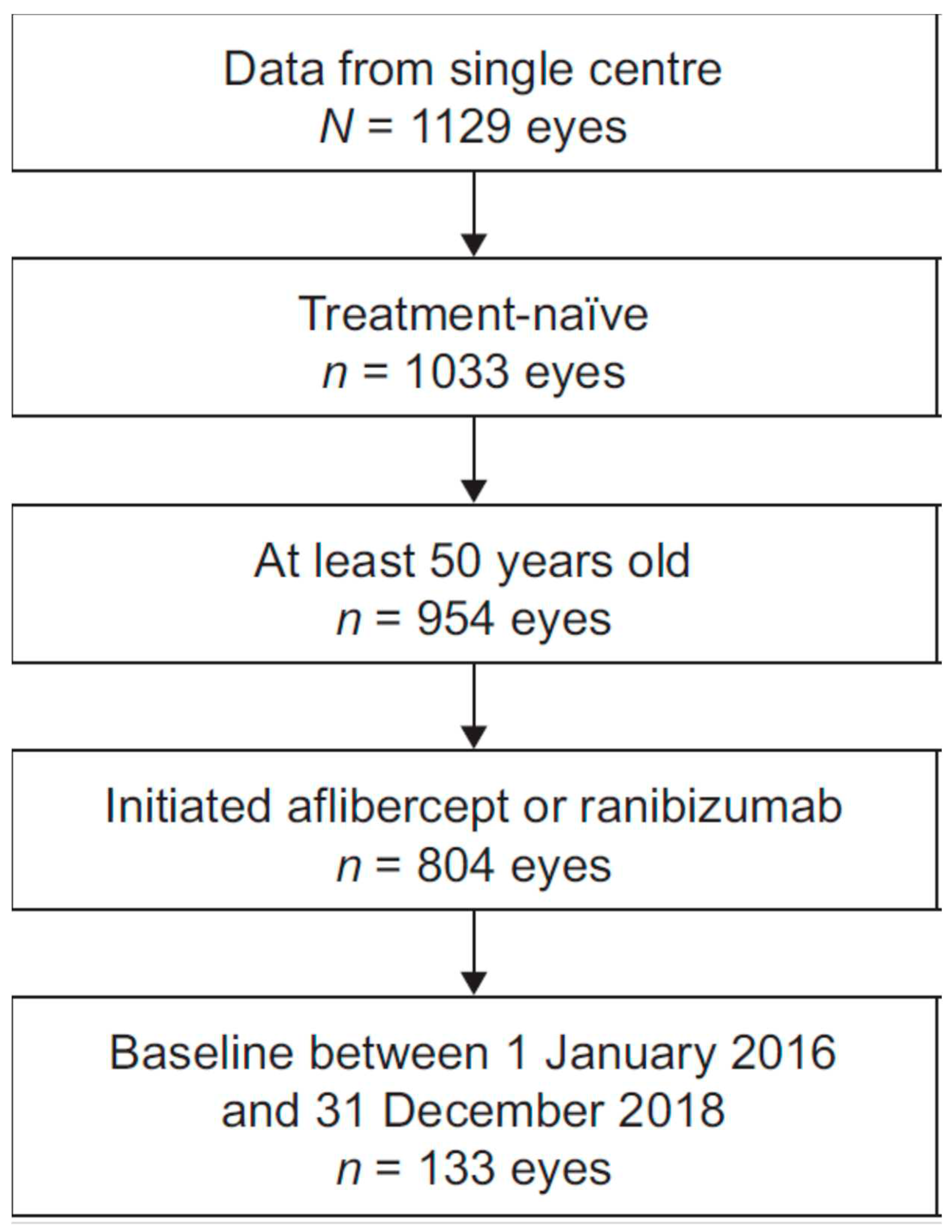
This retrospective, non-interventional, non-randomized, non-comparative study was designed to assess adherence to anti-VEGFs in treatment-naïve patients with nAMD who received their first ranibizumab or aflibercept injection between 1 January 2016 and 31 December 2018 (index period).
The study was conducted using data obtained from the Department of Ophthalmology at University Hospital Zurich, Zurich, Switzerland, which contributes data to the Fight Retinal Blindness (FRB!) International Registry. The FRB! Registry uses an electronic case report form to collect health-related data from patients receiving treatment for nAMD; treating institutions assign each patient a unique identifier code, which allows all patient data to be anonymized and encrypted (
18). All patients included in the study gave consent to be included in the registry. Ethical approval for the study was obtained from the Cantonal Ethics Committee, Zurich.
To be eligible for inclusion, patients (eyes) from the FRB! database with a diagnosis of nAMD were required to be 50 years of age or older on the date of their first injection (index date). Patients were excluded from the study if they had received any anti-VEGF other than ranibizumab or aflibercept prior to their index date.
In the instance of bilateral disease, both eyes were included in the analysis; therefore, “patient eye” is the unit of analysis. A patient eye was considered adherent if fewer than 20% of actual visits deviated from the dates of the scheduled visits by 14 days or more. Visits that deviated by more than 14 days from their scheduled date are described as “non-adherent visits” herein.
A minimum follow-up period of 12 months was required for all eyes. The 12-month study period was divided into the following time periods: 0–3 months (0–90 days); > 3 to 6 months (91–180 days); and > 6 to 12 months (181–420 days). Treatment intervals were categorized as either < 4 weeks (0–24 days), 4 to < 6 weeks (25–38 days), 6 to < 8 weeks (39–52 days), 8 to < 10 weeks (53–66 days), 10 to < 12 weeks (67–80 days) or ≥ 12 weeks (≥ 81 days).
Study objectives and endpoints
The primary objective of the study was to describe the real-world (non-)adherence rate to licensed anti-VEGF treatments in patients with nAMD during the first year of treatment; the primary endpoint was the rate of (non-)adherence. The secondary objectives of the study were to characterize the association between (non-)adherence and visual outcomes and look at treatment patterns. Secondary endpoints included: the number of days delay between scheduled and actual visits in (non-)adherent patient eyes during the first year of treatment; the proportion of treatment intervals that were < 4 weeks, 4 to < 6 weeks, 6 to < 8 weeks, 8 to < 10 weeks, 10 to < 12 weeks or ≥ 12 weeks (adherent vs non-adherent); the change in VA from baseline to months 3, 6, 9 and 12; and the number of injections, number of non-injection visits and total number of visits. VA was measured by the number of letters read on a logarithm of the minimum angle of resolution (logMAR) chart.
Statistical analyses
All analyses were conducted in R Version 3.6.2 (The R Project for Statistical Computing, Vienna, Austria). This was an exploratory study, and therefore no comparative analysis was carried out between adherent and non-adherent patient eyes. Continuous variables, including the number of injections, number of non-injection visits and total number of visits, were summarized using descriptive statistics, including means, medians, standard deviations (SDs), first and third quartiles, and two-sided 95% confidence intervals (CIs). Counts and proportions were provided for categorical variables with missing data considered as a separate category. No missing value imputation or sensitivity analyses were performed.
Results
Overall, 109 patients were included in the study (
Figure 1), 24 of whom had bilateral disease. Hence, 133 patient eyes were included; 129 (97.0%) were adherent and 4 (3.0%) were non-adherent. All non-adherent patients had unilateral disease. Baseline characteristics of patients are given in
Table 1. The mean age in the overall cohort was 80.1 years and 63.9% were women.
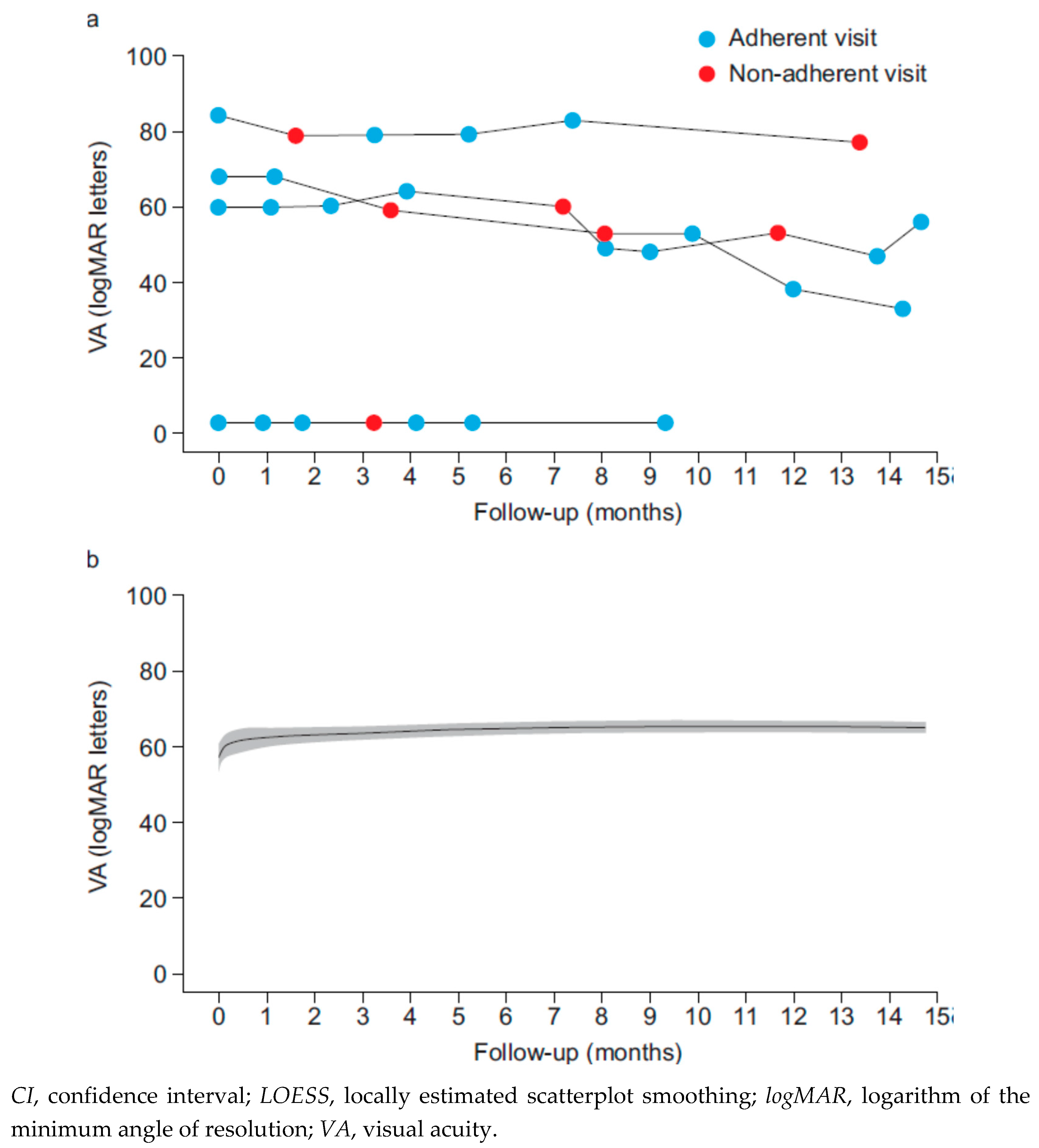
Mean (± SD) baseline VA was similar in adherent and non-adherent patient eyes (57.0 [± 23.6] logMAR letters vs 53.8 [± 35.3] letters, respectively;
Table 2). The mean (range) change in VA by month 12 of treatment was higher in adherent patient eyes (6.3 [−55–56] letters) than in non-adherent patient eyes (−11 [−30–0] letters).
Figure 2 shows VA over time in patient eyes. In all non-adherent eyes, VA decreased from index date to month 12.
In total, there were 1403 adherent visits (98.4%) and 23 non-adherent visits (1.6%), with 19 eyes having at least one non-adherent visit (
Table 3). Most non-adherent visits (
n = 17/23; 73.9%) occurred between the sixth and twelfth month of treatment. Adherent visits were associated with an overall mean increase in VA of 0.67 logMAR letters at each visit; the greatest increase (2.43 letters) was seen during the loading phase (months 0–3). On the contrary, non-adherence was associated with a mean decrease of −2.30 letters at each visit, with no VA gains from the previous visit during the loading phase and an overall decline in VA throughout months 3–12.
During the first year of treatment, the median number of injections in adherent patient eyes was 8; the corresponding figure for non-adherent patient eyes was 6 (
Table 2). Of the total 1426 visits, 1111 (77.9%) were associated with an injection. The median number of visits that did not result in an injection was one per patient eye in adherent patient eyes and 1.5 per patient eye in non-adherent patient eyes (
Table 2).
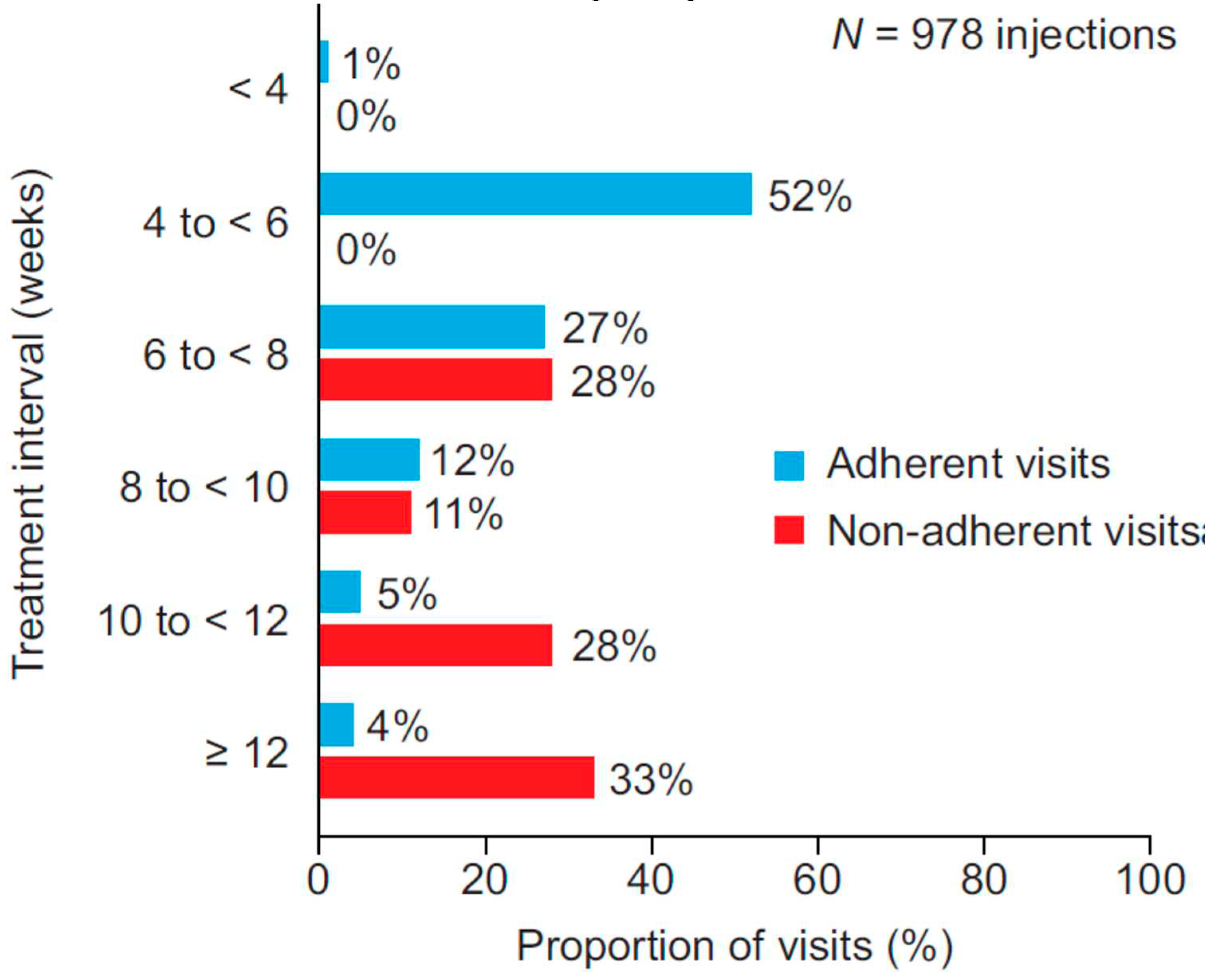
Injection intervals in adherent patient eyes tended to be shorter than those in non-adherent patient eyes. Most (78.2%) injection intervals for adherent visits were 4–8 weeks; however, for non-adherent visits most (72.2%) were 8 weeks or longer (
Figure 3).
Discussion
This non-interventional study found a high degree of adherence to treatment overall in a Swiss cohort of patients with nAMD and showed that adherence to anti-VEGF treatments was associated with favourable visual outcomes in real-world clinical practice.
Adherence to anti-VEGFs was found to be very high in the present study (97% of all patient eyes). This finding is consistent with previous studies, which have reported that patients with nAMD are more adherent to treatment than patients with other disorders of the macula, such as diabetic macular oedema (
19). Gillies et al. previously suggested that, owing to the paucity of other treatment options for patients with nAMD and the consequences of non-adherence, patients with nAMD may be particularly motivated to be adherent to treatment (
20).
Adherent patient eyes were shown to have a mean change in VA from baseline of 6.3 letters after 12 months of treatment – 17.3 letters more than non-adherent patient eyes. Eyes with adherent visits gained vision during the loading phase, with stability or further gains in the months following the loading phase. This is in contrast to patient eyes with non-adherent visits, in which overall there was no vision gain during the loading phase, followed by vision loss between months 3 and 12. Successful completion of a loading phase, as demonstrated here, has previously been shown to predict VA outcomes after 12 months of treatment (
21,
22). Together, these results demonstrate the positive impact of adherence to treatment on patients’ visual outcomes.
The real-world treatment burden of nAMD on patients and their carers is substantial due to the frequency of clinic visits; as such, real-world outcomes are rarely as favourable as those reported in randomized clinical trials (
23,
24). Nevertheless, the change in VA observed in adherent patients in the present study is within the range of that reported in randomized controlled trials, and higher than that often reported in real-world studies. The inclusion of the fellow eye, which typically has a higher baseline VA than the first-treated eye, and therefore less opportunity to improve vision, further highlights the noteworthiness of our results.
Overall, 78% of visits to the clinic were associated with an injection. The median number of visits not associated with an injection was only one. The minimal deviation between the number of visits and the number of injections suggests that, in the present study, physicians were successfully administering a treat and extend (T&E) protocol similar to that used in randomized controlled trials (
7,
8). Our results are in line with current literature, which suggests that in real-world clinical practice approximately 80% of visits are associated with an injection (
25).
As expected, most (78%) injection intervals for adherent visits were 4–8 weeks, whereas most (72%) were 8 weeks or longer for non-adherent visits. This suggests that an extended injection interval for patients receiving aflibercept or ranibizumab might decrease their likelihood of good visual outcomes. A recent study has similarly shown that delayed treatment has a detrimental effect on VA (
26), highlighting that with injection intervals of greater than 3 months, VA can be maintained only in a small minority of patients receiving aflibercept or ranibizumab. Thus, there is an unmet need for alternative treatment options that remain effective with longer injection intervals. This will in turn reduce the treatment burden on patients and will allow fewer adherent patients to maintain their VA gains following the loading phase. These findings emphasize that if patients are adherent to an adequate dosing regimen (i.e., T&E) and come to the clinic within the critical timeframe, then it is possible to achieve very good visual outcomes in real-world clinical practice. The consequences of non-adherence should be included in patients’ counselling when treatment starts.
The relatively short follow-up period (12 months) may be a limitation of this study; by not including patients with less than 12 months of follow-up, the likelihood of capturing the most adherent patients was increased. Given that only 10% of patients were included after implementation of the inclusion and exclusion criteria, the overall number of patients was low for a real-world study. Furthermore, only four patients were non-adherent; hence, results in non-adherent patients should be interpreted with caution. Finally, although there is the concern of limited generalizability of the results due to the small sample size, especially in the group of non-adherent patients, and the retrospective, single centre nature of the study, previous studies carried out in a single centre in Switzerland have demonstrated similar outcomes (
27).
Conclusions
The adherence rates to anti-VEGF treatment reported in this study were very high. Furthermore, good adherence was associated with favourable visual outcomes, likely to be associated with the use of a T&E protocol. These results demonstrate the importance of adhering to appropriate dosing regimens, such as T&E, for the treatment of nAMD. Physicians should emphasize to patients the importance of good adherence to treatment. Further studies are warranted on the effect of treatment adherence on visual outcomes in a real-world clinical practice over a longer period.
Author Contributions
All authors contributed to the drafting of the manuscript and approved the final version for submission. Daniel Barthelmes, Mark Gillies and Alexandros Sagkriotis contributed to study conception and design. Daniel Barthelmes, Mark Gillies and Sarah Steinmann contributed to the collection and interpretation of data for the work. Alexandros Sagkriotis contributed to the interpretation of data for the work. Vuong Nguyen contributed to the analysis and interpretation of data for the work.
Funding
This study was funded by Novartis Pharma AG.
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Cantonal Ethics Committee, Zurich. All patients included in the study gave consent to be included in the registry.
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Medical writing support was provided by Maxine Cox of PharmaGenesis London, London, UK, and funded by Novartis Pharma AG.
Competing interests
Daniel Barthelmes has received research grants from Novartis and Bayer and is a consultant for Alcon and Bayer. Mark Gillies is a consultant for and has received travel support, fees and research support from Bayer and Novartis. Alexandros Sagkriotis is a Novartis employee and shareholder. Vuong Nguyen and Sarah Steinmann have nothing to disclose.
References
- Birch, D.G.; Liang, F.Q. Age-related macular degeneration: a target for nanotechnology derived medicines. Int J Nanomedicine 2007, 2, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D.; Agarwal, A.; Lavina, A.M.; Tawansy, K.A. Focal inner retinal hemorrhages in patients with drusen: an early sign of occult choroidal neovascularization and chorioretinal anastomosis. Retina 2003, 23, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.; Price, H.; Taylor, H.; Leasher, J.; Keeffe, J.; Glanville, J.; Sieving, P.C.; Khairallah, M.; Wong, T.Y.; Zheng, Y.; et al. New systematic review methodology for visual impairment and blindness for the 2010 Global Burden of Disease study. Ophthalmic Epidemiol 2013, 20, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N Engl J Med 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Mones, J.; Richard, G.; Bandello, F.; et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol 2014, 98, 1144–1167. [Google Scholar] [CrossRef] [PubMed]
- Kovach, J.L.; Schwartz, S.G.; Flynn, H.W., Jr.; Scott, I.U. Anti-VEGF treatment strategies for wet AMD. J Ophthalmol 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006, 355, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Prenner, J.L.; Halperin, L.S.; Rycroft, C.; Hogue, S.; Williams Liu, Z.; Seibert, R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol 2015, 160, 725–731. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef]
- Mosen, D.M.; Schmittdiel, J.; Hibbard, J.; Sobel, D.; Remmers, C.; Bellows, J. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage 2007, 30, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Graffigna, G.; Barello, S.; Bonanomi, A. The role of Patient Health Engagement Model (PHE-model) in affecting patient activation and medication adherence: a structural equation model. PLoS One 2017, 12, e0179865. [Google Scholar] [CrossRef] [PubMed]
- Chong Teo, K.Y.; Saxena, N.; Gan, A.; Wong, T.Y.; Gillies, M.C.; Chakravarthy, U.; Gemmy Cheung, C.M. Detrimental Effect of Delayed Re-treatment of Active Disease on Outcomes in Neovascular Age-Related Macular Degeneration: The RAMPS Study. Ophthalmol Retina 2020, 4, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Ehlken, C.; Ziemssen, F.; Eter, N.; Lanzl, I.; Kaymak, H.; Lommatzsch, A.; Schuster, A.K. Systematic review: non-adherence and non-persistence in intravitreal treatment. Graefes Arch Clin Exp Ophthalmol 2020, 258, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Tadayoni, R.; Beatty, S.; Berger, A.R.; Cereda, M.G.; Hykin, P.; Staurenghi, G.; Wittrup-Jensen, K.; Nilsson, J.; Kim, K.; et al. Determinants of visual acuity outcomes in eyes with neovascular AMD treated with anti-VEGF agents: an instrumental variable analysis of the AURA study. Eye (Lond) 2016, 30, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.S.; Yu, Y.; VanderBeek, B.L. Association of Visit Adherence and Visual Acuity in Patients With Neovascular Age-Related Macular Degeneration: Secondary Analysis of the Comparison of Age-Related Macular Degeneration Treatment Trial. JAMA Ophthalmol 2020, 138, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yalamanchili, S.P.; Maatouk, C.M.; Enwere, D.U.; Conti, T.F.; Hom, G.L.; Briskin, I.N.; Greenlee, T.E.; Babiuch, A.S.; Singh, R.P. The Short-term Effect of a Single Lapse in Anti-Vascular Endothelial Growth Factor Treatment for Diabetic Macular Edema Within Routine Clinical Practice. Am J Ophthalmol 2020, 219, 215–221. [Google Scholar] [CrossRef]
- Gillies, M.C.; Walton, R.; Liong, J.; Arnold, J.J.; McAllister, I.; Morlet, N.; Hunyor, A.; Guymer, R.; Keeffe, J.; Essex, R.; et al. Efficient capture of high-quality data on outcomes of treatment for macular diseases: the Fight Retinal Blindness! project. Retina 2014, 34, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Ehlken, C.; Helms, M.; Bohringer, D.; Agostini, H.T.; Stahl, A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol 2018, 12, 13–20. [Google Scholar] [CrossRef]
- Gillies, M.C.; Walton, R.J.; Arnold, J.J.; McAllister, I.L.; Simpson, J.M.; Hunyor, A.P.; Guymer, R.; Essex, R.W.; Morlet, N.; Barthelmes, D. Comparison of outcomes from a phase 3 study of age-related macular degeneration with a matched, observational cohort. Ophthalmology 2014, 121, 676–681. [Google Scholar] [CrossRef]
- Clemens, A.; Sagkriotis, A.; Griner, R.; Durus, A.; Doyle, O.; Wintermantel, T.; Chakravarthy, U. Key confounders for translating results from non-interventional trials (NIS) to those observed in randomized controlled trials (RCTs): applying predictive analytics in neovascular age-related macular degeneration (nAMD). Proceedings of 19th EURETINA Congress, Paris, France., 5–8 September 2019. [Google Scholar]
- Holz, F.G.; Tadayoni, R.; Beatty, S.; Berger, A.; Cereda, M.G.; Cortez, R.; Hoyng, C.B.; Hykin, P.; Staurenghi, G.; Heldner, S.; et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015, 99, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.N.; Mehta, H.; Barthelmes, D.; Nguyen, V.; Gillies, M.C. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina 2016, 36, 1418–1431. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.Y.; Dubois, L.; Tadayoni, R.; Fajnkuchen, F.; Nghiem-Buffet, S.; Delahaye-Mazza, C.; Guiberteau, B.; Quentel, G. Results of one-year's treatment with ranibizumab for exudative age-related macular degeneration in a clinical setting. Am J Ophthalmol 2009, 148, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.C.; Nguyen, V.; Daien, V.; Arnold, J.J.; Morlet, N.; Barthelmes, D. Twelve-month outcomes of ranibizumab vs. aflibercept for neovascular age-related macular degeneration: data from an observational study. Ophthalmology 2016, 123, 2545–2553. [Google Scholar] [CrossRef]
- Teo, K.; Saxena, N.; Gan, A.; Wong, T.Y.; Gillies, M.C.; Chakravarthy, U.; Cheung, C. Detrimental effect of delayed retreatment of active disease on outcomes in neovascular age related macular degeneration – RAMPS study. Ophthalmol Retina 2020. [Google Scholar] [CrossRef]
- Parvin, P.; Zola, M.; Dirani, A.; Ambresin, A.; Mantel, I. Two-year outcome of an observe-and-plan regimen for neovascular age-related macular degeneration treated with Aflibercept. Graefes Arch Clin Exp Ophthalmol 2017, 255, 2127–2134. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).