1. Introduction
Phantom Limb Pain (PLP) is clinically defined as the perception of pain or discomfort in a limb that no longer exists [
1] and is commonly observed as a consequence of amputation, affecting as many as 80% of individuals who undergo this surgical procedure [
2]. The etiology of PLP appears to be predominantly linked to central neural changes; however, peripheral and psychological factors may also contribute to its manifestation.
The typical pain experienced in PLP is attributed to a dysfunction in the transmission of pain signals within the nervous system [
3]. Indeed, although the mechanisms underlying PLP remain unclear, it is known that sensitized and reorganized nerve endings and cell bodies within the peripheral limb affect the CNS, causing changes in somatosensory processing pathways [
3]. This process can result in the formation of neuromas, neoformations of nerve fibers of pathological nature, characterized by abnormal activity either spontaneously or as a result of mechanical stimulation of the stump. The pain may be severe at first, tending to ease over time; it is usually intermittent, but in some cases it can last for days or, in some people, become chronic and persists for years [
4]. Phantom sensation is a general term for a wide variety of symptoms [
5]. These sensations encompass positional, morphological, or kinetic attributes of the phantom, as well as pain, warmth, cold, itching, tingling, electric impulses, and other paraesthesias [
6]. After limb amputation, patients often report that the deafferented part of the body remains perceptually intact even in terms of pain or other sensations. This pain tends to be more intense in the distal parts and the characteristics of these sensations are often described as wounding, stabbing, throbbing, burning, aching, pinching, stinging and compressing [
2,
5].
Phantom pain can be associated with specific limb positions or movements and can be provoked or aggravated by various physical factors, such as changes in atmospheric conditions or pressure on the limb and psychological factors, such as emotional stress [
2,
7].
This type of chronic pain is often combined with symptoms such as sleep disturbances, anxiety, and depression [
8]. It is known that those emotional triggers contribute to the persistence and exacerbation of PLP leading to a central sensitization, a pathological condition characterized by increased neuronal activity, lowering of neuron activation threshold and spreading to other sites [
9]. The increased activity of peripheral nociceptors leads to changes in the structure of dorsal horn neurons, with increased responsiveness to stimuli and reduced inhibitory activity. In addition, peripheral nerve injury can result in degeneration of C-fibers in the lamina II of the spinal cord and subsequent colonization of A-beta fibers, which are responsible for the transmission of non-painful stimuli leading to misinterpretation. At the brain level, reorganization of the somatosensory cortex occurs as a result of the discrepancy between motor commands and proprioceptive feedback from the missing limb [
10]. Several studies have found a strong correlation between the degree of brain reorganization described above and the intensity of PLP [
2,
7].
The treatment of PLP is complex and recent studies have found limited evidence regarding the choice of pharmacological treatment. Some of these studies, however, have observed the effectiveness of morphine and gabapentin in reducing pain intensity while emphasizing the presence of side effects that limit their usability. Given the complexity of the pathological mechanism underlying chronic pain and the multitude of associated symptoms, additional therapeutic options beyond pharmacological treatment are deemed necessary, as pharmacotherapy alone is often insufficient [
11].
Whole Body Cryostimulation (WBC) is a therapeutic procedure involving exposure of the entire body to extremely cold air at temperatures as low as -140°C for 2-3 minutes. Widely recognized in the realm of sports for its well-documented pain-relieving and anti-inflammatory properties [
12], WBC is increasingly garnering attention within the clinical domains. In fact, several studies have shown beneficial effects in patients with various conditions characterized by chronic pain, fatigue and inflammation, such as obesity, fibromyalgia and neurological disorders [
13,
14,
15,
16,
17,
18]. WBC can slow down nerve conduction velocity, modulate the autonomic nervous system, stimulate endorphins secretion from the brain and induce exercise-mimicking molecular anti-inflammatory effects [
19], with direct implications in pain control [
20]. Therefore, we hypothesized that WBC may be beneficial in relieving symptoms of patients with PLP. To the best of our knowledge, no studies to date have addressed this specific issue so far.
2. Methods
2.1. Brief Case Description
Here we report the case of R.N., a 54-year-old male Paralympic athlete with Phantom Limb Syndrome secondary to right lower limb amputation in 2004. The patient was referred as an outpatient to the Rehabilitation Unit of Istituto Auxologico Italiano, in Piancavallo (VB), Italy, in August 2023 for a cycle of ten WBC sessions prescribed by a physiatrist (PC).
2.2. Clinical History
At the age of 15, he underwent open heart surgery for a congenital atrial septal defect. At the age of 20, following a car accident, he suffered an incomplete D7 spinal cord injury, which was managed conservatively with an orthosis and then bedridden for three months. He resumed daily activities with a spinal brace. At the age of 33, while working with a chainsaw, he suffered a muscle injury to the rectus muscle in the center of his thigh, which required 80 stitches with no other consequences. At the age of 35, while working on the family farm, the patient suffered an injury: his right lower limb was caught in the drive shaft of a tractor, which tore off the limb in the upper third of the tibia, causing it to be amputated. The patient was taken to the emergency department and underwent amputation at the mid-thigh of the right leg. The contralateral left lower limb also suffered muscle loss in the thigh and lower third of the leg, but subsequently recovered without residual anatomical or functional deficits. In the immediate post-operative period, pain was managed with opioid infusion, ketorolac and centrally acting analgesics (Gabapentin). PLP started soon after the amputation. Prosthetic fitting began two months after the incident. Upright posture and movement were restored in about one month. Three months after the accident, the patient was able to walk with a temporary mechanical prosthesis while waiting for an electronic prosthesis, which was provided after seven months. One year later, he resumed his previous job on the farm, but performance was below pre-injury levels. PLP lasted 18 months and gradually decreased in intensity. A remitting painful sensation persisted, typically lasting 4-5 days and triggered by weather changes. At the age of 45, he was abusing psychotropic (amphetamine), pharmacological (citalopram) substances and alcohol, and was diagnosed with myocarditis. Two years later, he managed to wean off addiction and started a canoeing and kayaking sports programme for people with disabilities. He also changed his job becoming a safety officer in a company. Over time, he achieved success in competitive sports. He is currently a multiple Italian paracanoe champion and two-time World champion in para-rafting with the National Amputee Team.
2.3. Clinical Examination
On examination, the patient presented with PLP and diffuse sports-related enthesitic pain in the right shoulder, right groin and chronic pain in the lower lumbar region radiating to the left hip, gluteus and anterior thigh. He reported persistent paresthesia or tingling, similar to numbness, occasionally accompanied by excruciating pain attacks described as 'shocks' or 'electric jolts'. This caused considerable discomfort and anger, which was difficult to manage since no effective remedies had been found over the years. This affected his emotional state and overall quality of life. The pain episodes typically lasted 4-5 days and were usually triggered by sudden temperature or weather changes. The patient also reported severe pain at night, which severely affected his mood and sleep quality. Subjectively, he described the pain as "coming from the brain", as if "the pain is in the brain because that's where the suffering comes from".
2.4. Symptoms Assessment
The clinical evaluations included assessments conducted both before and after the WBC cycle. Pain levels were assessed using the Numeric Rating Scale (NRS) [
21]. To discriminate between neuropathic and nociceptive pain, the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) was used [
22]. The quality and intensity of pain were analyzed using the Short-form-McGill Pain Questionnaire 2 (SF-MPQ-2) [
23]. Health-related quality of life was evaluated using the Short Form Health Survey 36 (SF-36) [
24]. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) [
25]. General subjective well-being was measured using the 5-item World Health Organization Well-Being Index (WHO-5) [
26]. Additionally, the severity of depression was determined using the Beck Depression Inventory(BDI) [
27].
2.5. Intervention
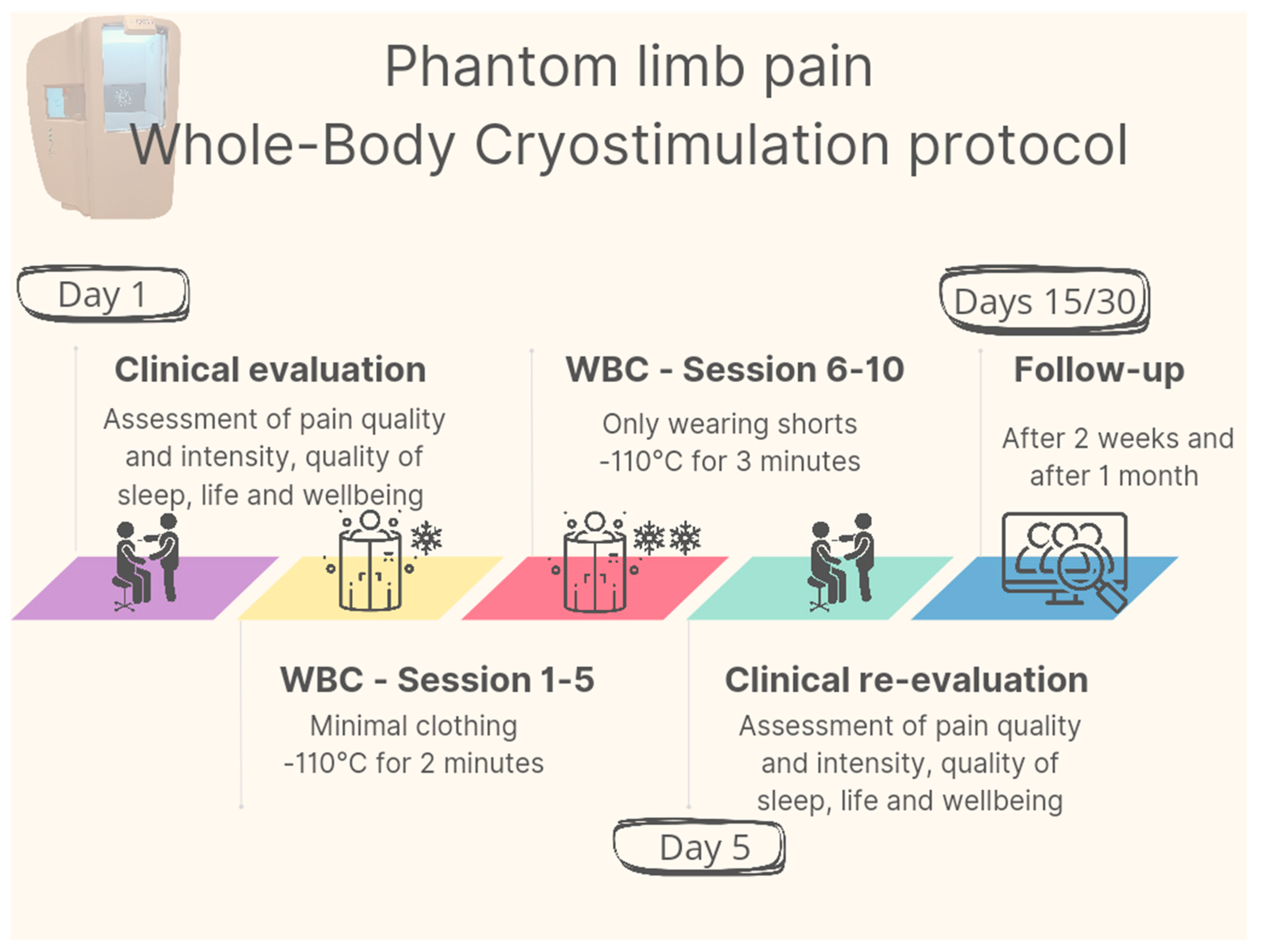
The intervention consisted of a total of 10 outpatient WBC sessions performed twice a day (at 9 am and 12 pm) from 21 August to 25 August 2023. No other physiotherapy treatment other than WBC were administred. After confirming the absence of contraindications to WBC treatment according to Bad Voslau contraindications list [
28], the patient underwent an initial 1-minute familiarization session, during which he entered the cryochamber (Arctic, CryoScience, Rome) wearing minimal clothing. He then underwent five 2-minute WBC sessions at -110°C; starting from the sixth session, the treatment duration was increased to 3 minutes and the patient was asked to remove the T-shirt to increase the body surface area exposed to the cryogenic cold stimulus. Each treatment was monitored by specially trained personnel. A graphical representation of the protocol is provided in
Figure 1.
3. Results
At the time of examination, pain sensation, assessed with the LANSS Pain Scale, scored 18/24, suggesting a probable neuropathic mechanism, as a score >12 indicates the presence of PLP.
The results from all the scales and questionnaires administered are shown in
Table 1 as pre (before WBC), post (after WBC) and range values.
At the end of the WBC cycle and for the following 2 weeks, the patient reported a noticeable reduction in PLP; in particular, pain exacerbations, sensation of electric current and tingling during thunderstorms had completely ceased. The Present Pain Intensity (PPI) score improved from “excruciating” before WBC to “discomforting” after WBC. The patient felt elated with the results achieved. The enthesitic pain completely disappeared, with complete recovery of function of all previously affected joints including in sports activities. He had regained the ability to drive long distances without the pain in the inguinal enthesitis in the healthy limb, and reported feeling generally better, sleeping restful nights, and feeling less anger.
After two weeks, the pain gradually reappeared with the same pattern of onset and duration as before the WBC cycle, but with less intensity.
One month after completion of the WBC cycle, the functional and clinical picture had further changed. The symptoms of widespread enthesitis were gradually returning after sport activity, but with less intensity and without the need to stop training. Improvements in the ability to drive pain-free for long distances remained constant. Stump sensitivity to atmospheric changes returned to previous levels but occurred much less frequently. The patient also reported a reduction in analgesic medications and night-time pain with a significant improvement in sleep quality compared with before WBC.
4. Discussion
This first case-study on the effects of WBC in PLP describes the positive effects on pain, function and quality of life in a Paralympic amputated athlete who had previously undergone various pharmacological, physical therapy and physiotherapy treatments without success. Previous “off label” prescriptions of WBC in specific conditions such as Post Covid Condition [
29,
30], have shown positive effects, likely due to the wide range of effects elicited by WBC on pain, fatigue, and mood [
16]. Given the multiple mechanisms at peripheral and central level implicated in the development of central sensitization characterizing PLP and considering the broad range of possible symptoms, treatment of PLP calls for interventions acting at multiple levels. WBC is a powerful physical modality that generates a thermal shock which represents an intense physical experience for the patient. The cold stress induces a series of physiological responses affecting the circulatory, nervous, endocrine, and immune systems. WBC could thus be described as an "adaptive therapy" since repeated cryogenic stimuli induce a stress that by training the homeostatic systems elicits adaptations that promote the restoration of homeostasis [
31]. Activation of the sympathetic nervous system through cold exposition leads to an increase in the release of noradrenaline, a hormone involved in modulating painful stimuli activating and enhancing descending inhibitory pathways [
14]. Another analgesic action stems from the slowing of nerve conduction velocity in C fibers, responsible for transmitting pain signals. Due to its action on the biological mechanisms involved in pain perception and modulation, anti-inflammatory properties [
32], and positive impact on fatigue, muscular pain, sleep quality, and anxiety-depressive disorders [
33], WBC has already been proposed as an adjuvant therapy in conditions where these functions are altered [
16]. Our hypothesis was that WBC could provide beneficial effects in a condition like PLP characterized by an altered perception of pain resulting from pathological sensitization of the central nervous system. The patient reported rapid benefits not only on PLP and enthesitic pain, but also on sleep, quality of life and well-being, starting from the very first WBC session and persisting for the following 2 weeks. WBC seems to have rapid but short-lasting analgesic effect on PLP, which may in turn suggest that repeated, intermittent WBC sessions when pain relapses could be considered to help modulating pain and following up symptoms. Despite symptoms relapsing after 2 weeks, albeit with lower intensity, the patient reported unprecedented benefits after WBC, particularly regarding PLP and sleep quality, compared to other previously experienced pharmacological and physical treatments. The patient's positive emotional involvement in the treatment and his satisfaction with a short, well-tolerated treatment without adverse effects may have played a role in the functional improvements observed and a placebo effect cannot be ruled out. Controlled studies with larger samples will be needed to confirm these findings and clarify the specific mechanisms of action of WBC in PLP.
5. Conclusions
Our preliminary findings, together with the low incidence of adverse events reported in the literature, suggest that WBC could be studied on a larger scale as a potentially adjuvant treatment to be used alongside existing therapies for pain conditions characterized by central sensitization. As this is the first case study on the effects of WBC in patients with PLP, further research and larger studies are needed to confirm the results obtained.
Author Contributions
Conceptualization, P.P.,I.S. and P.C.; methodology, P.P., I.S. and F.V; validation, P.C, P.P. and J.M.F; formal analysis, A.A and P.P.; investigation ,I.S. and P.P; data curation, P.P, F.V. and I.S.; writing—original draft preparation, P.P. ,I.S. ,A.A. and P.C.; writing—review and editing, P.P., J.M.F and P.C.; visualization, A.A.; supervision, P.C.; funding acquisition, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Italian Ministry of Health - Ricerca Corrente.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Istituto Auxologico Italiano (protocol code 2021_05_18_14, approved 18 May 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Hanyu-Deutmeyer AA, Cascella M, Varacallo M. Phantom Limb Pain. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Oct 19]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK448188/.
- Flor, H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. 2002, 1, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.L.; Russell, H.G.; Schumacher, P.J.; Robinson-Freeman, K.E.; O’Conor, E.C.; Gibney, K.D.; Yambem, O.; Dykes, R.W.; Waters, R.S.; Tsao, J.W. A review of current theories and treatments for phantom limb pain. J. Clin. Invest. 128 2168–2176. [CrossRef] [PubMed]
- Erlenwein, J.; Diers, M.; Ernst, J.; Schulz, F.; Petzke, F. Clinical updates on phantom limb pain. Pain Rep. 2021, 6, e888. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Seifert, D.; Seats, A.; Giacomazzi, S.; Kipp, M.; Orhurhu, V.; Kaye, A.D.; Viswanath, O. Treatment Strategies and Effective Management of Phantom Limb–Associated Pain. Curr. Pain Headache Rep. 2019, 23, 64. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Grossberg, G.T. Phantom limb pain: mechanisms and treatment approaches. Pain Res. Treat. 2011, 2011, 864605. [Google Scholar] [CrossRef] [PubMed]
- Nikolajsen, L.; Jensen, T.S. Phantom limb pain. Br. J. Anaesth. 2001, 87, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Padovani, M.T.; Martins, M.R.I.; Venâncio, A.; Forni, J.E.N. Anxiety, depression and quality of life in individuals with phantom limb pain. Acta Ortop. Bras. 2015, 23, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Baron, R. Mechanisms of disease: neuropathic pain--a clinical perspective. Nat. Clin. Pract. Neurol. 2006, 2, 95–106. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain Off. J. Am. Pain Soc. 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Hall, N.; Eldabe, S. Phantom limb pain: a review of pharmacological management. Br. J. Pain 2018, 12, 202–207. [Google Scholar] [CrossRef]
- Banfi, G.; Lombardi, G.; Colombini, A.; Melegati, G. Whole-body cryotherapy in athletes. Sports Med. Auckl. NZ 2010, 40, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Varallo, G.; Piterà, P.; Fontana, J.M.; Gobbi, M.; Arreghini, M.; Giusti, E.M.; Franceschini, C.; Plazzi, G.; Castelnuovo, G.; Capodaglio, P. Is Whole-Body Cryostimulation an Effective Add-On Treatment in Individuals with Fibromyalgia and Obesity? A Randomized Controlled Clinical Trial. J. Clin. Med. 2022, 11, 4324. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.M.; Bozgeyik, S.; Gobbi, M.; Piterà, P.; Giusti, E.M.; Dugué, B.; Lombardi, G.; Capodaglio, P. Whole-body cryostimulation in obesity. A scoping review. J. Therm. Biol. 2022, 106, 103250. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Kostka, J.; Włodarczyk, T.; Dugué, B. Whole-body cryostimulation (cryotherapy) provides benefits for fatigue and functional status in multiple sclerosis patients. A case-control study. Acta Neurol. Scand. 2016, 134, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, P.; Cremascoli, R.; Piterà, P.; Fontana, J.M. WHOLE-BODY CRYOSTIMULATION: A REHABILITATION BOOSTER. J. Rehabil. Med. Clin. Commun. 2022, 5, 2810. [Google Scholar] [CrossRef] [PubMed]
- Verme, F.; Scarpa, A.; Varallo, G.; Piterà, P.; Capodaglio, P.; Fontana, J.M. Effects of Whole-Body Cryostimulation on Pain Management and Disease Activity in Active Rheumatic Polymyalgia: A Case-Report. Biomedicines 2023, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.M.; Gobbi, M.; Piterà, P.; Giusti, E.M.; Capodaglio, P. Whole-Body Cryostimulation in Fibromyalgia: A Scoping Review. Appl. Sci. 2022, 12, 4794. [Google Scholar] [CrossRef]
- Lombardi, G.; Ziemann, E.; Banfi, G. Whole-Body Cryotherapy in Athletes: From Therapy to Stimulation. An Updated Review of the Literature. Front. Physiol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Louis, J.; Theurot, D.; Filliard, J.-R.; Volondat, M.; Dugué, B.; Dupuy, O. The use of whole-body cryotherapy: time- and dose-response investigation on circulating blood catecholamines and heart rate variability. Eur. J. Appl. Physiol. 2020, 120, 1733–1743. [Google Scholar] [CrossRef]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.B.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef]
- Bennett, M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001, 92, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Turk, D.C.; Revicki, D.A.; Harding, G.; Coyne, K.S.; Peirce-Sandner, S.; Bhagwat, D.; Everton, D.; Burke, L.B.; Cowan, P.; et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 2009, 144, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 2050312116671725. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ciervo, C.A.; Kabat, M. Use of the Beck Anxiety and Depression Inventories for Primary Care with Medical Outpatients. Assessment 1997, 4, 211–219. [Google Scholar] [CrossRef]
- Zimmer Medizin System. Consensus Declaration on Whole-Body Cryotherapy (WBCT). 2006.
- Piterà, P.; Gobbi, M.; Fontana, J.M.; Cattaldo, S.; Massucci, M.; Capodaglio, P. Whole-Body Cryostimulation: A Rehabilitation Booster in Post-COVID Patients? A Case Series. Appl. Sci. 2022, 12, 4830. [Google Scholar] [CrossRef]
- GOBBI, M.; TROTTI, G.; TANZI, M.; KASAP, F.; PITERÀ, P.; CAPODAGLIO, P. POST-COVID SYMPTOMS AND WHOLE-BODY CRYOTHERAPHY: A CASE REPORT. J. Rehabil. Med. - Clin. Commun. 2022, 5, 1000075. [Google Scholar] [CrossRef]
- Alito, A.; Quartarone, A.; Leonardi, G.; Tisano, A.; Bruschetta, A.; Cucinotta, F.; Milardi, D.; Portaro, S. Brown adipose tissue human biomarkers: Which one fits best? A narrative review. Medicine (Baltimore) 2022, 101, e32181. [Google Scholar] [CrossRef]
- Sadura-Sieklucka, T.; Sołtysiuk, B.; Karlicka, A.; Sokołowska, B.; Kontny, E.; Księżopolska-Orłowska, K. Effects of whole body cryotherapy in patients with rheumatoid arthritis considering immune parameters. Reumatologia 2019, 57, 320–325. [Google Scholar] [CrossRef]
- Rymaszewska, J.; Ramsey, D.; Chładzińska-Kiejna, S. Whole-body cryotherapy as adjunct treatment of depressive and anxiety disorders. Arch. Immunol. Ther. Exp. (Warsz.) 2008, 56, 63–68. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).