Submitted:
31 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Section
2.3. Calculation of Carbonation Performance
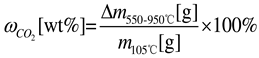
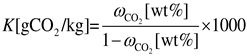
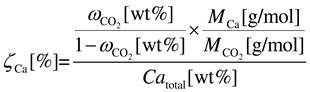
2.4. Sample Characterization
3. Results and Discussion
3.1. Carbonation Performance of Different Steel Slags
3.2. Sample Characterization
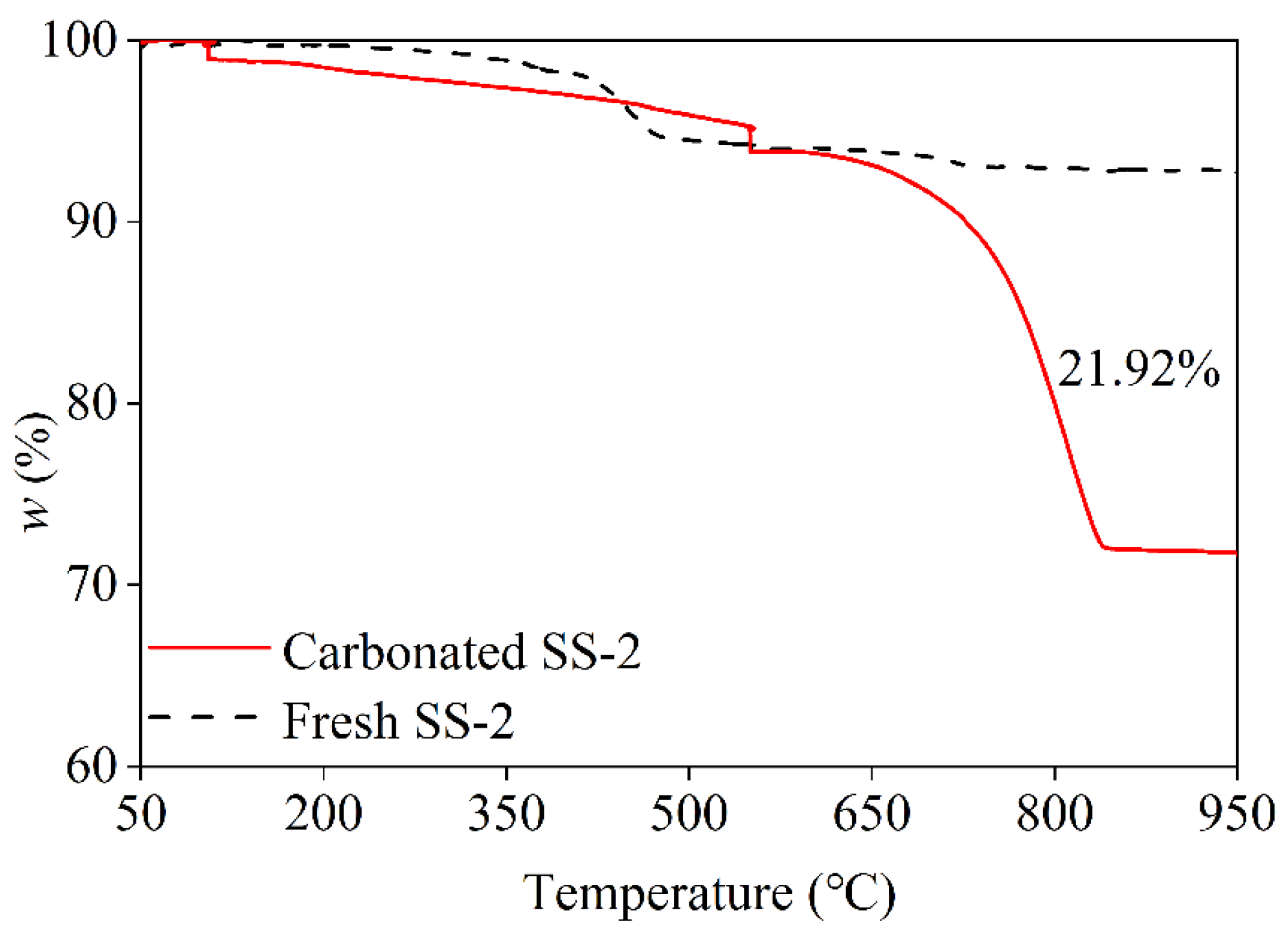
3.2.1. TG Analysis
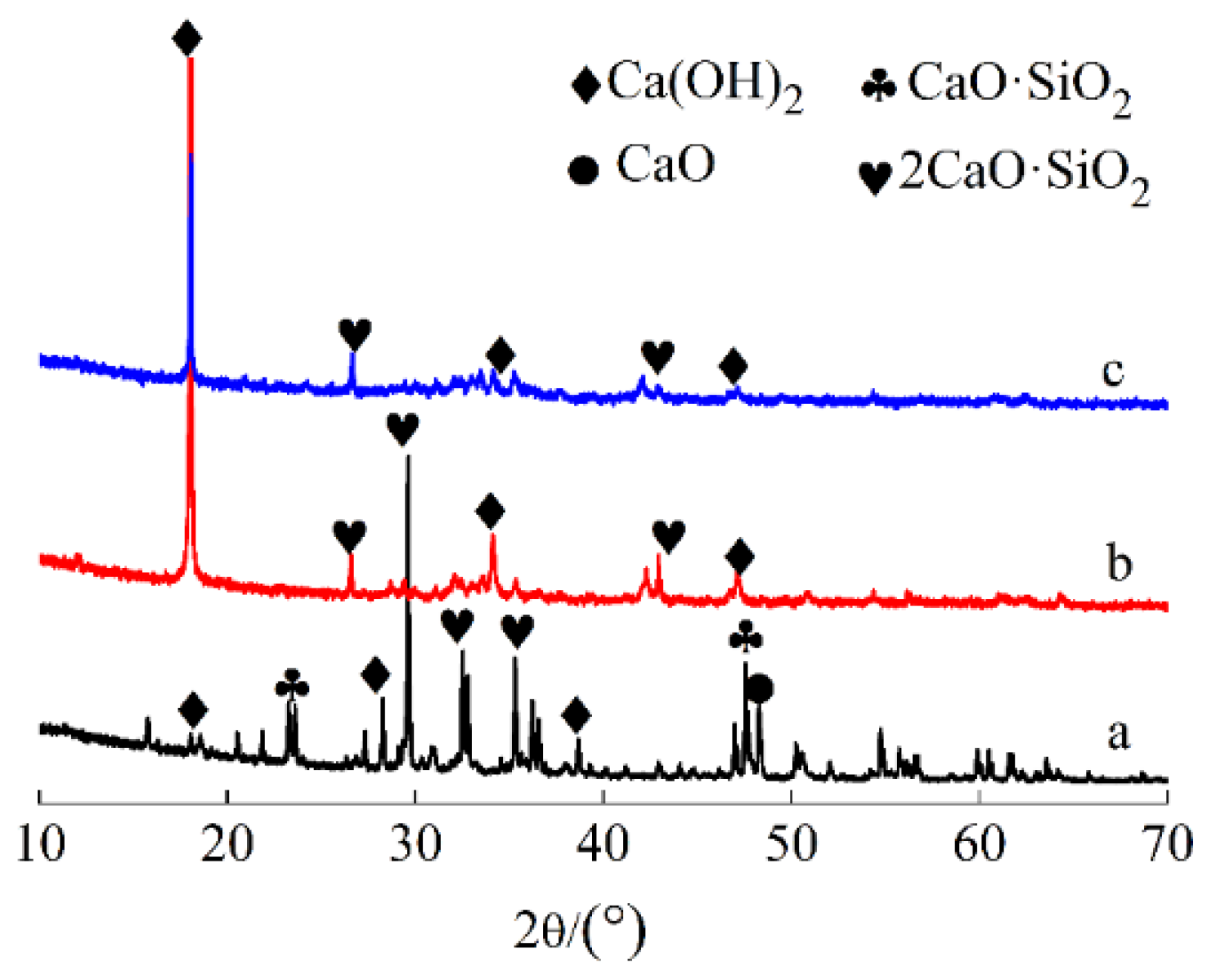
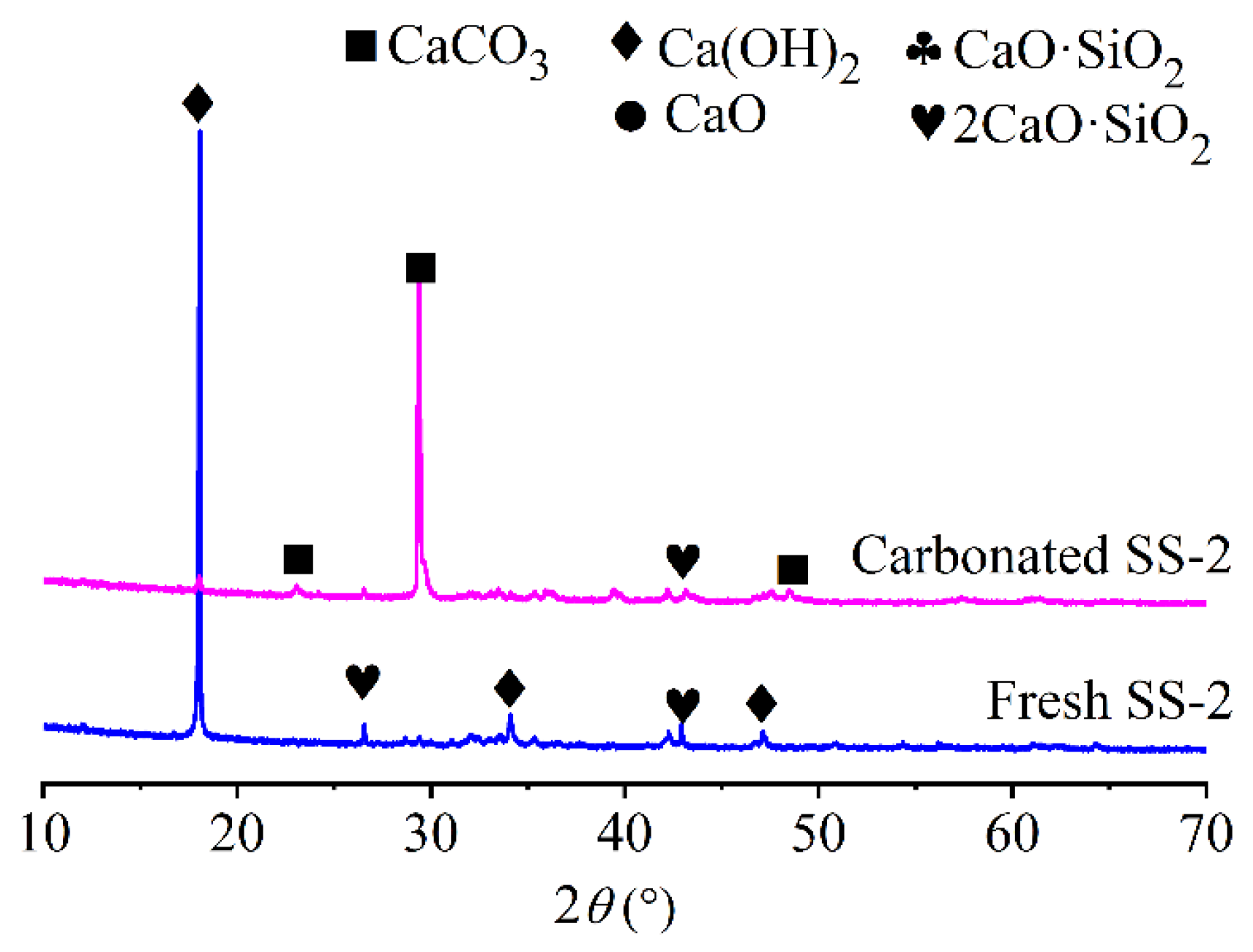
3.2.2. XRD Analysis
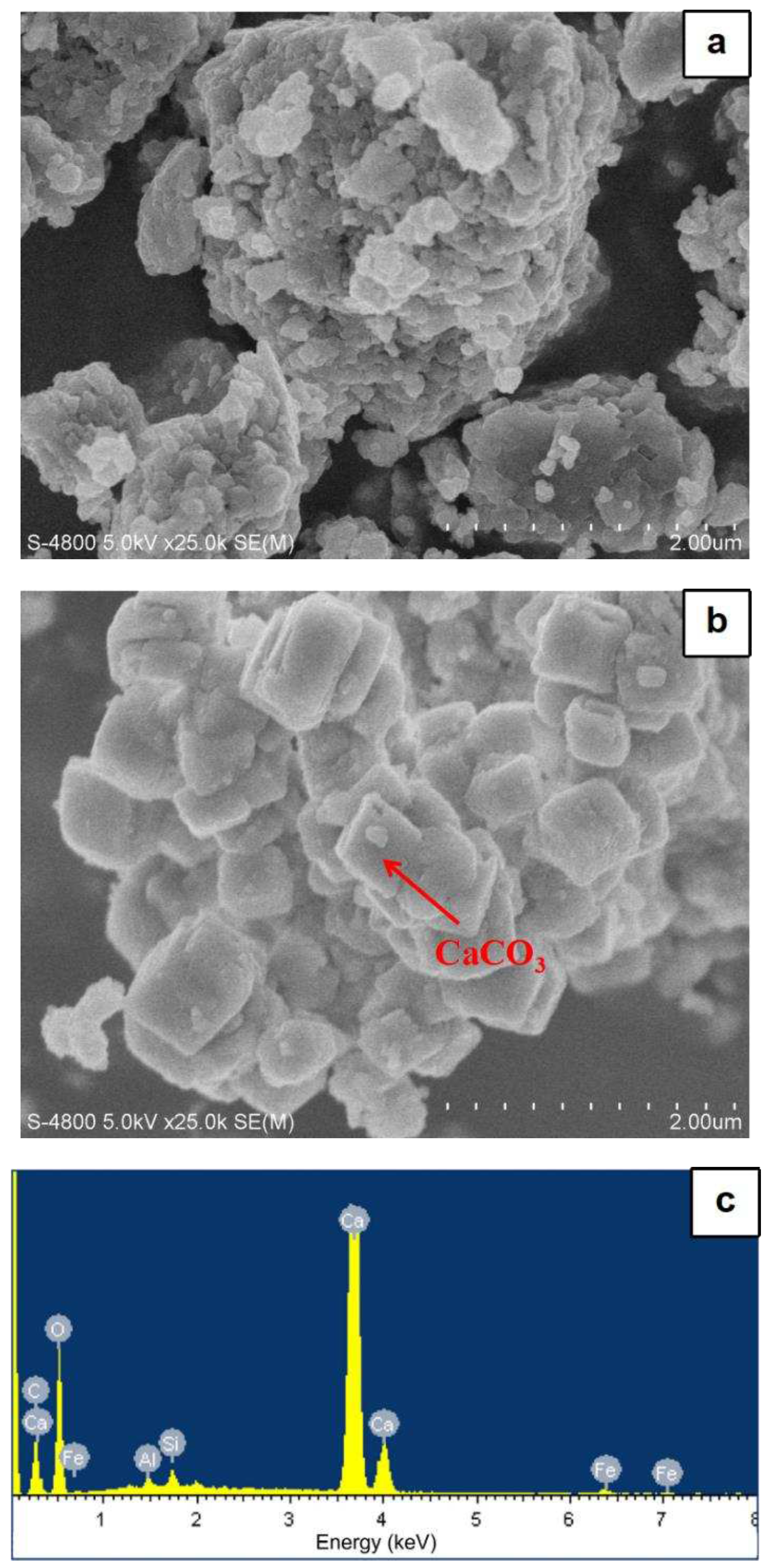
3.2.3. SEM‒EDS analysis
3.2.4. FTIR Spectra
3.3. Effect of Operating Parameters on the Carbonation of Steel Slag
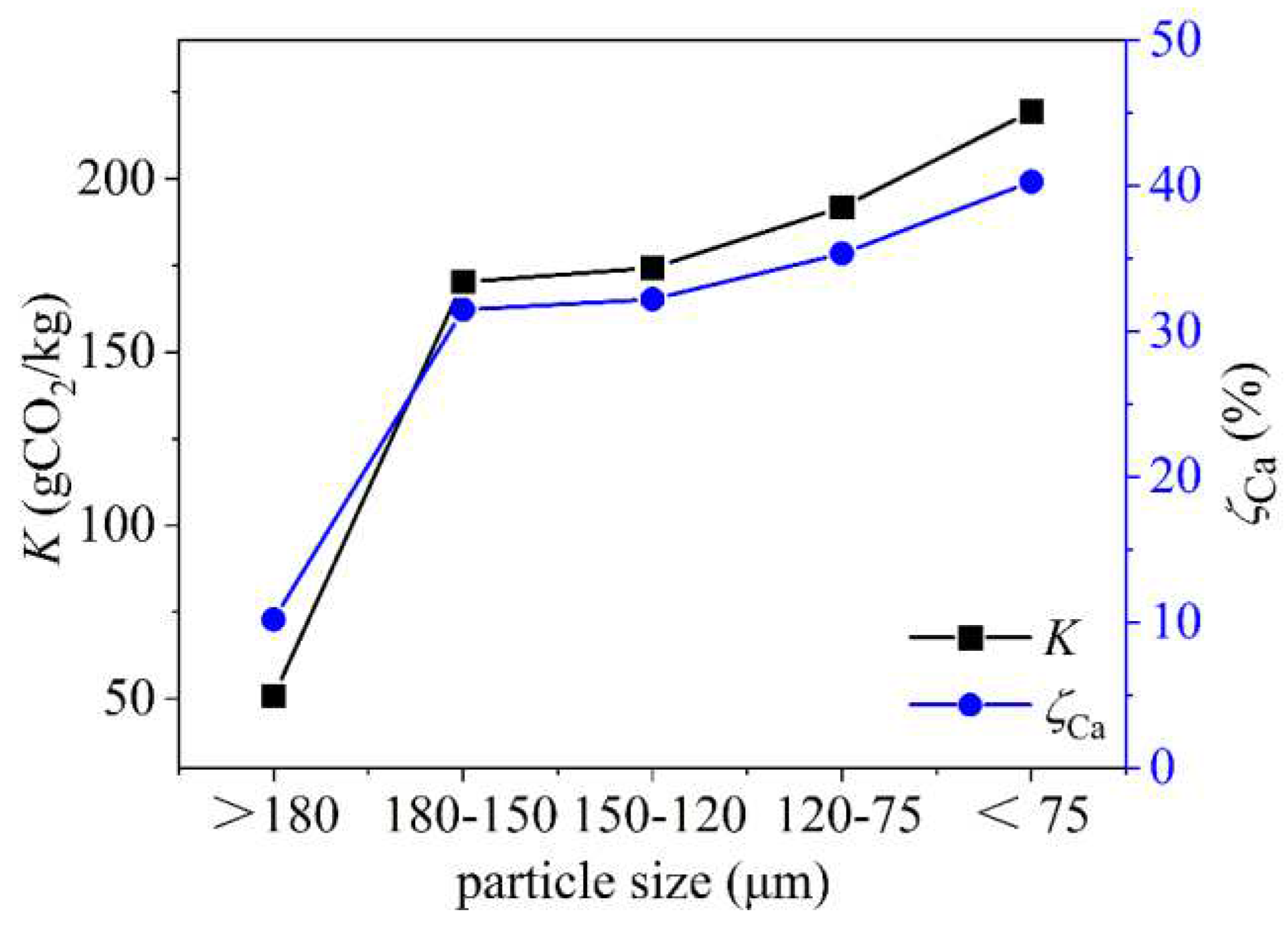
3.3.1. Effect of Particle Size
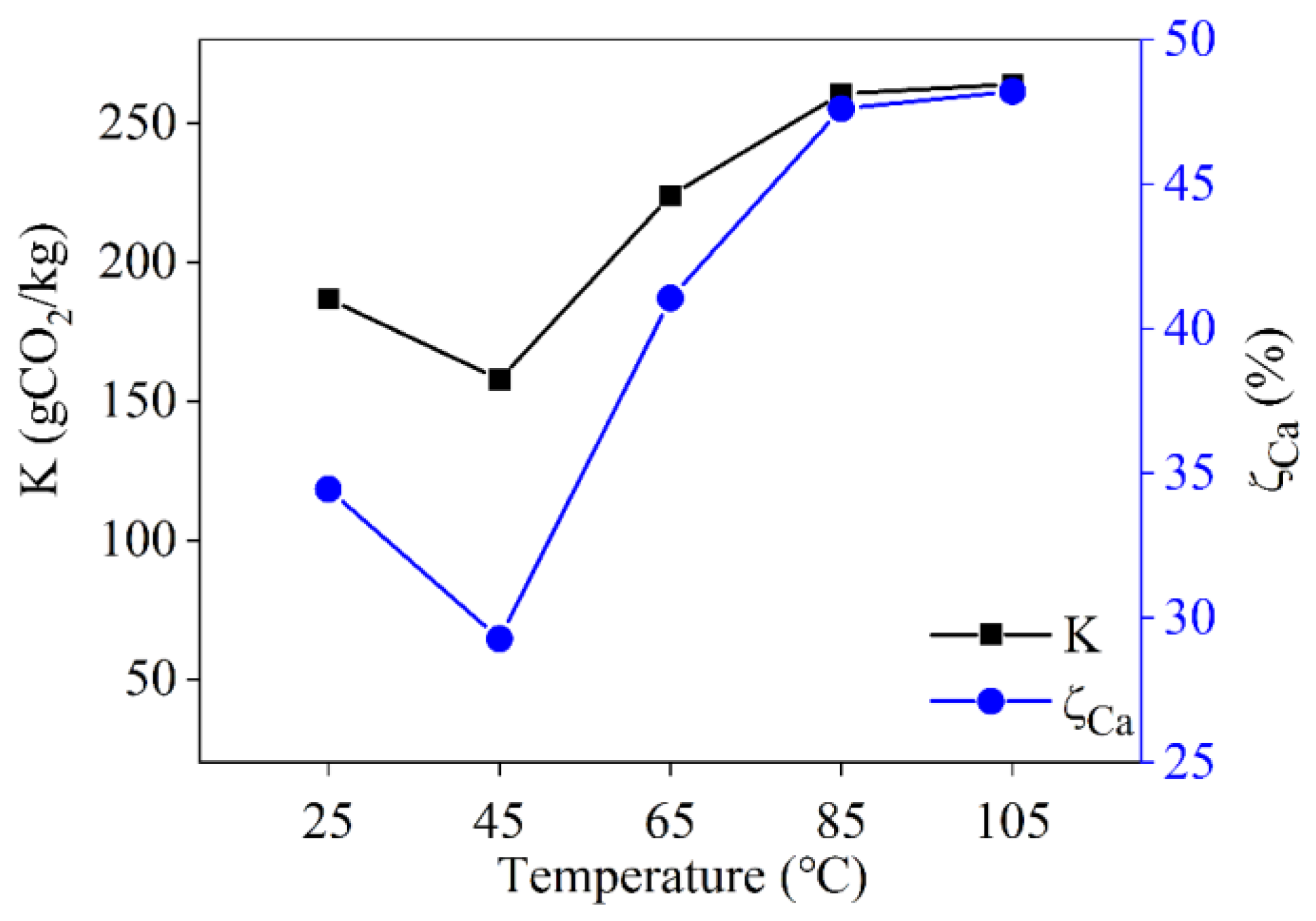
3.3.2. Effect of Reaction Temperature
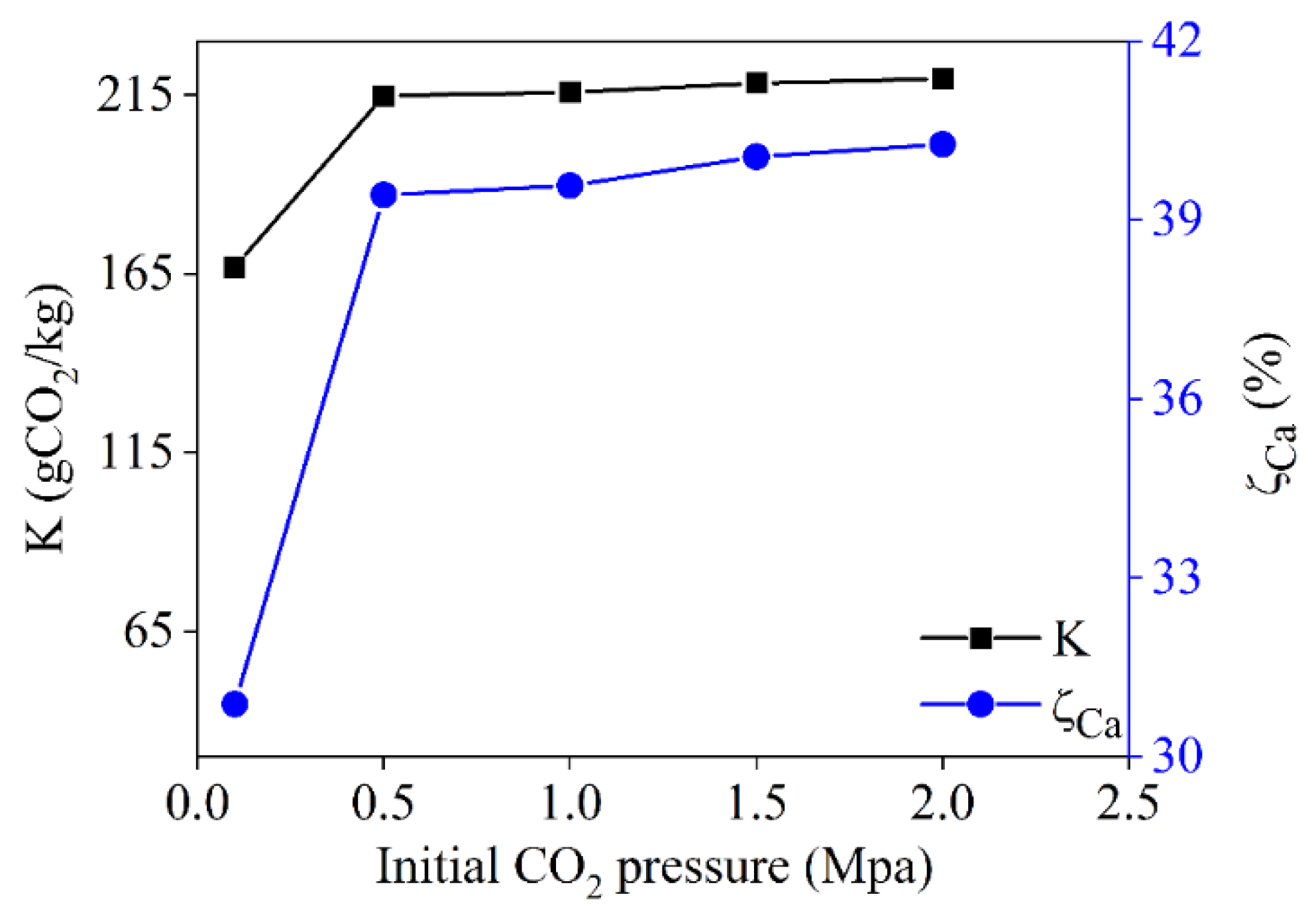
3.3.3. Effect of Initial CO2 Pressure
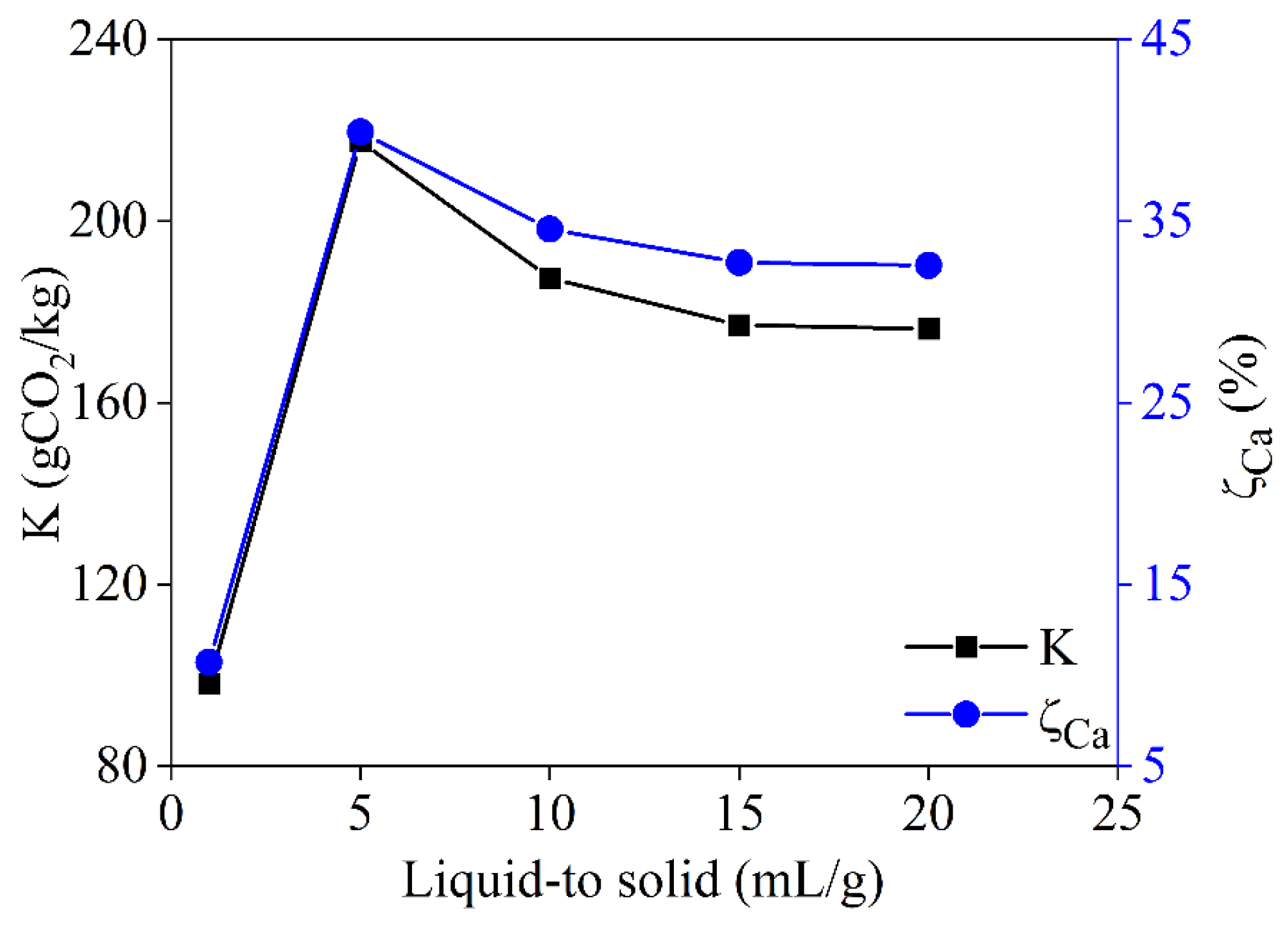
3.3.4. Effect of the Liquid-to-Solid Ratio
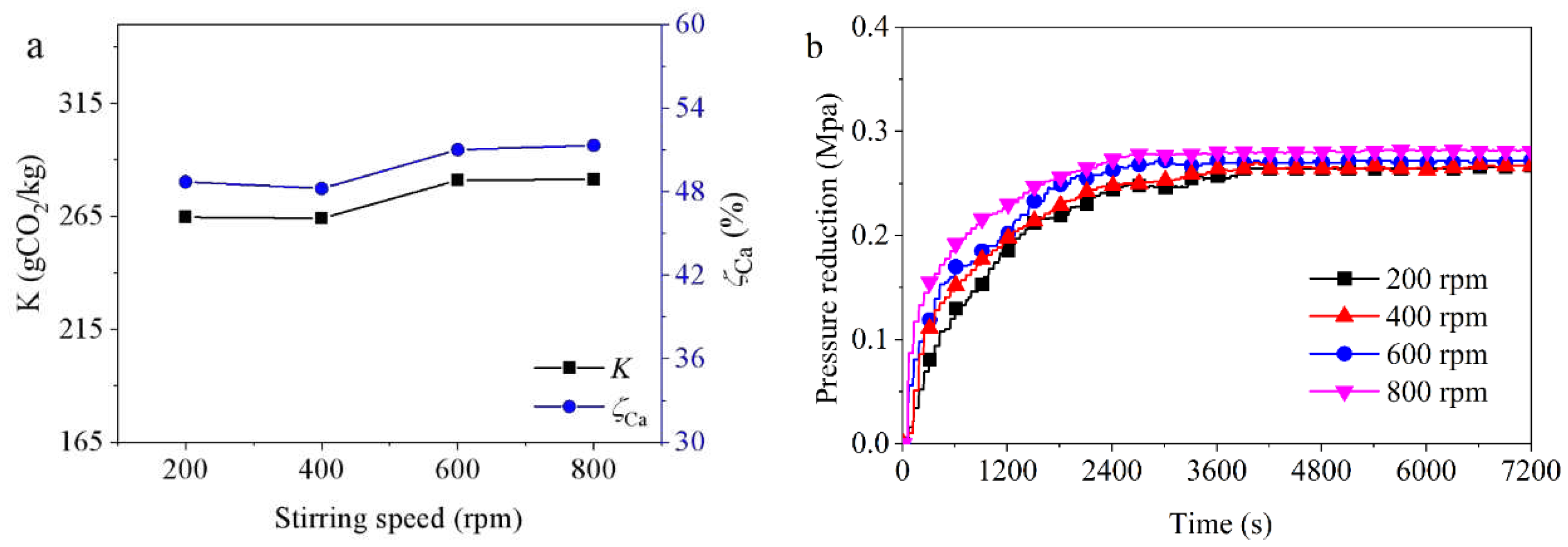
3.3.5. Effect of Stirring Speed
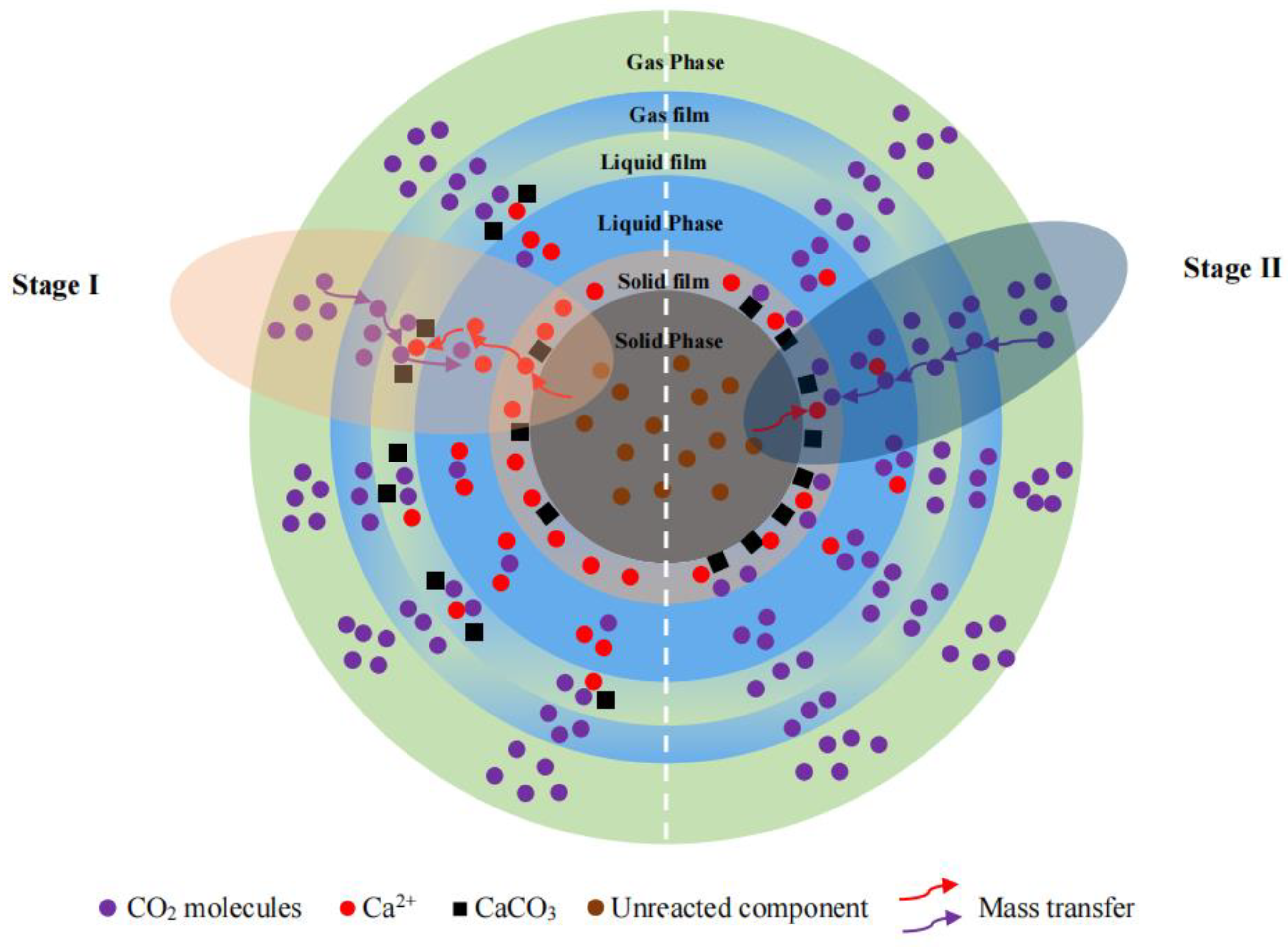
3.4. Mechanism Analysis of Steel Slag for CO2 Sequestration
4. Conclusions
References
- Rahmanihanzaki, M.; Hemmati, A., A review of mineral carbonation by alkaline solidwaste. International Journal of Greenhouse Gas Control 2022, 121, 103798. [CrossRef]
- Kamkeng, A. D. N.; Wang, M.; Hu, J.; Du, W.; Qian, F., Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chemical Engineering Journal 2021, 409, 128138. [CrossRef]
- Pan, S.-Y.; Chen, Y.-H.; Fan, L.-S.; Kim, H.; Gao, X.; Ling, T.-C.; Chiang, P.-C.; Pei, S.-L.; Gu, G., CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction. Nature Sustainability 2020, 3, (5), 399-405. [CrossRef]
- Lei, L.; Bai, L.; Lindbråthen, A.; Pan, F.; Zhang, X.; He, X., Carbon membranes for CO2 removal: Status and perspectives from materials to processes. Chemical Engineering Journal 2020, 401, 126084. [CrossRef]
- Ferrara, G.; Belli, A.; Keulen, A.; Tulliani, J.-M.; Palmero, P., Testing procedures for CO2 uptake assessment of accelerated carbonation products: Experimental application on basic oxygen furnace steel slag samples. Construction and Building Materials 2023, 406, 133384. [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K., Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [CrossRef]
- Liu, J.; Zeng, C.; Li, Z.; Liu, G.; Zhang, W.; Xie, G.; Xing, F., Carbonation of steel slag at low CO2 concentrations: Novel biochar cold-bonded steel slag artificial aggregates. Science of The Total Environment 2023, 902, 166065. [CrossRef]
- Zhang, Y.; Yu, L.; Cui, K.; Wang, H.; Fu, T., Carbon capture and storage technology by steel-making slags: Recent progress and future challenges. Chemical Engineering Journal 2023, 455, 140552. [CrossRef]
- Loria, P.; Bright, M. B. H., Lessons captured from 50 years of CCS projects. The Electricity Journal 2021, 34, (7), 106998. [CrossRef]
- Cheng, C.; Huang, W.; Xu, H.; Liu, Z.; Li, X.; Shi, H.; Yu, Y.; Qu, Z.; Yan, N., CO2 sequestration and CaCO3 recovery with steel slag by a novel two-step leaching and carbonation method. Science of The Total Environment 2023, 891, 164203. [CrossRef]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P., Research progress on CO2 capture and utilization technology. Journal of CO2 Utilization 2022, 66. [CrossRef]
- Santos, R. M.; Ling, D.; Sarvaramini, A.; Guo, M.; Elsen, J.; Larachi, F.; Beaudoin, G.; Blanpain, B.; Van Gerven, T., Stabilization of basic oxygen furnace slag by hot-stage carbonation treatment. Chemical Engineering Journal 2012, 203, 239-250. [CrossRef]
- Ghouleh, Z.; Guthrie, R. I. L.; Shao, Y., High-strength KOBM steel slag binder activated by carbonation. Construction and Building Materials 2015, 99, 175-183. [CrossRef]
- Li, Z.; Chen, J.; Lv, Z.; Tong, Y.; Ran, J.; Qin, C., Evaluation on direct aqueous carbonation of industrial/mining solid wastes for CO2 mineralization. Journal of Industrial and Engineering Chemistry 2023, 122, 359-365. [CrossRef]
- Ibrahim, M. H.; El-Naas, M. H.; Zevenhoven, R.; Al-Sobhi, S. A., Enhanced CO2 capture through reaction with steel-making dust in high salinity water. International Journal of Greenhouse Gas Control 2019, 91, 102819. [CrossRef]
- Chang, E. E.; Pan, S.-Y.; Chen, Y.-H.; Tan, C.-S.; Chiang, P.-C., Accelerated carbonation of steelmaking slags in a high-gravity rotating packed bed. Journal of Hazardous Materials 2012, 227-228, 97-106. [CrossRef]
- He, B.; Zhu, X.; Cang, Z.; Liu, Y.; Lei, Y.; Chen, Z.; Wang, Y.; Zheng, Y.; Cang, D.; Zhang, L., Interpretation and Prediction of the CO2 Sequestration of Steel Slag by Machine Learning. Environmental Science & Technology 2023. [CrossRef]
- Wang, Z.; Cui, L.; Liu, Y.; Hou, J.; Li, H.; Zou, L.; Zhu, F., High-efficiency CO2 sequestration through direct aqueous carbonation of carbide slag: determination of carbonation reaction and optimization of operation parameters. Frontiers of Environmental Science & Engineering 2023, 18, (1), 12. [CrossRef]
- Yadav, S.; Mehra, A., Experimental study of dissolution of minerals and CO2 sequestration in steel slag. Waste Management 2017, 64, 348-357. [CrossRef]
- Pan, S.-Y.; Hung, C.-H.; Chan, Y.-W., Integrated CO2 Fixation, Waste Stabilization, and Product Utilization via High-Gravity Carbonation Process Exemplified by Circular Fluidized Bed Fly Ash. ACS Sustainable Chemistry & Engineering 2016, 4, (6). [CrossRef]
- Huijgen, W. J. J.; Comans, R. N. J., Carbonation of steel slag for CO2 sequestration: leaching of products and reaction mechanisms. Environmental Science & Technology 2006, 40, (8), 2790-2796. [CrossRef]
- Qin, L.; Gao, X., Properties of coal gangue-Portland cement mixture with carbonation. Fuel 2019, 245, 1-12. [CrossRef]
- Yang, C.; Yang, X.; Zhao, T.; Liu, F., An indirect CO2 utilization for the crystallization control of CaCO3 using alkylcarbonate. Journal of CO2 Utilization 2021, 45, 101448. [CrossRef]
- Li, X.; Mehdizadeh, H.; Ling, T.-C., Environmental, economic and engineering performances of aqueous carbonated steel slag powders as alternative material in cement pastes: Influence of particle size. Science of The Total Environment 2023, 903, 166210. [CrossRef]
- Ukwattage, N. L.; Ranjith, P. G.; Yellishetty, M.; Bui, H. H.; Xu, T., A laboratory-scale study of the aqueous mineral carbonation of coal fly ash for CO2 sequestration. Journal of Cleaner Production 2015, 103, 665-674. [CrossRef]
- Omale, S. O.; Choong, T. S. Y.; Abdullah, L. C.; Siajam, S. I.; Yip, M. W., Utilization of Malaysia EAF slags for effective application in direct aqueous sequestration of carbon dioxide under ambient temperature. Heliyon 2019, 5, (10), e02602. [CrossRef]
- Tamilselvi Dananjayan, R. R.; Kandasamy, P.; Andimuthu, R., Direct mineral carbonation of coal fly ash for CO2 sequestration. Journal of Cleaner Production 2016, 112, 4173-4182. [CrossRef]
- Georgakopoulos, E.; Santos, R. M.; Chiang, Y. W.; Manovic, V., Influence of process parameters on carbonation rate and conversion of steelmaking slags – Introduction of the ‘carbonation weathering rate’. Greenhouse Gases: Science and Technology 2016, 6, (4), 470-491. [CrossRef]
- N, H. D.; S, G. J.; Komar, K. S.; C, E. T.; L, S. L., Carbon dioxide sequestration in cement kiln dust through mineral carbonation Environmental science & technology 2009, 43, (6). [CrossRef]
- Polettini, A.; Pomi, R.; Stramazzo, A., CO2 sequestration through aqueous accelerated carbonation of BOF slag: A factorial study of parameters effects. Journal of Environmental Management 2016, 167, 185-195. [CrossRef]
- Wang, C.; Xu, Z.; Lai, C.; Sun, X., Beyond the standard two-film theory: Computational fluid dynamics simulations for carbon dioxide capture in a wetted wall column. Chemical Engineering Science 2018, 184, 103-110. [CrossRef]
- Yang, J.; Liu, S.; Ma, L.; Zhao, S.; Liu, H.; Dai, Q.; Yang, Y.; Xu, C.; Xin, X.; Zhang, X.; Liu, J., Mechanism analysis of carbide slag capture of CO2 via a gas-liquid-solid three-phase fluidization system. Journal of Cleaner Production 2021, 279, 123712. [CrossRef]
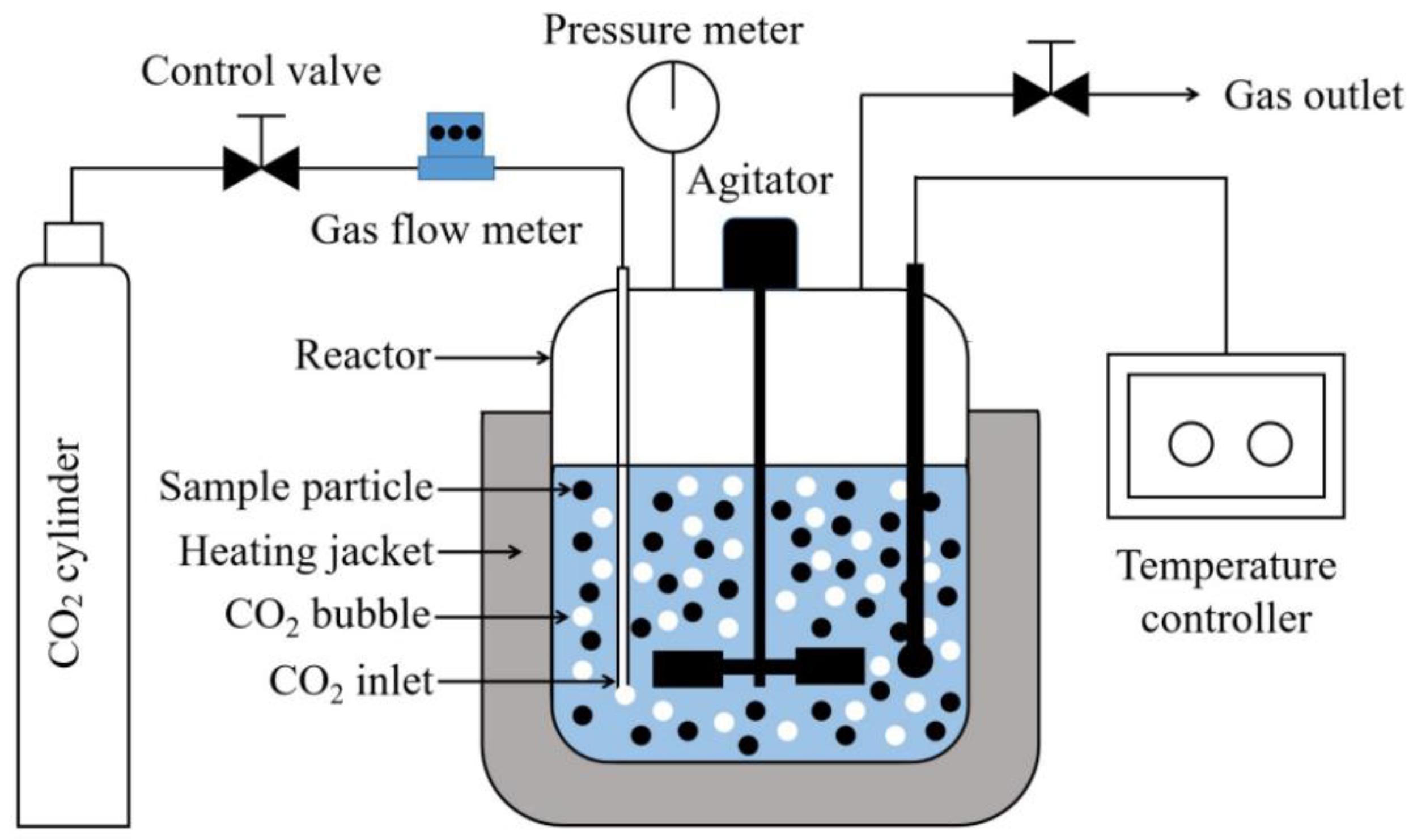

| Sample | wt(%) | |||||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | Na2O | K2O | P2O5 | SO3 | |
| SS-1 | 22.21 | 1.35 | 0.39 | 64.73 | 6.25 | 1.07 | 0.02 | 0.00 | 0.00 | 0.23 |
| SS-2 | 9.47 | 1.98 | 24.44 | 51.79 | 5.95 | 0.71 | 0.08 | 0.05 | 1.45 | 0.41 |
| SS-3 | 16.80 | 4.42 | 24.90 | 39.10 | 4.38 | 1.00 | 0.338 | 0.40 | 1.47 | 1.32 |
| Sample | K (gCO2/kg) |
| SS-1 | 106.8 |
| SS-2 | 191.9 |
| SS-3 | 136.9 |
| Phase | Reaction equation | |
| 65°C | ||
| CaO | CaO+CO2CaCO3 | -124.22 |
| Ca(OH)2 | Ca(OH)2+H2O+CO2→CaCO3+2H2O | -69.19 |
| CaO·SiO2 | CaO·SiO2+H2O+CO2→CaCO3+SiO2·H2O | -38.67 |
| 2CaO·SiO2 | (2CaO·SiO2)+H2O+2CO2→2CaCO3+SiO2·H2O | -57.17 |
| Sample Parameter | unit | >180 | 180~150 | 150~120 | 120~75 | <75 |
| BET surface area | m2/g | 2.0045 | 10.2273 | 10.9660 | 13.9087 | 14.1616 |
| Total pore volume | cm3/g | 0.005434 | 0.021842 | 0.023745 | 0.029356 | 0.037526 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




